Abstract
Lycopene, a red non-provitamin A carotenoid, mainly presenting in tomato and tomato byproducts, has the highest antioxidant activity among carotenoids because of its high number of conjugated double bonds. The objective of this study was to investigate the effect of lycopene supplementation in the diet on plasma lipid profile, lipid peroxidation and antioxidant defense system in feedlot lamb. Twenty-eight Bamei male lambs (90 days old) were divided into four groups and fed a basal diet (LP0, 40:60 roughage: concentrate) or the basal diet supplemented with 50, 100, and 200 mg/kg lycopene. After 120 days of feeding, all lambs were slaughtered and sampled. Dietary lycopene supplementation significantly reduced the levels of plasma total cholesterol (p<0.05, linearly), total triglycerides (TG, p<0.05) and low-density lipoprotein cholesterol (LDL-C, p<0.05), as well as atherogenic index (p<0.001), whereas no change was observed in high-density lipoprotein cholesterol (p>0.05). The levels of TG (p<0.001) and LDL-C (p<0.001) were decreased with the feeding time extension, and both showed a linear trend (p<0.01). Malondialdehyde level in plasma and liver decreased linearly with the increase of lycopene inclusion levels (p<0.01). Dietary lycopene intake linearly increased the plasma antioxidant vitamin E level (p<0.001), total antioxidant capacity (T-AOC, p<0.05), and activities of catalase (CAT, p<0.01), glutathione peroxidase (GSH-Px, p<0.05) and superoxide dismutase (SOD, p<0.05). The plasma T-AOC and activities of GSH-Px and SOD decreased with the extension of the feeding time. In liver, dietary lycopene inclusion showed similar antioxidant effects with respect to activities of CAT (p<0.05, linearly) and SOD (p<0.001, linearly). Therefore, it was concluded that lycopene supplementation improved the antioxidant status of the lamb and optimized the plasma lipid profile, the dosage of 200 mg lycopene/kg feed might be desirable for growing lambs to prevent environment stress and maintain normal physiological metabolism.
Keywords: Lycopene, Plasma, Lipid Profile, Antioxidant Enzymes, Malondialdehyde, Lamb
INTRODUCTION
There is an increase in the demand for lamb in China as people seek more protein and more diverse diets. Industrial agriculture based on large-scale monoculture is gradually becoming the dominant system of modern sheep farming, which implies that animals will face a variety of psychological and environmental stresses and consequent health risks. Due to cost and practicality, dietary modifications of ruminants are preferable to other methods of improving production and health. Recent studies have shown that diets enriched with antioxidant substances can be used to attenuate the negative effects of environmental stress, as the detrimental effects of environmental stress could be partly due to induction of oxidative stress (Bollengier-Lee et al., 1998; Seven et al., 2010; Chauhan et al., 2014).
Lycopene, a carotenoid antioxidant without provitamin-A activity, has recently received considerable scientific interest. Lycopene can provide a protection against damage caused by reactive oxygen species (ROS) (Di-Mascio et al., 1989; Jain et al., 1999), and thus prevent damage to cells and tissues as well as ameliorating genetic problems (Atish and Anil, 2013). Studies have showed that lycopene consumption has a cardioprotection effect in humans and animals (Arab and Steck, 2000; Rissanen, 2006) by up-regulating the redox status such as improving antioxidant enzyme activities and antioxidant vitamin contents (Luo and Wu, 2011), and optimizing the plasma lipid profile (Sahin et. al., 2006a; Upaganlawar and Balaraman, 2012). To our knowledge, the majority of lycopene-related studies have focused on humans and rodents (Jain et al., 1999; Atish and Anil, 2013; Aydin et al., 2013), while the cardioprotection and antioxidative effects exerted by lycopene have not previously been investigated in sheep.
A great quantity of by-products, mainly tomato peel and seeds, are generated by the tomato industry in Bayannaoer area of the autonomous province of Inner Mongolia, which is the second largest tomato planting and processing base after Xinjiang province. As an alterative solution for reducing the burden on environment, the agro-industrial byproducts are locally used as supplements in small ruminant diets and lambs on this diet perform very well in terms of growth and health. Tomato seeds and peel residues contain a great variety of biologically active substances, principally lycopene (Calvo et al., 2008), so whether the lycopene plays a positive role in the good effects of the byproduct is still not elucidated in sheep.
The objective of this study was, therefore, to investigate the effects of different levels of dietary tomato lycopene supplementation on plasma lipid profile, lipid peroxidation and antioxidant defense systems in feedlot Bamei lambs.
MATERIALS AND METHODS
The feeding trial was conducted at Fuchuan sheep farm, Bayannaoer city, Inner Mongolia, China (40°59′47″ N latitude and 107°34′50″ E longitude). The experiment lasted for 120 days, beginning on May 28, 2013 and ending on September 24, 2013. The climate of the location is classified as temperate continental monsoon climate. Maximum environmental temperatures registered throughout the experiment varied from 22°C to 36°C. The experiment was approved by the Institutional Animal Care Committee of China Agricultural University with the criteria in the Guide for the Care of Laboratory Animals (Beijing, China).
Animals and diets
Twenty-eight Bamei male lambs (a meat-type sheep developed in 2007) with average age of 3 months±10 days, an average initial live weight of 19.34±2.21 kg and the same genetic background were equally assigned, on the basis of body weight, into 4 dietary treatments of 7 animals each. All animals were kept in individual pens (2.5×1 m2) with sand bedding in an open-side barn, with a roof but without walls. All lambs were placed on the sunny side of shed in a line across the lateral direction and they were allowed visual contact with each other and had ad libitum access to water. The experimental period lasted 120 days and was preceded by an adaption period of two weeks.
Food grade lycopene powder (10.84% lycopene) was produced by Shanxi Sciphar Hi-tech Industry Co., Ltd, Xi’an, China. The control group (LP0) was fed a basal diet without lycopene supplementation. The other three groups were fed the basal diet with dietary lycopene supplementation at levels of 50 (LP50), 100 (LP100), and 200 (LP200) mg lycopene/kg feed, respectively. The ingredients and chemical composition of the basal diet, which was formulated according to NRC (2007), are shown in Table 1. The feed samples destined for chemical analyses were taken at the beginning, in the middle and at the end of the experimental period, then were pooled and analyzed in triplicate for crude protein (AOAC, 2000), neutral detergent fiber fractions and acid detergent fiber fractions (Van Soest et al., 1991). Gross energy content was measured using an adiabatic bomb calorimeter (Parr 1261, Parr Instrument Company, Moline, IL, USA). The crude fat was determined by extracting the sample with petroleum ether using an automatic soxhlet extractor (Gerhardt Analytical Systems, Konigswinter, Germany). During the trial, each lamb was fed twice daily at 6:00 and 17:00. Orts were recorded daily.
Table 1.
Ingredients and chemical composition of the basal diet fed to the lambs1
| Items | Basal diet |
|---|---|
| Ingredients (%, as fed basis) | |
| Chinese wildrye grass hay, ground | 32 |
| Alfalfa hay, ground | 8 |
| Corn | 34 |
| Soybean meal | 8.9 |
| Dried distilled grain solubles | 9.0 |
| Wheat bran | 5.5 |
| Salt | 0.5 |
| Limestone | 0.3 |
| Dicalcium phosphate | 0.5 |
| Sodium bicarbonate | 0.3 |
| Minerals and vitamin premix2 | 1.0 |
| Chemical composition (%, as-fed basis) | |
| Dry matter | 91.7 |
| Gross energy (MJ/kg) | 15.98 |
| Crude protein | 13.93 |
| Crude fat | 2.51 |
| Neutral detergent fiber | 35.98 |
| Acid detergent fiber | 17.89 |
Experimental diets were supplemented with 0, 50, 100, and 200 mg lycopene/1 kg basal diet.
Per kilogram of diet: 23 mg Fe, 28 mg Zn, 17 mg Mn, 6 mg Cu, 0.04 mg I, 0.2 mg Se, 0.1 mg Co, 3,700 IU vitamin A, 1,000 mg vitamin D3, 60 IU vitamin E.
Sample preparation
At day 30, 60, 90, and 120 of the trial period, 10 mL blood was taken from jugular vein of each lamb into heparin sodium tube (vacuum blood collection) at 6:00 AM prior to the morning feeding, and then immediately centrifuged at 3,000 r/m for 10 min at 4°C to obtain plasma. The plasma was divided into aliquots for storage at −20°C till analysis.
At the end of 120-day feeding trial, sheep were fasted overnight with free access to water and slaughtered in the local abattoir using Halal methods (Sen et al., 2006). The liver was sampled and stored at −80°C till analysis.
Analytical procedure
Concentrations of total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and total triglycerides (TG) in plasma were measured enzymatically on an automatic analyzer (Hitachi 7600, Tokyo, Japan); Atherogenic index (AI) was estimated using the formula of AI = (TC − HDL)/HDL.
The concentrations of Vitamin E and Vitamin A in plasma were determined by high performance liquid chromatography (HPLC), following precipitation of proteins by ethanol and extraction of the supernatant in hexane. The hexane layer was transferred to a test tube and evaporated to dryness in a 60°C water bath under a stream of nitrogen gas. The residue was re-dissolved immediately in 0.5 mL of methanol and transferred to an amber glass vial equipped with screw cap and PTFE/rubber septa. Samples were analyzed by normal phase HPLC (Dikma Diamonsil-C18, 250×4.6 mm, 5 μm, 100Å ) fitted with variable wavelength ultraviolet detector (Agilent Series 1200, Santa Clara, CA, USA). The mobile phase was methanol: water (98:2, v/v) at a constant flow rate of 1 mL/min. The injection volume was 20 μL. The detection wavelength was set to 230 nm and 265 nm for vitamin E and vitamin A respectively. Results were presented in mg/L.
Malondialdehyde (MDA) level of lipid peroxides, total antioxidant capacity (T-AOC), and activities of catalase (CAT), glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) in plasma and liver were determined using commercially available assay kits (Institute of Biological Engineering of Nanjing Jianchen, Nanjing, China). Liver homogenates were centrifuged at 2,500 r/m for 10 min at 4°C to remove crude fractions, and the resulting supernatants were used for the determination of enzymatic activities by spectrophotometric methods using a spectrophotometer (UV-1800PC, Shanghai mapada instruments Co., Ltd., Shanghai, China). All of the assays followed the instructions of the kits. Liver protein was measured by the method of coomassie brilliant blue and detected at 595 nm. The MDA level was presented in nmol/mL in plasma or nmol/mg protein in liver, and the enzyme activity was presented in unit/mL plasma or unit/mg protein.
Statistical analyses
Blood parameters data of lipid profile and antioxidant enzymes were analyzed using repeated measures of general linear model (GLM) in IBM SPSS Statistics 21.0 (Statistical Product and Service Solutions, IBM Corporation, New York, NY, USA), which consists of diet treatment, sampling time and treatment×sampling time interaction. Means were separated using least significant difference and presented as means±standard error in tabular form. When the interaction was not significant but the main effects of treatment and/or sampling time were significant, difference among all means was tested separately with least significant difference multiple-range tests. Statistical significance was declared at p≤0.05.
The content of plasma vitamins and activities of liver antioxidant enzymes were subjected to GLM procedures of SPSS. Differences among means were tested by least significant difference comparisons. Orthogonal polynomial contrasts were used to examine the linear effects of increasing levels of lycopene in the diet. In the orthogonal polynomial analysis, coefficients were corrected because of unequal spacing of treatments. A value of p≤0.05 was used to indicate statistical significance.
RESULTS
Plasma lipids profile
The effects of varying levels of dietary lycopene supplementation and sampling time on plasma lipid profile in sheep are presented in Table 2. The levels of plasma TC (p<0.01), TG (p<0.05) and LDL-C (p<0.05) were reduced in lycopene-supplemted groups, compared to the control group, and the level of TC showed a significant linear trend (p<0.05) with the increasing of lycopene levels. Compared to the control group, there was a significant decrease in AI (p<0.001) as the decrease of plasma TC and the numerical increment of HDL-C level in lycopene treatments, although no treatment effect was observed in HDL-C level (p>0.05). The levels of TG (p<0.01) and LDL-C (p<0.001) were decreased with the feeding time extension, and both showed a linear trend (p<0.01). There was no interaction in plasma lipid profile between treatment and sampling time.
Table 2.
Effects of varying levels of dietary lycopene supplementation and sampling time on plasma lipid profile in feedlot sheep
| TC (mmol/L) | TG (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) | AI1 | |
|---|---|---|---|---|---|
| LP2 | |||||
| LP0 | 1.68±0.09a | 0.64±0.02a | 0.59±0.03 | 0.61±0.04a | 2.00±0.20a |
| LP50 | 1.36±0.06b | 0.60±0.01ab | 0.61±0.03 | 0.51±0.04ab | 1.27±0.07b |
| LP100 | 1.44±0.06b | 0.61±0.01ab | 0.65±0.04 | 0.51±0.03ab | 1.24±0.08b |
| LP200 | 1.39±0.04b | 0.59±0.01b | 0.66±0.03 | 0.48±0.04b | 1.16±0.11b |
| Time3 | |||||
| Day 30 | 1.49±0.07 | 0.66±0.02a | 0.67±0.02 | 0.63±0.03a | 1.27±0.14 |
| Day 60 | 1.36±0.07 | 0.60±0.01b | 0.58±.03 | 0.56±0.03a | 1.46±0.16 |
| Day 90 | 1.42±0.08 | 0.57±0.01b | 0.60±.04 | 0.45±0.04b | 1.42±0.13 |
| Day 120 | 1.61±0.09 | 0.59±0.01b | 0.65±0.04 | 0.46±0.03b | 1.54±0.15 |
| Statistical significance | |||||
| LP | ** | * | NS | * | *** |
| Time | NS | *** | NS | *** | NS |
| LP×time | NS | NS | NS | NS | NS |
| Linear | |||||
| LP | * | NS | NS | NS | NS |
| Time | NS | ** | NS | *** | NS |
TC, total cholesterol; TG, total triglyceride; HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoprotein-cholesterol; AI, atherogenic index ; LP, lycopene; NS, not significant.
AI = (TC-HDL)/HDL.
LP0, no LP; LP50, 50 mg LP/kg feed; LP100, 100 mg LP/kg feed; LP200, 200 mg LP/kg feed.
Time means sampling time at day 30, 60, 90, and 120 of feeding.
Means with different small letters within LP or time in the same column are significantly different (p<0.05).
p<0.05;
p<0.01;
p<0.001.
Lipid peroxidation
In Table 3, plasma MDA level was significantly affected by treatment and sampling time, in which dietary lycopene decreased the concentration of plasma MDA (p<0.001), while sampling time showed the reverse pattern with the extension of the feeding time (p<0.05, linearly). In liver, dietary lycopene had the same positive effect for MDA as that in plasma (p<0.01), especially for LP200 treatment, significantly lower than the LP0 and LP50 treatments.
Table 3.
Effect of varying levels of dietary lycopene on plasma and liver malondialdehyde (MDA) levels in feedlot sheep
| Items | MDA | |
|---|---|---|
|
| ||
| Plasma (nmol/mL) | Liver (nmol/mg protein) | |
| Treatment1 | ||
| LP0 | 3.83±0.32a | 2.91±0.33a |
| LP50 | 2.45±0.19b | 2.70±0.32ab |
| LP100 | 2.59±0.15b | 1.81±0.14bc |
| LP200 | 2.68±0.22b | 1. 48±0.14c |
| p-value | *** | ** |
| Linear | ** | ** |
| Time2 | ||
| Day 30 | 2.05±0.13b | |
| Day 60 | 3.38±0.34a | |
| Day 90 | 2.93± 0.26a | |
| Day 120 | 3.21±0.14a | |
| p-value | ** | |
| Linear | * | |
LP, lycopene.
LP0, no LP; LP50, 50 mg LP/kg feed; LP100, 100 mg LP/kg feed; LP200, 200 mg LP/kg feed.
Time means sampling time at day 30, 60, 90 and 120 of feeding.
Means with different small letters in the same row are significantly different (p<0.05).
p<0.05;
p<0.01;
p<0.001.
Antioxidant defense system
As shown in Figure 1, plasma vitamin E level was largely increased with the increase of lycopene supplementation levels in the diets after supplementation for 120 days (p<0.001). The mean level of vitamin E increased linearly by 130% from LP0 treatment to LP200 treatment (p<0.001). No significant difference was observed among the treatments with respect to content of vitamin A (p>0.05).
Figure 1.
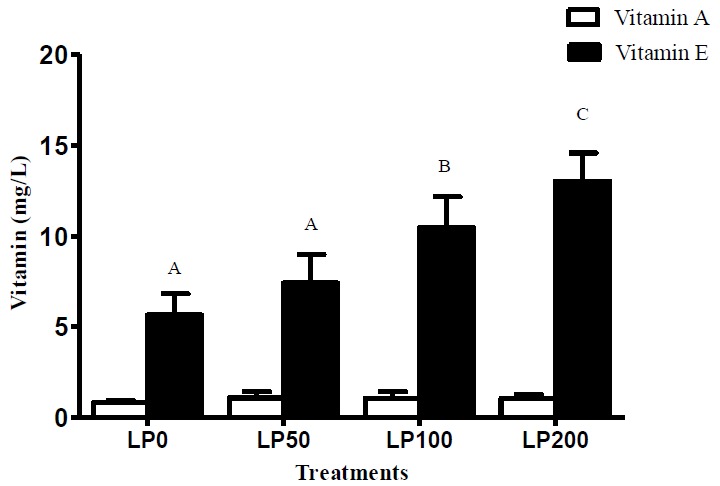
Effect of different dietary lycopene levels on plasma vitamin A and vitamin E levels in feedlot Bamei sheep. Values with different letters are highly significantly different (p<0.01). Vertical bars are standard deviations. LP, lycopene; LP0 = No LP; LP50, 50 mg LP/kg feed; LP100, 100 mg LP/kg feed; LP200, 200 mg LP/kg feed.
Effects of varying levels of dietary lycopene on T-AOC and antioxidant enzymes activities in plasma and liver are presented in Table 4 and 5, respectively. In Table 4, dietary lycopene intake linearly increased the plasma T-AOC (p<0.05), although no significant difference was found among all treatments (p>0.05). The activities of GSH-Px (p<0.05), CAT (p<0.01) and SOD (p<0.05) in lycopene-supplemented groups were all increased linearly, especially in LP100 and LP200 treatments, compared to the control group. As for sampling time, the activities of plasma T-AOC, GSH-Px, and SOD were decreased with the extension of the feeding time (Table 4). There was no variation for liver T-AOC and GSH-Px activity among the four treatment groups (Table 5). Similar to the situation in plasma, activities of liver CAT (p<0.05) and SOD (p<0.001) were linearly increased with the increase in dietary lycopene supplementation levels (Table 5), especially significantly in LP200 treatments, compared to LP0 group.
Table 4.
Effects of varying levels of dietary lycopene and sampling time on plasma T-AOC and antioxidant enzymes activities in sheep
| T-AOC | CAT | GSH-Px | SOD | |
|---|---|---|---|---|
| LP1 | ||||
| LP0 | 17.87±0.68 | 9.51±0.49b | 131.12±7.97b | 68.13±5.58a |
| LP50 | 19.40±0.86 | 10.70±0.64ab | 154.19±7.31ab | 81.89±4.41b |
| LP100 | 20.53±1.14 | 12.25±0.51a | 158.25±9.84ab | 85.16±5.34b |
| LP200 | 20.71±1.15 | 12.04±0.69a | 180.02±12.72a | 84.91±5.40b |
| Time3 | ||||
| Day 30 | 22.42±0.80a | 11.47±0.68 | 180.85±13.31a | 91.74±2.29a |
| Day 60 | 20.42±1.07ab | 11.21±0.54 | 154.02±8.15ab | 88.77±4.20a |
| Day 90 | 18.17±1.05b | 10.64±0.41 | 133.37±9.71b | 81.29±4.44a |
| Day 120 | 17.50±0.63b | 11.06±0.76 | 155.34±6.44ab | 58.29±6.31b |
| Statistical significance | ||||
| LP | NS | ** | ** | * |
| Time | ** | NS | * | *** |
| LP×time | NS | NS | NS | NS |
| Linear | ||||
| LP | * | ** | * | * |
| Time | *** | NS | NS | *** |
T-AOC, total antioxidant capacity; CAT, catalase; GSH-Px, glutathione peroxidase; SOD, superoxide dismutase; LP, lycopene; NS, not significant.
LP0, no LP; LP50, 50 mg LP/kg feed; LP100, 100 mg LP/kg feed; LP200, 200 mg LP/kg feed.
Time means sampling time at day 30, 60, 90, and 120 of feeding.
Means with different small letters within LP or Time in the same column are significantly different (p<0.05).
p<0.05;
p<0.01;
p<0.001.
Table 5.
Effect of varying levels of dietary lycopene on liver T-AOC and antioxidant enzymes activities (nmol/mg protein) in feedlot sheep
| Variables | Treatment1 | p value | Linear | |||
|---|---|---|---|---|---|---|
|
| ||||||
| LP0 | LP50 | LP100 | LP200 | |||
| T-AOC | 1.48±0.31 | 1.37±0.10 | 1.56±0.07 | 1.77±0.13 | NS | NS |
| CAT | 62.09±2.56 | 70.72±4.92 | 73.37±3.82 | 75.89±3.56 | NS | * |
| GSH-Px | 336.12±25.19 | 338.29±33.26 | 350.08±12.64 | 345.74±20.13 | NS | NS |
| SOD | 40.47±0.62b | 40.16±2.38b | 48.13±1.79ab | 54.86±3.76a | ** | *** |
LP, lycopene; T-AOC, total antioxidant capacity; NS, not significant; CAT, catalase; GSH-Px, glutathione peroxidase; SOD, superoxide dismutase.
LP0, no LP; LP50, 50 mg LP/kg feed; LP100, 100 mg LP/kg feed; LP200, 200 mg LP/kg feed.
Means with different small letters in the same row are significantly different (p<0.05).
p<0.05;
p<0.01;
p<0.001.
DISCUSSION
Sheep in intensive farming system are frequently exposed to a variety of stressors, including environmental and psychological ones. In the present study, our experiment was conducted in summer and the average maximum temperature was 29°C (extreme temperatures reached 36°C in July and August). Experimental animals were exposed to the sun from 9:30 am to 5:00 pm because the roof of the shed was 3 meters high and the animals were arranged on the sunny side of the barn. Sheep tend to be less susceptible to heat stress than other animal species such as swine and cattle due to the presence of the wool cover (Marai, 2007). We observed that the lambs were panting at the daytime. In addition, all lambs were kept in individual pens and they were observed to become very vocal and agitated when separated from their flock mates, which posed a great psychological or emotional distress as sheep are flock animals and strongly gregarious (Nowak et al., 2008). Both factors above are stressors for lambs and they are associated with increased ROS production (Mujahid et al., 2005; Wang et al., 2007; Sahin et al., 2011). Superfluous ROS induced by stress can disturb the balance between oxidant and antioxidant systems. Thus lambs need to be supplemented with adequate antioxidant by diets to improve the antioxidant status. Herbal antioxidants were shown to have the ability to scavenge excessive free radicals in vivo and thus affect antioxidant defense in sheep (Nieto et al., 2010; Ortuno et al., 2014). In our experiment, we examined the effects of lycopene supplementation on antioxidant defense enzymes like SOD, GSH-Px, and CAT and non-enzymes such as vitamins A and E. The results showed significant increasing tendencies for these enzymes activities and the content of antioxidant vitamin E, which demonstrated an improvement in antioxidant status of the sheep as a result of the supplementation with lycopene. Lycopene is the single most potent natural carotenoid oxygen quencher (Di-Mascio et al., 1989; Rao and Agarwal, 1999; Agarwal and Rao, 2000). It can inactivate hydrogen peroxide and nitrogen dioxide (Bohm et al., 1995). Because of the lack of studies regarding lycopene as dietary supplement in ruminant animals, the results of the present experiment are not comparable with literature. However, the positive impacts of lycopene supplementation on antioxidant status are in agreement with previous studies done in other animal species such as rat, poultry and fish (Breinholt et al., 2000; Sahin et al., 2006a; 2014). In sheep, Sgorlon et al. (2006) had confirmed that tomato pomace, containing 1.3% lycopene, was able to counterbalance oxidative stress in sheep by inducing specific transcriptional activity of genes involved in oxidant defenses. We previously found that dry matter intake had a linear increase with an increased addition of lycopene (p<0.05), which were 943, 989, 1,055, and 1,023 g/d for LP0, LP50, LP100, and LP200 groups respectively. The average daily weight gain increased by 8.54%, 12.20%, and 7.32% in LP50, LP100 and LP200 treatments, respectively, compared to LP0 group (unpublished data), also implying that lycopene supplementation seems to ameliorate the stress suffered by lambs. In addition, the observed decrease in activities of the antioxidant enzymes (T-AOC, GSH-Px, and SOD) with the extension of the feeding time suggests a lower oxidative status in lambs, the reason for which might be that animal gradually adapted to the stress and thus their anti-stress capability correspondingly enhanced with the growth.
Stress can disturb the balance between the production of ROS and antioxidant systems and results in oxidative injury such as lipid peroxidation (Sahin et al., 2014). Lipid peroxides and their products can cause damage to membrane-bound enzymes and other macromolecules, including DNA, and have been implicated in several disease processes (Sahin et al, 2006b; 2008). In the present study, the contents of plasma and liver MDA showed a significantly decrease trend in a linear manner with the increase of lycopene supplement levels. MDA is one of the most frequently used indicators of lipid peroxidation associated with oxidative stress (Aksu et al., 2010). The decrease in plasma and liver MDA levels could be due to the ability of lycopene to affect antioxidant defense system. Similar to our results, Sahin et al. (2006; 2008; and 2014) reported that quail and rainbow trout supplemented with lycopene had a significant reduction in MDA values in serum and liver.
Antioxidant properties of lycopene were thought to be primarily responsible for its biological effects (Sahin et al, 2006a; 2011; 2014), which may be important in the prevention from chronic diseases associated with oxidative stress such as cardiovascular diseases. Studies have provided evidence in support of the protective effect of lycopene on a favorable lipid profile (Sahin et al., 2006a; Napolitano et al., 2007; Ried and Fakler, 2011). In the present study, our data showed that the plasma TC, TG, and LDL-C levels (p<0.05) were reduced in lycopene supplemented groups, compared to the control group, meanwhile, the values of TG (p<0.01) and LDL-C maintained a decreased trend with the feeding time extension, which demonstrated the cardioprotection effect of lycopene in sheep. Amarenco et al. (2007; 2008) reported that free radicals were excessively generated in all living organisms as a result of increased metabolic activities induced by the stress and were presumed to trigger degenerative diseases like atherosclerosis, which is positively correlated with serum LDL-C and inversely correlated with HDL-C. Stress exposure also increases the levels of blood TC and TG and supplementation of antioxidants decreases these elevated levels (Sahin et al., 2006a; Marai, 2007). Similar effects of different antioxidants on the lipid metabolism have been reported (Oshima et al., 1996). To the best of our knowledge, this is the first study to evaluate the effect of lycopene supplementation on blood lipid parameters measured in sheep. Fuhrman et al. (1997) demonstrated that tomato lycopene, when used as dietary supplement at 60 mg/d, significantly decreased plasma LDL-C and TG concentration without affecting HDL-C in macrophages. Likewise, Silaste et al. (2007) found that the average plasma TC and LDL-cholesterol were reduced by 5.9% and 12.9%, respectively, in healthy normocholesterolemic adults after high daily dietary intake of tomato juice and ketchup for a period of 3 weeks, without any change in the concentrations of HDL-cholesterol and triglycerides. In addition to its antioxidant properties, lycopene has been proposed to reduce cholesterol levels by its suppression of cholesterol synthesis, increase of LDL degradation, and inhibition of the hydroxy-methyl-glutaryl-coenzyme, a (HMGCoA)-reductase enzyme (Fuhrman et al., 1997), which needs to be further investigated in sheep. In our experiment, we speculated that the positive effect on lipid metabolism for lycopene could be related to the antioxidative effect of lycopene, reflected by higher vitamin E levels and antioxidant enzyme activities as well as lower MDA concentration in lycopene-supplemented groups.
CONCLUSION
In conclusion, the present study showed that lycopene supplementation improved plasma and liver antioxidant capacities in feedlot Bamei lambs during summer. In addition, it reduced lipid peroxidation and improved the lipid profile, thus offering an effective strategy to decrease the risk of developing diseases related to oxidative stress. Based on the data in lipid profile, MDA and antioxidant defense system in the present study, the level of 200 mg/kg lycopene in the diet is recommended for growing lambs to counter environment stress and keep normal physiological metabolism.
ACKNOWLEDGMENTS
The authors wish to express their gratitude to Dr. Elaine Grings (South Dakota State University) for her comments and corrections on this manuscript. This research was supported by projects of the China Agricultural Ministry (CARS-39 and 200903060). We appreciate the assistance of Kun Liu and Zhaoyun Zuo in obtaining the samples.
REFERENCES
- Agarwal A, Rao AV. Tomato lycopene and its role in human health and chronic diseases. Can Med Assoc J. 2000;163:739–744. [PMC free article] [PubMed] [Google Scholar]
- Aksu DS, Aksu T, Ozsoy B, Baytok E. The effects of replacing inorganic with a lower level of organically complexed minerals (Cu, Zn and Mn) in broiler diets on lipid peroxidation and antioxidant defense systems. Asian Australas J Anim Sci. 2010;23:1066–1072. [Google Scholar]
- Amarenco P, Goldstein LB, Szarek M, Sillesen H, Rudolph AE, Callahan A, Hennerici M, Simunovic L, Zivin JA, Welch KMA. Effects of intense low-density lipoprotein cholesterol reduction in patients with stroke or transient ischemic attack: The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke. 2007;38:3198–3204. doi: 10.1161/STROKEAHA.107.493106. [DOI] [PubMed] [Google Scholar]
- Amarenco P, Labreuche J, Touboul PJ. High-density lipoprotein-cholesterol and risk of stroke and carotid atherosclerosis: A systematic review. Atherosclerosis. 2008;196:489–496. doi: 10.1016/j.atherosclerosis.2007.07.033. [DOI] [PubMed] [Google Scholar]
- AOAC. Official Methods of Analysis. 17th edn. Association of Official Analytical Chemists; Gaithersburg, MD, USA: 2000. [Google Scholar]
- Arab L, Steck S. Lycopene and cardiovascular disease. Am J Clin Nutr. 2000;71:1691S–1695S. doi: 10.1093/ajcn/71.6.1691S. [DOI] [PubMed] [Google Scholar]
- Atish P, Anil K. Lycopene protects against memory impairment and mito-oxidative damage induced by colchicine in rats: An evidence of nitric oxide signaling. Eur J Pharmacol. 2013;721:373–381. doi: 10.1016/j.ejphar.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Aydin S, Palabiyik SS, Erkekoqlu P, Sahin G, Basaran N, Giray BK. The carotenoid lycopene protects rats against DNA damage induced by Ochratoxin A. Toxicon. 2013;73:96–103. doi: 10.1016/j.toxicon.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Bohm F, Trinkler JH, Truscott TG. Carotenoids protect against cell membrane damage by the nitrogen dioxide radical. Nat Med. 1995;1:98–99. doi: 10.1038/nm0295-98. [DOI] [PubMed] [Google Scholar]
- Bollengier-Lee S, Mitchell MA, Utomo DB, Williams PEV, Whitehead CC. Influence of high dietary vitamin E supplementation on egg production and plasma characteristics in hens subjected to heat stress. Br Poult Sci. 1998;39:106–112. doi: 10.1080/00071669889466. [DOI] [PubMed] [Google Scholar]
- Breinholt V, Lauridsen ST, Daneshvar B, Jakobsen J. Dose-response effects of lycopene on selected drug-metabolizing and antioxidant enzymes in the rat. Cancer Lett. 2000;154:201–210. doi: 10.1016/s0304-3835(00)00401-8. [DOI] [PubMed] [Google Scholar]
- Calvo MM, Garcia ML, Selgas MD. Dry fermented sausages enriched with lycopene from tomato peel. Meat Sci. 2008;80:167–172. doi: 10.1016/j.meatsci.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Chauhan SS, Celi P, Leury BJ, Clarke IJ, Dunshea FR. Dietary antioxidants at supranutritional doses improve oxidative status and reduce the negative effects of heat stress in sheep. J Anim Sci. 2014;92:3364–3374. doi: 10.2527/jas.2014-7714. [DOI] [PubMed] [Google Scholar]
- Di-Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 1989;274:532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- Fuhrman B, Elis A, Aviram M. Hypocholesterolemic effect of lycopene and β-carotene is related to suppression of cholesterol synthesis and augmentation of LDL receptor activity in macrophages. Biochem Biophys Res Commun. 1997;233:658–662. doi: 10.1006/bbrc.1997.6520. [DOI] [PubMed] [Google Scholar]
- Jain CK, Agarwal S, Rao AV. The effect of dietary lycopene on bioavailability, tissue distribution, in vivo antioxidant properties and colonic preneoplasia in rats. Nutr Res. 1999;19:1383–1391. [Google Scholar]
- Luo C, Wu XG. Lycopene enhances antioxidant enzyme activities and immunity function in N-Methyl-N′-nitro-N-nitrosoguanidine-induced gastric cancer rats. Int J Mol Sci. 2011;12:3340–3351. doi: 10.3390/ijms12053340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marai IFM, EI-Darawany AA, Fadiel A, Abdel-Hafez MAM. Physiological traits as affected by heat stress in sheep-A review. Small Rumin Res. 2007;71:1–12. [Google Scholar]
- Mujahid A, Yoshiki Y, Akiba Y, Toyomizu M. Superoxide radical production in chicken skeletal muscle induced by acute heat stress. Poult Sci. 2005;84:307–314. doi: 10.1093/ps/84.2.307. [DOI] [PubMed] [Google Scholar]
- Napolitano M, De Pascale C, Wheeler-Jones C, Botham KM, Bravo E. Effects of lycopene on the induction of foam cell formation by modified LDL. Am J Physiol Endocrinol Metabol. 2007;293:E1820–1827. doi: 10.1152/ajpendo.00315.2007. [DOI] [PubMed] [Google Scholar]
- Nieto G, Diaz P, Banon S, Garrido MD. Dietary administration of ewe diets with a distillate from rosemary leaves (Rosmarinus officinalis L.): Influence on lamb meat quality. Meat Sci. 2010;84:23–29. doi: 10.1016/j.meatsci.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Nowak R, Porter RH, Blache D, Dwyer CM. Behaviour and the welfare of sheep. Anim Welf. 2008;6:81–134. [Google Scholar]
- Ortuno J, Serrano R, Jordan MJ, Banon S. Shelf life of meat from lambs given essential oil-free rosemary extract containing carnosic acid plus carnosol at 200 or 400 mg kg−1. Meat Sci. 2014;96:1452–1459. doi: 10.1016/j.meatsci.2013.11.021. [DOI] [PubMed] [Google Scholar]
- Oshima S, Ojima F, Sakamoto H, Ishiguro Y, Terao J. Supplementation with carotenoids inhibits singlet oxygen-mediated oxidation of human plasma low-density lipoprotein. J Agric Food Chem. 1996;44:2306–2309. [Google Scholar]
- Rao AV, Agarwal S. Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases: A review. Nutr Res. 1999;19:305–323. [Google Scholar]
- Ried K, Fakler P. Protective effect of lycopene on serum cholesterol and blood pressure: Meta-analyses of intervention trials. Maturitas. 2011;68:299–310. doi: 10.1016/j.maturitas.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Rissanen T. Lycopene and cardiovascular disease. In: Rao AV, editor. Tomatoes, Lycopene and Human Health. Caledonian Science Press; Stranraer, Scotland: 2006. pp. 141–152. [Google Scholar]
- Sahin K, Yazlak H, Orhan C, Tuzcu M, Akdemir F, Sahin N. The effect of lycopene on antioxidant status in rainbow trout (Oncorhynchus mykiss) reared under high stocking density. Aquaculture. 2014;418–419:132–138. [Google Scholar]
- Sahin K, Onderci M, Sahin N, Gursu MF, Khachik F, Kucuk O. Effects of lycopene supplementation on antioxidant status, oxidative stress, performance and carcass characteristics in heat-stressed Japanese quail. J Therm Biol. 2006a;31:307–312. [Google Scholar]
- Sahin K, Orhan C, Akdemir F, Tuzcu M, Ali S, Sahin N. Tomato powder supplementation activates Nrf-2 via ERK/Akt signaling pathway and attenuates heat stress-related responses in quails. Anim Feed Sci Technol. 2011;165:230–237. [Google Scholar]
- Sahin N, Orhan C, Tuzcu M, Sahin K, Kucuk O. The Effects of tomato powder supplementation on performance and lipid peroxidation in quail. Poult Sci. 2008;87:276–283. doi: 10.3382/ps.2007-00207. [DOI] [PubMed] [Google Scholar]
- Sahin N, Sahin K, Onderci M, Karatepe M, Smith MO, Kucuk O. Effects of dietary lycopene and vitamin E on egg production, antioxidant status and cholesterol levels in Japanese quail. Asian Australas J Anim Sci. 2006b;19:224–230. [Google Scholar]
- Sen AR, Santra A, Karim SA. Effect of dietary sodium bicarbonate supplementation on carcass and meat quality of high concentrate fed lambs. Small Rumin Res. 2006;65:122–127. [Google Scholar]
- Seven I, Aksu T, Seven PT. The effects of propolis on biochemical parameters and activity of antioxidant enzymes in broilers exposed to lead-induced oxidative stress. Asian Australas J Anim Sci. 2010;23:1482–1489. [Google Scholar]
- Sgorlon S, Stradaioli G, Zanin D, Stefanon B. Biochemical and molecular responses to antioxidant supplementation in sheep. Small Rumin Res. 2006;64:143–151. [Google Scholar]
- Silaste ML, Alfthan G, Aro A, Kesaniemi YA, Horkko S. Tomato juice decreases LDL cholesterol levels and increases LDL resistance to oxidation. Br J Nutr. 2007;98:1251–1258. doi: 10.1017/S0007114507787445. [DOI] [PubMed] [Google Scholar]
- Upaganlawar AB, Balaraman R. Cardioprotective effect of vitamin E in combination with lycopene on lipid Profile, lipid metabolizing enzymes and infarction size in myocardial infarction induced by isoproterenol. Pharmacologia. 2012;3:215–220. [Google Scholar]
- Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Wang L, Gong MX, Nishida H, Shirakawa C, Sato S, Konishi T. Psychological Stress-induced oxidative stress as a model of sub-healthy condition and the effect of TCM. Evid Based Complement Alternat Med. 2007;4:195–202. doi: 10.1093/ecam/nel080. [DOI] [PMC free article] [PubMed] [Google Scholar]


