Abstract
Purpose of review
Syndesmophytes are characteristic components of the spine pathology of ankylosing spondylitis (AS). Understanding their growth may reveal insights to pathogenesis and potential treatment. We review recent studies on rates of development of syndesmophytes, patient characteristics associated with more rapid syndesmophyte growth, local vertebral abnormalities that precede syndesmophytes, systemic biomarkers of syndesmophytes, and studies of medications.
Recent findings
New syndesmophytes develop in one-third of patients over two years. Consistent clinical predictors are male gender, elevated serum C-reactive protein levels, and pre-existing syndesmophytes. Concomitant vertebral inflammation and fat dysplasia on magnetic resonance imaging predict future syndesmophytes at the same vertebral location, but most syndesmophytes do not have recognized antecedents. Associations with serum levels of Wnt pathway proteins are inconsistent, as are the results of observational studies of tumor necrosis factor-alpha inhibitors.
Summary
Although there is better understanding of the frequency of syndesmophyte development, the pathogenesis of syndesmophytes remains unclear.
Keywords: ankylosing spondylitis, syndesmophytes, magnetic resonance imaging, computed tomography, tumor necrosis factor-alpha inhibitors
Introduction
Syndesmophytes are one of the main features of spinal structural damage in ankylosing spondylitis (AS). Extensive bridging of syndesmophytes across multiple vertebrae is pathognomonic of AS, making the study of their development key to understanding this disease. Here we review reports of the rates of development of syndesmophytes in cohort studies and of patient characteristics that are associated with more rapid development published since 2012. Next, we review studies of local factors in the vertebral bodies that predict syndesmophyte formation, and systemic factors in the circulation that have been tested for associations with syndesmophyte formation. Lastly, we review associations with medication use.
Rate of Syndesmophyte Development
The currently-accepted method for evaluating the progression of structural damage in AS is by the reading of cervical and lumbar spine radiographs using the modified Stoke AS Spinal Score (mSASSS) [1]. Although the mSASSS also includes vertebral squaring, sclerosis and erosions, it is heavily weighted by syndesmophytes and is therefore used as a proxy measure of syndesmophyte evolution. The mSASSS can increase by the development of new syndesmophytes or new bridging of existing syndesmophytes. The score range is 0–72. Two years is usually considered the minimum interval required for observing change.
Changes in mSASSS over time
In an update of the Outcome in Ankylosing Spondylitis International Study (OASIS) involving 186 patients up to 12 years, Ramiro et al. reported that the mean two-year mSASSS progression ranged from 1.8 to 2.5 [2••]. At the group level, progression conformed to a linear model with a mean rate of about 1 mSASSS unit/year. The two-year rate was somewhat higher than previous studies, and possibly due to cohort characteristics or the reading methodology that was not blinded to time sequence. Median changes were not reported, which is important because they might reveal whether the mean was influenced by a small subgroup with fast progression. Enrollment started in 1996 and the majority of patients were treated with nonsteroidal anti-inflammatory drugs (NSAIDs) only. In a study of 356 patients treated with a tumor necrosis factor-alpha inhibitor (TNFi), Braun et al. reported mSASSS progression from 0.9 to 1.6 over two-years, although the mean mSASSS at baseline was higher in this group than in the OASIS cohort [3]. In this study, the readers were blinded to the time sequence of the radiographs, making its results difficult to compare with those of OASIS. Braun et al. reported a median change of 0, indicating that at least one-half of patients did not progress over two years.
Development of new syndesmophytes over time
mSASSS progression due specifically to new syndesmophytes may be of special interest as the processes governing the development of new syndesmophytes may differ from those governing the growth of existing syndesmophytes. Ramiro et al. reported that new syndesmophytes were observed in 29%–33% of patients with at least one uninvolved vertebral corner at baseline over two years [2••]. Two studies of patients treated with TNFi reported very similar proportions (36.8% and 37% respectively) over two years [4, 5]. It should be noted that the majority of patients in Ramiro et al.’s study were not treated with TNFi. In contrast, Kang et al. reported that 13% developed new syndesmophytes, but they included only the lumbar spine, and studied only women, who are known to develop fewer syndesmophytes than men [6].
Is syndesmophyte growth continuous or saltatory?
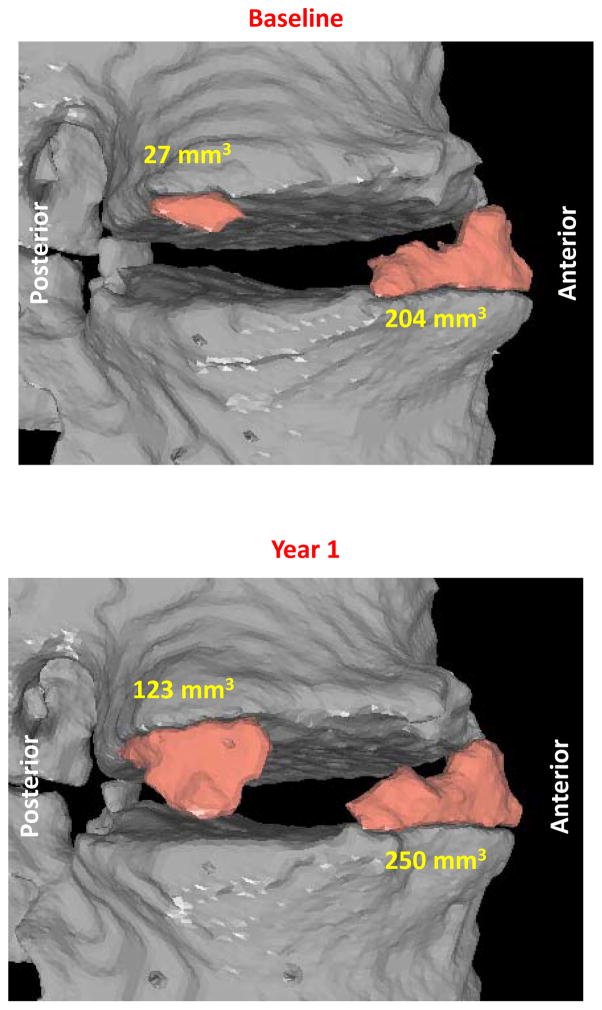
With the use of computed tomography to image syndesmophytes, we have been able to fully quantitate syndesmophyte volume around the entire vertebral rim [7••]. We found that syndesmophyte volume per patient grew on average by 18% over 2 years [8]. However, behind this mean, there was large heterogeneity among patients, among different intervertebral disk spaces of the same patient, and among individual syndesmophytes in the same disk space (Figure 1) [9•]. This heterogeneity suggests that local factors have an important influence on syndesmophyte growth, which could complicate the identification of systemic biomarkers of growth. Syndesmophyte growth was also nonlinear with respect to time, with many patients adding unequal amounts of bone in the first study year versus the second study year. Despite the group level results suggesting continuous linear progression in their 12 year study, Ramiro et al. also reported that periods of progression often alternated with periods of stability [2••]. This indicates that differences in individual growth patterns become obscured with increasingly summarized data.
Figure 1.
Longitudinal changes in syndesmophyte volume over one year on computed tomography scans. One syndesmophyte demonstrated marked increase in volume (noted in cubic millimeters) while a neighboring syndesmophyte had very little increase in volume.
Patient Characteristics associated with Syndesmophyte Growth
Recent investigations of patient characteristics associated with radiographic progression tend to corroborate previous studies [2••, 3, 6, 10–13•]. The strongest and most-consistently identified predictors of progression are the presence of existing syndesmophytes at baseline, male gender, and elevated serum levels of C-reactive protein (CRP) (Table 1). Additionally, using the 12-year data of OASIS, Ramiro et al reported a positive association between rates of change in the mSASSS and higher AS activity by several measures, in particular the AS disease activity score (ASDAS) [13•]. However, this association was only present among men. A dose-dependent association with cigarette smoking has also been reported [10]. A link between mSASSS severity and smoking was also stressed in a cross-sectional study of 647 patients [11].
Table 1.
Significant associations between patient characteristics and radiographic progression in 7 studies of 5 cohorts.
| Ramiro 2015 [2] | Ramiro 2014 [13] | Kang 2014 [6] | Braun 2013 [3] | Poddubnyy 2013 [10] | Chung 2012 [11]* | Tubergen 2012 [12] | ||
|---|---|---|---|---|---|---|---|---|
| Methods | Cohort | OASIS | OASIS | 67 women | GO-RAISE | GESPIC | DESIR | OASIS |
| Outcome | Change in mSASSS | Change in mSASSS | New syndesmophytes | Change in mSASSS | mSASSS and new syndesmophytes | mSASSS | New syndesmophytes | |
| Statistics | Multivariate | Univariate | Univariate | Univariate | Multivariate | Multivariate | Multivariate | |
| Results | Existing syndesmophytes | yes | yes | yes | yes | - | - | yes |
| CRP | - | yes | yes | yes | - | yes | no | |
| Male gender | yes | - | - | - | - | yes | - | |
| Smoking | - | - | - | - | yes | yes | - | |
| Older age | - | - | yes | - | - | - | no | |
| HLA-B27 | yes | no | - | - | - | no | no | |
| Disease duration | no | - | yes | - | - | - | no | |
| Symptom duration | no | - | - | - | - | - | no | |
| Family history | - | - | - | - | - | yes | - | |
| Sacroiliitis severity | - | - | yes | - | - | - | - | |
| Age at onset of IBP | - | - | - | - | - | yes | - | |
| BASDAI | - | yes | - | - | - | - | no | |
| ASDAS | - | yes | - | - | - | - | no | |
| Hip involvement | - | - | - | - | - | - | no | |
| Extra-articular manifestation | - | no | - | - | - | - | - | |
| ESR | - | no | - | - | - | - | no |
Indicates a cross-sectional study. Other studies are longitudinal.
(-) not studied or reported.
OASIS: Outcome in Ankylosing Spondylitis International Study; GO-RAISE: phase III clinical trial of Golimumab; GESPIC: German Spondyloarthritis Inception Cohort; DESIR: Devenir des Spondylarthropathies Indifférenciées Récentes; ; CRP: C-reactive protein; IBP: inflammatory back pain; BASDAI: Bath ankylosing spondylitis disease activity index; ASDAS: AS disease activity score; Extra-articular manifestations include psoriasis, inflammatory bowel disease and uveitis; ESR: erythrocyte sedimentation rate.
Local Factors in Syndesmophyte Initiation and Growth
New studies have continued to investigate associations between syndesmophyte formation and prior vertebral abnormalities on magnetic resonance imaging (MRI), including inflammation as reflected by bone marrow edema on MR short tau inversion recovery (STIR) sequences and fat dysplasia visible on T1-weighted images. Radiographic studies have examined associations with vertebral erosions and sclerosis.
Vertebral Bone Marrow Edema and Fat Dysplasia
Two recent follow-up studies of patients enrolled in TNFi clinical trials have reported on the relationship between MR imaging of inflammation and fat dysplasia and new syndesmophytes in 73 and 76 patients, respectively [4, 5]. MRI scans were performed at two time points one year or two years apart, to observe inflammation resolution and new fatty degeneration. Both studies reported that new syndesmophytes occurred about as frequently in vertebral corners which appeared normal at baseline as in vertebral corners which had either fat or inflammatory lesions (57% versus 43% respectively for Baraliakos et al., 46% versus 54% respectively for Maksymowych et al.). Maksymowych et al. distinguished early pure inflammatory lesions from mixed inflammatory/dysplastic/erosive lesions, and found that new syndesmophytes were much more associated with mixed lesions. Both studies also found that vertebral corners with both inflammation and fat degeneration at baseline had the highest risk of developing new syndesmophytes (relative risk of 3.3 [4] and odds ratio of 4.0 [5]), compared to vertebral corners without either abnormality at baseline. Persistent MRI inflammation seemed more conducive to new syndesmophyte formation. However, the sequence of ‘inflammation - new fat dysplasia - new syndesmophyte’ was rare, being observed only once among 1466 vertebral corners [4]. In 1345 vertebral corners, the sequence ‘new fat dysplasia - new syndesmophyte’ was observed only 4 times [5]. Preventing inflammatory lesions from reaching an advanced stage might be a promising strategy for inhibiting the development of syndesmophytes.
Vertebral Erosions and Sclerosis
A new study of the OASIS cohort explored the relationship between erosions or sclerosis and new syndesmophytes on radiographs [14]. Erosions and sclerosis were rare: 1%–2.5% of all vertebral corners had erosions and 0.7%–1.7% had sclerosis. Although the vast majority of new syndesmophytes occurred in previously-normal vertebral corners, the presence of erosions or sclerosis increased the probability of a new syndesmophyte at that site two years later. The likelihood of a new syndesmophyte was more strongly associated with sclerosis (odds ratio = 5) than erosions (odds ratio = 2). No significant association was found with vertebral squaring. This study adds new data supporting the hypothesis that inflammation (erosions) and possibly repair of inflammation (sclerosis) predict the development of syndesmophytes.
Positron Emission Tomography (PET) imaging studies
The 18F-fluoride radionuclide tracer is incorporated at sites of new bone formation and can be visualized in PET scans. Pilot cross-sectional studies have shown the feasibility of observing 18F-fluoride uptake in syndesmophytes and inflammatory lesions [15, 16]. Further work is needed to investigate if increased vertebral 18F-fluoride uptake predicts local syndesmophyte growth.
Systemic Factors in Syndesmophyte Initiation and Growth
Mediators of skeletal development and homeostasis have received attention as systemic biomarkers of syndesmophyte growth. The Wingless (Wnt) signaling pathway is a key regulator of cell fate and embryogenesis. Among other functions, certain Wnt proteins promote osteoblast development and enhanced bone formation. Inhibitors of this pathway include Dickkopf-1 (DKK-1), sclerostin, and the secreted Frizzled-related proteins. DKK-1 also stimulates osteoprotegerin to promote osteoclastogenesis. In animal models of arthritis, increased DKK-1 levels lead to bone resorption [17], and blockade of DKK-1 caused sacroiliac joint fusion [18]. In addition to its role in angiogenesis, vascular endothelial growth factor (VEGF) promotes bone repair. Adipokines, a family of pleotropic autokines, also have effects on bone.
Wnt pathway molecules
Based on its biological effects and animal studies, DKK-1 levels might be predicted to be lower in patients with AS than healthy controls, or lower in patients with more extensive syndesmophytes. Studies in patients with AS have reported seemingly conflicting results. In contrast to Diarra et al [17], Daoussis et al [19] found that serum DKK-1 levels were higher in patients with AS compared to healthy controls. Heiland et al [20] reported no difference total serum DKK-1 levels in patients with syndesmophytes versus those without syndesmophytes. However, functional DKK-1 levels were significantly lower in patients with more syndesmophyte growth. This association remained significant in a multivariate analysis that adjusted for known syndesmophyte risk factors. In this study, functional DKK-1 was considered a net measure of all Wnt pathway proteins and their antagonists.
This finding raises the question of the potential role of other Wnt pathway mediators, such as sclerostin and Wnt proteins. In an earlier study, Appel et al [21] reported that low serum levels of sclerostin were associated with the formation of new syndesmophytes. In a cross sectional study, Klingberg et al [22] found that patients with AS had significantly higher serum levels of Wnt-3a and lower levels of sclerostin than healthy controls, and elevated serum Wnt-3a levels were independently associated with higher mSASSS. Osteoprotegerin is another key molecule in bone metabolism that is an inhibitor of osteoclast formation. Its expression by osteoblasts is regulated by the Wnt pathway. Associations between osteoprotegerin and syndesmophyte formation in AS have not been carefully studied.
VEGF, adipokines and other molecules
In patients with axial spondyloarthritis included in German Spondyloarthritis Inception Cohort, Poddubnyy et al [23•] found that baseline serum VEGF levels were significantly higher in patients whose mSASSS increased by ≥ 2 units in two years compared to those who did not have similar increases, and were significantly higher in patients who had new syndesmophytes or new bridging compared to those who did not (odds ratio = 13.6).
Matrix metalloproteinase-3 (MMP3) has been implicated as a predictor of progression of structural damage in AS [24]. A further study of this pathway found that a circulating citrullinated and MMP–degraded fragment of vimentin (VICM) might have prognostic value for syndesmophyte progression [25]. Serum levels of VICM were significantly elevated in patients with AS compared to healthy controls. In 115 patients, baseline VICM serum levels were significantly associated with an increase in mSASSS in two years, after adjustment for age, sex, disease duration and baseline mSASSS.
Among studies of adipokines, Syrbe et al [26] found that serum levels of resistin and visfatin were significantly higher in patients with AS compared to healthy controls, and that baseline visfatin levels predicted worsening of mSASSS and new syndesmophyte formation over two years (Table 2).
Table 2.
Associations of systemic factors and radiographic progression.
| Study | Heiland 2012[20] | Appel 2009 [21] | Poddubnyy 2014 [23•] | Bey-Jensen 2013 [25] | Syrbe 2014 [26] |
|---|---|---|---|---|---|
| Biomarker | Functional DKK-1 | Sclerostin | VEGF | VICM | Visfatin |
| Patient group | AS | AS | axSpA | AS | AS |
| Definition of radiographic progression | new syndesmophyte formation or new bridging | new syndesmophyte formation or new bridging | new syndesmophyte formation or new bridging | new syndesmophyte or mSASSS change > 0 | new syndesmophyte formation |
| Nprogressor; Ntotal | 12;49 | 15;46 | 18;172 | NA;115 | 22;86 |
| Level in progressors* | 4.13 ± 2.10 pg/ml | NA | 579.4 ± 385.6 pg/ml | NA | 37.1 ± 55.3 ng/ml |
| Level in nonprogressors* | 6.78 ± 5.48 pg/ml | NA | 404.3 ± 306.8 pg/ml | NA | 15.3 ± 44.8 ng/ml |
| p | 0.002 | 0.007 | 0.041 | 0.0005 | 0.0023 |
Values are mean ± standard deviation
DKK-1: Dickkopf-1; VEGF: vascular endothelial growth factor; VICM: vimentin; AS: ankylosing spondylitis; axSpA: axial spondyloarthritis; Nprogressor: number of patients who had radiographic progression; Ntotal: total number of patients included in the analysis.
Although Wnt pathway molecules, VEGF, VICM, and adipokines have all been suggested to have an effect on radiographic progression, the independent and combined effects of these mediators on the development of new syndesmophytes is unclear. Definitions of radiographic progression differed among studies. It would be important to compare, in the same study, the predictive values of these factors, together with accepted clinical risk factors, such as baseline mSASSS and CRP, so their relative importance could be judged. Identifying systemic biomarkers of syndesmophyte formation will be challenging if much of the important effect is exerted by local mediators, and if levels of these systemic markers are influenced by treatments.
Associations with Treatment
Whether TNFi or NSAIDs can inhibit or slow syndesmophyte formation in AS remains unresolved. One hypothesis posits that because TNF can stimulate DKK-1 production, blocking TNF pharmacologically may actually stimulate new bone formation by decreasing this repressor [27]. A recent study of 22 patients treated for eight years with infliximab provided evidence against this hypothesis by not finding greater increases in mSASSS among the treated group than in historical controls not treated with TNFi [28]. The mean number of new syndesmophytes per patient at eight years was significantly lower in the infliximab-treated group (1.0±0.6 versus 2.7±0.8; p = 0.007).
Haroon et al [29••] reported the association of TNFi treatment with radiographic progression in a prospective cohort study of 334 patients with AS. TNFi use was associated with a lower likelihood of radiographic progression, defined as an increase in mSASSS ≥ 2 over two years, with an adjusted odds ratio of 0.47 (95% confidence interval 0.24–0.94). Results were similar in a sub-analysis that adjusted for propensity to have been prescribed TNFi. Among TNFi-treated patients, both a shorter duration of TNFi treatment and a delay in treatment initiation of more than 10 years was associated with greater mSASSS progression.
In an updated analysis of a clinical trial comparing continuous NSAIDs use (n=76) versus on-demand NSAIDs use (n=74), Kroon et al reported that lower increases in mSASSS over 2 years with continuous NSAIDs use was more pronounced in those patients with elevated time-averaged erythrocyte sedimentation rates, CRPs, and ASDAS [30]. The implication is that by reducing inflammation, continuous use of NSAIDs slows radiographic progression. In another study, high NSAIDs intake was also associated with a lower likelihood of radiographic progression (increase in mSASSS ≥ 2) in patients with AS (odds ratio 0.15; 95% confidence interval 0.02–0.96) but not in those with non-radiographic axial spondyloarthritis [31]. This protective effect was more pronounced in patients an elevated CRP. Conversely, in the studies of Haroon et al [29••] and Ramiro [2••], there were no significant differences in radiographic progression by measures of NSAID use. It is unclear if these different results are due to differences in patient characteristics, measures, or study designs. With the potential for numerous threats to validity, well-controlled observational studies of this topic are difficult to design. Given the slow rate of syndesmophyte development, clinical trials are not practical using radiographic endpoints, and would require more sensitive measures of syndesmophyte growth.
Conclusions
While there have been great advances in the symptomatic treatment of AS, proven disease-modifying treatments are not yet available. To achieve the goal of slowing the development of spinal fusion and to provide better answers about the effects of currently available medications, more sensitive and reliable outcome measures are needed, such as those provided by computed tomography. Focus on local vertebral factors is likely to yield greater insights than measurements done systemically, given the multiple factors other than syndesmophytes that can influence systemic factors.
Key Points.
Syndesmophytes in AS develop slowly, and one-third of patients develop new syndesmophytes visible on plain radiographs over two years.
Growth of syndesmophytes is heterogeneous, even among those in the same disk space, suggesting that local factors are important.
Vertebral inflammation and fat dysplasia on MRI predict future syndesmophytes, but are very insensitive predictors.
Studies of serum proteins have yet to identify any confirmed prognostic biomarkers.
Although some studies are suggestive, no medication has yet been established to slow syndesmophyte growth.
Acknowledgments
Financial support and sponsorship
This work was supported by the Intramural Research Program, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health.
Footnotes
Conflicts of Interest
None of the authors have conflicts of interest related to this work.
Contributor Information
Sovira Tan, Intramural Research Program, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health.
Runsheng Wang, Intramural Research Program, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health.
Michael M. Ward, Intramural Research Program, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health.
References
- 1.Creemers MC, Franssen MJ, van’t Hof MA, et al. Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis. 2005;64:127–129. doi: 10.1136/ard.2004.020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2••.Ramiro S, Stolwijk C, van Tubergen A, et al. Evolution of radiographic damage in ankylosing spondylitis: a 12 year prospective follow-up of the OASIS study. Ann Rheum Dis. 2015;74:52–59. doi: 10.1136/annrheumdis-2013-204055. Evolution of mSASSS in an established AS cohort followed for up to 12 years. [DOI] [PubMed] [Google Scholar]
- 3.Braun J, Baraliakos X, Hermann KGA, et al. The effect of two golimumab doses on radiographic progression in ankylosing spondylitis: results through 4 years of the GO-RAISE trial. Ann Rheum Dis. 2014;73:1107–1113. doi: 10.1136/annrheumdis-2012-203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baraliakos X, Heldmann F, Callhoff J, et al. Which spinal lesions are associated with new bone formation in patients with ankylosing spondylitis treated with anti-TNF agents? A long-term observational study using MRI and conventional radiography. Ann Rheum Dis. 2014;73:1819–25. doi: 10.1136/annrheumdis-2013-203425. [DOI] [PubMed] [Google Scholar]
- 5.Maksymowych WP, Morency N, Conner B, et al. Suppression of inflammation and effects on new bone formation in ankylosing spondylitis: evidence for a window of opportunity in disease modification. Ann Rheum Dis. 2013;72:23–28. doi: 10.1136/annrheumdis-2011-200859. [DOI] [PubMed] [Google Scholar]
- 6.Kang KY, Kwok SK, Ju JH, et al. The predictors of development of new syndesmophytes in female patients with ankylosing spondylitis. Scand J Rheumatol. 2014 Oct 29;:1–4. doi: 10.3109/03009742.2014.938693. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7••.Tan S, Yao J, Flynn JA, et al. Quantitative measurement of syndesmophyte volume and height in ankylosing spondylitis using CT. Ann Rheum Dis. 2014;73:544–50. doi: 10.1136/annrheumdis-2012-202661. Computed tomography can accurately and reliably quantitate syndesmophyte volume and height. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan S, Yao J, Flynn JA, et al. Quantitative syndesmophyte measurement in ankylosing spondylitis using CT: longitudinal validity and sensitivity to change over 2 years. Ann Rheum Dis. 2015;74:437–443. doi: 10.1136/annrheumdis-2013-203946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9•.Tan S, Yao J, Flynn JA, et al. Dynamics of syndesmophyte growth in AS as measured by quantitative CT: heterogeneity within and among vertebral disc spaces. Rheumatology. 2014 doi: 10.1093/rheumatology/keu423. Epub ahead of print. Syndesmophyte growth is heterogeneous, even among those in the same disk space. [DOI] [PMC free article] [PubMed]
- 10.Poddubnyy D, Haibel H, Listing J, et al. Cigarette smoking has a dose-dependent impact on progression of structural damage in the spine in patients with axial spondyloarthritis: results from the German SPondyloarthritis Inception Cohort (GESPIC) Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-203148. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Chung HY, Machado P, van der Heijde D, et al. Smokers in early axial spondyloarthritis have earlier disease onset, more disease activity, inflammation and damage, and poorer function and health-related quality of life: results from the DESIR cohort. Ann Rheum Dis. 2012;71:809–816. doi: 10.1136/annrheumdis-2011-200180. [DOI] [PubMed] [Google Scholar]
- 12.van Tubergen A, Ramiro S, van der Heijde D, et al. Development of new syndesmophytes and bridges in ankylosing spondylitis and their predictors: a longitudinal study. Ann Rheum Dis. 2012;71:518–523. doi: 10.1136/annrheumdis-2011-200411. [DOI] [PubMed] [Google Scholar]
- 13•.Ramiro S, van der Heijde D, van Tubergen A, et al. Higher disease activity leads to more structural damage in the spine in ankylosing spondylitis: 12-year longitudinal data from the OASIS cohort. Ann Rheum Dis. 2014;73:1455–1461. doi: 10.1136/annrheumdis-2014-205178. Increases in mSASSS were associated with higher cumulative AS disease activity. [DOI] [PubMed] [Google Scholar]
- 14.Ramiro S, van Tubergen A, van der Heijde D, et al. Erosions and sclerosis on radiographs precede the subsequent development of syndesmophytes at the same site: A twelve-year prospective follow up of patients with ankylosing spondylitis. Arthritis Rheumatol. 2014;66:2773–2779. doi: 10.1002/art.38775. [DOI] [PubMed] [Google Scholar]
- 15.Bruijnen STG, van der Weijden MAC, Klein JP, et al. Bone formation rather than inflammation reflects Ankylosing Spondylitis activity on PET-CT: a pilot study. Arthritis Res Ther. 2012;14(2):R71. doi: 10.1186/ar3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SG, Kim IJ, Kim KY, et al. Assessment of bone synthetic activity in inflammatory lesions and syndesmophytes in patients with ankylosing spondylitis: the potential role of 18F-fluoride positron emission tomography-magnetic resonance imaging. Clin Exp Rheumatol. 2015 Jan 9; Epub ahead of print. [PubMed] [Google Scholar]
- 17.Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 18.Uderhardt S, Diarra D, Katzenbeisser J, et al. Blockade of Dickkopf (DKK)-1 induces fusion of sacroiliac joints. Ann Rheum Dis. 2010;69:592–597. doi: 10.1136/ard.2008.102046. [DOI] [PubMed] [Google Scholar]
- 19.Daoussis D, Liossis SN, Solomou EE, et al. Evidence that Dkk-1 is dysfunctional in ankylosing spondylitis. Arthritis Rheum. 2010;62:150–158. doi: 10.1002/art.27231. [DOI] [PubMed] [Google Scholar]
- 20.Heiland GR, Appel H, Poddubnyy D, et al. High level of functional dickkopf-1 predicts protection from syndesmophyte formation in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71:572–574. doi: 10.1136/annrheumdis-2011-200216. [DOI] [PubMed] [Google Scholar]
- 21.Appel H, Ruiz-Heiland G, Listing J, et al. Altered skeletal expression of sclerostin and its link to radiographic progression in ankylosing spondylitis. Arthritis Rheum. 2009;60:3257–3262. doi: 10.1002/art.24888. [DOI] [PubMed] [Google Scholar]
- 22.Klingberg E, Nurkkala M, Carlsten H, Forsblad-d’Elia H. Biomarkers of bone metabolism in ankylosing spondylitis in relation to osteoproliferation and osteoporosis. J Rheumatol. 2014;41:1349–1356. doi: 10.3899/jrheum.131199. [DOI] [PubMed] [Google Scholar]
- 23•.Poddubnyy D, Conrad K, Haibel H, et al. Elevated serum level of the vascular endothelial growth factor predicts radiographic spinal progression in patients with axial spondyloarthritis. Ann Rheum Dis. 2014;73:2137–2143. doi: 10.1136/annrheumdis-2013-203824. Higher serum VEGF levels predicted new syndesmophyte formation. [DOI] [PubMed] [Google Scholar]
- 24.Maksymowych WP, Landewe R, Conner-Spady B, et al. Serum matrix metalloproteinase 3 is an independent predictor of structural damage progression in patients with ankylosing spondylitis. Arthritis Rheum. 2007;56:1846–1853. doi: 10.1002/art.22589. [DOI] [PubMed] [Google Scholar]
- 25.Bay-Jensen AC, Karsdal MA, Vassiliadis E, et al. Circulating citrullinated vimentin fragments reflect disease burden in ankylosing spondylitis and have prognostic capacity for radiographic progression. Arthritis Rheum. 2013;65:972–980. doi: 10.1002/art.37843. [DOI] [PubMed] [Google Scholar]
- 26.Syrbe U, Callhoff J, Conrad K, et al. Adipokine serum levels in patients with ankylosing spondylitis and their relation to clinical parameters and radiographic spinal progression. Arthritis Rheumatol. 2014 doi: 10.1002/art.38968. [DOI] [PubMed] [Google Scholar]
- 27.Maksymowych WP, Chiowchanwisawakit P, Clare T, et al. Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis: evidence of a relationship between inflammation and new bone formation. Arthritis Rheum. 2009;60:93–102. doi: 10.1002/art.24132. [DOI] [PubMed] [Google Scholar]
- 28.Baraliakos X, Haibel H, Listing J, et al. Continuous long-term anti-TNF therapy does not lead to an increase in the rate of new bone formation over 8 years in patients with ankylosing spondylitis. Ann Rheum Dis. 2014;73:710–715. doi: 10.1136/annrheumdis-2012-202698. [DOI] [PubMed] [Google Scholar]
- 29••.Haroon N, Inman RD, Learch TJ, et al. The impact of tumor necrosis factor alpha inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum. 2013;65:2645–2654. doi: 10.1002/art.38070. Tumor necrosis factor-alpha inhibitor use was associated with lower likelihood of an increase in mSASSS in this large observational study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroon F, Landewe R, Dougados M, van der Heijde D. Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71:1623–1629. doi: 10.1136/annrheumdis-2012-201370. [DOI] [PubMed] [Google Scholar]
- 31.Poddubnyy D, Rudwaleit M, Haibel H, et al. Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis. 2012;71:1616–1622. doi: 10.1136/annrheumdis-2011-201252. [DOI] [PubMed] [Google Scholar]