Abstract
Background:
Anaplasmosis is an important issue for animal breeders in terms of economic losses as well as a health concern to human. Ticks are considered as the main vector of this disease. Lack of documented information about Anaplasma species in Iran was the scope of this study to determine the population of ticks and the presence of Anaplasma in ticks, domestic ruminants and also human beings in northern Iran.
Methods:
A total of 101 unengorged hard ticks, 78 domestic ruminants and 40 human blood samples collected from Ghaemshahr, Mazandaran Province, northern Iran were tested by nested PCR against 16s rRNA gene of Anaplasma species.
Results:
Positive PCR was found in 50 ticks, 28 sheep, 2 cattle, one goat, and 10 human specimens. Sequence analysis of the PCR products confirmed presence of A. ovis in two Rhipicephalus sanguineus and two Ixodes ricinus ticks, one human and 4 sheep samples. Moreover one Boophilus annulatus tick and one sheep sample were infected with A. bovis. Furthermore one sample of sheep was infected with A. centrale.
Conclusion:
This study is the first report of tick infection to A. ovis, A. bovis and human infection to A. ovis in Iran. The result of this study is a survey of Anaplasma infections from ticks, domestic animals and human in Iran which help to have appropriate prevention measures for anaplasmosis.
Keywords: Anaplasma, Human, Iran, Livestock, Tick
Introduction
Anaplasmosis, a disease caused by various species of Anaplasma, poses important economic constraints to animal breeders. Besides the costs of the additional veterinary care, anaplasmosis causes abortion in animals, reduction of milk production, body weight, and frequently leads to death (Stuen et al. 2003).
Members of the genus Anaplasma are obligatory intracellular gram negative bacteria that infect blood cells of mammals. Six Anaplasma species are currently recognized (Dumler et al. 2001). Vertebrates are main reservoirs of the Anaplasma bacteria, however in many cases bacteria from the genus Anaplasma cause diseases in domestic animals and human. Anaplasma ovis invades and reproduces within erythrocytes. This bacterium induces acute anemia in sheep and goats (Splitter et al. 1956). Anaplasmosis in cattle is caused by A. bovis infecting monocytes (Uilenberg 1993), or by A. marginale and A. centrale which parasitize and replicate in red blood cells (Kuttler 1966). Anaplasma bovis is reported mostly from cattle, but also detected in small ruminants which could be a reservoir of this bacterium (Goethert et al. 2003).
Ixodid ticks play an important role in maintaining Anaplasma species in nature. It is evidenced that various species of Ixodes, Dermacentor, Rhipicephalus and Amblyomma genera are the main vectors of the Anaplasma bacteria in different regions of the world. Rhipicephalus sanguineus, a common tick vector for Anaplasma, has been reported from India, the United States, all regions of Africa, and around the Mediterranean Basin (Stafford 2007).
Agriculture and animal husbandry is the main activities of people in Mazandaran Province, northern Iran, and anaplasmosis is one of the major veterinary health problems there. However, there have been only a few studies to detect tick anaplasmosis infections in the country (Bashiribod et al. 2004, Spitalska et al. 2005). These two studies reported A. phagocytophilum and Ehrlichia ovina infection in ticks in north and counter parts of Iran.
Due to the lack of documented information about Anaplasma species in ticks, animals and also human beings in the country and having found clinical features and laboratory findings similar to anaplasmosis in some shepherds in suburban areas of Ghaemshahr County in northern Iran during recent years, we conducted the present study to understand more about the Anaplasma infections in Iran.
Materials and Methods
Sampling
The study was carried out in Ghaemshahr County in north of Iran, in which a number of suspected cases of anaplasmosis were reported (Mahmoudi 2004). The collection site was villages of suburban forest area in Ghaemshahr in which the climate is subtropical with cold winters and moderate summers.
About 425 domestic ruminants (361 sheep, 54 goats and 10 available cattle) from 18 herds of nine villages in Ghaemshahr were inspected for tick infestation. The whole body of each animal was inspected and the ticks were manually removed from animals’ body. The sampling was done through 2008 in a period corresponding to seasonal tick activity. Collected ticks from infested animals were kept in dry plastic tubes containing few fresh grass leaves covered by a lid containing several minute holes. Tubes were labeled and conditioned under room temperature for a few days, and then they were dispatched to the laboratory. The purpose of this procedure was to maintain ticks alive inside the tubes until the laboratory taxonomic identification. Ticks species identification was done by using the criteria key described by Hoogstraal (Hoogstraal 1979). Totally 323 ticks were collected from which 101 unengorged ticks were selected for pathogen detection by PCR examination.
Blood samples were additionally taken from both domestic ruminants and corresponding voluntary shepherds. Blood samples were taken from jugular vein of sheep and goat and from caudal vein of cattle. A total of 78 blood samples including 65 from sheep, nine from cattle and four from goats were collected, from which 38 were from animals with tick infestation and 40 from animals without any tick infestation.
Forty samples were taken from median cubital vein of corresponding voluntary shepherds from different age groups. All animals and humans had not any clinical signs. All tissue had been obtained with consent given according to the institutional guidelines.
DNA extraction and PCR amplification-sequencing
DNA extraction carried out from ticks and blood samples using the G-spin™ Genomic DNA Extraction Kit (iNtRON Biotechnology, Korea) and i-genomic Blood DNA Extraction Mini Kit (iNtRON Biotechnology, Korea), respectively following the manufacturer protocol.
Anaplasma DNA was detected by means of nested PCR with primers derived from the 16S rRNA gene of Anaplasma species. PCR was performed in 20 μl reaction mixture containing 10 mm Tris-HCl (Ph 9.0), 30 mm KCl, 1.5 mm MgCl2, 250 mm each dNTP, 0.5 mm each sense and antisense primers, 1 U Taq DNA polymerase and 2 μl of DNA. The conditions for PCR included an initial denaturation at 94 °C for 3 min followed by 35 cycles of amplification (1 min denaturation at 94 °C, 1 min annealing at 57 °C and 1 min elongation at 72 °C) (Rar et al. 2008). The primer pair used for PCR were Ehr1 (5′-GAACGAACGCT GGCGGCAAGC-3′) and Ehr2 (5′-AGTA [T/C]CG[A/G]ACCAGATAGCCGC-3′). Nested PCR was performed with 2 μl of the first PCR reaction using primer pair Ehr3 (5′-TGCATAGGAATCTACCTAGTAG-3′) and Ehr4 (5′-CTAGGAATTCCGCTATCC TCT-3′) and resulted in a 524-bp product. The PCR procedure was with exception of annealing temperature (60 °C), the same as described above (Rar et al. 2008).
As positive control we used Anaplasma DNA obtained from Department of Parasitology, Faculty of Veterinary Medicine, University of Tehran (Noaman et al. 2009), and double distilled water as negative control was used. The PCR analysis was performed on agarose gel using ethidium bromide and UV condition. The PCR products were directly subjected to sequencing by Seqlab (GmbH, Germany).
The nucleotide sequences were then blasted by BLASTN (http://www.ncbi.nlm.nih.gov/BLAST) program.
Results
Blood and ticks infected by Anaplasma species
Overall 102 (24%) out of 425 inspected animals and in particular 28.25% (88/361) sheep, 22.22% (12/54) goats and 20% (2/10) cattle were infested with ticks. Altogether 323 ticks were collected categorized into four genera and six species: Rhipicephalus sanguineus (266, 82.35%), R. bursa (1, 0.31 %), Ixodes ricinus (49, 15.17%), Boophilus annulatus (4, 1.24%), Haemaphysalis punctata (1, 0.31%) and H. numidiana (2, 0.62%). Rhipicephalus sanguineus with 82.35% was observed as the most abundant tick species found in the study area. Totally 101 unengorged ticks were examined by nested PCR for presence of Anaplasma species.
A 524-bp 16S rRNA gene fragment of Anaplasma species was identified in 49.5% of examined ticks (50 out of 101), including R. sanguineus (34/54, 62.96%), I. ricinus (14/39, 35.9%), B. annulatus (1/4, 25%), and H. punctata (1/1, 100%).
Similarly, blood samples from ruminants also contained Anaplasma DNA. Genome of Anaplasma species was detected in 39.74% (31/78) blood samples, and in particular 43.08% (28/65) sheep, 22.22% (2/9) cattle, and 25% (1/4) goats were infected (Table 1, Fig. 1).
Table 1.
Results of nested PCR for detection of Anaplasma species in ticks, domestic animal and human blood samples collected from Ghaemshahr, Iran, 2008
| Male | Total | |
|---|---|---|
| Ticks | ||
| Rhipicephalus sanguineus | 11/ 16 (68.75) | 34/ 54 (62.96) |
| Rhipicephalus bursa | 0 | 0/1 (0) |
| Ixodes ricinus | 1/6 (16.67) | 14/39 (35.9) |
| Boophilus annulatus | 0/1 (0) | 1/4 (25) |
| Haemaphysalis punctata | 1/1 (100) | 1/1 (100) |
| Haemaphysalis numidiana | 0 | 0/2 (0) |
| Domestic ruminants | ||
| Sheep | 9/15 (60) | 28/65 (43.08) |
| Goat | 0 | 1/4 (25) |
| Cattle | 0 | 2/9 (22.22) |
| Human age groups | ||
| < 20 years | 0/3 (0) | 1/5 (20) |
| 20–30 years | 1/4 (25) | 1/7 (14.29) |
| 30–40 years | 0/3 (0) | 0/8 (0) |
| 40–50 years | 1/5 (20) | 3/9 (33.33) |
| 50–60 years | 1/2 (50) | 3/6 (50) |
| > 60 years | 1/1 (100) | 2/5 (40) |
Fig. 1.
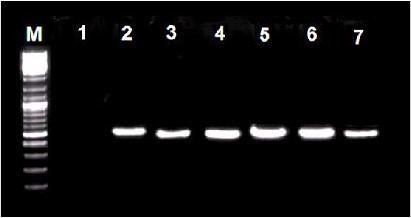
Nested PCR amplicons of a 524-bp Anaplasma 16S rRNA gene fragment from biological samples (tick and blood). M, 100-bp size marker (Fermentase, Germany), lane 1, negative (water) control, lane 2, positive control, lane 3–7, Anaplasma positive samples of tick, sheep, cattle, goat and human blood, respectively
Thirty eight out of 78 tested blood samples, belonged to the ruminants infested with ticks. Nineteen out of 38 (50%) were infected with Anaplasma, and in 16 cases, Anaplasma DNA was detected in both the tick and its host simultaneously. Of 78 blood samples, 40 were collected from ruminants without tick infestation; in 12/40 (30%) infection to the bacteria were revealed.
This study also is the first which tested human blood by molecular methods for the presence of Anaplasma species In Iran.
Blood samples from 40 people between 15 and 78 years old were examined by nested PCR with the same primers. DNA of Anaplasma species was detected in 25% (10/40) blood samples, and in particular six of 22 (27.27%) female and four of 18 (22.22%) male. Most of the infected cases were from shepherds older than 40 years old and in contact with livestock for most of their lives (Table 1, Fig. 1).
Sequencing
A number of positive PCR samples against Anaplasma in ticks separated from sheep (5), sheep blood (6), and human blood (1) were sequenced by the forward and reverse primers and the consensus sequences were submitted to GenBank.
All nucleotide sequences were registered in GenBank under accession numbers JF51 4503, JF514504, JF514505, JF514506, JF49 5135, JF514507, JF514510, JF514511, JF51 4512, JF514513, JF514508 and JF514509.
Among the specimens, two R. sanguinus (JF514510, JF495135), two I. ricinus (JF514 503, JF514511), three sheep (JF514504, JF 514505, JF514506) and one human (JF5145 07) with 484 bp length were 100% identical to A. ovis present in GenBank database. One specimen originated from sheep (JF514512) was identical to A. ovis except for one nucleotide transmission (C/T).
Nucleotide sequences of A. bovis determined in one female B. annulatus tick (JF 514508) and one sheep (JF514513) were identical to each other. Also one blood sample from a female sheep was infected with A. centrale (JF514509).
The present study for the first time demonstrated the presence of A. ovis in human, R. sanguineus and I. ricinus, and A. bovis in B. annulatus in Iran.
Discussion
The main vectors of the Anaplasma bacteria are ticks, especially the genera Ixodes, Dermacentor, Rhipicephalus and Amblyomma.
During our study six species of hard ticks were identified: R. sanguineus, R. bursa, I. ricinus, B. annulatus, H. punctata, and H. numidiana. Half of these species are known as Anaplasma vectors. Out of 323 collected ticks from animals, a significant number of 266 R. sanguineus were identified. This species stands out as being the most prevalent tick species comprising 82.35% of the ticks collected from domestic ruminants in Ghaemshahr. A total of 101 unengorged ticks were assayed by nested PCR for presence of Anaplasma species. Most of (34/50, 68%) the infected ticks belonged to R. sanguineus species. Therefore R. sanguineus with a high level of infestation of domestic ruminants, and the prevalence of Anaplasma DNA (62.96%) could be the most abundant vector of Anaplasma species in Ghaemshahr. The nucleotide sequence determination deriving from one male and one female R. sanguineus were identical to that of the gene of A. ovis (JF495135, JF514510). This is in agreement with the results of other researches indicating the role of R. sanguineus in Anaplasma transmission. Previous study in Africa had shown presence of A. platys DNA in a female R. sanguineus (Sanogo et al. 2003). In a study in Turkey A. ovis 16S rRNA gene fragment was detected in two R. sanguineus ticks (Aktas et al. 2009).
In the present study A. ovis 16S rRNA was detected in two I. ricinus ticks making this tick species as a probable vector of A. ovis in Ghaemshahr. The tick I. ricinus has been reported from Europe and Northern Africa (Sarih et al. 2005), and was evidenced to be the vector of Anaplasma and in particular A. phagocytophilum in Europe (Parola et al. 2005, Silaghi et al. 2008, Aktas et al. 2009, Portillo et al. 2011). In Iran, I. ricinus ticks were found only in Caspian Sea regions in north part of Iran (Rahbari et al. 2007, Razmi et al. 2007). In Ghaemshahr, Iran A. phagocytophilum 16S rRNA was detected in 5 (5.1%) of tested I. ricinus ticks. It showed a high probability of human granulocytotropic ehrlichiosis (HGE) in some stock-farmer patients from Ghaemshahr suburban areas with similar clinical and laboratory findings to HE (Bashiribod et al. 2004).
In Brazil, A. marginale DNA was detected in B. microplus ticks. B. annulatus could experimentally transmit this bacterium to calves (Shimada et al. 2004). We detected a 524 bp 16S rRNA gene fragment of A. bovis in one female B. annulatus collected from sheep in Ghaemshahr.
We could detect Anaplasma sp. in one H. punctata tick collected from a sheep. H. punctata was experimentally able to transmit Babesia major to calves. Anaplasma phagocytophilum and A. bovis was detected in H. megaspinosa and H. longicornis in Japan and Korea, respectively (Oh et al. 2009, Yoshimoto et al. 2010).
We examined two groups of blood samples obtained from animals with and without tick infestation. Fifty percent (19/38) of samples from animals with tick infestation were Anaplasma positive compared to 30% (12/40) positive samples taken from animals without any ticks on their bodies. These results demonstrate the importance of ticks in Anaplasma transmission to domestic animals in the studied area.
A few studies on the infectivity of animal blood samples to Anaplasma have been done in Iran; in Khorasan Province, north east of Iran 80.3% of sheep and 38.92% of goats blood smears were infected with A. ovis (Razmi et al. 2006). In Isfahan Province in the center of Iran, 16S rRNA gene fragment of A. marginale was detected in 50 % of cattle without any clinical signs (Noaman et al. 2009). In the present study 39.7% of blood samples were infected with Anaplasma. These blood samples were obtained from domestic ruminants without any clinical signs showing their role as reservoirs of Anaplasma.
During our study, A. ovis and A. bovis DNA was detected in sheep blood samples. A. bovis is a bacterium detected mainly in cattle and small mammals (Goethert et al. 2003), but in our study was detected in sheep. These results make sheep also as potential reservoirs of this bacterium.
Of 40 blood samples taken from people from different age groups 15–78 years, 10 (25%) were infected. There was not any significant difference between males and females showing equal cooperation of women and men in livestock husbandry in studied area. Most of the infected cases were from shepherds older than 40 years old and in contact with livestock for most of their lives. One blood sample obtained from a 55-year-old woman without any clinical symptoms was infected with A. ovis.
Some researches in Cyprus showed Anaplasma spp. infections in humans (Psaroulaki et al. 2008, Chochlakis et al. 2009). In another laboratory testing of human blood samples by universal primers against all Anaplasma species in Cyprus, A. ovis 16S rRNA was found in one human blood sample taken from a 27-year-old woman with thrombocytopenia and elevated levels of transminses (Chochlakis et al. 2010).
In Iran, A. ovis was identified in sheep (Spitalska et al. 2005, Razmi et al. 2006). Since sheep are reservoirs of A. ovis, infection of humans with this pathogen may occur, but transmission of A. ovis to humans is uncertain. R. sanguineus and I. ricinus are dominant tick species in sheep in northern Iran (Rahbari et al. 2007, Hosseini Vasoukolaei et al. 2010). Results revealed that R. sanguineus highly infested domestic ruminants and A. ovis DNA was detected in this tick species. Ixodes ricinus role as a vector of A. phagocytophilum to human has been suggested (Bashiribod et al. 2004). However, potential role of R. sanguineus and I. ricinus as vectors of A. ovis to human is unknown.
Conclusion
This study is the first to report molecular detection of A. ovis from human in Iran, whereas does not show A. ovis human pathogenicity. However, further epidemiological, clinical and pathological investigations are needed to understand A. ovis potential to infect human.
Infection of ticks, ruminants and humans to Anaplasma species demonstrated the potential endemicity and circulation of the pathogen among different tick species found in the region. Ticks of R. sanguineus are probably the main vector of Anaplasma in northern part of Iran. Application of control measures with emphasizing on control of R. sanguineus could prevent Anaplasma transmission in the region.
Acknowledgements
This study was funded by the School of Public Health of Tehran University of Medical Sciences (grant No. 8768-63-02-88). We are thankful to staff of Ghaemshahr hospital for their sundry precious contribution. The authors declare that there is no conflict of interests.
References
- 1. Aktas M, Altay K, Dumanli N, Kalkan A. ( 2009) Molecular detection and identification of Ehrlichia and Anaplasma species in ixodid ticks. Parasitol Res. 104: 1243– 1248. [DOI] [PubMed] [Google Scholar]
- 2. Bashiribod H, Kazemi B, Eslami G, Bigdeli S, Bandehpour M, Rahbarian N, Ramezani Z. ( 2004) First molecular detection of Anaplasma phgocytophilum in Ixodes ricinus ticks in Iran. J Med Sci. 4: 282– 286. [Google Scholar]
- 3. Chochlakis D, Koliou M, Ioannou I, Tselentis Y, Psaroulaki A. ( 2009) Kawasaki disease and Anaplasma sp. infection of an infant in Cyprus. Int J Infect Dis. 13( 2): 71– 73. [DOI] [PubMed] [Google Scholar]
- 4. Chochlakis D, Ioannou I, Tselentis Y, Psaroulaki A. ( 2010) Human anaplasmosis and Anaplasma ovis variant. Emerg Infect Dis. 16( 6): 1031– 1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, Rikihisa Y, Rurangirwa FR. ( 2001) Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 51( 6): 2145– 2165. [DOI] [PubMed] [Google Scholar]
- 6. Goethert HK, Telford SR., III ( 2003) Enzootic transmission of Anaplasma bovis in Nantucket cottontail rabbits. J Clin Microbiol. 41( 8): 3744– 3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hosseini-Vasoukolaei N, Telmadarraiy Z, Vatandoost H, Yaghoobi-Ershadi MR, Hosseini Vasoukolaei M, Oshaghi MA. ( 2010) Survey of tick species parasiting domestic ruminants in Ghaemshahr County, Mazandaran Province, Iran. Asian Pac J Trop Med. 3( 10): 804– 806. [Google Scholar]
- 8. Hoogstraal H. ( 1979) The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol. 15( 4): 307– 417. [DOI] [PubMed] [Google Scholar]
- 9. Kuttler KL. ( 1966) Clinical and hematologic comparison of Anaplasma marginale and Anaplasma centrale infections in cattle. Am J Vet Res. 27( 119): 941– 946. [PubMed] [Google Scholar]
- 10. Mahmoudi F. ( 2004) Suspected cases of human ehrlichiosis? The 12th Iranian congress on infectious diseases and tropical medicine, 17–21 January 2004, Tehran, Iran. [Google Scholar]
- 11. Noaman V, Shayan P, Amininia N. ( 2009) Molecular diagnostic of Anaplasma marginale in carrier cattle. Iran J Parasitol. 4( 1): 26– 33. [Google Scholar]
- 12. Oh JY, Moon BC, Bae BK, Shin EH, Ko YH, Kim YJ, Park YH, Chae JS. ( 2009) Genetic identification and phylogenetic analysis of Anaplasma and Ehrlichia species in Haemaphysalis longicornis collected from Jeju island, Korea. J Bacteriol Virol. 39( 4): 257– 267. [Google Scholar]
- 13. Parola P, Davoust B, Raoult D. ( 2005) Tick and flea-borne rickettsial emerging zoonoses. Vet Res. 36( 3): 469– 492. [DOI] [PubMed] [Google Scholar]
- 14. Portillo A, Perez-Martinez L, Santibanez S, Santibanez P, Palomar AM, Oteo JA. ( 2011) Anaplasma spp. in wild mammals and Ixodes ricinus from the north of Spain. Vector-Borne Zoonot. 11( 1): 3– 8. [DOI] [PubMed] [Google Scholar]
- 15. Psaroulaki A, Koliou M, Chochlakis D, Ioannou I, Mazeri S, Tselentis Y. ( 2008) Anaplasma phagocytophilum infection in a child. Pediatr Infect Dis J. 27( 7): 664– 666. [DOI] [PubMed] [Google Scholar]
- 16. Rahbari S, Nabian S, Shayan P. ( 2007) Primary report on distribution of tick fauna in Iran. Parasitol Res. 101( 2): 175– 177. [DOI] [PubMed] [Google Scholar]
- 17. Rar VA, Livanova NN, Panov VV, Kozlova IV, Pukhovskaya NM, Vysochina NP, Tkachev SE, Ivanov LI. ( 2008) Prevalence of Anaplasma and Ehrlichia species in Ixodes persulcatus ticks and small mammals from different regions of the Asian part of Russia. Int J Med Microbiol. 298( 1): 222– 230. [Google Scholar]
- 18. Splitter EJ, Anthony HD, Twiehaus MJ. ( 1956) Anaplasma ovis in the United States; experimental studies with sheep and goats. Am J Vet Res. 17( 64): 487– 491. [PubMed] [Google Scholar]
- 19. Razmi GR, Dastjerdi K, Hossieni H, Naghibi A, Barati F, Aslani MR. ( 2006) An epidemiological study on Anaplasma infection in cattle, sheep, and goats in Mashhad suburb, Khorasan Province, Iran. Ann NY Acad Sci. 1078( 1): 479– 481. [DOI] [PubMed] [Google Scholar]
- 20. Razmi GR, Glinsharifodini M, Sarvi S. ( 2007) Prevalence of ixodid ticks on cattle in Mazandaran Province, Iran. Korean J Parasitol. 45( 4): 307– 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanogo YO, Davoust B, Inokuma H, Camicas JL, Parola P, Brouqui P. ( 2003) First evidence of Anaplasma platys in Rhipicephalus sanguineus (Acari: Ixodida) collected from dogs in Africa. Onderstepoort J Vet Res. 70( 3): 205– 212. [PubMed] [Google Scholar]
- 22. Sarih M, M'Ghirbi Y, Bouattour A, Gern L, Baranton G, Postic D. ( 2005) Detection and identification of Ehrlichia spp. in ticks collected in Tunisia and Morocco. J Clin Microbiol. 43( 3): 1127– 1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shimada MK, Yamamura MH, Kawasaki PM, Tamekuni K, Igarashi M, Vidotto O, Vidotto MC. ( 2004) Detection of Anaplasma marginale DNA in larvae of Boophilus microplus ticks by polymerase chain reaction. Ann NY Acad Sci. 1026( 1): 95– 102. [DOI] [PubMed] [Google Scholar]
- 24. Silaghi C, Gilles J, Hohle M, Fingerle V, Just FT, Pfister K. ( 2008) Anaplasma phagocytophilum infection in Ixodes ricinus, Bavaria, Germany. Emerg Infect Dis. 14( 6): 972– 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spitalska E, Namavari MM, Hosseini MH, Shaddel F, Amrabadi OR, Sparagano OAE. ( 2005) Molecular surveillance of tick-borne diseases in Iranian small ruminants. Small Ruminant Res. 57( 2): 245– 248. [Google Scholar]
- 26. Stafford KC., III ( 2007) Ticks of the Northeastern United States. Tick management handbook. EPS Printing II, LLC South Windsor, Connecticut. [Google Scholar]
- 27. Stuen S, Nevland S, Moum T. ( 2003) Fatal cases of tick-borne fever (TBF) in sheep caused by several 16S rRNA gene variants of Anaplasma phagocytophilum. Ann NY Acad Sci. 990( 1): 433– 434. [DOI] [PubMed] [Google Scholar]
- 28. Uilenberg G. ( 1993) Other ehrlichiosis of ruminants. In: Woldehiwet Z, Ristic M. (Eds): Rickettsial and chlamydial diseases of domestic animals. Oxford Pergamon Press, Oxford, pp. 293– 332. [Google Scholar]
- 29. Yoshimoto K, Matsuyama Y, Matsuda H, Sakamoto L, Matsumoto K, Yokoyama N, Inokuma H. ( 2010) Detection of Anaplasma bovis and Anaplasma phagocytophilum DNA from Haemaphysalis megaspinosa in Hokkaido, Japan. Vet Parasitol. 168( 1–2): 170– 172. [DOI] [PubMed] [Google Scholar]


