Abstract
Background:
Mosquitoes are well known as vectors of several disease causing pathogens. The extensive use of synthetic insecticides in the mosquito control strategies resulted to the development of pesticide resistance and fostered environmental deterioration. Hence in recent years plants become alternative source of mosquito control agents. The present study assessed the larvicidal and oviposition altering activity of six different plants species-Alstonia scholaris, Callistemon viminalis, Hyptis suaveolens, Malvastrum coromandelianum, Prosopis juliflora, Vernonia cinerea against Aedes albopictus mosquito in laboratory.
Methods:
Leaf extracts of all the six plants species in five different solvents of various polarities were used in the range of 20–400ppm for larval bioassay and 50,100 and 200ppm for cage bioassay (for the study of oviposition behavior) against Ae. albopictus. The larval mortality data were recorded after 24 h and subjected to Probit analysis to determine the lethal concentrations (LC50), while OAI (Oviposition activity index) was calculated for oviposition altering activity of the plant extracts.
Results:
Vernonia cinerea extract in acetone and C. viminalis extract in isopropanol were highly effective against Aedes albopictus larvae with LC50 value 64.57, 71.34ppm respectively. Acetone extract of P. juliflora found to be strong oviposition-deterrent which inhibited >2 fold egg laying (OAI-0.466) at 100ppm.
Conclusion:
Vernonia cinerea and C. viminallis leaf extracts have the potential to be used as larvicide and P. juliflora as an oviposition-deterrent for the control of Ae. albopictus mosquito.
Keywords: Larvicide, Oviposition activity, Aedes albopictus, Plant extracts, Vector Management
Introduction
Mankind and mosquitoes must have lived in close association since our ancestors first evolved. The ‘Asian Tiger mosquito’ Ae. albopictus (Skuse) (Deptera: Culicidae) is an important vector of Dengue, Chikungunya and other viruses (Pialoux et al. 2007, Rezza 2012). Approximately 3.5 billion people live in dengue endemic countries located in the tropical and subtropical regions of the world (WHO 2011). The invasion of Aedes mosquito is very close to human habitat and the preferable breeding sites are small water bodies near the vicinity of human, like water tanks, waste pots, tyres etc., therefore a need has arisen to control their reproduction by using such means, and those are not harmful to living environment unlike using synthetic insecticides. The extensive use of synthetic insecticides in the mosquito control strategies resulted to the development of pesticide resistance and fostered environmental deterioration. Hence in recent years plants become alternative source of mosquito control agents. The use of plant products for vector control has several appealing features as they are easily degradable, less hazardous and rich stock house of chemicals of diverse biological activity and economical as well as practical in application. Therefore botanicals have attracted considerable interest in integrated pest management programs in the recent time (Koul and Walia 2009). Plant products especially the secondary metabolites can be used, either as insecticides for killing larvae or adult mosquitoes, as repellents for protection against mosquito bites or as oviposition deterrents, depending on the type of activity they possess (Sukumar et al. 1991). The control of mosquitoes at larval stage is considered as an efficient way in the integrated vector management (Rutledge et al. 2003). Furthermore altering the behavior of mosquitoes for oviposition may also reduce the breeding of mosquitoes. Some plant metabolites may also inhibit the egg laying of female mosquito which can be used as oviposition deterrents in integrated vector management. Many studies have been carried out to investigate the oviposition altering behavior of plants secondary metabolites against mosquitoes (Prajapati et al. 2005, Waliwitya et al. 2009).
The present investigation has been carried out to explore the larvicidal and oviposition activity of six different plants viz. Alstonia scholaris, Callistemon viminalis, Hyptis suaveolens, Malvastrum coromandelianum, Prosopis juliflora, Vernonia cinerea.
Alstonia scholaris (Apocynaceae) is an evergreen tree, native to the Indian subcontinent and Southeast Asian countries. The plant is used in Ayurvedic, Unani and Siddha/Tamil types of alternative medicinal systems (Khare 2007). Callistemon viminalis (Myrtaceae) is an ornamental shrub, widely distributed to Asian countries. It has been reported to possess antimicrobial properties and extensively used for intestinal illness (Delahaye et al. 2009). Jiji et al. (2012) investigated the insecticidal activity of this plant against mosquito larvae. Hyptis suaveolens (Lamiaceae) is an aggressive annual weed, showed effective repellency against adult An. gambiae mosquito (Palsson and Jaenson 1999). Malvastrum coromandelianum (Malvaceae) has been reported for its antimicrobial activity (Islam et al. 2010) and larvicidal property against Cx. quinquefasciatus (Nazar et al. 2009). Prosopis juliflora (Fabaceae) is a drought resistant, wide spread exotic weed in semi arid areas of India and it is well reported for insecticidal activities against mosquitoes (Senthilkumar et al. 2009). Vernonia cinerea (Asteraceae) is an annual weed and is extensively used in indigenous medicine as stomachic and for cold, asthma and bronchitis (Kirtikar and Basu 2000). Further more this plant is also possesses insect antifeedent property (Tandan et al. 1999).
Several studies on the bioactivity of these plants have been documented in literatures, both the larvicidal and oviposition altering activities of these plants against Ae. albopictus mosquito are not yet explored extensively.
Materials and Methods
Plant Material
The green leaves of six plant species Alstonia scholaris, Callistemon viminalis, Hyptis suaveolens, Malvastrum coromandelianum, Prosopis juliflora, Vernonia cinerea, were collected during September-December, 2011 from the Gwalior region (Lat. 26.2215° N, Long. 78.1780° E) Madhya Pradesh, India. Each specimen was identified with the help of expert from School of Studies in Botany, Jiwaji University, Gwalior, India. Plant material dried under shade for 15 days.
Plant extraction
Dried plant leaves were powdered by using an electric blender. Powder was separately subjected to solvent extraction (solvents of different polarity-hexane, methanol, acetone, isopropanol, di-methyl-sulphoxide) with Soxhlet apparatus (Make-Borosil). Totally, 200gm of powdered material in 500ml solvent was extracted for 8h at boiling point range 50–80 °C. The extracts were filtered through a Buchner funnel with Whatman filter paper number 1. Crude extracts were dried using rotary evaporator, stored in glass vials. Stock solution of each plant extracts were prepared (as per WHO 2005 norms with slight modifications) in their respective solvents except of dried hexane extract, which was dissolved in acetone (hexane has its own larval toxicity). Test solutions of five concentrations-20, 50, 100, 200, 400ppm, were prepared by diluting the stock solution with distilled water.
Mosquitoes
The Aedes albopictus mosquitoes were taken from the colony, being maintained in our laboratory (Defence Research and Development Establishment, Gwalior, India). Cyclic generations of vector mosquitoes were reared at 27±2 °C room temperature and 70± 5% relative humidity. Larval stages were maintained in bowls (2.5L) and fed on yeast powder. Adult mosquitoes were reared in wooden cages (30×30×30 inches) and were provided cotton soaked with 10% sugar solution. Females were offered rabbit blood once in a week. Moist filter paper was kept in beaker inside the cage for female mosquito to lay their eggs.
Larval bioassay
Bioassay for the larvicidal activity was carried out as per WHO (2005) procedure. A total of 20 early third instar larvae of Ae. albopictus were introduced in glass beakers (250ml) containing 100ml tap water. One ml of test solutions of different concentrations was introduced in beakers for treatment along with control which was treated with the respective solvents (acetone is used for hexane extract). Four replicates were set up for each test concentration and larval mortality was recorded after 24h. To determine the lethal concentration for each plant species, data were analyzed by Probit analysis (Finney 1971) using POLO PC software. The corrected mortality was estimated by Abbott’s formula (1925) and the LC50 values were calculated.
Oviposition activity
Oviposition altering activity of acetone extract of four easily available plants- C. viminalis, H. suaveolens, P. juliflora and V. cinerea was studied against Ae. albopictus mosquitoes. Three concentrations- 50, 100 and 200 of test solution of each plant extract were prepared in acetone solvent for the treatment. Oviposition behavior of mosquitoes, in four replicates for each concentration was tested using dual choice oviposition bioassay according to Xue et al. (2003) method with few modifications. A total of 20 gravid females were released in a cage of size 60×60×45 cm and two white plastic bowl of 500ml capacity were filled with 100ml distilled water, one as control and another as treatment bowl and the filter paper strip of size 30× 5cm was placed inside each bowl for oviposition. Treatment bowl contained test solution and control bowl was treated with acetone. Thirty minutes after treatment, bowls were placed in cages in diagonal position approx. Fifteen inches apart from each other. Egg laying in treated as well as control bowls were observed after 24h of treatment. Oviposition activity Index (OAI) was estimated using (Karmer and Mulla 1979), OAI= (Nt−Nc)/ (Nt+Nc), where Nt is number of eggs laid in treatment, Nc-No. of eggs laid in control bowls.
Results
Larvicidal Effect
The toxicity of crude extracts of all the six plants were evaluated against third instar larvae of Ae. albopictus and the results of larval susceptibility are presented in Table 1 and Fig. 1a–f. Alstonia scholaris extract showed larval mortality with almost similar LC50 values, ranged from 394.26–473.78ppm for all the solvents. It caused only 35% mortality in hexane extract at highest concentration (Fig. 1a). Callistemon viminalis extract exhibited variations in larval mortality for different solvents. Isopropanol, acetone and methanol extracts were highly effective (LC50 values- 71.34, 110.35, 115.33 respectively) as compare to hexane and DMSO extracts. Larvae of Ae. albopictus were almost equally susceptible towards H. suaveolens and M. coromandelianum with very high range of LC50 in each solvent extract. Methanol extract of P. juliflora was relatively more effective with LC50 value- 80.22ppm as compared to other solvent extracts and it showed 90% larval mortality at highest concentration of 400ppm. Vernonia cinerea was comparatively more effective among the plant used for larval toxicity, as it exhibited maximum mortality (45–90% larval mortality at 200ppm) in all the solvent extracts except DMSO extract (55% mortality at 400ppm). Results revealed that acetone extract of V. cinerea was most effective with LC50 value- 64.57ppm.
Table 1.
LC50 and X2 value of larval bioassay of six plant extracts using different solvents
| Plant Species | Solvent for extraction | LC50 (95% lower and upper limit) | X2 Value |
|---|---|---|---|
| Alstonia scholaris | *Hexane | - | 1.195 |
| Isopropanol | 394.26 (242.21–853.65) | 0.183 | |
| Methanol | 473.19 (339.31–754.13) | 0.785 | |
| Acetone | 459.81 (328.81–732.33) | 0.883 | |
| DMSO | 443.78 (267.28–1113.98) | 0.048 | |
| Callistemon viminalis | Hexane | 308.77 (199.34–619.48) | 0.534 |
| Isopropanol | 71.34 (40.92–111.50) | 0.503 | |
| Methanol | 115.33 (62.24–244.27) | 0.593 | |
| Acetone | 110.35 (76.56–161.71) | 1.094 | |
| *DMSO | - | 0.512 | |
| Hyptis suaveolens | Hexane | 689.69 (402.39–1989.57) | 0.436 |
| Isopropanol | 349.77 (237.62–570.54) | 0.613 | |
| Methanol | 310.47 (208.72–556.74) | 0.435 | |
| Acetone | 258.39 (177.51–422.71) | 0.670 | |
| DMSO | 569.73 (349.95–1543.41) | 1.437 | |
| Malvastrum Coromandelianum | *Hexane | - | 0.030 |
| Isopropanol | 410.22 (279.35–707.06) | 0.372 | |
| Methanol | 337.27 (249.12–478.590) | 1.419 | |
| Acetone | 371.45 (255.76–607.49) | 0.843 | |
| *DMSO | - | 0.302 | |
| Prosopis juliflora | Hexane | 393.07 (253.17–916.88) | 0.014 |
| Isopropanol | 218.15 (132.65–435.48) | 1.340 | |
| Methanol | 80.22 (57.84–114.94) | 0.353 | |
| Acetone | 130.74 (83.33–227.46) | 0.738 | |
| DMSO | 244.67 (139.29–606.93) | 0.880 | |
| Vernonia cinerea | Hexane | 250.36 (154.93–508.61) | 0.320 |
| Isopropanol | 162.10 (114.33– (252.41) | 0.192 | |
| Methanol | 198.27 (141.83–286.98) | 2.071 | |
| Acetone | 64.57 (45.68–90.04) | 0.549 | |
| * DMSO | - | 0.667 |
LC50 could not accessible due to less mortality
Fig. 1a.
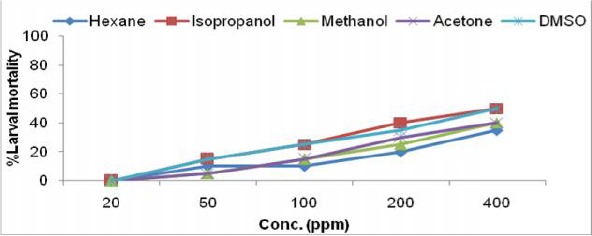
Larvicidal activity of Alstonia scholaris leaf extract in different solvents against IIIrd instar larvae of Ae. albopictus.
Fig. 1f.
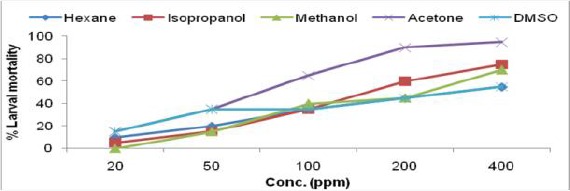
Larvicidal activity of Vernonia cinerea leaf extract in different solvents against IIIrd instar larvae of Ae. albopictus
Fig. 1b.
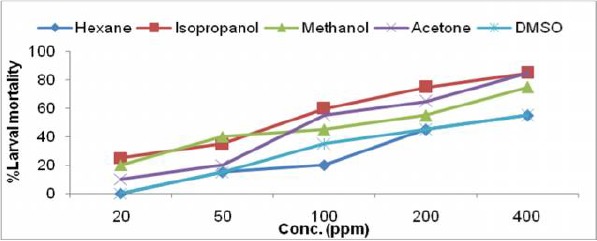
Larvicidal activity of Callistemon viminalis leaf extract in different solvents against IIIrd instar larvae of Ae. albopictus
Fig. 1c.
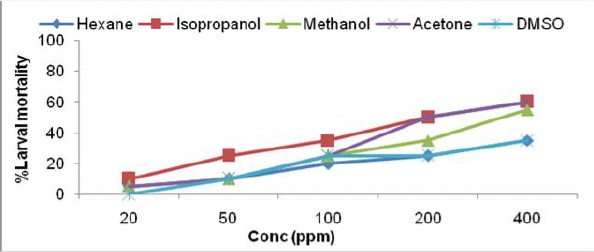
Larvicidal activity of Hyptis suaveolens leaf extract in different solvents against IIIrd instar larvae ofAe. albopictus
Fig. 1d.
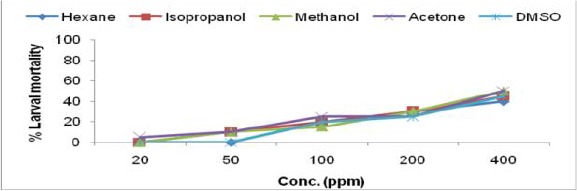
Larvicidal activity of Malvastrum coromandelianum leaf extract in different solvents against IIIrd instar larvae of Ae. albopictus
Fig. 1e.
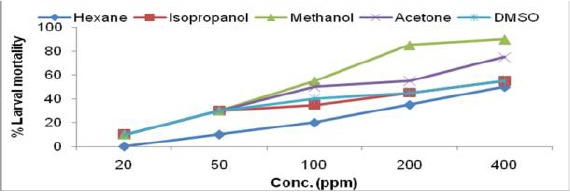
Larvicidal activity of Prosopis juliflora leaf extract in different solvents against IIIrd instar larvae of Ae. albopictus
According to Fig. 2 among all the solvents used for extraction, acetone and methanol extracts were most effective as compared to the other solvent extracts. Isopropanol extract exhibited moderate larval mortality followed by Hexane and DMSO extract of all the plants tested.
Fig. 2.
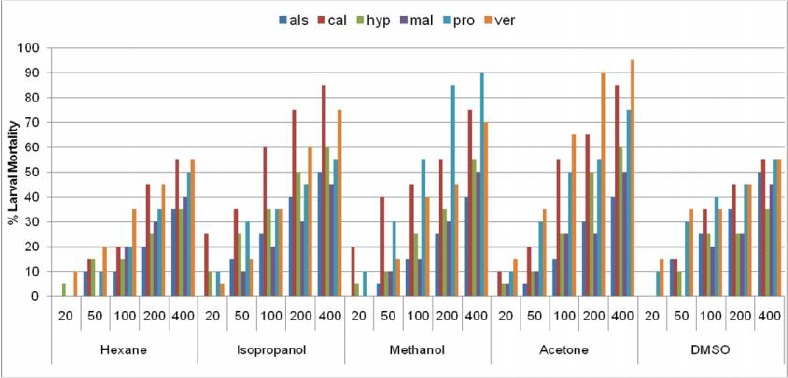
Comparative Larvicidal activity of solvent extracts (als- A. scholaris, cal-C. viminalis, hyp-H. suaveolens, mal-M. coromandalianum, pro-P juliflora, ver-V. cinerea) at different concentration against Ae. albopictus larvae.
Oviposition activity
Four plant species were selected for the evaluation of oviposition activity. Callistemon viminalis extract showed attractancy towards the gravid females with maximum egg laying in treated container as compared to untreated (Table 2). In case of H. suaveolens and V. cinerea, egg laying was not significantly different in treated and untreated containers, however P. juliflora was found to be effective in preventing egg laying at 50 and 100ppm concentration with almost equal OAI value −0.460 and −0.466 (Table 2 and Fig. 3).
Table 2.
Oviposition activity of acetone extract of selected plants against Aedes albopictus females
| Plant Species | Conc. (ppm) | Mean No. of eggs laid (±SE) | OAI (Oviposition activity Index) | |
|---|---|---|---|---|
| (Treatment) | (Control) | |||
| Callistemon viminalis | 50 | 94.00(17.66) | 39.66 (10.47) | 0.406 |
| 100 | 133.75 (11.79) | 86.66 (8.81) | 0.213 | |
| 200 | 125.25 (25.5) | 100.00 (23.62) | 0.112 | |
| Hyptis suaveolens | 50 | 101.25 (18.3) | 131.00 (16.09) | −0.128 |
| 100 | 77.00 (12.70) | 121.00 (14.97) | −0.22 | |
| 200 | 115.75 (30.17) | 97.33 (4.631) | −0.086 | |
| Prosopis juliflora | 50 | 36.00 (10.49) | 98.33 (27.28) | −0.46 |
| 100 | 55.25 (17.221) | 152.00 (35.15) | −0.466 | |
| 200 | 87.00 (38.31) | 133.00 (11.01) | −0.209 | |
| Vernonia cinerea | 50 | 54.25 (15.63) | 75.00 (15.69) | 0.160 |
| 100 | 101.50 (16.17) | 91.00 (36.66) | 0.054 | |
| 200 | 102.25 (37.65) | 95.66 (32.06) | 0.332 | |
Fig. 3.
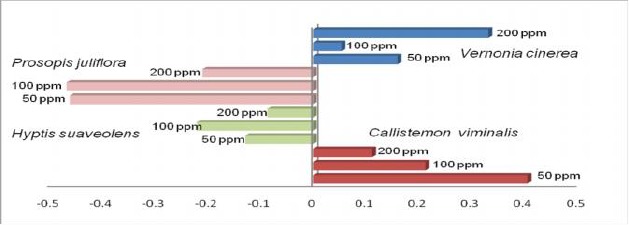
Oviposition activity index (OAI) values for selected plant extracts at different concentration.
Discussion
The present study focused on the larvicidal as well as the oviposition altering activity of crude plants extracts. Among all the plants V. cinerea was comparatively more effective than other plants against the larvae of Ae. albopictus. In a study Arivoli et al. (2011) had reported the larvicidal property of this plant in ethyl acetate extract against Cx. quinquefasciatus larvae with an LC50 value of 1.63 g/L. Vernonia cinerea is a member of Asteraceae family which possesses various types of phytochemical compounds (flavonoids, sesquiterpines, thiophene derivatives) those have been found to be toxic to insects including mosquito larvae (Ribeiro et al. 1994).
Significant results in larval mortality were also observed for C. viminalis. In the preliminary study Jiji et al. (2012) reported the larvicidal efficacy of aqueous extract of this plant against Culex quinquefasciatus. Khambay et al. (1999) isolated two compounds viminadione A and viminadione B from hexane extract of C. viminalis and viminadione A found to be effective against house flies, aphids and thrips.
The present study revealed that the methanolic leaf extract of P. juliflora was effective against larvae of Aedes mosquito. Senthilkumar et al. (2009) also evaluated the larvicidal and adulticidal efficacy of 10% leaves extracts of P. juliflora and showed that it was quite effective (LC50- 9.3 mg/L) against larvae of An. stephensi. Recently Bansal et al. (2012) studied the methanol extract of P. juliflora and reported LC50 value 128 ppm against Ae. aegypti larvae.
Hyptis suaveolens is well known for its repellent property against mosquitoes. Palsson and Jaenson (1999) observed that H. suaveolens provided approximately 84% protection for 2 hrs against An. gambaie. Although it is good repellent but not showed significant larvicidal activity. Our findings agree with the results of Cavalcanti et al. (2004) who showed LC50 value 261ppm, while contradicts with Amusan et al. (2005), who revealed high mortality rate (80%) in ethanol extract of H. suaveolens at conc. 0.9ppm against Ae. aegypti larvae.
Moderate larval mortality was observed in M. coromandelianum and A. scholaris plant extracts. Nazar et al. (2009) has reported similar mortality rate in methanolic extract of M. coromandelianum against Cx. quinquefasciatus larvae. Kaushik and Saini (2009) while screening some plants for larvicidal activity against Ae. aegypti, found that acetone extract of A. scholaris showed its efficacy with the LC50 value 239.9ppm.
In the present investigation acetone extract of V. cinerea, isopropanol extract of C. viminalis and methanol extract of P. juiflora showed maximum efficacy, while DMSO and hexane extracts of all the plant species did not cause significant toxic effect on larvae. The dose dependent response in larval mortality was noticed in the present study. Larvicidal activities of crude plant extracts varied according to the plant species and solvents used. Crude extract in these solvents might have some complex mixture of biocidal active compounds, including phenolics, terpenoides, flavonoids and alkaloids which may jointly or independently contribute to produce mortality of larvae. Phytochemicals limonin and nomilin from some citrus plants were reported to have larvicidal activity against mosquitoes (Bilal et al. 2012). Acetone extract contains maximum quantity of phenols and flavonols, while methanol extract contains flavones, terpenoids, tannins and polyphenols (Tiwari et al. 2011) and these phytochemicals are reported for their insecticidal activity. The higher activity of the methanol and isopropanol extracts as compared to the hexane and DMSO extract can be attributed to the presence of higher amounts of polyphenols.
Different solvent types can significantly affect the potency of extracted plant compounds (Karmegam et al. 1997) because of the polarity range. Roots extract of Derris elliptica in different solvent was evaluated for its larvicidal activity against Ae. aegypti, indicating that among the crude residues acetone extract was the most effective (Ameen et al. 1985).
The mode of action and site of effect for larvicidal phytochemicals has received little attention. Ray et al. (1999) and David et al. (2000) found that botanical derivatives primarily affect the midgut epithelium and secondarily affect the gastric caeca and the malpighian tubules in mosquito larvae.
Phytochemicals can also influence the ovipositional behavior of mosquitoes. Present study reported that among four plants tested for oviposition behavior, P. juliflora exhibited significant results and suggested the repellency towards gravid females with 3 fold egg laying in untreated bawls at 100ppm. H. suaveolens and V. cinerea did not show any significant difference in oviposition whereas C. viminalis showed slightly attractancy at 50ppm with almost 2 fold egg laying in treated bowls. This suggests that these extracts produce some volatile compounds, which may act as repellents or attractants at specific concentrations.
Aedes is a container breeder mosquito, follows visual and olfactory cues to find appropriate oviposition sites and then both physical and chemical factors of water, to assess the suitability of potential larval habitats in order to maximize the fitness of offspring (Sumba et al. 2004). Rajkumar and Jebanesan (2005) found that 0.01, 0.025, 0.05, 0.075 and 0.1 % ethanol leaf extract of Solanum trilobatum reduced egg laying by An. stephensi, from 18–99% in treated containers. The oviposition deterrent activity of four essential oils derived from chamomile (Matricaria recutita), sesame (Sesamum indicum), jojoba (Simmondsia chinensis) and ginger (Zingiber officinalis), were evaluated against the gravid females of the filarial vector Culex pipiens under laboratory conditions at three doses (0.5, 5 and 50ppm) exhibited potent deterrent activity against gravid female mosquitoes with various degrees of repellency (ranging from 48.73–100%) (El-Gendy and Shaalan 2012). Tawatsin et al. (2006) studied 18 essential oils and reported their oviposition deterrent activity against Ae. aegypti ranging from 16.6 to 94.7%.
Many plants from different families possess promising phytochemicals for mosquito control which are much economical and environmental friendly (Sivagnaname and Kalyanasundaram 2004, Bakar et al. 2012). These phytochemicals like phenolics, alkaloids and terpenoids exist in plants extracts may jointly or independently contribute to the mosquitoes as larvicidal/oviposition deterrents/ repellents.
Further more use of plant products in vector control is target specific, efficacious, safe and appropriate for use at the community level, widely available at no or limited cost and withstand the risk of vector developing resistance against them. In the present study potentially effective plants included some weeds, this may also contribute to assess the possibility of using large biomass of weeds available in the wastelands of northern India as potential insecticides in spite of using other medicinal or cultivable plants. For improving the potency and stability of the plant products, in depth investigation on the active fraction or compound of these plant species are necessary to be further elucidated.
Conclusion
Our results revealed that crude extract of V. cinerea and C. viminalis were significantly effective as larvicide and P. juliflora as oviposition deterrent among the tested plants. These plants can be used as natural insecticides in integrated vector control programs.
Acknowledgements
The authors are thankful to Prof (Dr) MP Kaushik Director Defence Research and Development Establishment (DRDE), Gwalior, for providing necessary research facilities and support in the study. The authors declare that there is no conflict of interest.
References
- 1. Abbott SW. ( 1925) A method of computing the effectiveness of an insecticide. J Econ Entomol. 18: 265– 267. [Google Scholar]
- 2. Amusan AAS, Idowu AB, Arowolo FS. ( 2005) Comparative toxicity of bush tea leaves ( Hyptis suaveolens) and orange peel ( Citrus sinensis) oil extract on larvae of the yellow fever mosquito Aedes aegypti. Tanzan Health Res Bull. 7( 3): 174– 178. [PubMed] [Google Scholar]
- 3. Ameen M-ul, Shahjahan RM, Khan HR, Chowdhury AKA. ( 1985) Larvicidal effect of indigenous Derris elliptica root on Aedes aegypti (Diptera: Culicidae). Int J Entomol. 1: 39– 43. [Google Scholar]
- 4. Arivoli S, Tennyson S, Martin JJ. ( 2011) Larvicidal efficacy of Vernonia cinerea (L.) (Asteraceae) leaf extracts against the filarial vector Culex quinquefasciatus Say (Diptera: Culicidae). J Biopest. 4: 37– 42. [Google Scholar]
- 5. Bakar AA, Sulaiman S, Omar B, Ali RM. ( 2012) Evaluation of Melaleuca cajuputi (Family: Myrtaceae) Essential Oil in Aerosol Spray Cans against Dengue Vectors in Low Cost Housing Flats. J Arthropod-Borne Dis. 6( 1): 28– 35. [PMC free article] [PubMed] [Google Scholar]
- 6. Bansal SK, Karam SV, Sharma S, Sherwani MRK. ( 2012) Laboratory observations on the larvicidal efficacy of three plant species against mosquito vectors of malaria, Dengue/Dengue Hemorrhagic Fever (DF/DHF) and lymphatic filariasis in the semi-arid desert. J Environ Biol. 33( 3): 617– 621. [PubMed] [Google Scholar]
- 7. Bilal H, Akram W, Ali-Hassan S. ( 2012) Larvicidal Activity of Citrus Limonoids against Aedes albopictus Larvae. J Arthropod-Borne Dis. 6( 2): 104– 111. [PMC free article] [PubMed] [Google Scholar]
- 8. Cavalcanti ESB, Maia de Moraisde S, Ashley ALM, Santana EWP. ( 2004) Larvicidal activity of essential oils from Brazilian plants against Aedes aegypti L. Memó Insti Oswaldo Cruz. 99( 5): 541– 544. [DOI] [PubMed] [Google Scholar]
- 9. David JP, Rey D, Pauntou MP, Meyran JC. ( 2000) Differential toxicity of leaf litter to dipteran larvae of mosquito developmental sites. J Invertebr Pathol. 75( 1): 9– 18. [DOI] [PubMed] [Google Scholar]
- 10. Delahaye C, Rainford L, Nicholson A, Mitchell S, Lindo J, Ahmad M. ( 2009) Antibacterial and antifungal analysis of crude extracts from the leaves of Callistemon viminalis. J Med Bio Sci. 3( 1): 190. [Google Scholar]
- 11. El-Gendy NA, Shaalan EA. ( 2012) Oviposition deterrent activity of some volatile oils against the filarial mosquito vector Culex pipiens. J Entomol . 9: 435– 441. [Google Scholar]
- 12. Finney DJ. ( 1971) Probit analysis. III Edition Cambridge University Press, London, pp. 20– 42. [Google Scholar]
- 13. Islam M, Ali E, Saeed MA, Jamshaid M, Khan MTJ. ( 2010) Antimicrobial and irritant activities of the extracts of Malva parviflora L., Malvastrum coromandelianum L. and Amaranthus viridis L.-A preliminary investigation. Pak J Pharmacy. 20: 3– 6. [Google Scholar]
- 14. Jiji T, Shonima GM, Pratheesh PT, Muraleedhara KG. ( 2012) Larvicidal activity of indigenous plants against Culex quinquefasciatus. J Environ Res and Dev. 7( 1A): 354– 360. [Google Scholar]
- 15. Karmegan N, Sakthivadivel M, Anuradha V, Daniel T. ( 1997) Indigenous plant extracts as larvicidal agents against Culex quinquefasciatus Say. Biores Technol. 5: 137– 140. [Google Scholar]
- 16. Karmer WL, Mulla MS. ( 1979) Oviposition attractant and repellents of mosquitoes: Oviposition response of Culex mosquitoes to organic infusions. Environ Entomol. 8: 1111– 1117. [Google Scholar]
- 17. Kaushik R, Saini P. ( 2009) Screening of some semi-arid region plants for larvicidal activity against Aedes aegypti mosquitoes. J Vector Borne Dis. 46: 244– 246. [PubMed] [Google Scholar]
- 18. Khambay BPS, Beddie DG, Hooper AM, Simmonds MSJ, Green PWC. ( 1999) New Insecticidal Tetradecahydroxanthenediones from Callistemon viminalis. J Nat Prod. 62( 12): 1666– 1667. [Google Scholar]
- 19. Khare CP. ( 2007) Indian Medicinal Plants: An Illustrated Dictionary. Springer-Verlag BerlinHeidelberg; New York. [Google Scholar]
- 20. Kirtikar KR, Basu BD. ( 2000) Indian Medicinal Plants. Vol 6 Sri Satguru Publication, New Delhi, pp. 1828– 1831. [Google Scholar]
- 21. Koul O, Walia S. ( 2009) Comparing impacts of plant extracts and pure allelochemicals andimplications for pest control. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources, 4, 049, Available from: http://www.cabi.org/cabreviews.
- 22. Nazar S, Ravikumar S, Williams GP, Ali MS, Suganthi P. ( 2009) Screening of Indian coastal plant extracts for larvicidal activity of Culex quinquefasciatus. Ind J Sc and Technol. 3: 24– 27. [Google Scholar]
- 23. Palsson K, Jaenson GT. ( 1999) Plant products used as mosquito repellents in Guinea Bissau, West Africa. Acta Topica. 72( 1): 39– 52. [DOI] [PubMed] [Google Scholar]
- 24. Pialoux G, Gauzere BA, Jaurequiberry S, Strobel M. ( 2007) Chikungunya, an epidemic arbovirosis. Lancet Infec Dis. 7: 319– 327. [DOI] [PubMed] [Google Scholar]
- 25. Prajapati V, Tripathi AK, Aggarwal KK, Khanuja SPS. ( 2005) Insecticidal, repellent and oviposition deterrent activity of selected essential oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Bioresour Technol. 96: 1749– 1757. [DOI] [PubMed] [Google Scholar]
- 26. Rajkumar S, Jebanesan A. ( 2005) Oviposition deterrent and skin repellent activities of Solanum trilobatum leaf extract against the malarial vector Anopheles stephensi. J Insect Sc. 5: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rey D, Cuany A, Pautou MP, Meyran JC. ( 1999) Differential sensitivity of mosquito texa to vegetable tannins. J Chem Ecol. 25( 3): 537– 548. [Google Scholar]
- 28. Rezza G. ( 2012) Aedes albopictus and the reemergence of Dengue. BMC Public Health, 12, 72, Available at: http://www.biomedcentral.com/1471-2458/12/72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ribeiro A, Santos LMST, Romanaha AJ, Veloso DP, Zani CL. ( 1994) Flavonoids from Trixis vauthieri (Asteraceae) extract active in vitro against trypomastigote forms of Tripanosoma cruzi. Memó Inst Oswaldo Cruz. 89: 188. [Google Scholar]
- 30. Rutledge CR, Clark F, Curtis A, Sackett S. ( 2003) Larval mosquito control. Technical Bull Florida Mosq Cont Assoc. 4: 16– 19. [Google Scholar]
- 31. Senthilkumar N, Varma P, Gurusubramanian G. ( 2009) Larvicidal and adulticidal activities of some medicinal plants against the malarial vector, Anopheles stephensi (Liston). Parasitol Res. 104: 237– 244. [DOI] [PubMed] [Google Scholar]
- 32. Sivagnaname N, Kalyanasundaram M. ( 2004) Laboratory Evaluation of Methanolic Extract of Atlantia monophylla (Family: Rutaceae) against Immature Stages of Mosquitoes and Non-target Organisms. Memó Inst Oswaldo Cruz. 99( 1): 115– 118. [DOI] [PubMed] [Google Scholar]
- 33. Sukumar K, Perich MJ, Boobar LR. ( 1991) Botanical derivatives in mosquito control: A review. J Am Mosq Cont Assoc. 7: 210– 237. [PubMed] [Google Scholar]
- 34. Sumba LA, Okoth K, Deng AL, Githure J, Knols BGJ, Beier JC, Hassanali A. ( 2004) Daily oviposition patterns of the African malaria mosquito Anopheles gambiae Giles (Diptera: Culicidae) on different types of aqueous substrates. J Circadian Rhythms. 2: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tandon M, Shukla YN, Prapathi AK, Singh SC. ( 1999) Insect antifeedant principles from Vernonia cinerea. Phytotherapy Res. 12: 195– 199. [Google Scholar]
- 36. Tawatsin A, Asavadachanukorn P, Thavara U, Wongsinkongman P, Bansidhi J, Boonruad T, Chavalittumrong P, Soonthornchareonnon N, Komalamisra N, Mulla MS. ( 2006) Repellency of essential oils extracted from plants in Thailand against four mosquito vectors (Diptera: Culicidae) and oviposition deterrent effects against Aedes aegypti (Diptera: Culicidae). Southeast Asian J Trop Med Public Health. 37( 5): 915– 931. [PubMed] [Google Scholar]
- 37. Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. ( 2011) Phytochemical screening and Extraction: A Review. Internationale Pharmaceutica Sciencia. 1( 1): 98– 106. [Google Scholar]
- 38. Waliwitya R, Kennedy CJ, Lawenberger CA. ( 2009) Larvicidal and oviposition altering activity of monoterpenoids, trans- anethol and rosemary oil to the yellow fever mosquito Aedes aegypti (Diptera: Culicidae). Pest Manag Sci. 65: 241– 248. [DOI] [PubMed] [Google Scholar]
- 39. WHO ( 2005) Guidelines for laboratory and field testing of mosquito larvicides, WHO/CDS/WHOPES/GCDPP/2005.1 3. [Google Scholar]
- 40. WHO ( 2011) Guidelines on the quality, safety and efficacy of dengue tetravalent vaccine (live attenuated) WHO/DRAFT, (DEN) 1– 93. [Google Scholar]
- 41. Xue R, Barnard DR, Ali A. ( 2003) Laboratory evaluation of 18 repellent compounds as oviposition deterrents of Aedes albopictus and as larvicides of Aedes aegypti, Anopheles quadrimaculatus, and Culex quinquefasciatus. J Am Mosq Control Assoc. 19( 4): 397– 403. [PubMed] [Google Scholar]


