Abstract
Four vaccines for feline leukemia virus (FeLV) are available in the United States. This study's purpose was to compare the efficacy of Nobivac feline 2-FeLV (an inactivated, adjuvanted whole-virus vaccine) and PureVax recombinant FeLV (a live, canarypox virus-vectored vaccine) following FeLV challenge. Cats were vaccinated at 9 and 12 weeks with Nobivac feline 2-FeLV (group A, n = 11) or PureVax recombinant FeLV (group B, n = 10). Group C (n = 11) comprised unvaccinated controls. At 3 months postvaccination, cats were immunosuppressed and challenged with FeLV-A/61E. The outcomes measured were persistent antigenemia at 12 weeks postchallenge (PC) and proviral DNA and viral RNA at 3 to 9 weeks PC. Persistent antigenemia was observed in 0 of 11 cats in group A, 5 of 10 cats in group B, and 10 of 11 cats in group C. Group A was significantly protected compared to those in groups B (P < 0.013) and C (P < 0.0001). No difference was found between groups B and C (P > 0.063). The preventable fraction was 100% for group A and 45% for group B. At 9 weeks PC, proviral DNA and viral RNA were detected 1 of 11 cats in group A, 6 of 10 cats in group B, and 9 of 11 cats in group C. Nucleic acid loads were significantly lower in group A than in group C (P < 0.01). Group A had significantly lower proviral DNA loads than group B at weeks 6 to 9 (P < 0.02). The viral RNA loads were significantly lower in group A than in group B at weeks 7 to 9 (P < 0.01). The results demonstrate that Nobivac feline 2-FeLV-vaccinated cats were fully protected against persistent antigenemia and had significantly smaller amounts of proviral DNA and plasma viral RNA loads than PureVax recombinant FeLV-vaccinated cats and unvaccinated controls.
INTRODUCTION
Feline leukemia virus (FeLV) is a retroviral infection of cats that is transmitted mainly through saliva, although other body fluids can transmit the virus as well (1, 2). Infected cats demonstrate a wide range of clinical signs, including cytoproliferative disorders (lymphoid or myeloid tumors), cytosuppressive disorders (infectious diseases associated with immunosuppression, anemia, myelosuppression), inflammatory disorders, neurological disorders, abortions, enteritis, and more (3, 4).
The outcome of FeLV exposure is dependent on a variety of factors, including host immune status, host age, viral strain, viral load, and exposure route. Previous classifications, including the classifications of persistent antigenemia, transient antigenemia, and elimination of infection, were mainly defined by tests for antigenemia (p27 enzyme-linked immunosorbent assay [ELISA]), virus isolation, and immunofluorescence assays (3, 5). Tests for antigenemia are highly useful in clinical applications and in determining whether the clinical disease is a result of actively circulating virus.
The use of PCR testing for FeLV infection has altered the understanding of the pathophysiology and clinical course of FeLV infection (6–9). PCR can detect low levels of viral RNA circulating in the bloodstream as well as low levels of proviral DNA integrated into the cat's genome, resulting in more sensitive assays for FeLV status. Because of PCR testing, it is now generally accepted that there are three major outcomes for cats that are exposed to FeLV: progressive, regressive, and abortive infections (2, 6, 10).
Progressive infection is characterized by an inadequate immune response to FeLV infection. In these cats, the virus will actively replicate and circulate in blood, bone marrow, and tissues. FeLV is spread in the saliva of these cats, and they typically succumb to FeLV infection within years. These cats will test positive for FeLV antigenemia (p27 antigen) by ELISA and test positive for viral RNA and proviral DNA by PCR (2, 6, 10).
Regressive infection is characterized by an effective immune response, with viral containment occurring shortly after infection. There may be transient antigenemia, as tested by p27 ELISA. These cats do test positive for proviral DNA and may test transiently or persistently positive for viral RNA. These cats are at little risk for succumbing to FeLV-associated disease and do not spread the virus (2). In fact, some of these cats that have low concentrations of proviral DNA with little to no circulating viral RNA may completely clear the infection with time, resembling an abortive infection (6). However, there may be an association of persistently high proviral DNA and viral RNA concentrations with reactivation of the infection (6, 7, 11, 12).
Cats that develop an abortive infection are able to completely clear the virus and will test negative for the p27 antigen, viral RNA, and proviral DNA. To date, there are no large-scale prevalence studies in North America based on PCR results and on the classification of cats as developing regressive, progressive, or abortive infections. All current prevalence studies are based on the detection of persistent antigenemia by p27 ELISA. The most recent large-scale seroprevalence study conducted in the United States demonstrated that 2.3% of 18,000 cats tested seropositive for FeLV (13). Based on this and previous studies, the rates of FeLV infection appear to be decreasing, most likely due to effective vaccination protocols (13, 14). However, different vaccines have been shown to have various degrees of efficacy (15). To demonstrate true efficacy, vaccines should be tested by a challenge model, and FeLV testing using at least p27 ELISA, PCR for viral RNA, and PCR for proviral DNA should be conducted. PCR testing is of paramount importance in determining the true FeLV status in challenged vaccinated or unvaccinated cats.
Four vaccines for FeLV are available in the United States, including two whole-virus adjuvanted killed vaccines; a dual-adjuvanted, multiple-antigen vaccine; and a nonadjuvanted, canarypox virus-vectored vaccine. Previous work has demonstrated the efficacy of whole-virus adjuvanted killed vaccines after challenge, including testing vaccinated and unvaccinated cats for viral RNA, proviral DNA, FeLV antibodies, and the p27 antigen (16–18). There are limited data evaluating the efficacy of the nonadjuvanted recombinant FeLV vaccine available for use (19). Therefore, the purpose of this study was to compare the efficacy of two commercially available feline leukemia vaccines, Nobivac feline 2-FeLV (an inactivated whole-virus vaccine) and PureVax recombinant FeLV (a live canarypox virus-vectored vaccine) following challenge with virulent feline leukemia virus.
MATERIALS AND METHODS
Study animals.
Thirty-two 8-week-old cats were enrolled in the study. All cats tested negative for FeLV infection by p27 ELISA (optical density [OD], <0.200; IDEXX, Westbrook, ME) prior to vaccination, booster, and challenge. Cats were housed separately during the vaccination phase of the study. During the challenge phase, placebo cats were randomly divided and housed with each of the vaccinated groups. All cats were housed to meet the 9 CFR USDA Animal Welfare Regulations (chapter 1) (20) and the Institutional Animal Care and Use Committee (IACUC) guidelines. Cats were observed daily throughout the course of the study and were euthanized if they became unwell or at the end of the study. All male cats were castrated at 21 weeks of age according to standard veterinary procedures.
Immunization.
The vaccines were administered subcutaneously to cats at 9 and 12 weeks of age with the Nobivac feline 2-FeLV vaccine (group A, n = 11) or the PureVax recombinant FeLV vaccine (group B, n = 10), per the manufacturer's label. Cats in group C (n = 11) served as age-matched, unvaccinated controls. Injection site and systemic reactions were noted (if present) 4 to 8 h after vaccination, daily for 2 days following vaccination, and 1 to 2 times per week thereafter until challenge. The Nobivac feline 2-FeLV vaccine is a whole-virus adjuvanted killed vaccine that contains FeLV subtype A/Rickard strain. The PureVax recombinant FeLV is a nonadjuvanted canarypox virus-vectored vaccine that contains mutated env, gag, and part of the pol proteins of the FeLV subtype A/Glasgow-1 strain.
Challenge.
Viral challenge occurred 3 months after the second vaccination. Blood was collected for PCR testing prior to challenge. During the entire challenge phase of the study, the placebo-vaccinated cats were randomly divided and comingled with the commercially vaccinated groups. Commercially vaccinated groups remained separate from each other. On the day of challenge, all cats were administered methylprednisolone acetate (MPA) intramuscularly at a dose of 10 mg/kg of body weight ≥4 h prechallenge. Three ml of feline leukemia virus (105.5 PFU/ml), strain 61E (subtype A) grown on NCE-F161 cells, was administered via the oronasal route. Pre- and postchallenge samples of the virus were back titrated to check the concentration on clone 81 cells. One week postchallenge, all cats were administered 10 mg/kg of methylprednisolone acetate (MPA) intramuscularly. From alignment and analysis of amino acid sequences of vaccine and challenge viruses, this is considered a heterologous challenge.
Sample collection and monitoring.
The cats were observed daily for clinical signs of illness. Blood samples were collected weekly in acid-citrate-dextrose (ACD) tubes for 3 to 10 weeks postchallenge, in EDTA tubes for 3 to 9 weeks postchallenge, and in serum-separating tubes (SSTs) on weeks 11 and 12 postchallenge. Blood from SSTs was allowed to clot at room temperature and centrifuged to obtain serum. Serum and ACD blood samples were tested for antigenemia by p27 ELISA (PetCheck; IDEXX, Portland, OR; performed by Merck Animal Health, Elkhorn, NE). Blood samples from EDTA tubes were tested by quantitative PCR (qPCR) for viral RNA and proviral DNA (Zoologix, Inc., Chatsworth, CA). All personnel testing laboratory samples and performing clinical observations were blinded to the treatment groups.
ELISA to detect antibodies to FeLV.
FeLV antibody detection on serum samples was performed by indirect ELISA. Briefly, plates were coated with a capture antigen (1:1,000 dilution, swine pox virus expressing FeLV gp70 glycoprotein). Plates were washed once with phosphate-buffered saline Triton X-100 (PBST) and incubated with a blocking buffer (5% fish gelatin) for 90 min at 36°C. Plates were washed once with PBST and incubated with the positive-control, negative-control, and test samples (diluted 1:250) in triplicate at 36°C for 90 min. Plates were washed 4 times with PBST and incubated with goat anti-cat IgG(H+L) peroxidase conjugate (Kirkegaard and Perry Laboratories, Inc.) as a 1:10,000 dilution at 36°C for 90 min. Plates were washed 4 times with PBST, and a 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Kirkegaard and Perry Laboratories, Inc.) was added to each well. The plates were incubated at 36°C for 20 min. Then, 100 μl of 1.5 M phosphoric acid was added to the wells to stop the reaction. The absorbance of each well was measured at 650 nm, and the mean OD of the blank wells was subtracted from the controls and samples. The status of a sample was evaluated by the sample-to-positive ratio (S/P ratio). The sample-to-positive ratio was calculated as follows: S/P ratio = [(sample OD − negative-control OD)/(positive-control OD − negative-control OD)].
ELISA to detect FeLV p27 antigen.
FeLV p27 antigen testing on serum samples was performed by ELISA (PetCheck; IDEXX, Portland, OR). Briefly, 50 μl of serum or plasma was added to anti-p27 antigen-coated wells; then, 50 μl of horseradish peroxidase conjugate was added to each well. Plates were incubated for 5 min at 15°C to 30°C and then washed 5 times with wash buffer. The TMB substrate (100 μl per well) was then added, and the plate was incubated for 5 min at 15°C to 30°C. Following incubation, 50 μl of stop solution was then added to each well, plates were read visually, and absorbance was measured. The development of a distinct blue color was considered positive for FeLV p27 antigen as a visual measurement. The absorbance of each well was also measured at 650 nm, and samples were considered positive if the OD was >0.200.
Real-time reverse transcription PCR to detect FeLV viral RNA and real-time PCR to detect FeLV DNA.
FeLV proviral DNA and plasma viral RNA loads were quantified using real-time PCR (qPCR) and real-time reverse transcription PCR (RT-qPCR), respectively. For DNA extraction, 200 μl of EDTA whole blood was extracted using the Qiagen blood DNA minikit (Qiagen, Valencia, CA). DNA was eluted in a Tris-EDTA (TE) buffer. For RNA extraction, approximately 600 μl of EDTA whole blood was spun down to separate out the plasma from the cell layer. A total of 140 μl of plasma was used for RNA extraction using the Qiagen Viral RNA extraction kit (Qiagen, Valencia, CA). RNA was eluted in sterile water.
Proviral DNA was quantitated by real-time PCR (Zoologix, Inc., Chatsworth, CA). The limit of detection was approximately 400 copies of DNA/1 ml. Plasma viral RNA was quantitated by real-time RT-PCR in a one-step reaction (Zoologix, Inc., Chatsworth, CA). The limit of detection was approximately 1,000 copies of RNA/1 ml. Primers and TaqMan probes were the same as used in previous studies and methodology (11, 21). The primers and probes were directed against the unique region (U3) of the long terminal repeat (LTR) of FeLV types A, B, and C. Endogenous FeLV sequences are not detected based on this primer selection.
The viral load of each sample was determined by comparing the sample threshold cycle (CT) with the coamplified standard curve. Both RNA and DNA standards were cloned U3 regions of FeLV subtype A/Glasgow-1, as per previous studies and methodology (11, 21). Ten-fold dilutions from 109 to 10−2 copies/5 μl were used to generate the standard curve.
Necropsy.
Three cats were euthanized during the challenge phase of the study due to adverse reactions to MPA administration (weight loss, dehydration, and lethargy). One cat did not recover from anesthesia during the course of the study shortly after blood collection. Necropsy was performed on all of these cats at the Cornell University Animal Health Diagnostic Center. The remaining cats were euthanized at the conclusion of the study.
In vivo infectivity analysis.
Following challenge, blood was collected from persistently viremic control cats. Peripheral blood mononuclear cells (PBMCs) were isolated and stimulated for 4 days with Con-A and then cocultured with Crandell-Rees feline kidney (CRFK) cells. The supernatant was harvested and stored in liquid nitrogen until use. Five nonvaccinated control cats were then challenged oronasally with 105.7 PFU/cat for 2 consecutive days. At ≥4 h prechallenge and 1 week postchallenge, cats were administered 10 mg/kg of methylprednisolone acetate (MPA) intramuscularly. Persistent viremia was measured by ELISA to detect the p27 antigen (see “ELISA to detect FeLV p27 antigen,” above).
Data analysis.
Persistent FeLV antigenemia was defined as the presence of the FeLV p27 antigen in serum or plasma, detected by ELISA, for 3 consecutive weeks between the 3rd and 12th week postchallenge or for ≥5 occasions, consecutive or not. The incidence of persistent FeLV antigenemia was compared between the vaccinated groups and between the controls and vaccinated groups using Fisher's exact test in SAS 9.3, where P values of <0.05 indicate a significant difference. Further statistical analysis for each week was performed between the vaccinated groups and between the controls and vaccinated groups using the Wilcoxon exact rank sum test (Mann-Whitney U test). Statistical significance was set at a P value of <0.05. Statistical analysis using the Wilcoxon exact rank sum test was performed between the vaccinated groups and the vaccinated groups and controls (P < 0.05).
Plasma viral RNA (viremia) and proviral DNA were analyzed by qRT-PCR and qPCR, respectively. The log10 DNA titer and the log10 RNA titer were analyzed separately by the Wilcoxon exact rank sum test (also called the Mann-Whitney U test) between the vaccinated groups and between the controls and vaccinated groups. Statistical significance was set at a P value of <0.05. Additionally, the percentage of positive results (DNA or RNA titer > 0) between vaccinated groups and between the controls and vaccinated groups was analyzed by Fisher's exact test. Statistical significance was set at a P value of <0.05. The prevented fraction to determine vaccine efficacy was calculated as follows: 1 − [(incidence of persistent antigenemia in vaccinates)/(incidence of persistent antigenemia in controls)].
RESULTS
Antibodies to FeLV postvaccination and prechallenge.
Antibodies (IgG) to FeLV were detected using indirect ELISA. All cats were negative for the FeLV antibody on study day 0, prior to vaccination. Ten of 11 cats in the Nobivac feline 2-FeLV group were positive for the FeLV antibody on study days 20 (S/P ratio, 3.0544 ± 1.007) and 111 (S/P ratio, 1.002 ± 0.48). One cat was not tested on study day 20 due to a low serum sample volume. All cats in the PureVax recombinant FeLV group as well as the control group remained negative for the FeLV antibody through the entire vaccination phase. Levels of the IgG antibody in cats that received Nobivac feline 2-FeLV were significantly higher at days 20 and 111 than in those that received Purevax recombinant FeLV (P = 0.00) and in controls (P = 0.00) (Fig. 1).
FIG 1.
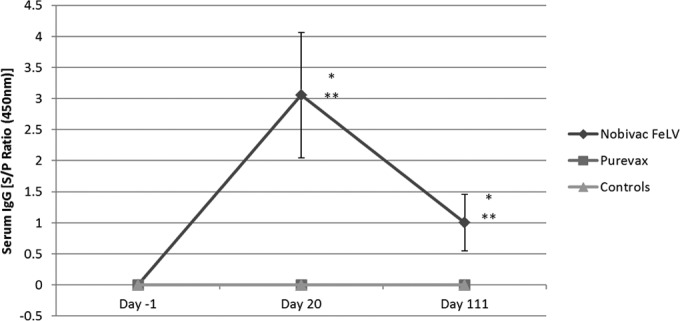
Serum IgG antibodies to FeLV vaccination phase. All cats were negative for FeLV antibody prior to vaccination on study day 0. Ten of 11 cats in the Nobivac feline 2-FeLV group were positive for FeLV antibody on study days 20 (average S/P ratio, 3.0544 ± 1.007) and 111 (average S/P ratio, 1.002 ± 0.48). The PureVax recombinant FeLV-vaccinated cats and control-group cats remained antibody negative during the vaccination phase of the study. Statistically significant (P = 0.00) differences were seen between the Nobivac feline 2-FeLV group and the Purevax recombinant FeLV group (*) and the control group (**).
FeLV p27 antigen (persistent antigenemia) and vaccine efficacy.
FeLV persistent antigenemia was determined by p27 ELISA to detect circulating viral antigen in blood. The development of a distinct blue color combined with an OD of >0.200 was considered positive. Following challenge, 10 of 11 (91%) control cats developed persistent antigenemia (average challenge phase OD, 0.726). No cats (0 of 11) in the Nobivac feline 2-FeLV group became persistently viremic (average challenge phase OD, 0.047). In comparison, 5 of 10 cats (50%) vaccinated with Purevax recombinant FeLV became persistently FeLV viremic (average OD, 0.445) (Fig. 2 and 3).
FIG 2.
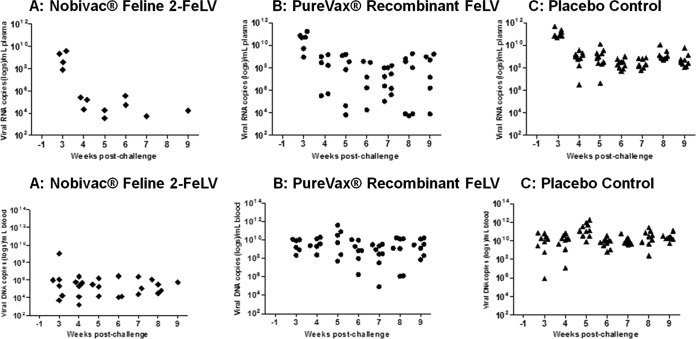
p27 antigen, proviral DNA, and viral RNA results by weeks postchallenge. All cats were tested for p27 antigen, FeLV plasma viral RNA, and FeLV proviral DNA. Cats in the Nobivac feline 2-FeLV group were negative for p27 antigen throughout the postchallenge period; 50% of the PureVax-vaccinated cats and 91% of the control-group cats became persistently antigenemic postchallenge. At week 9 postchallenge, plasma viral RNA was detected in 1 of 11 (9%) of the Nobivac feline 2-FeLV-vaccinated cats, in 6 of 10 (60%) of the PureVax recombinant FeLV-vaccinated cats, and in 9 of 11 (82%) of the unvaccinated control cats. From weeks 7 to 9, viral RNA loads were significantly lower in the Nobivac group than in the PureVax recombinant FeLV group (P < 0.01). Proviral DNA was detected in 1 of 11 (9%) of the Nobivac feline 2-FeLV-vaccinated cats, in 6 of 10 (60%) of the PureVax recombinant FeLV-vaccinated cats, and in 9 of 11 (82%) of the unvaccinated control cats. From weeks 6 to 9, cats in the Nobivac feline 2-FeLV group had significantly lower proviral DNA loads than those in the PureVax recombinant FeLV group (P < 0.02).
FIG 3.
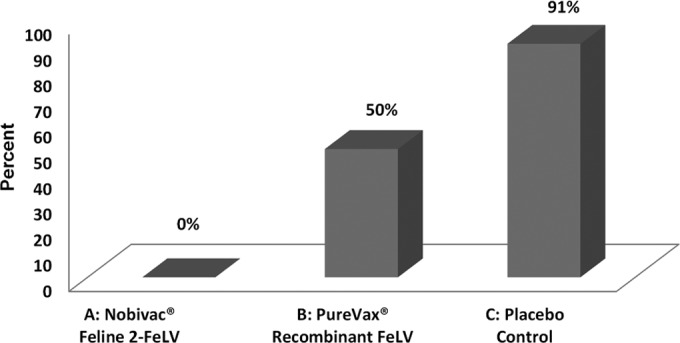
FeLV persistent antigenemia. FeLV p27 antigen ELISA to detect persistent antigenemia in the three groups (group A, Nobivac feline 2-FeLV; group B, PureVax recombinant FeLV; group C, control). Optical densities (OD) ≥0.200 were considered positive for the FeLV p27 antigen. Testing was performed during both the vaccination and challenge phases of the study. Percentages of cats persistently antigenemic were calculated at 0% for the Nobivac feline 2-FeLV group, 50% for the PureVax recombinant FeLV group, and 91% for the control group.
Nobivac feline 2-FeLV had a demonstrated vaccine efficacy of 100% (prevented fraction) compared to the control group. The prevented fraction for the PureVax recombinant FeLV group was calculated at 45% compared to the control group. Compared to both the control group and the PureVax recombinant FeLV group, the protection conferred by Nobivac Feline 2-FeLV was significantly higher (P < 0.0001 and P = 0.0124, respectively). There was no significant difference between the PureVax recombinant FeLV group and the control group (P = 0.0635) (Table 1).
TABLE 1.
Prevented fraction based on persistent antigenemia
| Group | Treatment | Persistent antigenemia (%) | Prevented fraction (%)a |
|---|---|---|---|
| A | Nobivac feline 2-FeLV | 0 | 100 |
| B | PureVax recombinant FeLV | 50 | 45 |
| C | Placebo control | 91 |
Prevented fraction was calculated for all groups using the equation 1 − [(incidence of persistent antigenemia in vaccinates)/(incidence of persistent antigenemia in controls)]. Statistically significant differences in protection were seen when the Nobivac feline 2-FeLV group was compared to the control group and to the PureVax recombinant FeLV group (P < 0.0001 and P = 0.0124, respectively). There was no significant difference between the PureVax recombinant FeLV group and the control group (P = 0.0635).
Persistent antigenemia data were statistically analyzed using the Wilcoxon exact rank sum test. The groups were compared on day −1 (prechallenge) and then weekly postchallenge from weeks 3 to 12. Higher levels of p27 antigen were seen in the Purevax recombinant FeLV vaccinates and controls than in the Nobivac feline 2-FeLV vaccinates at all time points. Statistically significant differences were seen between the Nobivac feline 2-FeLV and PureVax recombinant FeLV groups at weeks 3, 4, 6 to 9, and 11 postchallenge (P < 0.01). Statistically significant differences were seen between the Nobivac feline 2-FeLV and control groups at all time points (P < 0.03). No statistically significant differences were seen between PureVax recombinant FeLV groups and control groups at any time point (P > 0.08) (Fig. 4).
FIG 4.
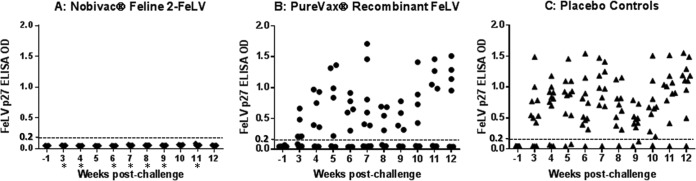
FeLV p27 antigenemia ELISA data. FeLV p27 antigen ELISA to detect persistent antigenemia in the three groups (group A, Nobivac feline 2-FeLV; group B, PureVax recombinant FeLV; group C, control group). Optical densities (OD) ≥ 0.200 were considered positive for the FeLV p27 antigen. Statistically significant differences were seen between the Nobivac feline 2-FeLV and control groups at all time points (P < 0.03). Statistically significant differences were seen between the Nobivac feline 2-FeLV and PureVax recombinant FeLV groups at weeks 3, 4, 6 to 9, and 11 postchallenge (*, P < 0.01). No statistically significant differences were seen between the PureVax recombinant FeLV and control groups at any time points (P > 0.08).
Plasma viral RNA.
The circulating plasma viral RNA load was determined by a real-time RT-PCR assay from 3 to 9 weeks postchallenge to determine whether FeLV vaccination would prevent circulating viral nucleic acid persistence (active replication). At the end of the challenge, plasma viral RNA was detected in 1 of 11 (9%) of the Nobivac feline 2-FeLV-vaccinated cats, in 6 of 10 (60%) of the PureVax recombinant FeLV-vaccinated cats, and in 9 of 11 (82%) of the unvaccinated control cats. Viral RNA concentrations were low for the Nobivac feline 2-FeLV-vaccinated group at <1 × 106 copies/ml at all time points except for week 3 (median, 1.5 × 105 copies of RNA/ml; range, 3.3 × 103 to 3.7 × 109 copies of RNA/ml). In contrast, 5 of the 9 PureVax recombinant FeLV-vaccinated cats had plasma viral RNA concentrations exceeding 1 × 106 copies/ml for the majority of the time points that they were positive (median, 1.0 × 108 copies of RNA/ml; range, 5.3 × 103 to 1.7 × 1011 copies of RNA/ml). Ten of 11 control cats tested positive for plasma viral RNA at concentrations exceeding 1 × 106 copies/ml for the majority of testing (median, 6.5 × 108 copies of RNA/ml; range, 5.9 × 107 to 4.7 × 1011 copies of RNA/ml). Concentrations of viral RNA between the groups were compared using the Wilcoxon exact rank sum test. Plasma viral RNA loads were significantly lower in the Nobivac feline 2-FeLV-vaccinated group than in the control group at all time points (P < 0.01). Plasma viral RNA loads were significantly lower in the Nobivac feline 2-FeLV-vaccinated group than in the PureVax recombinant FeLV-vaccinated group from weeks 7 to 9 (P < 0.01). There was a strong association seen between plasma viral RNA loads and persistent antigenemia (Fig. 2 and 5).
FIG 5.
FeLV plasma viral RNA and proviral DNA results by PCR. Quantitative detection of FeLV virus in proviral DNA by real-time PCR and in plasma viral RNA by reverse transcription real-time PCR. The limit of detection for DNA was approximately 400 copies of DNA/1 ml. The limit of detection for RNA was approximately 1,000 copies of RNA/1 ml. At the end of the challenge, proviral DNA and plasma viral RNA were detected in 1 of 11 (9%) of the Nobivac feline 2-FeLV-vaccinated cats, in 6 of 10 (60%) of the PureVax recombinant FeLV-vaccinated cats, and in 9 of 11 (82%) of the unvaccinated control cats. Statistically significant differences were seen between the Nobivac feline 2-FeLV and control groups at all time points for plasma viral RNA and proviral DNA (both P < 0.01). Statistically significant differences were seen between the Nobivac feline 2-FeLV and PureVax recombinant FeLV groups between weeks 7 and 9 for plasma viral RNA (P < 0.01) and between weeks 6 and 9 for proviral DNA (P < 0.02).
Plasma proviral DNA.
Circulating proviral DNA loads were determined by quantitative PCR assay from 3 to 9 weeks postchallenge to determine whether FeLV vaccination would prevent nucleic acid persistence. At the end of the challenge, proviral DNA was detected in 1 of 11 (9%) of the Nobivac feline 2-FeLV-vaccinated cats, in 6 of 10 (60%) of the PureVax recombinant FeLV-vaccinated cats, and in 9 of 11 (82%) of the unvaccinated control cats. All cats except one in the Nobivac feline 2-FeLV-vaccinated group had proviral DNA concentrations below 1 × 106 copies/ml (median, 2.2 × 105 copies of proviral DNA/ml; range, 1.5 × 103 to 1 × 109 copies of proviral DNA/ml). In contrast, 5 of the 10 proviral DNA positive PureVax recombinant FeLV-vaccinated cats had proviral concentrations >1 × 106 for the majority of the study (median, 2.1 × 109 copies of proviral DNA/ml; range, 8.6 × 104 to 4.1 × 1011 copies of proviral DNA/ml). Ten of 11 control cats tested positive for proviral DNA at concentrations exceeding 1 × 106 copies/ml for the majority of testing (median, 1.7 × 1010 copies of proviral DNA/ml; range, 8.3 × 105 to 1.8 × 1012 copies of proviral DNA/ml). Concentrations of proviral DNA between the groups were compared using the Wilcoxon exact rank sum test. Proviral DNA loads were significantly lower in the Nobivac feline 2-FeLV-vaccinated group than in the control group (P < 0.01). In addition, the Nobivac feline 2-FeLV-vaccinated group had significantly lower proviral DNA loads than the PureVax recombinant FeLV-vaccinated group from weeks 6 to 9 (P < 0.02). Proviral DNA loads were strongly associated with persistently viremic cats (Fig. 2 and 5).
Adverse events and clinical disease.
One cat (group B, PureVax recombinant FeLV) did not recover from anesthesia following the week 7 blood collection. Additionally, 3 cats (2 in group B [PureVax recombinant FeLV] and 1 in group C [control group]) were euthanized during the course of the study due to weight loss, dehydration, and lethargy secondary to MPA administration. No fever or clinical signs were observed in any of the vaccinated or control groups due to FeLV infection.
In vivo infectivity analysis.
Four of the 5 naive control cats (80%) demonstrated persistent antigenemia as measured by p27 ELISA for the infectivity analysis.
DISCUSSION
This study reinforced previous work demonstrating the efficacy of killed, whole-virus adjuvanted vaccines in protecting against a feline leukemia virus challenge (16–18). In this study, the FeLV p27 antigen could not be detected in any of the cats vaccinated with Nobivac feline 2-FeLV, a killed whole-virus adjuvanted FeLV vaccine, at any point in the 12 weeks postchallenge. The prevented fraction calculated for the Nobivac feline 2-FeLV vaccine was 100%. This is consistent with prevented fractions in previous studies using this vaccine, which ranged from 90% to 100% (16–18). In contrast, 5 of 10 (50%) cats that were vaccinated with the nonadjuvanted, live canarypox-vectored vaccine (PureVax recombinant FeLV) tested positive for the FeLV p27 antigen. The prevented fraction calculated for the PureVax recombinant FeLV vaccine was 45%. This prevented fraction was higher than that seen in a previous report with a recombinant canarypox virus-vectored vaccine, which was 20% (19). Ten of 11 (91%) control cats were positive for the FeLV p27 antigen, demonstrating a strong viral challenge. Significantly higher levels of p27 antigen were measured at weeks 3, 4, 6 to 9, and 11 postchallenge (P < 0.01) in the PureVax recombinant FeLV-vaccinated cats compared to the Nobivac feline 2-FeLV-vaccinated cats. Furthermore, it was found that vaccination with the Nobivac feline 2-FeLV vaccine produced detectable levels of IgG directed against FeLV prechallenge. Ten of 11 cats in this group tested positive for FeLV-specific antibodies at day 20 and day 111. No antibodies could be detected in either the PureVax recombinant FeLV-vaccinated or control groups, and this difference was statistically significant (P = 0.00).
These results support the finding that vaccination with killed, whole-virus adjuvanted vaccines can help to prevent persistent antigenemia. Clinically, this is highly relevant based on the availability of in-clinic diagnostic tests relying on the presence of a viral antigen. Vaccination with the nonadjuvanted, live canarypox-vectored vaccine did not confer the same level of protection, based on persistent antigenemia and immunoglobulin testing. In this study, it appears that vaccination with the killed, whole-virus adjuvanted Nobivac feline 2-FeLV vaccine can help to prevent active viral replication after severe challenge.
Both the vaccines and the challenge virus contained FeLV subtype A. Only FeLV subtype A is considered infectious (Other subtypes arise from mutation within the cat after infection) (22). The Nobivac feline 2-FeLV vaccine is a whole-virus, adjuvanted killed vaccine that contains FeLV subtype A/Rickard strain. PureVax recombinant FeLV is a nonadjuvanted, canarypox virus-vectored vaccine that contains the pol and env proteins of the FeLV subtype A/Glasgow-1 strain. The envelope proteins of Nobivac feline 2-FeLV and Purevax recombinant FeLV contain amino acids that differ significantly from the envelope protein of the challenge strain FeLV subtype A/61E (data not shown). Thus, the challenge for both vaccines would be considered a heterologous challenge.
The requirement for virus-neutralizing antibodies for protection from FeLV is controversial. The passive transfer of virus-specific antibodies has been shown to provide protection against disease after FeLV exposure (23). Other studies have shown that the development of virus-neutralizing antibodies after FeLV exposure may be important in long-term FeLV challenge outcomes. In one study, high titers of virus-neutralizing antibodies developed in challenged cats that were able to overcome infection but not in challenged cats that became persistently viremic (24). In this same study, the development of cytotoxic T lymphocytes (CTLs) occurred before virus-neutralizing antibodies, and the adoptive transfer of these CTLs was able to lower the FeLV viremic load (24). This lends support to the fact that other immune mechanisms may be involved in protection against FeLV. This is highlighted in a previous study in which vaccination with a DNA construct vaccine containing env/gag/pol and a gene adjuvant containing interleukin 12 (IL-12) and IL-18 conferred protection against FeLV challenge without the development of virus-neutralizing antibodies until after challenge (25). Thus, the development of detectable levels of IgG seen in this study with Nobivac feline 2-FeLV prechallenge may or may not have significance compared to the PureVax recombinant FeLV vaccinated or control groups, which did not develop detectable levels of IgG. The IgG ELISA data should be evaluated in combination with the PCR data, persistent antigenemia data, and challenge outcome to compare efficacies of the vaccines.
The limit of detection for viral RNA and proviral DNA in this study, at 1,000 copies/ml and 400 copies/ml, respectively, was similar to other studies. This similarity includes studies detecting the proviral DNA load from 5 copies of DNA in qPCR and 5 proviral copies/106 cells to 1,150 RNA copies/ml and 2,250 viral RNA copies/ml of plasma (8, 18, 26). Caution should always be used when comparing results across different studies, as different PCR techniques may be employed at different facilities. However, based on the limits of detection of the assays used in this study, it can be concluded that the sensitivity of this assay was high, consistent with other PCR assays for FeLV virus detection, and even very low limits of viral integration and replication were detected.
Previous studies have shown that vaccination does not confer sterilizing immunity, and no vaccine to date has been shown to completely prevent proviral DNA integration and plasma viral RNA circulation after challenge. However, the concentration of viral RNA and proviral DNA, the duration of active infection as detected by PCR, and the degree and length of persistent antigenemia are all factors that may be used in predicting the outcome of viral infection and likelihood of viral reactivation. In a study examining both naturally and experimentally infected cats, cats that were p27 antigen positive and progressively infected had significantly higher proviral DNA loads than cats that were p27 antigen negative and regressively infected. In the naturally infected cats, the mean proviral load in the regressively infected cats was 300 times lower than that in the progressively infected cats (11). This was reflected in another study, in which cats that recovered from FeLV infection had lower proviral DNA burdens than cats that were persistently infected (24).
These results are mirrored when plasma viral RNA loads are examined. In multiple studies looking at infection outcome (recrudescence) and viral RNA loads, higher viral RNA concentrations were significantly associated with viral reactivation, even more so if the cats were persistently viremic (7, 12). One study demonstrated differences in viral RNA concentration and infection outcome and classified the cats as regressively infected without antigenemia (median, 1.8 × 103 copies/ml of plasma), regressively infected with antigenemia (median, 8.4 × 104 copies/ml of plasma), or progressively infected (median, 4.7 × 107 copies/ml of plasma) and correlated these outcomes to long-term (12-year) survival (Worse survival rates were seen in cats with higher concentrations of virus and antigenemia) (12). Similarly, cats that test antigen negative but remain plasma viral RNA positive may be at higher risk of FeLV reactivation than cats that become viral RNA negative (8). This positive-to-negative viral RNA status may be a result of the virus replicating initially in peripheral immune cells (such as monocytes and lymphocytes) before being contained by an effective, vaccine-induced immune response. Thus, it can be supposed that, while vaccination may not confer sterilizing immunity, viral RNA and proviral DNA concentrations as well as persistent antigenemia status should be examined when trying to determine the degree of protection that a vaccine offers from infection.
Throughout this study, Nobivac feline 2-FeLV-vaccinated cats had significantly lower proviral DNA and plasma viral RNA loads and were positive for much shorter periods of time than the PureVax recombinant FeLV-vaccinated cats. In addition, no Nobivac feline 2-FeLV-vaccinated cat ever tested positive for persistent antigenemia, while half of the PureVax recombinant FeLV-vaccinated cats were persistently viremic. A median concentration of 1.5 × 105 copies of RNA/ml and 2.2 × 105 copies of proviral DNA/ml was seen in the Nobivac feline 2-FeLV-vaccinated cats (with all time points except week 3 at <1 × 106 copies/ml). Median concentrations of 1.0 × 108 copies of RNA/ml and 1.7 × 1010 copies of proviral DNA/ml were seen in the PureVax recombinant FeLV-vaccinated cats (with all cats at >1 × 106 copies/ml for the majority of the time points). In the Nobivac feline 2-FeLV-vaccinated group, 7 of 11 cats tested positive for circulating plasma viral RNA and/or proviral DNA at least once in the 3 to 9 weeks postchallenge, but only 1 of these cats remained positive for both plasma viral RNA and proviral DNA at the conclusion of testing (9 weeks). In contrast, 9 of 10 PureVax recombinant FeLV-vaccinated cats tested positive for circulating plasma viral RNA and/or proviral DNA at least once in the 3 to 9 weeks postchallenge, with 6 of these cats remaining positive for both viral RNA and proviral DNA at the conclusion of the study. Ten of 11 control cats tested positive for both proviral DNA and viral RNA at concentrations exceeding 1 × 106 copies/ml for the majority of testing (median, 6.5 × 108 copies of RNA/ml; median, 1.7 × 1010 copies of proviral DNA/ml).
From this data, it appears that Nobivac feline 2-FeLV-vaccinated cats either experienced abortive infection (in 4 cats that never tested PCR positive) or regressive infection that was contained by an effective, vaccine-induced immune response (in 7 cats that had low viral nucleic acid concentrations but were never viremic). Although long-term outcomes were not examined, these cats with low PCR loads and no persistent antigenemia might be less likely to reactivate infection and may eventually clear the infection (12). In contrast, PureVax recombinant FeLV-vaccinated cats that were PCR positive with high PCR loads (9 cats total) and were persistently (4 cats) or transiently (1 cat) viremic would be much more likely to remain progressively infected or become regressively infected, with a higher likelihood of viral reactivation (12). However, because bone marrow samples were not obtained and analyzed, the ability of the two vaccines to prevent latency cannot be established. The Nobivac feline-2 FeLV vaccine provided excellent protection in the face of a severe viral challenge accompanied by immunosuppression. Nobivac feline 2-FeLV-vaccinated cats were fully protected against persistent antigenemia and had significantly smaller amounts of proviral DNA and plasma viral RNA loads than PureVax recombinant FeLV-vaccinated cats and unvaccinated control cats. In this study, it appears that Nobivac feline 2-FeLV (a whole-virus adjuvanted killed vaccine) provided superior protection against FeLV infection compared to PureVax recombinant FeLV (a nonadjuvanted, canarypox virus-vectored vaccine). Effective vaccination is an important means of controlling FeLV, and the efficacy of the chosen vaccine should be examined closely to obtain the best protection possible.
ACKNOWLEDGMENTS
The study was supported by funds from Merck Animal Health. The authors are employees of Merck Animal Health, the company that markets Nobivac Feline 2-FeLV.
M. Patel and M. Stahl designed the study. M. Patel, K. Carritt, and J. Lane supported the in-life phase, data acquisition, laboratory work, and vaccine acquisition. M. Patel and H. Jayappa interpreted the data. M. Bourgeois wrote the article and contributed to the design of the study. The manuscript was read and approved by all named authors.
We thank L. Deng for data analysis and K. Heaney for providing valuable input during article writing. We thank A. Torres for evaluating the study protocol.
All animal research was run at a contracted, independent research facility (Liberty Research) by blinded investigators. All PCR analyses were run at a contracted, independent research facility (Zoologix, Inc.) by blinded investigators. Merck employees, who were blinded, performed the ELISA analysis.
REFERENCES
- 1.Pacitti AM, Jarrett O, Hay D. 1986. Transmission of feline leukemia virus in the milk of a nonviraemic cat. Vet Rec 118:381–384. doi: 10.1136/vr.118.14.381. [DOI] [PubMed] [Google Scholar]
- 2.Levy J, Crawford C, Hartmann K, Hofmann-Lehmann R, Little S, Sundahl E, Thayer V. 2008. American Association of Feline Practitioners' feline retrovirus management guidelines. J Feline Med Surg 10:300–316. doi: 10.1016/j.jfms.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoover EA, Mullins JI. 1991. Feline leukemia virus infection and diseases. J Am Vet Med Assoc 199:1287–1297. [PubMed] [Google Scholar]
- 4.Levy JK, Crawford PC. 2005. Feline leukemia virus. In Ettinger SJ, Feldman EC (ed), Textbook of veterinary internal medicine, 6th ed WB Saunders, Philadelphia, PA. [Google Scholar]
- 5.Rojko JL, Kociba GJ. 1991. Pathogenesis of infection by the feline leukemia virus. J Am Vet Med Assoc 199:1305–1310. [PubMed] [Google Scholar]
- 6.Torres AN, Mathiason CK, Hoover EA. 2005. Re-examination of feline leukemia virus: host relationships using real-time PCR. Virology 332:272–283. doi: 10.1016/j.virol.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann-Lehmann R, Tandon R, Boretti FS, Meli ML, Willi B, Cattori V, Gomes-Keller MA, Ossent P, Golder MC, Flynn JN, Lutz H. 2006. Reassessment of feline leukemia virus (FeLV) vaccines with novel sensitive molecular assays. Vaccine 24:1087–1094. doi: 10.1016/j.vaccine.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Hofmann-Lehmann R, Cattori V, Tandon R, Boretti FS, Meli ML, Riond B, Lutz H. 2008. How molecular methods change our views of FeLV infection and vaccination. Vet Immunol Immunopathol 123:119–123. doi: 10.1016/j.vetimm.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Torres AN, O'Halloran KP, Larson LJ, Schultz RD, Hoover EA. 2008. Development and application of a quantitative real-time PCR assay to detect feline leukemia virus RNA. Vet Immunol Immunopathol 123:81–89. doi: 10.1016/j.vetimm.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Major A, Cattori V, Boenzli E, Riond B, Ossent P, Meli ML, Hofmann-Lehmann R, Lutz H. 2010. Exposure of cats to low doses of FeLV: seroconversion as the sole parameter of infection. Vet Res 41:17. doi: 10.1051/vetres/2009065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofmann-Lehmann R, Huder JB, Gruber S, Boretti F, Sigrist B, Lutz H. 2001. Feline leukemia provirus load during the course of experimental infection and in naturally infected cats. J Gen Virol 82:1589–1596. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann-Lehmann R, Cattori V, Tandon R, Boretti FS, Meli ML, Riond B, Pepin AC, Willi B, Ossent P, Lutz H. 2007. Vaccination against the feline leukemia virus: outcome and response categories and long-term follow-up. Vaccine 25:5531–5539. doi: 10.1016/j.vaccine.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Levy JK, Scott HM, Lachtara JL, Crawford PC. 2006. Seroprevalence of feline leukemia virus and feline immunodeficiency virus infection among cats in North America and risk factors for seropositivity. J Am Vet Med Assoc 228:371–376. doi: 10.2460/javma.228.3.371. [DOI] [PubMed] [Google Scholar]
- 14.Lee IT, Levy JK, Gorman SP, Crawford PC, Slater MR. 2002. Prevalence of feline leukemia virus infection and serum antibodies against feline immunodeficiency virus in unowned free-roaming cats. J Am Vet Med Assoc 220:620–622. doi: 10.2460/javma.2002.220.620. [DOI] [PubMed] [Google Scholar]
- 15.Sparkes AH. 1997. Feline leukemia virus: a review of immunity and vaccination. J Small Anim Pract 38:187–194. doi: 10.1111/j.1748-5827.1997.tb03339.x. [DOI] [PubMed] [Google Scholar]
- 16.Hines DL, Cutting JA, Dietrich DL, Walsh JA. 1991. Evaluation of efficacy and safety of an inactivated virus vaccine against feline leukemia virus infection. J Am Vet Med Assoc 199:1428–1430. [PubMed] [Google Scholar]
- 17.Pedersen NC. 1993. Immunogenicity and efficacy of a commercial feline leukemia virus vaccine. J Vet Intern Med 7:34–39. doi: 10.1111/j.1939-1676.1993.tb03166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres AN, O'Halloran KP, Larson LJ, Schultz RD, Hoover EA. 2010. Feline leukemia virus immunity induced by whole inactivated virus vaccination. Vet Immunol Immunopathol 134:122–131. doi: 10.1016/j.vetimm.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuke K, King V, Southwick K, Stoeva MI, Thomas A, Winkler MT. 2014. Efficacy of an inactivated FeLV vaccine compared to a recombinant FeLV vaccine in minimum age cats following virulent FeLV challenge. Vaccine 32:2599–2603. doi: 10.1016/j.vaccine.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Code of Federal Regulations. 1989. Title 9. Animal Welfare. Chapter 1. US Department of Agriculture Animal and Plant Health Inspection Service. 9 CFR Part 1. http://awic.nal.usda.gov/final-rules-animal-welfare-9-cfr-parts-1-2-and-3.
- 21.Cattori V, Hofmann-Lehmann R. 2008. Absolute quantitation of feline leukemia virus proviral DNA and viral RNA loads by TaqMan real-time PCR and RT-PCR. In Methods in molecular biology, vol 429: molecular beacons: signalling nucleic acid probes. Humana Press, Inc, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- 22.Greggs WM, Clouser CL, Patterson SE, Mansky LM. 2011. Broadening the use of antiretroviral therapy: the case for feline leukemia virus. Ther Clin Risk Manag 7:115–122. doi: 10.2147/TCRM.S17731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haley PJ, Hoover EA, Quackenbush SL, Gasper PW, Macy DW. 1985. Influence of antibody infusion on pathogenesis of experimental feline leukemia virus infection. J Natl Cancer Inst 74:821–827. [PubMed] [Google Scholar]
- 24.Flynn JN, Dunham SP, Watson V, Jarrett O. 2002. Longitudinal analysis of feline leukemia virus-specific cytotoxic T lymphocytes: correlation with recovery from infection. J Virol 76:2306–2315. doi: 10.1128/jvi.76.5.2306-2315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanlon L, Argyle D, Bain D, Nicolson L, Dunham S, Golder MC, McDonald M, McGillivray C, Jarrett O, Neil JC, Onions DE. 2001. Feline leukemia virus DNA vaccine efficacy is enhanced by coadministration with interleukin-12 (IL-12) and IL-18 expression vectors. J Virol 75:8424–8433. doi: 10.1128/JVI.75.18.8424-8433.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tandon R, Cattori V, Gomes-Keller MA, Meli ML, Golder MC, Lutz H, Hofmann-Lehmann R. 2005. Quantitation of feline leukemia virus viral and proviral loads by TaqMan real-time polymerase chain reaction. J Virol Methods 130:124–132. doi: 10.1016/j.jviromet.2005.06.017. [DOI] [PubMed] [Google Scholar]