Abstract
Background:
Anopheles culicifacies is an important vector of malaria in Southeast Asia, contributing to almost 70% of malaria cases in India. It exists as a complex of five morphologically indistinguishable species A, B, C, D and E with varied geographical distribution patterns. In India, 8% of the total population of Madhya Pradesh (Central India) contributes about 30% of total malaria cases, 60% of total falciparum cases and 50% of malaria deaths. An. culicifacies is the major malaria vector in this state. Vector control mainly relies on the proper identification and distribution of vector species exists in a particular area. The present study was carried out to identify the distribution of An. culicifacies sibling species in certain endemic district of Central India, Madhya Pradesh.
Methods:
The An. culicifacies mosquitoes collected from the study districts were identified morphologically. The genomic DNA was isolated from the mosquitoes and subjected to Allele specific PCR targeting D3 domain of 28S ribosomal DNA.
Results:
The mean prevalence of An. culicifacies during the study period was in the range of 8–120 per man per hour (PMH). From the study areas species B was identified from Jabalpur, Chindwara and Hoshangabad, Species C from Hoshangabad only, Species D from Narsinghpur and Khandwa and sibling species E from Mandla, Chindwara and Hoshangabad respectively.
Conclusion:
This is the first report to detect species E from Madhya Pradesh region which necessitate for reconsideration of species distribution of each An. culicifacies sibling species that would enable to develop required vector control strategies.
Keywords: Malaria, Madhya Pradesh, Anopheles culicifacies, Sibling species, AS-PCR
Introduction
Malaria is a major public health problem in tropical and subtropical countries including India and its dynamics vary from place to place. Among all anopheline vectors, Anopheles culicifacies (Diptera: Culicidae) is a principal malaria vector in rural, periurban and tribal settings (Mishra et al. 2012). An. culicifacies has a wide distribution that extends from Ethiopia, Yemen and Iran in the west via Afghanistan, Pakistan, India, Bangladesh, Myanmar and Thailand, to Laos and Vietnam and Combodia in the east (Harrison 1980, Van Bortel et al. 1984, Subbarao 1988, Zaim and Javaherian 1991, Surendran et al. 2000, Mahmood et al. 2002, Vatandoost et al. 2011). To the north it is found in Nepal and southern China, and in the south in Sri Lanka. It is responsible for about 65–70% malaria cases in India (Goswami et al. 2006). An. culicifacies is a complex of 5 isomorphic types which are designated as species A and B (Green and Miles 1980), species C (Subbarao et al. 1983), species D (Subbarao et al. 1988, Suguna et al. 1989, Vasantha et al. 1991) and species E (Kar et al. 1999) with varying biological characteristics such as feeding preference, biting activity, and susceptibility to commonly used insecticides in public health programs (Joshi et al. 1988, Subbarao et al. 1988, Raghavendra et al. 1991, Subbarao et al. 1997) which are relevant for the transmission of the disease and control.
Madhya Pradesh is situated in the central part of India with an area of 308 thousand km2 of which forest cover 76,429 km2 (about 25% of the total land area). Madhya Pradesh (population 72.6 Million) along with other states like Orrisa (population 42 Million), Jharkhand (population 33 Million), and Chhattisgarh (population 25.5 Million) contributes for more than 60% of reported (confirmed) malaria cases in India. According to National Vector Borne Diseases Control Program (NVBDCP) epidemiological data for 2010 from predominantly these tribal states with a total population of 173.1 million (out of a total of the country population i. e., 1.21 billion) represent 14.3 % population show persistent malaria transmission with high API (annual parasite incidence), slide positivity rate (SPR) and very high Pf% (Sharma 2012). Madhya Pradesh alone account for 6% of the total population of the country but contributes to 8.6% of the total malaria cases. Malaria is complex in Madhya Pradesh because of vast tracts of forest with tribal settlement (20% of state population) (Singh et al. 2004, Anon 2007). The magnitude of the problem can be accessed from an estimate made in 1987, that 54 million individuals of various ethnic origins residing in forested area of India and accounting for 8% of the total population contributed 30% of total malaria cases, 60% of total falciparum cases and 50% of malaria deaths in the country (Sharma 1996).
The reasons for such a high diseases prevalence in Madhya Pradesh is mainly due to locations of the villages in the deep forest and is characterized by rocky undulation interspersed with ravines and foothills. Another reason is the innumerable streams which flow into the river, Narmada. These streams flow continuously and provide ample breeding sites covered with dense aquatic vegetation for production of number of anophelines particularly An. culicifacies (Singh 2006).
Therefore malaria control in these areas requires specific approaches and control strategies which includes the proper surveillance for distribution of An. culicifacies members and their identification (Pattanayak et al. 1994). Since sibling species A, B, C, D and E of An. culicifacies are morphologically indistinguishable at any stage of life and due to practical difficulties associated with classical cytotaxonomic method for the identification of members of the complex, a molecular method using an allele-specific polymerase chain reaction (AS-PCR) assay targeted to the D3 domain of 28S ribosomal DNA was used to distinguish these sibling species (Singh et al. 2004, Goswami et al. 2006). The assay discriminates An. culicifacies species at two tier level diagnosis. Firstly in D3-PCR the species complex is distinguished in two groups i.e., A and D in one group and species B, C and E in the second group. In second tier involves AD-PCR assay which distinguishes species A from species D, whereas the BCE-PCR assay distinguishes species B, C and E with each other. With combination of these two tier PCR assays it is possible to identify individual mosquito of the An. culicifacies complex.
The present study was aimed to find the distribution of members of An. culicifacies species in various districts of Madhya Pradesh, India. In the earlier reports from this region species A, B, C and D were identified but species E reported from southern parts of India only was not reported from this area. Noticeably in our study we encountered species E from some of the districts in co-habitation with species B which indicates that all the five members of An. culicifacies species complex occurs in this part of central India.
Materials and Methods
Collection of Mosquitoes
The adult Anopheline mosquito species were collected from different districts of Madhya Pradesh ie, Mandla (Dungaria village), Jabalpur (Barela village), Chindwara (Chakarpat and Chikhla villages), Hoshangabad (Dhadav and Padav villages), Narsinghpur (Chinki village) and Khandwa (Chighdhalia) (Fig. 1 and Table 1). These sites were selected on the basis as they represent the tribal belt along the streams of Narmada River and also show high incidence of malaria (Singh 2004, 2006, 2009, Sharma 2012). The collections were made during the transmission period i.e. February–March and August–September in the morning period between 0600 h to 0800 h using mouth aspirator and battery operated torch. The fed anophelines were captured at various collection sites including human dwellings, cattle sheds, mixed dwelling and random collection sites. The fed mosquitoes were captured so that F1 generation of these mosquitoes can be utilized for further use after egg lay. All adult mosquitoes were brought to the lab for their identification by using standard keys (Christopher 1933, Wattal and Kalra 1961, Das et al. 1990, Nagpal and Sharma 1995). Each representative sample was pinned as a voucher specimen and kept in laboratory as a reference collection. From these collection the An. culicifacies female were separated and allowed for egg laying and the adult emerged from them are used for further standardization and identification of mosquito sibling species using allele specific polymerase chain reaction (AS-PCR) (Goswami et al. 2006, Singh et al. 2006).
Fig. 1.
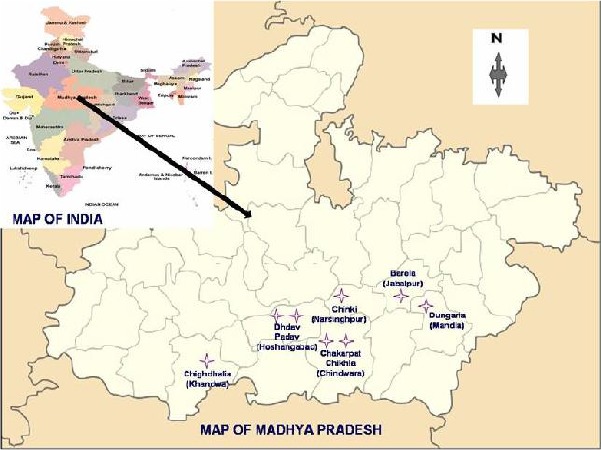
Map showing different collection sites in the study
Table 1.
List of collection sites and result using AS-PCR assay of An. culicifacies mosquito
| Village (District) (Latitude, Longitute) | Number of houses sampled | Number of An. culicifacies samples subjected | Species Identified |
|---|---|---|---|
| Dungaria (Mandla) (22° 60′ N, 80° 38′ E) | 7 | 54 | E (All) |
| Barela (Jabalpur) (23° 10′ N, 79° 59′ E ) | 5 | 26 | B (All) |
| Chakarpat, Chikhla (Chindwara) (22° 03′ N, 78° 59′ E ) | 8 | 56 | B (21), E (35) |
| Dhdav, Padav (Hoshangabad) (22° 46′ N, 77° 45′ E ) | 10 | 124 | B (40), C (16), E (68) |
| Chinki (Narsinghpur) (22° 57′ N, 79° 15′ E ) | 7 | 85 | D (All) |
| Chighdhalia (Khandwa) (21° 49′ N, 76° 22′ E ) | 8 | 74 | D (All) |
DNA Isolation
The DNA extraction was done by using method as described in our previous publication (Sharma et al. 2009, 2010). Each single adult mosquito was homogenized in the micro centrifuge tube by adding 100 μl lysis buffer. The homogenate was immediately kept on ice for 10 minutes and followed by heat treatment at 65 °C for 30 minutes. Subsequently, 30 μl 5M potassium acetate was added and immediately transferred to ice for one hour followed by centrifugation at 13,000 rpm for 15 minutes at 10 °C. To the supernatant obtained, a double volume of absolute chilled ethanol was added for precipitation of DNA and kept tubes at −20 °C for overnight. After centrifugation at 13,000 rpm for 15 minutes at 10 °C, the precipitated DNA was washed in 70% ethanol twice. The DNA pallet was allowed to air dry and finally dissolved in 50 μl TE buffer.
Allele Specific PCR (AS-PCR)
D3-PCR
The targeted region, D3 domain of 28 S rDNA, was amplified by PCR using universal primers, D3A and D3B designed for platyhelminth (Litvaitis et al. 1994) and later used for An. minimus (Sharpe et al. 1999) and for both An. fluviatilis and An. culicifacies (Singh et al. 2004a, b). Another set of allele specific primers namely ACA and ACB which are specific to species A/D and species B/C/E respectively were selected for design of multiplex AS-PCR. The sequences for the primers used were given in the Table 2 with their annealing temperatures.
Table 2.
List of primer used for molecular identification of An. culicifacies sibling species
| Sequence no. | Primer | Sequence (5′-3′) | Tm |
|---|---|---|---|
| 1 | D3A | GAC CCG TCT TGA AAC ACG GA | 67.3 |
| 2 | D3B | TCG GAA GGA ACC AGC TAC TA | 61.3 |
| 3 | ACA | GCC GTC CCC ATA CAC TG | 62.7 |
| 4 | ACB | CCG TAA TCC CGT GAT AAC TT | 60.2 |
| 5 | ADF | CTA ATC GAT ATT TAT TAC AC | 45.5 |
| 6 | ADR | TTA CTC CTA AAG AAG GC | 48.8 |
| 7 | DF | TTA GAG TTT GAT TCT TAC | 42.9 |
| 8 | BCEF | AAA TTA TTT GAA CAG TAT TG | 48.4 |
| 9 | BCR | TTA TTT ATT GGT AAA ACA AC | 48.6 |
| 10 | CR | AGG AGT ATT AAT TTC GTC T | 49.3 |
| 11 | ER | GTA AGA ATC AAA TTC TAA G | 45.1 |
The amplification was performed in a total of 15 μl of reaction mixture consisting of Tris. HCl 10 mM pH 9.0, KCl 50 mM Mg Cl2 2 mM, dNTP 0.2 mM 10 pmoles of primer 0.5 U of Taq DNA polymerase (MBI Fermentas) and 10ng of genomic DNA. Reactions were performed in a (BIORAD PCR System iCycler) thermal cycler. The PCR condition consisted of initial Denaturation step for 5 min at 95 °C followed by 35 cycles of 30 sec at 95 °C, 30 sec at 55 °C, and 60 sec at 72 °C. A final extension step was performed at 72 °C for 7 min.
AD-PCR and BCE-PCR
A total of seven primers of which three primers ADF, ADR, and DF were used in the AD-PCR assay differentiating sibling species A from D, and the other four set of primers BCEF, BCR, CR and ER were used in the BCE-PCR assay for the dedifferentiating species B, C and E from each other (Table 2).
Optimized condition for AD-PCR assay includes 35 cycles of the initial denaturation temperature at 95 °C for 40 s, annealing at 50 °C for 40 s, and extension 68 °C for 40 s, followed by a final extension at 72 °C for 10 min. The PCR reaction was comprised of ADF, ADR and DF primers each at 25 pmol, 200μmol/L of each of the dNTP, 1.5 mmol/ L MgCl2, 20 mmol/L (NH4) SO4, 75 mmol/ L Tris-HCL pH 9.0 and 0.625 unit of Taq DNA polymerase. Whereas the condition for BCE-PCR assay are similar as described for the AD-PCR assay except for the primer concentration of 25 pmol BCEF primer, 12 pmol BCR primer, 25 pmol ER primer and 30 pmol CR primer respectively.
Results
During the collection period a large number of Anopheles mosquitoes were collected from the various collection sites (Fig. 1 and Table 1) and per man per hour (PMH) count estimate was also made from each site. A total of 45 houses including cattle sheds were sampled for collection of mosquitoes. The mean prevalence of An. culicifacies during the study period was found to be in the range of 8–120 PMH, with a high density during August-September (90–120 PMH) and to a low density in February–March (8–50 PMH).
The AS-PCR assay using different primers, the A/D specific primer (ACA) in conjunction with D3B produces 313 bp amplification product and B/C/E-specific primer (ACB) forms 133 bp product with D3A. Additionally, the external primers D3A and D3B form common product in all the samples with 382bp products in species A and D whereas 385bp in species B/C/E serving as positive control (Fig. 2). For further distinguishing the sibling species in A/D and B/C/E individually, a total of seven primers of which three primers ADF, ADR, and DF were used in the AD-PCR assay differentiating sibling species A from D, and the other four set of primers BCEF, BCR, CR and ER were used in the BCE-PCR assay differentiating sibling species B, C and E from each other. In AD-PCR, the sibling species A and D produced the bands of 359 bp for D species and 359 bp and 166 bp for sibling species A. On the other hand in BCE-PCR, the products are 248 bp for B, 248 bp and 95 bp for C and 248 and 178 for sibling species E respectively (Fig. 3).
Fig. 2.
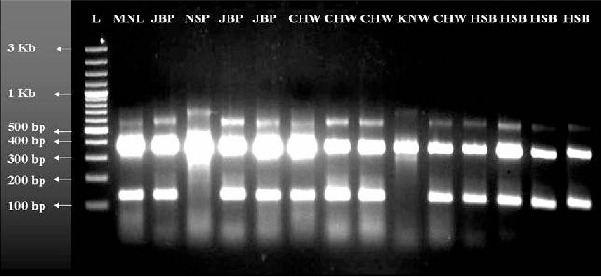
D3-PCR showing the different bands to differentiate A/D and B/C/E sibling species of An. culicifacies
Fig. 3.
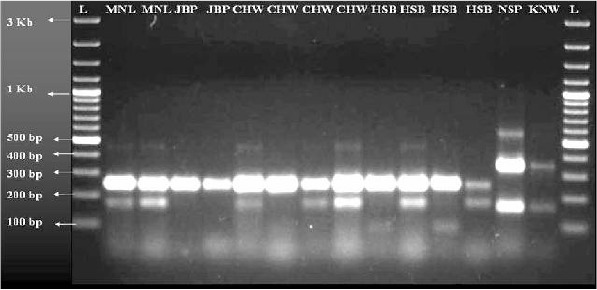
Allele specific multiplex AD-BCE-PCR showing different bands for each An. culicifacies sibling species
The An. culicifacies collected from different districts from Madhya Pradesh ie, Mandla, Jabalpur, Chindwara, Hoshangabad, Narsinghpur and Khandwa respectively. The collected mosquitoes were subjected to two tier PCR assays. From the D3-PCR only Narsinghpur and Khandwa samples were identified in A/D group whereas all the other districts samples were identified in and identified in B/C/E group (Fig. 2). Results obtained from AD-BCE PCR showed the presence of sibling species B from Jabalpur, Chindwara and Hoshangabad, C from Hoshangabad only, D from Narsinghpur and Khandwa and sibling species E from Mandla, Chindwara and Hoshangabad respectively (Fig. 3).
Discussion
The accurate identification and distribution pattern of anopheline mosquitoes is necessary for planning effective vector control strategies and for a better understanding of their potential role in malaria transmission. Wattal and Kalra in 1961 described 32 species of female anophelines in different regions of India. They divided India in six regions and included Madhya Pradesh in Hyderabad Region where about 28 species were described from this region (Wattal and Kalra 1961). In our collection we have also encountered about 11 species of anopheles from the study area which includes An. culicifacies, An. fluviatilis, An. stephensi, An. subpictus, An. annularis, An. barbirostris, An. varuna, An. jamesi, An. jeyporiensis, An. tessellates and An. theobaldi respectively. But in the study area malaria is mainly transmitted by two efficient vectors i.e., An. culicifacies and An. fluviatilis, moreover the density of An. culicifacies is very high throughout the year (Singh 2006). An. culicifacies being a major vector of malaria in India is responsible for approximately 65% of total malaria cases (Sharma 1998). In India all five species of An. culicifacies have been found among these species B was found throughout the country whereas species E was reported only from the southern parts of India. All these sibling species poses a number of biological variations among them. They may be different in the feeding pattern an important characteristics that influence vectorial capacity. Distinct difference were observed in laboratory studies with reference to insemination rate, fecundity, longevity, oviposition, gonotropic cycle, egg hatching, larval mortality rates and adult emergence time have been observed in different species of An. culicifacies (Ansari et al. 1997, Subbarao et al. 1998).
Attempts to find morphological markers for the members of species complex have not been successful so far except the variation in spermatheca of sibling species A and B (Das 1990) but this difference still need to be reconfirmed by other techniques. The classical technique of cytotaxonomic is difficult and has limited use as this requires semi gravid females only and moreover it requires highly skilled personnel. With the advent of DNA based technology we are now able to differentiate members of An. culicifacies complex. The DNA based technology includes PCR assay which are simple and sensitive at the same time they are applicable to all stages and either sexes of mosquitoes (Goswami et al. 2006).
As reported by Sharma (2012) in his review that the epidemiological indices of malaria in Madhya Pradesh revealed a very dismal picture of malaria. An international team of experts reported a very high incidence of malaria in pregnancy (MiP). For example in Madhya Pradesh (rural) 183,000–1.5 million per year contract malaria in pregnancy, and result in 73,000–629,000 lost foetus and 1,500 to 12,600 maternal deaths. Authors state “Plausible estimate of 220,000 MiP cases per year (136,000–305,000), 95,800 lost fetus (56,800–147,600) and 1,000 maternal deaths (650–1,600)” (Diamond et al. 2009).
In our study An. culicifacies mosquito species were collected from different malaria endemic district of Madhya Pradesh by using AS-PCR we were able to identify four species B, C, D and E from these areas. Species E was not reported earlier from these areas as this species is prevalent only in southern peninsular part of India. Recently species E was reported by Das et al. (2013) from Odisha, eastern India also showing its vectorial role. Species E is highly anthropophagic and possesses high sporozoite rate up to 20% and also known as vector in southern India and Srilanka (Kar et al. 1999). In our study we encountered species E from Mandla, Chindwara and Hoshangabad districts respectively which have high disease prevalence and represent the tribal belts (Singh 2004, 2006, 2009, Sharma 2012). Noticeably this species E was found sympatric with a non vector species B from Chindwara and Hoshangabad District, but in Jabalpur District only species B and in Mandla only species E were identified. Topographically the villages under study from Mandla and Jabalpur are very close to each other. Although species B is a poor vector of malaria in India but through examination of mitotic Y chromosome morphology (Kar et al. 1999), that what was reported as B on the Sri Lanka island is really a sympatric mixture of B and E (Surendran et al. 2000). Moreover these two sibling species in Sri Lanka differ in longevity and in their susceptibility to malaria parasite infection and common insecticides (Surendran et al. 2006b).
It is evident from the literature that species E cannot be differentiated from species B because they have homosequential polytene chromosome arrangements. Species E requires mitotic chromosome examination of male progeny and/or vectorial potential needs to be established for distinction from species B (Kar et al. 1999). In the absence of either of these, identification of species E may not be accurate. After screening several enzyme systems, electrophoretic variation at the Lactate dehydrogenase (Ldh) locus was useful. It could group species A and D in one category and species B and C in another category (Adak et al. 1994). Species E showed the same Ldhs allele as in species B and C (Kar et al. 1999). With the identification of species E, the paradox that species B females from northern India are not vectors, but homosequential females from Rameshwaram Island are vectors is resolved. Similar studies are needed to be carried out to see the vectorial capacity and disease transmission in the studied districts of Madhya Pradesh. As in mainland areas close to Rameshwaram, species E were found, populations in other parts of Tamilnadu state where species B has been identified also should be examined (Kar et al. 1999). An. culicifacies populations identified as species B in Sri Lanka also should be examined immediately for Y-chromosome variations and correlated with malaria infection (Surendran et al. 2000, 2006a). Recently, Adak et al. (1997) has reported acrocentric and submetacentric Y-chromosomes within species B but no epidemiological or dissection data is available that indicates that species B is a vector. Thus, there is an urgent need to develop suitable markers that can differentiate species B and E and also to see the other biological characteristics of these two species in such cohabiting areas to conclude that which species is responsible for disease transmission.
Moreover new areas should be explored for the presence of species E as it is a potent vector of malaria. Hence there is an urgent need for nationwide surveillance and identification of vector sibling species distribution once again so that modified species pattern in these areas could be established. Apart from confirming their identity, distribution pattern and their differential malaria vector status, it will be important to determine the susceptibility of these sibling species to insecticides in each part of the states/ country as this affects the efficiency of vector control operations in the malaria control programs in India.
Conclusion
In conclusion we can say that as we encountered a new sibling species E of An. culicifacies from the study sites where it was not reported earlier. Existence of such modified distribution of sibling species may exist in other areas also which necessitate for reconsidering the sibling species distribution in newer area. Knowledge of proper identification and distribution pattern of sibling species may further help us in development of vector control strategies.
Acknowledgements
The authors are thankful to Prof (Dr) MP Kaushik, Outstanding Scientist and Director, Defence Research and Development Establishment, Gwalior, Madhya Pradesh, India for his keen interest and providing all necessary facility to conduct this research work. Sincere thanks also due to the scientists and supportive staff of Vector Management Division for their kind cooperation for carrying out the above work. The authors declare that there is no conflict of interest.
References
- 1. Adak T, Subbarao SK, Sharma VP, Rao SRV. ( 1994) Lactate dehydrogenase allozyme differentiation of species in the Anopheles culicifacies complex. Med Vet Entomol. 8: 137– 140. [DOI] [PubMed] [Google Scholar]
- 2. Adak T, Kaur S, Wattal S, Nanda N, Sharma VP. ( 1997) Y-chromosome polymorphism in species B and C of Anopheles culicifacies complex. J Am Mosq Control Assoc. 13: 379– 383. [PubMed] [Google Scholar]
- 3. State wise malaria situation during 1997–2006 ( 2007) National Vector Borne Disease Control Programme 22- Sham Nath Marg Delhi-110054. [Google Scholar]
- 4. Ansari MA, Mani TR, Sharma VP. ( 1977) A preliminary note on the colonization of Anopheles culicifacies Giles. J Comm Dis. 9: 206– 207. [Google Scholar]
- 5. Christophers SR. ( 1933) The fauna of British India including Ceylon and Burma, Diptera, Vol. IV. Family Culicidae, Tribe Anopheline. Taylor and Francis, London. [Google Scholar]
- 6. Das BP, Rajagopal R, Akiyama J. ( 1990) Pictorial key to Indian anopheline mosquitoes. Zoology. 2: 132– 162. [Google Scholar]
- 7. Das BP. ( 1990) Morphological difference between sibling species of An. culicifacies: A preliminary report. Mosquito-Borne diseases Bulletin. 7: 131– 133. [Google Scholar]
- 8. Das M, Das B, Patra AP, Tripathy HK, Mohapatra N, Kar SK, Hazra RK. ( 2013) Anopheles culicifacies sibling species in Odisha, eastern India: First appearance of Anopheles culicifacies E and its vectorial role in malaria transmission. Trop Med Int Health. 18: 810– 821. [DOI] [PubMed] [Google Scholar]
- 9. Diamond-Smith N, Singh N, Dasgupta RK, Dash A, Thimasarn K, Campbell OM, Chandramohan D. ( 2009) Estimating the burden of malaria in pregnancy: a case study from rural Madhya Pradesh, India. Malar J. 8: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goswami G, Singh OP, Nanda N, Raghavendra K, Gakhar SK, Subbarao SK. ( 2006) Identification of all members of the Anopheles culicifacies complex using allele-specific polymerase chain reaction assays. Am J Trop Med Hyg. 75: 454– 460. [PubMed] [Google Scholar]
- 11. Green CA, Miles SJ. ( 1980) Chromosomal evidence for sibling species of the malaria vector Anopheles (Cellia) culicifacies Giles. J Trop Med Hyg. 83: 75– 78. [PubMed] [Google Scholar]
- 12. Harrison BA. ( 1980) The Myzomyia series of Anopheles ( Cellia) in Thailand, with emphasis on intra-specific variations (Diptera: Culicidae). Contr Am Entomol Inst (Ann Arbor). 17: 1– 195. [Google Scholar]
- 13. Joshi H, Vasantha K, Subbarao SK, Sharma VP. ( 1988) Host feeding patterns of Anopheles culicifacies species A and B. J Am Mosq Control Assoc. 4: 248– 251. [PubMed] [Google Scholar]
- 14. Kar I, Subbarao SK, Eapen A, Ravindran J, Satyanarayana TS, Raghavendra K, Nanda N, Sharma VP. ( 1999) Evidence for a new malaria vector species, species E, within the Anopheles culicifaciesc omplex (Diptera: Culicidae). J Med Entomol. 36: 595– 600. [DOI] [PubMed] [Google Scholar]
- 15. Litvaitis MK, Nunn G, Thomas WK, Kocher TD. ( 1994) A molecular approach for the identification of Meiofaunal turbellarians (Platyhelminthes, Turbellaria). Marine Biol. 120: 437– 442. [Google Scholar]
- 16. Mahmood F, Sakai RK, Akhtar K. ( 1984) Vector incrimination studies and observations of species A and B of the taxon Anopheles culicifacies in Pakistan. Trans Roy Soc Trop Med Hyg. 78: 607– 616. [DOI] [PubMed] [Google Scholar]
- 17. Mishra AK, Chand SK, Barik TK, Dua VK, Raghavendra K. ( 2012) Insecticide resistance status in Anopheles culicifacies in Madhya Pradesh, central India. J Vector Borne Dis 49: 39– 41. [PubMed] [Google Scholar]
- 18. Nagpal BN, Sharma VP. ( 1995) Indian Anophelines. Oxford and IBH Publishing Co. Pvt Ltd, Mohan Primlani, N Delhi. [Google Scholar]
- 19. Pattanayak S, Sharma VP, Kalra NL, Orlov VS, Sharma RS. ( 1994) Malaria paradigms in India and control strategies. Indian J Malariol. 31( 4): 141– 199. [PubMed] [Google Scholar]
- 20. Raghavendra K, Vasantha K, Subbarao SK, Pillai MKK, Sharma VP. ( 1991) Resistance in Anopheles culicifacies sibling species B and C to malathion in Andhra Pradesh and Gujarat states in India. J Am Mosq Control Assoc. 7: 255– 259. [PubMed] [Google Scholar]
- 21. Sharma AK, Mendki MJ, Tikar SN, Chandel K, Sukumaran D, Parashar BD, Veer V, Agarwal OP, Prakash S. ( 2009) Genetic Variability in geographical populations of Culex quinquefasciatus Say (Diptera: Culicidae) from India based on random amplified polymorphic DNA analysis. Acta Trop. 112: 71– 76. [DOI] [PubMed] [Google Scholar]
- 22. Sharma AK, Mendki MJ, Tikar SN, Kulkarni G, Veer V, Prakash S, Shouche YS, Parashar BD. ( 2010) Molecular phylogenetic study of Culex quinquefasciatus mosquito from different geographical regions of India using 16S rRNA gene sequences. Acta Trop. 116: 89– 94. [DOI] [PubMed] [Google Scholar]
- 23. Sharpe RG, Hims MM, Harbach RE, Butlin RK. ( 1999) PCR-based methods for the identification of species of the Anopheles minimus group: allele specific amplification and single strand conformation polymorphism, Med Vet Entomol. 13: 265– 273. [DOI] [PubMed] [Google Scholar]
- 24. Sharma VP. ( 1996) Re-emergence of malaria in India. Indian J Med Res. 103: 26– 45. [PubMed] [Google Scholar]
- 25. Sharma VP. ( 1998) Fighting malaria in India. Curr Sci. 75: 1127– 1140. [Google Scholar]
- 26. Sharma VP. ( 2012) Battling malaria iceberg incorporating strategic reforms in achieving Millennium Development Goals and malaria elimination in India. Indian J Med Res. 136( 6): 907– 925. [PMC free article] [PubMed] [Google Scholar]
- 27. Singh N, Das AP, Varun BM, Kataria OM. ( 2004) Tribal Malaria. ICMR Bulletin. 34: 1– 10. [Google Scholar]
- 28. Singh N. ( 2006) Tribal Malaria an Update on Changing Epidemiology. Proceedings of National symposium on Tribal Health, RMRCT, ICMR, Jabalpur, October 19–20, pp. 47– 54. [Google Scholar]
- 29. Singh N, Dash AP, Thimasarn K. ( 2009) Fighting malaria in Madhya Pradesh (Central India): Are we loosing the battle? Malar J. 8: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singh OP, Chandra D, Nanda N, Raghavendra K, Sunil S, Sharma SK, Dua VK, Subbarao SK. ( 2004a) Differentiation of members of the Anopheles fluviatilis species complex by an allele-specific polymerase chain reaction based on 28S ribosomal DNA sequences. Am J Trop Med Hyg. 70: 27– 32 [PubMed] [Google Scholar]
- 31. Singh OP, Goswami G, Nanda MN, Raghavendra K, Chandra D, Subbarao SK. ( 2004b) An allele-specific polymerase chain reaction assay for the differentiation of members of the Anopheles culicifacies complex. J Biosci. 29: 275– 280. [DOI] [PubMed] [Google Scholar]
- 32. Subbarao SK, Vasantha K, Adak T, Sharma VP. ( 1983) Anopheles culicifacies complex: evidence for a new sibling species, species C. Ann Entomol Soc Am. 76: 985– 986. [Google Scholar]
- 33. Subbarao SK, Vasantha K, Sharma VP. ( 1988) Response of Anopheles culicifacies species A and B to DDT and HCH in India: implications in malaria control. Med Vet Entomol. 2: 219– 223. [DOI] [PubMed] [Google Scholar]
- 34. Subbarao SK, Sharma VP. ( 1997) Anopheline species complexes and malaria control. Indian J Med Res. 106: 164– 173. [PubMed] [Google Scholar]
- 35. Subbarao SK. ( 1998) Anopheline Species Complexes in South-East Asia. World Health Organization Regional Office for South-East Asia, New Delhi, India. [Google Scholar]
- 36. Suguna SG, Tewari SC, Mani TR, Hiryan J, Reuben R. ( 1989) A cytogenetic description of a new species of the Anopheles culicifacies species complex. Genetica. 78: 225– 230. [DOI] [PubMed] [Google Scholar]
- 37. Surendran SN, Abhayawardana TA, De Silva BGDNK, Ramasamy MS, Ramasamy R. ( 2000) Anopheles culicifacies Y chromosome dimorphism indicates the presence of sibling species (B and E) with different malaria vector potential in Sri Lanka. Med Vet Entomol. 14: 437– 440. [DOI] [PubMed] [Google Scholar]
- 38. Surendran SN, Hawkes NJ, Steven A, Hemingway J, Ramasamy R. ( 2006a) Molecular studies of Anopheles culicifacies (Diptera: Culicidae) in Sri Lanka: sibling species B and E show sequence identity at multiple loci. Eur J Entomol. 103: 233– 237. [Google Scholar]
- 39. Surendran SN, Ramasamy MS, De Silva BGDNK, Ramasamy R. ( 2006b) Anopheles culicifacies sibling species B and E in Sri Lanka differ in longevity and their susceptibility to malaria parasite infection and common insecticides. Med Vet Entomol. 20: 153– 156. [DOI] [PubMed] [Google Scholar]
- 40. Van Bortel W, Sochanta T, Harbach RE, Socheat D, Roelants P, Backeljau T, Coosemans M. ( 2002) Presence of Anopheles culicifacies B in Cambodia established by the PCR-RFLP assay developed for the identification of Anopheles minimus species A and C and four related species. Med Vet Entomol. 16: 329– 334. [DOI] [PubMed] [Google Scholar]
- 41. Vasantha K, Subbarao SK, Sharma VP. ( 1991) Anopheles culicifacies complex: population cytogenetic evidence for species D (Diptera: Culicidae). Ann Entomol Soc Am. 84: 531– 536. [Google Scholar]
- 42. Vatandoost H, Emami SN, Oshaghi MA, Abai MR, Raeisi A, Piazzak N, Mahmoodi M, Akbarzadeh K, Sartipi M. ( 2011) Ecology of malaria vector Anopheles culicifacies in a malarious area of Sistan va Baluchestan Province, southeast Islamic Republic of Iran. East Mediterr Health J. 17( 5): 439– 445. [PubMed] [Google Scholar]
- 43. Wattal BL, Kalra NL. ( 1961) Region wise keys to the female Indian anophelines. Bull Natl Soc. Indian Mal Mosq Dis. 9: 85– 138. [Google Scholar]
- 44. Zaim M, Javaherian Z. ( 1991) Occurrence of Anopheles culicifacies species A in Iran. J Am Mosq Control Assoc. 7( 2): 324– 326. [PubMed] [Google Scholar]


