Abstract
Current influenza vaccines elicit primarily antibody-based immunity. They require yearly revaccination and cannot be manufactured until the identification of the circulating viral strain(s). These issues remain to be addressed. Here we report a phase Ib trial of a vaccine candidate (FLU-v) eliciting cellular immunity. Thirty-two males seronegative for the challenge virus by hemagglutination inhibition assay participated in this single-center, randomized, double-blind study. Volunteers received one dose of either the adjuvant alone (placebo, n = 16) or FLU-v (500 μg) and the adjuvant (n = 16), both in saline. Twenty-one days later, FLU-v (n = 15) and placebo (n = 13) volunteers were challenged with influenza virus A/Wisconsin/67/2005 (H3N2) and monitored for 7 days. Safety, tolerability, and cellular responses were assessed pre- and postvaccination. Virus shedding and clinical signs were assessed postchallenge. FLU-v was safe and well tolerated. No difference in the prevaccination FLU-v-specific gamma interferon (IFN-γ) response was seen between groups (average ± the standard error of the mean [SEM] for the placebo and FLU-v, respectively, 1.4-fold ± 0.2-fold and 1.6-fold ± 0.5-fold higher than the negative-control value). Nineteen days postvaccination, the FLU-v group, but not the placebo group, developed FLU-v-specific IFN-γ responses (8.2-fold ± 3.9-fold versus 1.3-fold ± 0.1-fold higher than the negative-control value [average ± SEM] for FLU-v versus the placebo [P = 0.0005]). FLU-v-specific cellular responses also correlated with reductions in both viral titers (P = 0.01) and symptom scores (P = 0.02) postchallenge. Increased cellular immunity specific to FLU-v correlates with reductions in both symptom scores and virus loads. (This study has been registered at ClinicalTrials.gov under registration no. NCT01226758 and at hra.nhs.uk under EudraCT no. 2009-014716-35.)
INTRODUCTION
For over 50 years, influenza public health programs have relied on vaccines that elicit prophylactic immunity via neutralizing antibodies against hemagglutinin (HA) and neuraminidase (NA). These vaccines, however, have three shortcomings. First, the HA and NA antigens are highly variable among strains and no single vaccine formulation provides universal protection. As new strain variants of the virus emerge year after year, vaccines must be reformulated and populations revaccinated (1). Second, mass manufacturing of HA/NA-based vaccines for newly emerged pandemic viruses can start only once the virus is identified. This causes a delay of 6 months or more between an influenza outbreak and a vaccine being available (2). Finally, the efficacy of HA/NA-based vaccines is limited. A report by Osterholm and colleagues (3) suggests that their efficacy rate is as low as 59% in 18- to 64-year-olds, falling to 35% in those over 65 years of age. Also, individuals suffering from chronic conditions such as chronic obstructive pulmonary disease, who are considered to have an increased risk of influenza virus infection, have been reported to suffer from deficient distribution of protective antibody in their airways (4). This may further limit the efficacy of traditional HA/NA vaccines in these individuals, making them more dependent on the cellular antiviral immune response.
Many novel influenza vaccine candidates that aim at totally or partially addressing these shortcomings are being developed (5). Most approaches still target the generation of neutralizing antibodies against capsid antigens (e.g., HA, NA, M2e). A few, based on the known key role played by cellular immune responses during natural infection (6), target the induction of cellular immunity against capsid and/or internal antigens (e.g., NP, M1, M2) (7).
We have developed a synthetic polypeptide-based influenza vaccine (FLU-v) and shown (8), like others (9–11), that CD8+ T-cell responses can, in the absence of neutralizing antibodies, protect mice against a lethal challenge with influenza virus. FLU-v has successfully completed a phase I study (12), and we now describe the results of a phase Ib challenge study to investigate the capacity of the vaccine to induce protective T-cell responses.
MATERIALS AND METHODS
Study population.
FLU-v was assessed in a randomized, double-blind single-center study (Retroscreen Virology Ltd.). One-hundred fifty-three healthy male subjects aged 18 to 45 years with no clinically significant abnormal findings (i.e., physical examination, electrocardiogram [ECG], medical history, or laboratory results) and no medical history of influenza-like illness in the prior 12 months were assessed for enrollment. Only those who had hemagglutination inhibition (HAI) titers of ≤10 to the challenge virus A/Wisconsin (H3N2) and did not receive a seasonal influenza vaccination in the previous 3 years were enrolled in this study. Thirty-two volunteers met the inclusion criteria.
Vaccine description.
FLU-v is a sterile equimolar mixture of four polypeptides encoding immunoreactive conserved regions within the influenza virus (8). The following sequences were synthetically manufactured (Bachem AG, Switzerland) in accordance with current good manufacturing practice (GMP): M1, DLEALMEWLKTRPILSPLTKGILGFVFTLTVP (32 amino acids [aa]); NPA, DLIFLARSALILRGSVAHKSC (21 aa); NPB, PGIADIEDLTLLARSMVVVR (20 aa); M2, IIGILHLILWILDRLFFKCIYRLF (24 aa).
FLU-v was administered subcutaneously in a 1.0-ml volume as a single 500-μg dose in saline emulsified (1:1) with adjuvant ISA-51 (Seppic, France). The placebo was saline emulsified with ISA-51. The adjuvant is composed of a light mineral oil and a surfactant system designed to make a water-in-oil emulsion. Functionally, ISA-51 is not known to preferentially favor the induction of Th1-like responses (13).
Study procedures.
Assessment of prospective volunteers started in May 2010. Recruited volunteers were vaccinated between August and October 2010, and all viral challenges and follow-up assessments were completed by December 2010.
At vaccine administration, a total of 32 eligible volunteers were randomized (1:1 ratio) into treatment and placebo groups. Both volunteers and clinical staff were blinded as to the nature of the formulations.
Participants were immunized on day −21 and entered quarantine on day −2. On day 0, they were challenged by nasal instillation with 1 ml of a solution containing approximately 105.25 50% tissue culture infective doses (TCID50)/ml of a GMP grade live A/Wisconsin/67/2005 (H3N2) virus provided by Retroscreen Ltd. From day 5 to day 7, volunteers received antiviral treatment (oseltamivir) before being discharged from quarantine on day 7. All participants were monitored until day 28.
Physical examination and clinical laboratory tests (clinical chemistry, hematology, urinalysis, etc.) were performed at screening, on day −21, daily from day −2 to day 7, and on day 28. ECGs and vital signs were assessed at screening, predose (day −21), and postdose on days −21, −2, 3, 6, and 28. Volunteer self-recorded observations from days −21 to −14 and −2 to 7, as well as a scripted symptom questionnaire completed on day −14, were assessed by the clinical staff. Identified abnormalities were entered into the adverse-event (AE) database.
Regulatory approval and ethical considerations.
This study was conducted in accordance with Good Clinical Practice, the Declaration of Helsinki (1964 and 2008), and applicable regulatory requirements. It was approved by the Plymouth Independent Ethics Committee under Research Ethics Committee reference no. 10/IEC04/1. Written informed consent was obtained from all participants.
Study objectives and endpoints.
The study objectives were to confirm the safety and tolerability of FLU-v, evaluate the cellular immune response to the vaccine, and assess the protective efficacy of FLU-v against an influenza A virus challenge.
Responses to the challenge virus were determined by HAI assay at recruitment and on days −21 (prevaccination), −2 (prechallenge), and 28 (postchallenge) as described previously (14). An HAI titer of >10 was considered positive for the challenge virus.
Cellular immunity.
(i) Cytokine ELISA.
Peripheral blood mononuclear cells (PBMCs) were isolated on the day of blood collection and frozen. Thawed PBMCs were seeded at 2 × 105/well (96-well plate) in RPMI 1640 (Sigma) supplemented with 25 mM HEPES, penicillin (100 U/ml), streptomycin (100 μg/ml), 10% fetal calf serum (FCS), and the test antigen, i.e., 4 μM FLU-v, 1 μg/ml concanavalin A (ConA; Sigma), or 1 μg/ml bovine serum albumin (BSA; Sigma). Each test antigen was tested in triplicate. After 24 h of incubation at 37°C and 5% CO2, IFN-γ production in the cell supernatant for each of the test antigens was determined with a validated enzyme-linked immunosorbent assay (ELISA; human IFN-γ kit 555142; BD). Response levels were calculated in picograms of IFN-γ produced per milliliter against a standard provided with the assay kit. The minimum IFN-γ level detected by the assay is 9 pg/ml.
Positive IFN-γ responses were defined as previously described (12). Briefly, volunteers showing a prevaccination FLU-v-specific IFN-γ response at least two times the individual response to the negative control (BSA and medium) were considered to have preexisting FLU-v cellular responses. Volunteers showing a postvaccination (prechallenge) FLU-v-specific IFN-γ response at least twice the individual response to both the negative control (BSA and medium) and their prevaccination IFN-γ FLU-v response were considered to have responded to the vaccination. All cytokine ELISAs were carried out by Huntingdon Life Sciences. Shortcomings at the contractor's facility resulted in the loss of immunogenicity data for five volunteers in the placebo group and six volunteers in the FLU-v group.
(ii) Enzyme-linked immunospot (ELISPOT) assay.
Frozen PBMCs were thawed and rested overnight at 37°C and 5% CO2 in RPMI 1640 (Sigma) supplemented with 25 mM HEPES, penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% FCS (all from Sigma). Cultures were harvested, washed by centrifugation (250 × g, 5 min), and resuspended at 106 cells/ml in the same medium as above but now also containing human T-activator Dynabeads (Life Technologies) prepared according to the manufacturer's instructions, 5 ng/ml interleukin-7 (IL-7; BioLegend), 1 ng/ml IL-2 (BioLegend), and 8 μM FLU-v. Following 5 days of incubation at 37°C and 5% CO2, individual cell cultures were harvested and washed once by centrifugation (250 × g, 5 min), resuspended at 106 cells/ml, and split into two equal fractions. One of the fractions was subjected to two rounds of CD8 depletion with CD8 Dynabeads (Life Technologies) according to the manufacturer's instructions. The other fraction was left untouched. The efficacy of the CD8 depletion process was assessed by flow cytometry.
CD8-depleted, as well as untouched, fractions were seeded into the wells of a human IFN-γ ELISPOT assay 96-well plate (Mabtech) at 105 cells/well in RPMI 1640 (Sigma) supplemented with 25 mM HEPES, penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% FCS and containing anti-CD3 monoclonal antibody (Mabtech), 8 μM FLU-v, or nothing (i.e., medium alone). After 24 h of incubation at 37°C and 5% CO2, IFN-γ-positive spots were counted. ELISPOT assays were carried out by SEEK.
Flow cytometry assessment of CD8 depletion.
Untouched and CD8-depleted PBMCs were harvested and washed in cold (4°C) blocking buffer by centrifugation (350 × g, 5 min) before being resuspended in cold blocking buffer supplemented with Trustain FcR block (BioLegend). After 10 min of incubation, a fluorescein isothiocyanate (FITC)-conjugated anti-human CD3 antibody (BioLegend), a phycoerythrin (PE)-conjugated anti-human CD8 antibody (BioLegend), or the respective isotype control (BioLegend) was added in cold blocking buffer. Following 30 min of incubation in the dark, cells were washed twice with cold blocking buffer by centrifugation (350 × g, 5 min), resuspended in cold fixation buffer (BD), and incubated for 20 min in the dark on ice. After the cell suspensions were washed twice with cold blocking buffer by centrifugation (350 × g, 5 min), they were resuspended in FACS Flow (BD) and analyzed by flow cytometry. These assays were carried out by SEEK.
Symptom scoring, virology, and HAI tests.
Symptom scores were determined with a standardized scoring system (14, 15) based on subject self-assessment and a clinician's examination. The parameters assessed (scored 0 to 3, corresponding to absent to severe) were runny nose, stuffy nose, sneezing, sore throat, earache, malaise, cough, shortness of breath, headache, and muscle/joint ache. The total score of a given individual is the sum of the daily scores of all of the parameters assessed, from day 1 to day 7 postchallenge. The mean total score is the average of all of the individual total scores within a group.
Viral shedding in the nasopharyngeal samples was determined by TCID50 assay as described in the WHO manual for the laboratory diagnosis and virological surveillance of influenza (16) and used elsewhere (14). Briefly, serial 10-fold dilutions of virus-containing nasal lavage samples from days 1 to 7 were inoculated into the wells of 96-well microtiter plates seeded with Madin-Darby canine kidney cells and incubated for 5 to 6 days at 37°C. Cytopathic effects in individual wells were determined via light microscopy. A titer greater than 101.5 was considered positive. The total viral shedding of a given individual is the sum of the daily viral shedding readings from days 1 to 5 postchallenge (volunteers were treated with an antiviral on days 6 and 7 postchallenge). The mean total viral shedding is the average of all of the individual total viral shedding measurements within a group. All virology was carried out by Retroscreen Ltd.
HA-specific antibody titers against A/Wisconsin/67/2005 (H3N2) virus in the serum samples were determined by HAI assay with chicken erythrocytes as described in the WHO manual for the laboratory diagnosis and virological surveillance of influenza (16) and used elsewhere (14). HAI analyses were carried out by Retroscreen Ltd.
Statistical analysis.
Intergroup differences were determined with Mann-Whitney tests. Correlations between variables for the placebo and FLU-v groups were determined with the Spearman rank correlation test.
RESULTS
Safety of FLU-v.
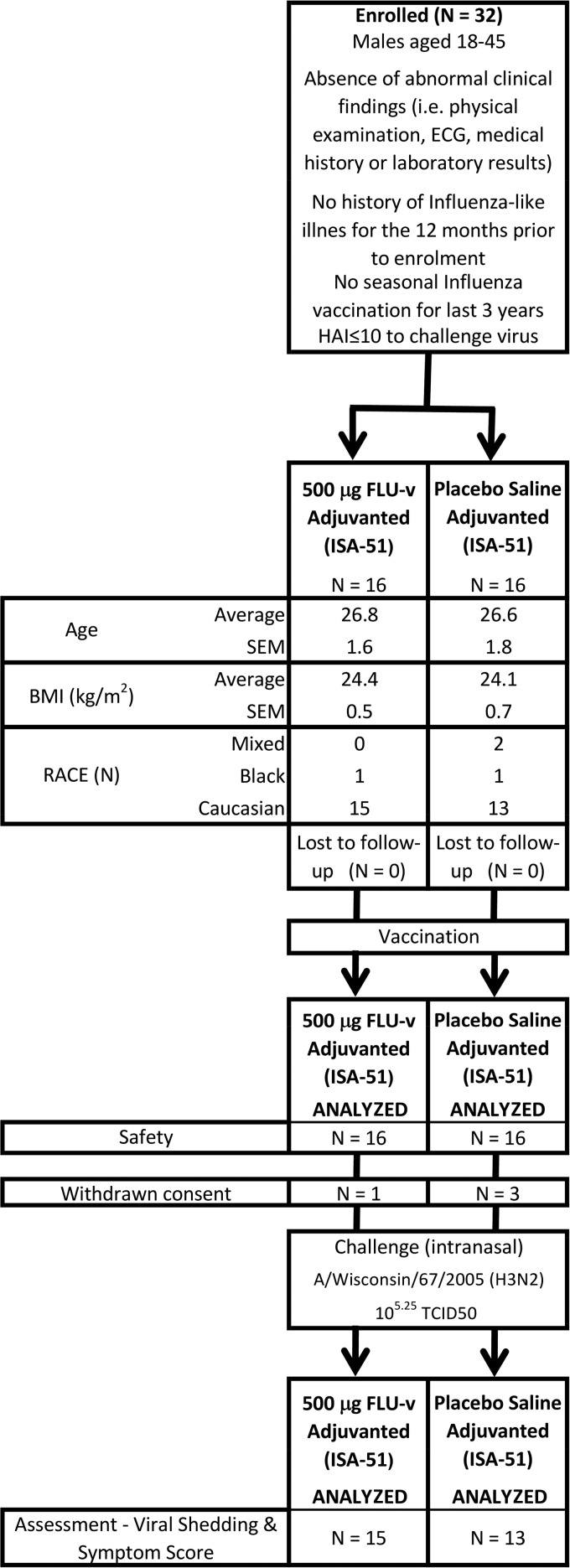
Following the assessment of potential candidates, 32 volunteers were enrolled and vaccinated. Trial profile and baseline clinical and demographic data are reported in Fig. 1.
FIG 1.
Consort profile. Trial profile and baseline demographic and clinical data for enrolled volunteers. Average indicates the arithmetic mean of the sample. IFN-γ values correspond to the cytokine production determined by ELISA following in vitro stimulation of PBMCs with FLU-v for 24 h, with each volunteer's sample tested in triplicate. BMI, body mass index.
Postvaccination, 16 individuals in the placebo group (100%) reported mild AEs, while 3 (18.8%) and 1 (6.3%) reported moderate and severe AEs, respectively. In the FLU-v group, 15 volunteers (93.8%) reported mild AEs and 6 (37.5%) reported moderate AEs. No volunteers (0%) reported severe AEs. Influenza-related symptoms that developed after challenge with the H3N2 influenza virus test strain were not considered on the list of AEs. No statistically significant difference in the incidence of AEs between groups postvaccination (prechallenge) was seen.
The majority of mild AEs were in the general disorders and administration site conditions class (30 [93.8%] of 32 volunteers). The most common were pain (12 [75%] of 16 in the placebo group versus 13 [81.2%] of 16 in the FLU-v group), erythema (5 [31.3%] of 16 versus 10 [62.5%] of 16), swelling (2 [12.5%] of 16 versus 11 [68.8%] of 16), and pruritus (2 [12.5%] of 16 versus 3 [18.8%] of 16) at the injection site. Other frequent mild AEs not in that class were rhinorrhea (2 [12.5%] of 16 versus 3 [18.8%] of 16) and myalgia (2 [12.5%] of 16 versus 3 [18.8%] of 16). The most common moderate AE was injection site pain (0 [0%] of 16 versus 6 [37.5%] of 16), and the only severe AE reported was one case (6.3%) of presyncope in the placebo group. Differences in severity for swelling and pain at the site of injection were found to be statistically significant (P = 0.003 and P = 0.017, respectively), but the clinical review did not raise this increment as a significant clinical concern.
There were no clinically significant findings in the individual or mean laboratory measurements, vital signs, or ECG measurements among the volunteers.
Immunogenicity of FLU-v and adjuvant.
Vaccine immunogenicity analysis was limited to the determination of cellular IFN-γ responses specific to the FLU-v formulation. No assessment of vaccine antibody responses was carried out, since earlier preclinical (8) and phase I (12) results have shown that FLU-v vaccination induces no significant antibody response.
HAI responses to the challenge virus (A/Wisconsin/67/2005 H3N2) were assessed prevaccination (day −21 prechallenge), postvaccination (day −2 prechallenge), and postchallenge (day 28). No HAI titers of >10 against the challenge virus were detected in the recruited volunteers before the challenge, either pre- or postvaccination.
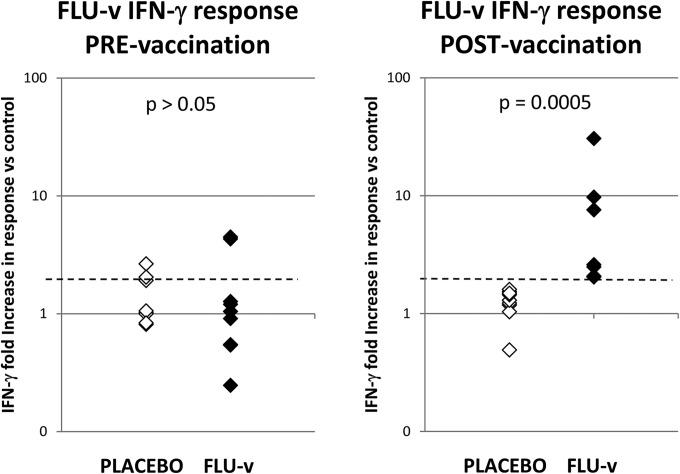
There were no differences between the placebo and FLU-v group cellular immune responses to the BSA-medium control at any time during this study (placebo versus FLU-v group [mean ± SEM], 99.2 ± 25.7 versus 80.6 ± 12.9 pg/ml). Both groups also responded in a dose-dependent manner to stimulation with ConA, a lectin that preferentially stimulates T cells and was used as a positive control (placebo versus FLU-v, 311 ± 85 versus 378 ± 35 pg/ml). The average and median prevaccination (day −21) FLU-v-specific cellular responses were similar and below the positivity threshold (i.e., ≥2-fold higher than the negative-control value) in both the placebo and FLU-v groups (average ± SEM, 1.4-fold ± 0.2-fold and 1.6-fold ± 0.5-fold increases versus the BSA-medium control; median fold increases of 1.0 and 1.0 in the placebo and FLU-v groups, respectively; Fig. 2). Despite the absence of a group response, two individuals in each group displayed prevaccination FLU-v-specific IFN-γ responses beyond the positive response threshold (i.e., ≥2-fold increase versus the BSA-medium control).
FIG 2.
FLU-v-specific cellular immune responses pre- and postvaccination. Values are fold increases in the in vitro IFN-γ response to FLU-v over that to the negative control (medium plus BSA). The IFN-γ responses to the negative control pre- and postvaccination in both groups are 99.2 ± 25.7 versus 80.6 ± 12.9 pg/ml (average ± SEM). The IFN-γ responses to the positive control (ConA) pre- and postvaccination in both groups are 311 ± 85 versus 378 ± 35 pg/ml (average ± SEM). The IFN-γ responses to FLU-v pre- and postvaccination in the placebo group are 153 ± 49 versus 111 ± 47 pg/ml (average ± SEM). The IFN-γ responses to FLU-v pre- and postvaccination in the FLU-v group are 122 ± 37 versus 251 ± 55 pg/ml (average ± SEM). The dotted line represents the threshold point for a positive IFN-γ response to FLU-v (i.e., a ≥2-fold increase over the negative-control value). Statistical significance is represented by the P value determined by Mann-Whitney analysis.
Nineteen days postvaccination (day −2; Fig. 2), no positive cellular responses to FLU-v were seen in any of the volunteers tested in the placebo group (1.3-fold ± 0.1-fold versus the BSA-medium control; range, 0.5- to 1.6-fold; median, 1.3-fold). A positive FLU-v response postvaccination was defined as an increase of at least 2-fold in both the individual's prevaccination FLU-v IFN-γ response and the individual's postvaccination IFN-γ response to the negative control (BSA and medium). In the FLU-v group, positive FLU-v-specific cellular responses of various intensities were found in all of the individuals tested (8.2-fold ± 3.9-fold versus the BSA-medium control; range, 2.0- to 30.6-fold; median, 2.6-fold). This result is consistent with our phase I data (12) and shows that vaccination with a single dose of adjuvanted FLU-v induces a vaccine-specific cellular response (P = 0.0005), even though in many individuals this response did not appear to be very strong (i.e., <4-fold increase), as reflected in the difference between the FLU-v group median (2.6) and average responses (8.2).
Our existing preclinical data (8) show that the protective IFN-γ response elicited in mice by FLU-v vaccination is mediated primarily by CD8+ T cells, but our IFN-γ ELISA data cannot corroborate those results in humans. A comprehensive post hoc analysis of the phenotype of the responding cells in the volunteers was not possible, as frozen PBMC cultures were still available for only six volunteers (i.e., three each in the placebo and FLU-v groups). Nonetheless, we believe that some anecdotal evidence on the nature of the responding cells can still be obtained from this limited number of samples. Therefore, we tested CD8-depleted and untouched fractions of these six PBMC samples for their FLU-v-specific IFN-γ responses by ELISPOT assay.
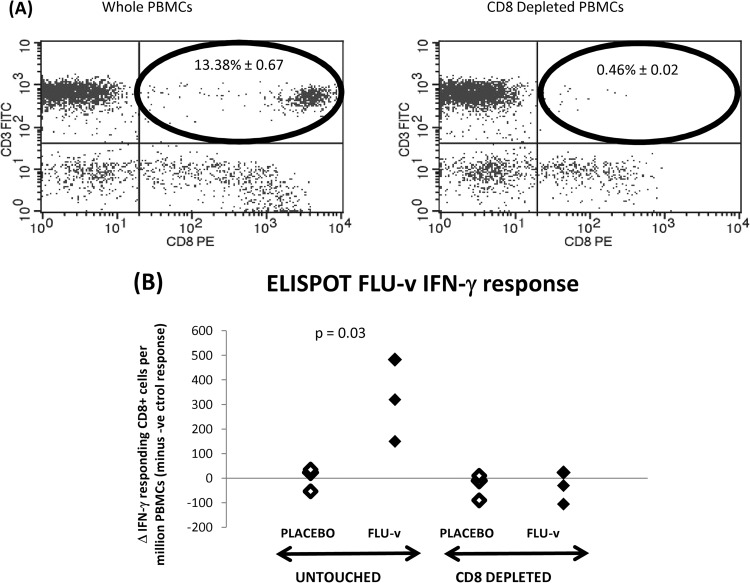
As shown in Fig. 3A, CD8 depletion removed >90% of the CD8+ T cells. CD3 stimulation (positive control) of untouched and CD8-depleted PBMCs resulted in a number of IFN-γ-responding cells (>940 cells/million PBMCs) significantly higher than that produced by the negative control in all six volunteers tested (i.e., placebo and FLU-v groups).
FIG 3.
FLU-v-specific cellular immune responses postvaccination—CD8 depletion—determined by ELISPOT assay and flow cytometry. (A) Effect of CD8 depletion in the PBMC population postvaccination as analyzed by flow cytometry with FITC-conjugated anti-CD3 and PE-conjugated anti-CD8 antibodies. Untreated and CD8-depleted PBMCs are represented, respectively, in the left and right graphs. The location of the CD8 T-cell population is circled in black. The percentages of CD8 T cells (± SEM) left in untreated and depleted populations are shown within the circles. (B) Postvaccination (prechallenge) in vitro IFN-γ ELISPOT assay response to FLU-v in PBMC suspensions either depleted of CD8 T cells or left untreated. Statistical significance was established by Mann-Whitney analysis.
Figure 3B shows that stimulation of untouched PBMC samples from the FLU-v group with FLU-v resulted in a significantly higher number of CD8+ responding cells than in the placebo group (average ± SEM, 318 ± 96 versus 2 ± 28 cells/million PBMCs in the FLU-v and placebo groups; P = 0.03). In contrast, stimulation of CD8-depleted PBMC samples with FLU-v revealed no significant difference in the number of CD8+ responding cells between the FLU-v and placebo groups (average ± SEM, −30 ± 31 versus −37 ± 37 cells/million PBMCs; P > 0.05).
The observed loss of FLU-v IFN-γ response following CD8 depletion suggests that, in the three FLU-v volunteers tested, the FLU-v-specific IFN-γ response is mediated by CD8+ T cells.
Immunization with FLU-v correlates with reduced virus shedding and symptom scores postchallenge.
Of all of the vaccinated volunteers, one individual in the FLU-v group and three in the placebo group decided to withdraw from this study before the challenge with live A/Wisconsin/67/2005 virus. No significant differences between the infection rates of the placebo and FLU-v groups were observed (8/13 versus 11/15, i.e., 61.5% versus 73.3%; P > 0.05). For clarification, the infection rate was determined as the percentage of challenged volunteers per group with at least one positive reading for viral shedding (i.e., a titer of ≥101.5 by TCID50 assay) between days 1 and 5 postchallenge.
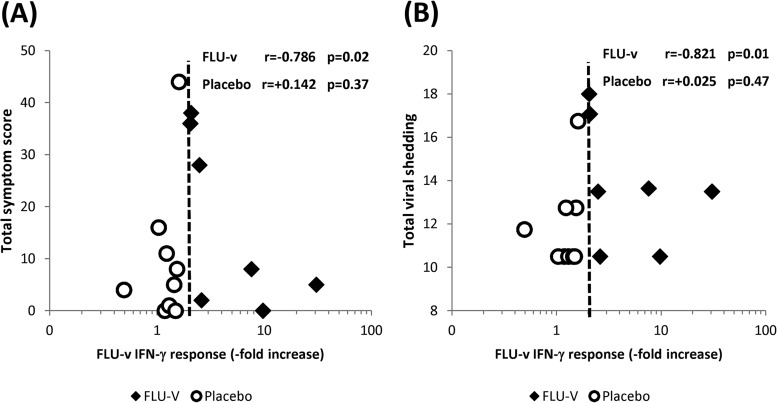
We found no significant differences (P > 0.05) in the mean total viral shedding or mean total symptom score between the placebo and FLU-v groups postchallenge. Nonetheless, we did find a significant correlation between increased IFN-γ responses to FLU-v and reductions in both total viral shedding (r = −0.821; P = 0.01) and the total symptom score (r = −0.786; P = 0.02) (Fig. 4A and B).
FIG 4.
Correlations between cellular IFN-γ responses and illness severity. Correlations between cellular IFN-γ responses specific to FLU-v prechallenge with A/Wisconsin/67/2005 (H3N2) and measurements of influenza severity postchallenge, i.e., symptom scores (A) and viral shedding (B). The magnitude of an individual's FLU-v-specific IFN-γ responses is represented as the fold increase in the response over the negative-control value. The dotted line represents the threshold point for a positive IFN-γ response to FLU-v (i.e., a ≥2-fold increase over the negative-control value). An individual's total symptom score represents the total sum of the daily scores (days 1 to 7 postchallenge). An individual's total viral shedding represents the total sum of daily viral shedding measurements by TCID50 (days 1 to 5 postchallenge). All of the analyses were carried out with the Spearman rank correlation test.
DISCUSSION
This study evaluated the ability of a synthetic polypeptide vaccine targeting conserved immunogenic regions of the NP, M1, and M2 proteins (FLU-v) to induce protective cellular responses to a live influenza virus challenge in humans.
Consistent with our earlier phase I trial results, vaccination with a single 500-μg dose of adjuvanted FLU-v was found, in general, to be safe and well tolerated, despite some increased reactogenicity signs (i.e., swelling and pain at the site of injection). The AE pattern and lack of differences between the placebo and FLU-v are consistent with the use of this adjuvant in both groups.
The chosen subcutaneous route combines ease of delivery and increased exposure to dermal antigen-presenting cells. Both the intramuscular and intradermal routes were rejected early in development after due consideration. Intramuscular vaccinations are easy to perform but do not usually result in strong cellular responses. Intradermal vaccinations, in contrast, elicit strong cellular responses but require a significant level of skill (17).
In our experience, past (12, 18) and present, ISA-51 is a safe and well-tolerated adjuvant in humans that induces strong immune responses. We did observe increased severity of swelling and pain at the site of injection of adjuvanted FLU-v but never to the unacceptable levels reported in other studies (19, 20). Direct comparisons of those studies and ours are difficult, since the antigen, the doses used, and the immune response sought (T and/or B cell) were all different. Nonetheless, it is noteworthy that in all of the those previous reports, high levels of local reactogenicity to ISA-51 were associated with intramuscular administration, while we have always used subcutaneous administration. Adjuvants are known to cause exaggerated local reactions if not injected properly into the muscle mass (21).
Prevaccination, vaccine-specific cellular responses were identified in only two volunteers in both the placebo and FLU-v groups. This percentage (12.5%) is higher than that seen in the phase I study (4%) but still shows that sustained historical exposure to influenza virus does not appear to elicit strong cellular immunity to FLU-v antigens. Cytokine ELISA remains a robust and reliable method (22, 23) that has been used regularly to determine antigen-specific cellular responses in both animal (24, 25) and human (26, 27) studies. Nonetheless, it is possible that a higher frequency of prevaccination FLU-v responders would have been detected if a different assay, such as an ELISPOT assay, had been used. The ELISPOT assay is significantly more sensitive than a standard cytokine ELISA, and it has the additional benefit of allowing the analysis of cytokine production at the single-cell level. A post hoc ELISPOT analysis of a very small number of samples from this trial (six samples, three from each group) still failed to show preexisting FLU-v-specific responses in either the placebo or FLU-v volunteers tested. Obviously, the small number of samples analyzed by ELISPOT assay can provide only anecdotal evidence, but the absence of preexisting FLU-v IFN-γ responses, both in these samples and in others from a previous phase I trial (12; data not shown), seems consistent with our view that sustained historical exposure to influenza virus does not appear to elicit strong cellular immunity to FLU-v antigens.
Postvaccination, all of the FLU-v group volunteers tested developed a FLU-v IFN-γ response, but in most cases, this response was relatively weak (i.e., between 2- and 4-fold higher than the negative-control value). This universal FLU-v response did not translate into a significant difference in symptom score or viral shedding from the placebo after a live influenza virus challenge. Nonetheless, we did find a significant correlation between the level of the IFN-γ response to FLU-v postvaccination and viral shedding and symptom score reductions postchallenge. These combined results suggest that only high FLU-v IFN-γ responses (i.e., >4-fold increases) have the potential to mediate clinically relevant viral shedding and symptom score reductions during infection. As one single dose of 500 μg of adjuvanted FLU-v was not able to induce a consistently high FLU-v IFN-γ response in all of the volunteers tested, higher doses or an increased number of immunizations may be required to achieve this goal.
As for the cell population providing the FLU-v IFN-γ response, we cannot answer that question with absolute certainty. As indicated earlier, ELISA data do not allow the analysis of cytokine production at the single-cell level and we had only enough PBMCs left from six volunteers (three each in the FLU-v and placebo groups) to carry out a post hoc phenotypic analysis of the FLU-v responding cells (i.e., IFN-γ ELISPOT assay). Undeniably, this sample size can provide only anecdotal evidence, but it is noteworthy that all three of the FLU-v volunteers tested displayed a CD8+ T-cell-driven FLU-v IFN-γ response. This is the same cell population that we found to be responsible for inducing protection against a lethal influenza virus challenge in mice after vaccination with FLU-v (8).
We accept that certain elements of our data may suggest alternative interpretations to some. For example, in the correlation graphs, some placebo group volunteers show lower total viral shedding and symptom scores postchallenge than their FLU-v group counterparts with high FLU-v IFN-γ responses (i.e., a 4-fold increase). This, together with the lack of differences in total viral shedding and symptom score outcomes between the placebo and FLU-v groups postchallenge, may suggest to some that the correlations we found between the FLU-v IFN-γ response level and viral shedding and symptom score reductions are simply accidental. If we were to accept this interpretation, we believe we would also have to accept that both groups controlled the infection by directing a similar cellular response (CD8 or CD4) to the same dominant viral epitopes in the challenge virus (the absence of an HAI antibody response to the challenge virus in all the volunteers and the identical rate of infection in both groups rule out a preinfection defense mechanism). Although we cannot exclude the possibility that both groups had similar preexisting cellular responses to the challenge virus (we, like others [14], never measured this response), two experimental observations suggest that this premise is not correct. First, the frequency of volunteers with high preexisting (i.e., prevaccination) IFN-γ responses (i.e., a 4-fold increase over the negative-control value) to serologically unrelated influenza virus strains was significantly lower in the FLU-v group than in the placebo group (data not shown). In contrast, after vaccination but still prechallenge, the frequency of these high heterosubtypic responders was the same in both groups. Second, although this previous observation appears to suggest that the increase in FLU-v-specific cellular responses in the FLU-v group may have also led to the increase in influenza virus heterosubtypic responses postvaccination, we know that the reverse is not true. That is, despite high preexisting heterosubtypic IFN-γ responses, we found no significant preexisting IFN-γ response to FLU-v in the placebo group. Both of these observations, together with the fact that FLU-v epitopes are presented to CD8+ T cells in influenza virus-infected human cells (8), appear consistent with FLU-v vaccination eliciting cellular responses to mainly subdominant epitopes shared by multiple influenza virus strains. This would be in agreement with at least two previous studies (28, 29) in which vaccination with subdominant epitopes mediated protection against respiratory viral infections.
In summary, we believe that our data are consistent with the general view (14, 30–32) that high levels of cellular immunity to influenza virus, even in the absence of an antibody responses, can mediate reductions in influenza virus shedding and symptom scores.
ACKNOWLEDGMENTS
We are grateful to all of the volunteers and clinical staff and Retroscreen Virology Ltd. for their participation and involvement in this study.
This study was fully funded by the SEEK group (PepTcell Ltd.). Olga Pleguezuelos, Stuart Robinson, Ana Fernández, Gregory A. Stoloff, and Wilson Caparrós-Wanderley are all employees of PepTcell Ltd. (SEEK is a trademark of PepTcell Ltd.). S.R., G.A.S., and W.C.-W. also own stock in PepTcell Ltd. Alex Mann, Anthony Gilbert, Ganesh Balaratnam, and Robert Lambkin-Williams are all employees of Retroscreen Virology Ltd. John Oxford is a non-executive director of Retroscreen Virology Ltd. A.M., R.L.-W., A.G., G.B., and J.O. hold stock and/or stock options in Retroscreen Virology. Tom Wilkinson is an independent academic who has collaborated in the past with Retroscreen Ltd. and John Oxford on clinical studies on influenza virus T cell-mediated responses in humans.
REFERENCES
- 1.Carrat F, Flahault A. 2007. Influenza vaccine: the challenge of antigenic drift. Vaccine 25:6852–6862. doi: 10.1016/j.vaccine.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 2.Gerdil C. 2003. The annual production cycle for influenza vaccine. Vaccine 21:1776–1779. doi: 10.1016/S0264-410X(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 3.Osterholm MT, Kelley NS, Sommer A, Belongia EA. 2012. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 4.Polosukhin VV, Cates JM, Lawson WE, Zaynagetdinov R, Milstone AP, Massion PP, Ocak S, Ware LB, Lee JW, Bowler RP, Kononov AV, Randell SH, Blackwell TS. 2011. Bronchial secretory immunoglobulin A deficiency correlates with airway inflammation and progression of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 184:317–327. doi: 10.1164/rccm.201010-1629OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert LC, Fauci AS. 2010. Influenza vaccines for the future. N Engl J Med 363:2036–2044. doi: 10.1056/NEJMra1002842. [DOI] [PubMed] [Google Scholar]
- 6.Kreijtz JH, Fouchier RA, Rimmelzwaan GF. 2011. Immune responses to influenza virus infection. Virus Res 162:19–30. doi: 10.1016/j.virusres.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Lillie PJ, Berthoud TK, Powell TJ, Lambe T, Mullarkey C, Spencer AJ, Hamill M, Peng Y, Blais ME, Duncan CJ, Sheehy SH, Havelock T, Faust SN, Williams RL, Gilbert A, Oxford J, Dong T, Hill AV, Gilbert SC. 2012. Preliminary assessment of the efficacy of a T-cell-based influenza vaccine, MVA-NP+M1, in humans. Clin Infect Dis 55:19–25. doi: 10.1093/cid/cis327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoloff GA, Caparrós-Wanderley W. 2007. Synthetic multi-epitope peptides identified in silico induce protective immunity against multiple influenza serotypes. Eur J Immunol 37:2441–2449. doi: 10.1002/eji.200737254. [DOI] [PubMed] [Google Scholar]
- 9.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, DeWitt CM, Friedman A. 1993. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 10.Graham MB, Braciale TJ. 1997. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J Exp Med 186:2063–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen JP, Doherty PC, Branum KC, Riberdy JM. 2000. Profound protection against respiratory challenge with a lethal H7N7 influenza A virus by increasing the magnitude of CD8(+) T-cell memory. J Virol 74:11690–11696. doi: 10.1128/JVI.74.24.11690-11696.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pleguezuelos O, Robinson S, Stoloff GA, Caparrós-Wanderley W. 2012. Synthetic Influenza vaccine (FLU-v) stimulates cell mediated immunity in a double-blind, randomised, placebo-controlled phase I trial. Vaccine 30:4655–4660. doi: 10.1016/j.vaccine.2012.04.089. [DOI] [PubMed] [Google Scholar]
- 13.Shibaki A, Katz SI. 2002. Induction of skewed Th1/Th2 T-cell differentiation via subcutaneous immunization with Freund's adjuvant. Exp Dermatol 11:126–134. doi: 10.1034/j.1600-0625.2002.110204.x. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, Lambkin-Williams R, Gilbert A, Oxford J, Nicholas B, Staples KJ, Dong T, Douek DC, McMichael AJ, Xu XN. 2012. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med 18:274–280. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- 15.Hayden FG, Fritz R, Lobo MC, Alvord W, Strober W, Straus SE. 1998. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Invest 101:643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization Global Surveillance Network. 2011. Manual for the laboratory diagnosis and virological surveillance of influenza. http://whqlibdoc.who.int/publications/2011/9789241548090_eng.pdf.
- 17.Siegrist CA. 2012. Vaccine immunology, p 17–36. In Plotkin S, Orenstein W, Offit P (ed), Vaccines, 6th ed Elsevier, Amsterdam, Netherlands. [Google Scholar]
- 18.Boffito M, Fox J, Bowman C, Fisher M, Orkin C, Wilkins E, Jackson A, Pleguezuelos O, Robinson S, Stoloff GA, Caparrós-Wanderley W. 2013. Safety, immunogenicity and efficacy assessment of HIV immunotherapy in a multi-centre, double-blind, randomised, placebo-controlled phase Ib human trial. Vaccine 31:5680–5686. doi: 10.1016/j.vaccine.2013.09.057. [DOI] [PubMed] [Google Scholar]
- 19.Graham BS, McElrath MJ, Keefer MC, Rybczyk K, Berger D, Weinhold KJ, Ottinger J, Ferarri G, Montefiori DC, Stablein D, Smith C, Ginsberg R, Eldridge J, Duerr A, Fast P, Haynes BF, AIDS Vaccine Evaluation Group. 2010. Immunization with cocktail of HIV-derived peptides in montanide ISA-51 is immunogenic, but causes sterile abscesses and unacceptable reactogenicity. PLoS One 5:e11995. doi: 10.1371/journal.pone.0011995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, Fay MP, Narum D, Rausch K, Miles AP, Aebig J, Orcutt A, Muratova O, Song G, Lambert L, Zhu D, Miura K, Long C, Saul A, Miller LH, Durbin AP. 2008. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS One 3:e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atkinson W, Wolfe S, Hamborsky J (ed). 2012. Epidemiology and prevention of vaccine-preventable diseases, 12th ed Appendix D: vaccine administration guidelines. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 22.Tanguay S, Killion JJ. 1994. Direct comparison of ELISPOT and ELISA-based assays for detection of individual cytokine-secreting cells. Lymphokine Cytokine Res 13:259–263. [PubMed] [Google Scholar]
- 23.Favre N, Bordmann G, Rudin W. 1997. Comparison of cytokine measurements using ELISA, ELISPOT and semi-quantitative RT-PCR. J Immunol Methods 204:57–66. doi: 10.1016/S0022-1759(97)00033-1. [DOI] [PubMed] [Google Scholar]
- 24.Sarawar SR, Sangster M, Coffman RL, Doherty PC. 1994. Administration of anti-IFN-gamma antibody to beta 2-microglobulin-deficient mice delays influenza virus clearance but does not switch the response to a T helper cell 2 phenotype. J Immunol 153:1246–1253. [PubMed] [Google Scholar]
- 25.Ulmer JB, Fu TM, Deck RR, Friedman A, Guan L, DeWitt C, Liu X, Wang S, Liu MA, Donnelly JJ, Caulfield MJ. 1998. Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA. J Virol 72:5648–5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katial RK, Sachanandani D, Pinney C, Lieberman MM. 1998. Cytokine production in cell culture by peripheral blood mononuclear cells from immunocompetent hosts. Clin Diagn Lab Immunol 5:78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Furman SA, Bradford CR, Chang AE. 1999. Expanded tumor-reactive CD4+ T-cell responses to human cancers induced by secondary anti-CD3/anti-CD28 activation. Clin Cancer Res 5:461–469. [PubMed] [Google Scholar]
- 28.Oukka M, Manuguerra JC, Livaditis N, Tourdot S, Riche N, Vergnon I, Cordopatis P, Kosmatopoulos K. 1996. Protection against lethal viral infection by vaccination with non-immunodominant peptides. J Immunol 157:3039–3045. [PubMed] [Google Scholar]
- 29.Cole GA, Hogg TL, Coppola MA, Woodland DL. 1997. Efficient priming of CD8+ memory T cells specific for a subdominant epitope following Sendai virus infection. J Immunol 158:4301–4309. [PubMed] [Google Scholar]
- 30.McMichael AJ, Gotch FM, Noble GR, Beare PA. 1983. Cytotoxic T-cell immunity to influenza. N Engl J Med 309:13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- 31.Hillaire ML, van Trierum SE, Kreijtz JH, Bodewes R, Geelhoed-Mieras MM, Nieuwkoop NJ, Fouchier RA, Kuiken T, Osterhaus AD, Rimmelzwaan GF. 2011. Cross-protective immunity against influenza pH1N1 2009 viruses induced by seasonal influenza A (H3N2) virus is mediated by virus-specific T-cells. J Gen Virol 92(Pt 10):2339–2349. doi: 10.1099/vir.0.033076-0. [DOI] [PubMed] [Google Scholar]
- 32.Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, Bean T, Barclay W, Deeks JJ, Lalvani A. 2013. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med 19:1305–1312. doi: 10.1038/nm.3350. [DOI] [PubMed] [Google Scholar]