Abstract
Presently, the seroprevalence of human papillomavirus (HPV) minor capsid antigen L2-reactive antibody is not well understood, and no serologic standard exists for L2-specific neutralizing antibodies. Therefore, we screened a total of 1,078 serum samples for HPV16 L2 reactivity, and these were obtained from four prior clinical studies: a population-based (n = 880) surveillance study with a high-risk HPV DNA prevalence of 10.8%, a cohort study of women (n = 160) with high-grade cervical intraepithelial neoplasia (CIN), and two phase II trials in women with high-grade vulvar intraepithelial neoplasia (VIN) receiving imiquimod therapy combined with either photodynamic therapy (PDT) (n = 19) or vaccination with a fusion protein comprising HPV16 L2, E7, and E6 (TA-CIN) (n = 19). Sera were screened sequentially by HPV16 L2 enzyme-linked immunosorbent assay (ELISA) and then Western blot. Seven of the 1,078 serum samples tested had L2-specific antibodies, but none were detectably neutralizing for HPV16. To develop a standard, we substituted human IgG1 sequences into conserved regions of two rodent monoclonal antibodies (MAbs) specific for neutralizing epitopes at HPV16 L2 residues 17 to 36 and 58 to 64, creating JWW-1 and JWW-2, respectively. These chimeric MAbs retained neutralizing activity and together reacted with 33/34 clinically relevant HPV types tested. In conclusion, our inability to identify an HPV16 L2-specific neutralizing antibody response even in the sera of patients with active genital HPV disease suggests the subdominance of L2 protective epitopes and the value of the chimeric MAbs JWW-1 and JWW-2 as standards for immunoassays to measure L2-specific human antibodies.
INTRODUCTION
Persistent infection with a high-risk human papillomavirus (hrHPV) is a necessary, although insufficient cause of cervical cancer and subsets of other anogenital and oral-pharyngeal cancers (1, 2). Despite the licensure of two HPV vaccines based on L1 virus-like particles (VLP) that have been shown to be highly effective, cervical cancer remains the third most common cancer worldwide, with 80% of cases occurring in the developing world (3). This disparity reflects both limited vaccine implementation and certain technical and logistic issues in cervical cancer screening in the developing world.
The first L1 VLP vaccines licensed (Gardasil [Merck & Co.] and Cervarix [GSK]) targeted the two most problematic hrHPV genotypes, HPV16 and HPV18, which together cause ∼70% of all cervical cancer cases. Gardasil also contains L1 VLP types derived from HPV6 and HPV11 and provides protection from benign genital warts caused by these viruses (4). Recognizing the importance of a greater breadth of coverage, a nonavalent vaccine targeting 7 of the 15 high-risk HPV types (5) was recently approved by the FDA. Emerging data suggest that the genotype distribution of HPV that causes cervical pathology differs by country, ethnicity, and in HIV+ individuals (6–8). However, increasing vaccine valency to further expand coverage will raise manufacturing costs and complexity. Importantly, >85% of the cervical cancer disease burden lies in low-resource settings, reflecting in part the insufficient resources for screening programs and HPV vaccination (9). Although some cross-protection against very closely related HPV types has been identified in current vaccines, it does not cover all hrHPV types, and its longevity is uncertain (10, 11). Thus, there remains a clear need to develop an affordable vaccine that broadly protects against all hrHPV types. Finally, none of these vaccines target cutaneous HPV genotypes associated with common warts or betapapillomaviruses associated with nonmelanoma skin cancer in persons with epidermodysplasia verruciformis (EV) or who are immunocompromised.
Vaccination with the minor capsid protein L2 has potential as an approach in comprehensive and inexpensive HPV vaccination, as the N-terminal protective epitope sequences are well conserved. Preclinical vaccine studies demonstrate that this region of L2 can elicit protection against diverse papillomavirus types (12–14). Further, the passive transfer of L2-specific neutralizing antibodies into naive animals is sufficient for protection from experimental challenge, providing evidence to support their central role in protective immunity. Additionally, because the neutralizing epitopes of L2 are linear and conserved, in contrast to the type-restricted conformational epitopes of L1 VLP vaccines, cross-reactive L2 vaccines can be produced as a single antigen in bacteria, which may reduce manufacturing costs compared to those of the licensed L1 VLP vaccines. However, while L2-specific antibodies are broadly reactive, their ability to neutralize less evolutionarily related types is weaker than for the cognate type. Therefore, we and others have sought to enhance cross-protection by either the concatenation of L2 epitopes derived from several HPV types or the display of L2 epitopes on VLPs. Both approaches can provide broad protection in vaccinated animals against experimental challenge with diverse HPV types (15–19).
To lay the groundwork for L2 vaccine trials, the prevalence of L2-specific neutralizing antibodies should be understood in both healthy patients and those with HPV disease. Preclinical studies suggest that L2 is immunologically subdominant to L1 in the context of the capsid, suggesting that responses to infection with genital HPV are likely to be rare and/or weak when they are detectable. To date, studies addressing L2-specific antibody responses to HPV infection are both limited in number and inconsistent with respect to seroprevalence (20–26). Some of these inconsistencies may reflect reliance upon a single assay format and/or the use of bacterially expressed L2 antigens only (20–26). To address this, we examined the serologic responses to HPV16 L2 in both an unscreened healthy population at risk for HPV infection and in patients with high-grade cervical or vulvar intraepithelial neoplasia grade 2/3 (CIN2/3 or VIN2/3, respectively), using HPV16 L2 enzyme-linked immunosorbent assay (ELISA), followed by a confirmatory Western blot analysis (i.e., a two-step approach). We also examined whether the topical application of a Toll-like receptor 7 (TLR7) agonist, imiquimod, at the lesion site and either ablation of the lesion with photodynamic therapy or intramuscular vaccination with HPV16 L2E7E6 fusion protein (TA-CIN) elicit serum antibody responses to L2.
In addition to seroprevalence, validated serologic assessment methods that are both functionally sensitive and high throughput are required to assess the neutralizing antibody responses in patients. Recently, several new in vitro assays with enhanced sensitivity to L2-specific neutralizing antibodies were developed (27–29). A key requirement for their routine validation is a reproducible positive control (30). Indeed, the need for the standardization of assays for assessing responses to HPV L1 VLP vaccination led the World Health Organization (WHO) to develop international serologic standards for HPV16 and HPV18 L1 VLP reactive antibodies using serum pooled from HPV-infected women (30, 31). Here, we sought to use a similar approach in generating a standard for future L2-based vaccination validation studies by identifying patient sera with L2-reactive neutralizing antibodies.
MATERIALS AND METHODS
Human samples.
The studies using human sera were reviewed and approved by the Johns Hopkins University institutional review board. Human serum samples were collected previously from women in four published clinical cohorts: (i) a 38% random sample (n = 880) of serum samples collected as part of a population-based (n = 2,331) surveillance study in India with a high-risk HPV DNA prevalence of 10.8% (Community Access to Cervical Health [CATCH] study) (32) (see Tables 1 and 2 for patient demographics), (ii) patients (n = 160) with high-grade cervical intraepithelial neoplasia (CIN) (33), (iii) patients (n = 19) with high-grade vulvar intraepithelial neoplasia (VIN) enrolled in a phase II study and who were treated with imiquimod for 8 weeks, followed by photodynamic therapy (34), and (iv) high-grade VIN patients (n = 19) who were treated with imiquimod for 8 weeks, followed by vaccination three times at monthly intervals with a fusion protein comprising HPV16 L2, E6, and E7 (TA-CIN) (35). In both phase II trial studies, sera were obtained prior to and after imiquimod treatment (weeks 0 and 10, respectively) and after PDT/TA-CIN vaccination (week 26).
TABLE 1.
Characteristics of 880 CATCH study patients who provided a sample for the serology study
| Characteristic | No. (%)a |
|---|---|
| Age (yr) | |
| 30–34 | 174 (19.6) |
| 35–39 | 121 (13.6) |
| 40–44 | 95 (37.1) |
| 45–49 | 54 (6.1) |
| 50–54 | 50 (5.6) |
| 55–59 | 29 (3.3) |
| 60+ | 36 (4.0) |
| Not reported | 34 (3.8) |
| Cytology | |
| Normal | 763 (86.5) |
| ASCUS or more severe | 119 (13.5) |
| HPV | |
| Negative | 787 (89.2) |
| Positive | 95 (10.8) |
| Histology | |
| Normal | 222 (24.9) |
| CIN1 | 2 (0.22) |
| CIN2 | 5 (0.56) |
| CIN3 | 3 (0.34) |
| Cancer | 4 (0.45) |
| Other | 6 (0.67) |
| No biopsy performed | 648 (72.8) |
This 38% sample was younger than the total enrolled population (P < 0.01) but did not differ by enrollment cytology result (P = 0.18) or hrHPV result (P = 0.56).
TABLE 2.
Summary of L2 ELISA-screened patient seraa
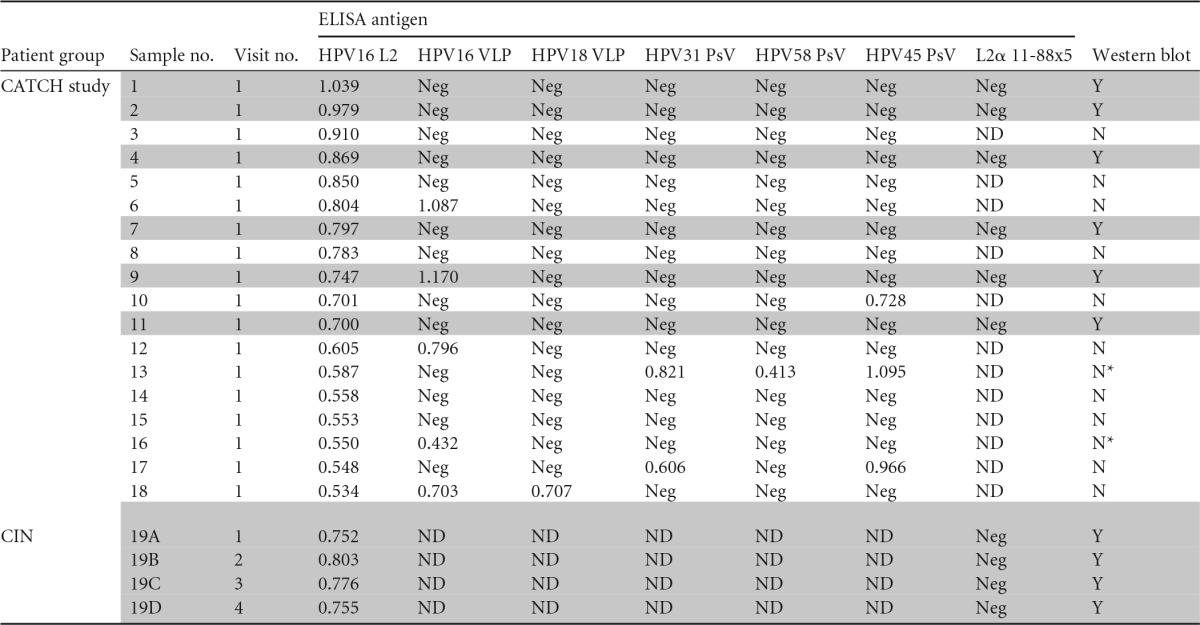
Shaded rows show the patients (n = 7/19) whose serum samples displayed reactivity for full-length HPV16 L2 by both ELISA and Western blot. Serum numbers 1 to 18 were from the CATCH study near Hyderabad, Andhra Pradesh (AP), India, and serum 19 was from the CIN cohort study at Johns Hopkins University, Baltimore, MD, USA. Neg, negative; ND, not done; Y, yes; N, no; N*, serum was limiting, and Western blot analysis could be done only at a dilution of 1:5,000.
Enzyme-linked immunosorbent assays and neutralization assays.
Immobilon plates (Nunc) were coated with 500 ng/well purified baculovirus-derived HPV16/18 L1 VLPs (36), HPV16/31/45/58 pseudovirions (PsVs), or bacterially expressed and 6His-tagged HPV16 L2 (37), or L2α 11-88x5 multimer polypeptide (17). The plates were incubated overnight at 4°C. The wells were then blocked with 1% bovine serum albumin (BSA)–phosphate-buffered saline (PBS) for 1 h at 37°C and incubated with human serum at a 1:50 dilution for 1 h at 37°C. Following a washing step with PBS–0.01% (vol/vol) Tween 20, peroxidase-labeled sheep anti-human (GE Healthcare) antibody diluted 1:5,000 in 1% BSA–PBS was incubated for 1 h. The plates were then washed and developed with 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid solution (Roche) for 10 min, and the absorbance at 405 nm was measured. For JWW-1 and WW-1 ELISA reactivity comparisons to HPV16 L2 and L2α 11-88x5, a total of 400 ng of antibody in 100 μl/well (26.7 nM) was utilized as the starting concentration. A neutralizing assay comparison between JWW-1, JWW-2, and control IgG was done using our recently described furin-cleaved pseudovirion-based neutralization assay (FC-PBNA) (38).
SDS-PAGE and Western blotting.
Four to 15% gradient SDS-PAGE gels were loaded with 40 μg of lysate of 293TT cells transfected with expression vectors for either enhanced green fluorescent protein (eGFP) or HPV16 L2. The gels were run at 110 V for 1.5 to 2 h. The proteins were then transferred onto a nitrocellulose membrane for 90 min at 50 V. The membranes were blocked for 1 h at room temperature (RT) with 5% nonfat dried milk in phosphate-buffered saline containing 0.1% (vol/vol) Tween 20 (PBST). The membranes were then incubated with serum diluted at ≥1:300 (highest, 1:5,000, due to limiting sera) in 5% milk in PBST overnight at 4°C. The membranes were then washed for 10 min three times with PBST. The membranes were incubated with anti-human IgG horseradish peroxidase (HRP) secondary antibody diluted 1:5,000 to 10,000 in 5% milk in PBST and incubated for 1 h at RT. The membranes were then washed three times with PBST, for 10 min each time. Chemiluminescent substrate was added to develop the membranes.
Design and generation of JWW-1 and JWW-2 expression plasmids.
The rat monoclonal antibody WW-1 is broadly neutralizing and reactive with HPV16 L217–36 (29). The WW-1 heavy- and light-chain sequences were obtained by sequencing the hybridoma cDNA (Aldevron, Fargo, MN, USA). Variable region sequences for the mouse monoclonal antibody MAb24B were derived from Nakao et al. (39). Both the constant light- and heavy-chain regions of WW-1 and MAb24B were replaced with the constant light- and heavy-chain regions of human IgG1 sequence and directly synthesized (Bio Basic, Inc., Ontario, Canada). The chimeric heavy and light chains of WW-1 and MAb24B were each cloned into a double expression vector, pVITRO1-neo-mcs (InvivoGen, San Diego, CA), to generate the JWW-1 and JWW-2 plasmids, respectively. The plasmids and cDNA sequence information to produce these human chimeric monoclonal antibodies are available from Addgene (see https://www.addgene.org/Richard_Roden/).
Expression and purification of JWW-1 and JWW-2.
For initial testing, 5 × 106 293TT cells were seeded into a 6-well plate the day before transfection. The cells were transfected with either pVITRO1-neo-mcs empty vector template (mock transfection) or JWW-1/JWW-2 using Mirus TransIT-2020 (Mirus Bio, Madison, WI), according to the manufacturer's protocol, and maintained in Opti-MEM reduced-serum medium (Gibco, Life Technologies, Grand Island, NY). After 72 h, the supernatant was clarified by centrifugation (1,600 rpm for 4.5 min at room temperature) and passage through a 0.2-μm pore filter (Millipore, Billerica, MA) to remove cellular debris. The filtrate was used for Western blot studies at a 1:100 dilution to determine reactivity. For large-scale purification, the cell line Expi293 was utilized, as per the manufacturer's instructions (Life Technologies), and purified using HiTrap protein G high-performance (HP) columns (GE Healthcare Bio-Sciences, Pittsburgh, PA). The quantity of antibody was determined using a Pierce BCA protein assay kit, per the manufacturer's instructions. Purity was assessed by SDS-PAGE and Coomassie blue staining.
Statistics and data analysis.
For all ELISAs, the mean absorbance and standard deviation (SD) of the entire study cohort were first calculated. Subsequently, a positive ELISA value for HPVL2 was defined as an optical density (OD) value greater than the mean + 3 SD. The results of the ELISA screens were plotted using R package ggplot2. The FC-PBNA neutralization titer was defined as the reciprocal of the dilution that caused a 50% reduction in luciferase activity. FC-PBNA comparisons of RG-1, JWW-1, and JWW-2 were done in triplicate and titrated 2-fold with a starting concentration of 200 nM. The values were plotted on GraphPad Prism 6 and fit using the nonlinear model y = bottom + (top − bottom)/(1 + 10(logEC50 − x) × Hill slope), where EC50 is the titer or concentration reducing signal by half estimated.
RESULTS
Prevalence of HPV16 L2-specific antibody in sera of an unscreened population at high risk for HPV infection.
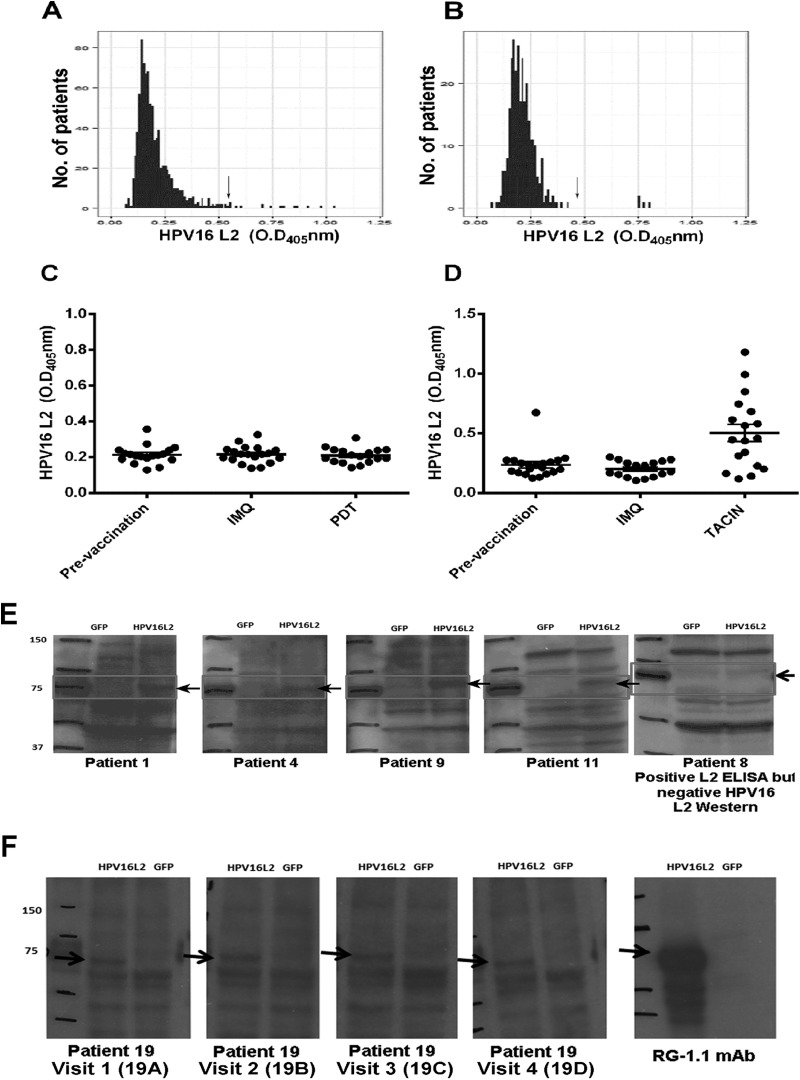
Since the WHO reference reagents for human PV L1 antibodies to HPV16 and HPV18 are based on serum pooled from several infected patients, we attempted to screen for L2-reactive sera from patients who are at high risk for or have active HPV genital disease. We first assessed the prevalence of HPV16 L2-reactive serum antibody responses in a random subset of women enrolled in the Community Access to Cervical Health (CATCH) study (Table 1) designed to evaluate the feasibility and impact of introducing cervical screening methods in a previously unscreened population in Medchal Mandal, Andhra Pradesh, India (32). Using ELISAs, serum samples from 880 women enrolled in the CATCH study were screened for reactivity with full-length HPV16 L2 protein. When employing a cutoff of mean OD + 3 SD by our L2 ELISA, 18 of the 880 serum samples screened were considered potentially positive (Table 2, patients 1 to 18). Since the recombinant HPV16 L2 antigen used in the ELISA was purified from Escherichia coli, there was a possibility that bacterial protein-specific antibody responses might contribute to false positives by reacting with bacterial contaminants in the HPV16 L2 antigen. Therefore, the 18 serum samples putatively positive for HPV16 L2-specific antibody by ELISA were rescreened in a Western blot-based assay, in which lysates of 293TT cells transfected with either an expression vector for HPV16 L2 or with GFP as a negative control were probed with individual patient serum and a peroxidase-linked secondary antibody specific to human IgG. While the presence of the ∼70-kDa HPV16 L2 protein in the 293TT lysates was confirmed by Western blotting using the mouse monoclonal antibody RG-1 (Fig. 1E and F), only 6/18 putative positive serum samples reacted specifically with a band of ∼70 kDa in the lysates of 293TT cells expressing HPV16 L2 (Fig. 1E and summarized in Table 2). This finding suggests that despite a cervical high-risk HPV DNA prevalence of 10.8% (as determined by Qiagen hc2 assays) in the CATCH study patients (Table 1), only a minor (0.68%) fraction of their serum was reactive with HPV16 L2 (by both ELISA and Western blot, Fig. 1E).
FIG 1.
Screening of patient sera for HPV16 L2-specific antibody. Optical density values plotted for HPV16 L2 ELISA measurements of the serological responses of previously unscreened CATCH study patients (n = 880), of which 10.3% were positive for high-risk HPV (A), high-grade CIN patients (n = 160) with 1 to 4 visits (n = 298) (B), high-grade HPV+ VIN patients (n = 19) who underwent 10 weeks of topical imiquimod therapy, which activates TLR7-dependent innate responses (IMQ) before being treated with photodynamic therapy (PDT) in a phase II study (34) (C), and high-grade HPV+ VIN patients (n = 19) who underwent 10 weeks of topical imiquimod therapy (INN), followed by three vaccinations with 125 μg of TA-CIN at monthly intervals in a prior phase II study prior to serum collection (35) (D). The absorbance values to the right of the arrow in panels A and B (mean + 3 SD cutoff) were considered putative positive responses and rescreened in a Western blot assay. (A) Eighteen data points corresponding to 18 different patients were above the cutoff (arrow). Representative Western blot analyses from 4 out of 6 of these 18 high-risk HPV patients were HPV16 L2 ELISA positive (E). (B) The 4 data points larger than the cutoff (arrow) correspond to four serum samples collected at different visits of a single HPV16+ CIN2 patient, and each was also reactive for HPV16 L2 by Western blotting (F). These results are summarized in Table 2.
HPV-infected individuals frequently mount a type-specific L1 VLP-reactive serum antibody response (30). Therefore, the serum samples of these 18 patients from the CATCH study identified in the HPV16 L2 ELISA screen were also tested for reactivity in two ELISAs using either baculovirus-derived HPV16 or HPV18 L1 VLP and previously defined cutoffs and standards (36). Importantly, only 1/6 of the verified HPV16 L2-reactive serum samples was also reactive in the HPV16 L1 VLP ELISA. None of the 6 patients also displayed reactivity against HPV18 L1 VLP (Table 2).
Given that L2 is highly conserved, it is possible that the negative HPV16 and HPV18 L1 VLP ELISA results were due to infection by other related HPV types. To explore this possibility, we performed HPV31/45/58 pseudovirion ELISAs with the patient sera. However, none of these 6 HPV16 L2-reactive serum samples (determined by both ELISA and Western blot) of the CATCH study patients reacted with these additional HPV genotypes either.
HPV16 L2 seroreactivity of women with HPV16+ CIN.
We next assessed for HPV16 L2-reactive serum antibodies a cohort of patients at Johns Hopkins Hospital (n = 160; mean age, 29.5 years) with cervical intraepithelial neoplasia grade 2/3 (CIN2/3) (Table 3) enrolled in a longitudinal cohort protocol, in which serially collected (≤4) blood samples were obtained. A total of 298 serum samples from this cohort were available for testing. Although the cervical swabs of 99 of the 160 patients were HPV16+ (62%), only one of their serum samples (Table 2, sample 19) was reactive to HPV16 L2 by both ELISA and Western blot assays (Fig. 1B and F). The serum sample was provided by a patient diagnosed with an HPV16+ CIN2 at study entry. Her cone excision at study week 16 was benign, suggesting that her lesion underwent spontaneous regression. A year later, she was diagnosed with an HPV16− CIN3, which also regressed. She had donated serum samples at four visits over this period, and each was similarly reactive to HPV16 L2 both via ELISA and Western blot (Table 2, samples 19A to D) but none to either HPV16 or HPV18 L1 VLP by ELISA (Table 2). Together, these data suggest that a systemic antibody response to HPV16 L2 was uncommon even in women with HPV16+ CIN2/3.
TABLE 3.
Characteristics of the cohort of 160 women with high-grade CIN enrolled in a natural history study (33) and treated at Johns Hopkins University, Baltimore, MD, USA
| Diagnosisa | No. (%) of patients |
|---|---|
| HGSIL | 132 (82.5) |
| Benign/CCSM | 11 (6.9) |
| ASCUS | 2 (1.3) |
| ASC-H | 2 (1.3) |
| AIM | 1 (0.6) |
| LSIL-H | 1 (0.6) |
| LSIL | 11 (6.9) |
| HPV16 status (HGSILs only) | |
| HPV16+ | 87 (54.4) |
| HPV16− | 42 (26.3) |
| Not done | 3 (1.9) |
| HPV16 status (all patients) | |
| HPV16+ | 99 (61.9) |
| HPV16− | 53 (33.1) |
| Not done | 7 (4.4) |
| Indeterminate | 1 (0.6) |
| Race/ethnicity | |
| White | 109 (68.1) |
| Black | 37 (23.1) |
| Asian | 5 (3.1) |
| Hispanic | 9 (5.6) |
| Age group (yr) | |
| 18–20 | 16 (10.0) |
| 21–30 | 86 (53.8) |
| 31–40 | 39 (24.4) |
| 41–50 | 13 (8.1) |
| ≥50 | 6 (3.8) |
Serum samples were collected at the screening visit and at subsequent treatment and follow-up visits when available. HGSIL, high-grade squamous intraepithelial lesion; CCSM, cervical cancer-specific mortality; ASC-H, high-grade atypical squamous cells; AIM, atypical immature squamous metaplasia; LSIL-H; high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion.
Neither imiquimod nor PDT elicits an HPV16 L2 antibody response in VIN patients.
The low prevalence of a serum antibody response to HPV16 L2 detectable in the cohort of women with HPV16+ CIN2/3 may reflect HPV infection that is confined to the mucosal epithelium. To address the possibility that infection at a different site, such as the vulva, might be more immunogenic, serum samples of 19 patients with high-grade vulvar intraepithelial neoplasia 2/3 (VIN2/3), of which 15 were HPV16+, obtained during a phase II study, were tested for HPV16 L2-specific antibody (34). None were positive (Fig. 1C and D).
The lack of an L2 antibody response might also reflect the localized and nonlytic replication of HPV and thus the minimal inflammation associated with genital HPV16 infection. Therefore, we reasoned that topical application of the TLR7 agonist imiquimod at the lesion site might act as an adjuvant that would promote a serologic response to L2. The patients were treated subsequently with imiquimod applied to the lesion once in the first week, twice in the second week, and three times weekly for six subsequent weeks. At week 10, 1 week after completing the imiquimod regimen, a peripheral blood sample was obtained. No L2-specific serologic response was detected in any of the subjects. Following imiquimod treatment, the patients received photodynamic therapy (using methyl aminolevulinate cream and red light via an Aktilite 128 [Photocure ASA] set to deliver a dose of 50 J/cm2) at weeks 12 and 16 of the study (34). Peripheral blood was obtained at week 26. No significant differences in L2 ELISA reactivity were identified in within-subject comparisons before versus after treatment (Fig. 1C and D). This finding suggests that any potential adjuvant effect caused by local innate stimulation with imiquimod, even upon lesion damage with photodynamic therapy, was insufficient to elicit detectable systemic L2 responses in the clinical setting of persistent HPV16+ VIN2/3.
Vaccination elicits an HPV16 L2 antibody response in VIN patients.
The inability to detect a serologic response against HPV16 L2 in patients with HPV16+ high-grade anogenital intraepithelial neoplasia (AGIN) may reflect some technical issue with the assay or that AGIN patients have a genetic background that is incompatible with the induction of an L2 antibody response. To further investigate, HPV16 L2-specific serologic responses in high-grade VIN patients (n = 19) treated with imiquimod for 10 weeks, followed by three vaccinations with 125 μg of a fusion protein composed of HPV16 L2, E7, and E6 (TA-CIN) were assessed. Consistent with an earlier VIN cohort study, no HPV L2 responses were induced following topical imiquimod application (Fig. 1D). However, after vaccination with TA-CIN, 63% of the patient group (12/19) developed an HPV16 L2 response (Fig. 1D) that was also detectable by Western blotting (data not shown). Thus, the failure to detect an L2 antibody response in HPV16+ VIN patients prior to L2 vaccination does not reflect either a technical issue with the assay or the inability of most VIN patients to mount an L2 antibody response.
L2 antibody responses induced by HPV infection are not detectably neutralizing.
Our screening efforts yielded 7 patients (see Table 2) with HPV16 L2 antibody verified by both ELISA and Western blot. As 6/7 were also HPV16 L1 VLP ELISA negative, we next assessed if their L2 responses had neutralizing potential. Using the standard in vitro HPV neutralization assay against HPV16 pseudovirions, none were detectably neutralizing (all EC50 titers, <1:50). It was surprising that sample 9 was not detectably neutralizing, but this may reflect a greater sensitivity of the HPV16 L1 VLP ELISA. The key neutralizing epitopes of HPV16 L2 have been mapped to its first 88 residues (40). However, none of the full-length HPV16 L2-reactive sera reacted in an ELISA with the L2α 11-88x5, an antigen composed of amino acids 11 to 88 from the L2 of HPV6/16/18/31/39 (17). This negative ELISA result was consistent with the inability of these sera to neutralize HPV16 pseudovirions (Table 2) despite their reactivity toward full-length HPV16 L2 by ELISA and Western blot. Taken together, these findings suggest that the HPV16 L2 antibody responses identified by ELISA are directed to nonneutralizing epitopes outside the region of amino acids 11 to 88.
Development of chimeric human monoclonal antibodies JWW-1 and JWW-2 as HPV L2 serological standards.
The inability to identify any naturally occurring neutralizing L2 sera subsequently led us to graft two rodent monoclonal antibodies, each targeting a different L2 neutralizing epitope, onto a human IgG1 backbone, which results in a chimeric human monoclonal antibody. Chimeric human monoclonal antibodies to L2 represent an alternate approach to developing a serological standard and have the additional benefit of reproducibility, ready quantification, and limitless supply. It has been reported that HPV16 L2 contains conserved neutralizing epitopes between residues 17 and 36 (14), 58 and 81 (39), and 108 and 120 (41), as defined by rodent monoclonal antibodies and peptide mapping. The neutralizing monoclonal antibodies WW-1 (29) and MAb24B (39) are particularly broadly reactive. WW-1 is a rat monoclonal IgG2a antibody that binds to HPV16 L217–36 but neutralizes more HPV genotypes than the first-generation mouse monoclonal antibody RG-1 (29). The mouse monoclonal antibody MAb24B developed by Nakao et al. (39) neutralizes several genital HPV types and recognizes HPV16 L2 residues 58 to 64. Their heavy- and light-chain sequences were obtained by sequencing WW-1 hybridoma cDNA or as described by Nakao et al. (39). Human chimeric antibodies were then directly synthesized for each, whereby the variable regions were retained, but the constant regions of the parental rodent antibodies in both their heavy and light chains were replaced with the equivalent constant regions of a human IgG1. The resultant human chimeric sequences encoding both chains derived from WW-1 or MAb24B then were subcloned separately into a double expression vector and renamed JWW-1 and JWW-2, respectively.
The chimeric antibodies were expressed in serum-free cultures of human 293 cells after transfection with JWW-1 or JWW-2 and purified from the conditioned medium using protein G-coupled beads. To test whether JWW-1 and JWW-2 chimeric antibodies both retained reactivity with L2 and were recognized by a peroxidase-linked secondary antibody to human IgG alongside them, each, along with their respective parental rodent monoclonal antibodies, was tested in an ELISA for reactivity to L2α 11-88x5, an antigen that contains both of their neutralizing epitopes. In addition, the WHO standard serum for HPV16 (05/134), which reacts with HPV16 L1 VLP, and the single serum sample from the HPV16+ CIN2 patient, which recognized HPV16 L2 (Table 2, sample 19) outside residues 11 to 88, were also tested as controls. Both JWW-1 and JWW-2 bound to L2α 11-88x5, and this was reflected by the strong recognition via a peroxidase-linked secondary antibody to human IgG in this ELISA. However, neither the HPV16+ CIN2 patient serum (Table 2, sample 19) nor WHO standard serum 05/134 reacted in this ELISA (Fig. 2A and B), further suggesting non-L2 reactivity for the WHO standard and the recognition of L2 epitopes outside the 11 to 88 region for sample 19. The chimeric antibodies and patient sera were each subsequently tested for reactivity by Western blotting with 293TT cells transfected with either full-length HPV16 L2 or GFP expression vectors. As expected, JWW-1, JWW-2, and the HPV16+ CIN2 patient serum (Table 2, sample 19A) reacted with an ∼70-kDa band consistent with HPV16 L2 when probed with a peroxidase-linked secondary antibody specific for human IgG, whereas 05/134 was not reactive (data not shown).
FIG 2.
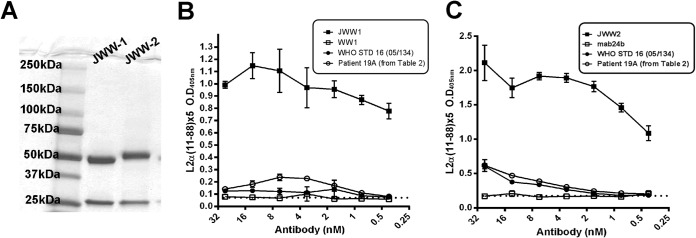
Reactivity of JWW-1 and JWW-2 human chimeric monoclonal antibodies with L2 by ELISA. (A) Coomassie blue-stained SDS-PAGE gel analysis of JWW-1 and JWW-2 monoclonal antibodies. (B) L2α 11-88x5 multimer ELISA using purified JWW-1 (closed squares), rat monoclonal WW-1 (open squares), WHO standard (STD) HPV16 serum 05/134 (closed circles), and serum from an HPV16+ CIN2 patient (visit 1, open circles) containing HPV16 L2-reactive antibodies. (C) L2α 11-88x5 multimer ELISA using purified JWW-2 (closed squares), mouse monoclonal MAb24b (open squares), WHO standard HPV16 serum 05/134 (closed circles), and serum from an HPV16+ CIN2 patient (visit 1, open circles) containing HPV16 L2-reactive antibodies. (B and C) ELISAs were tested with anti-human IgG secondary antibodies to test for human specificity of the chimeric MAbs JWW-1 and JWW-2 (P < 0.001) versus their parental antibodies (WW-1 or MAb24), which were used as negative controls.
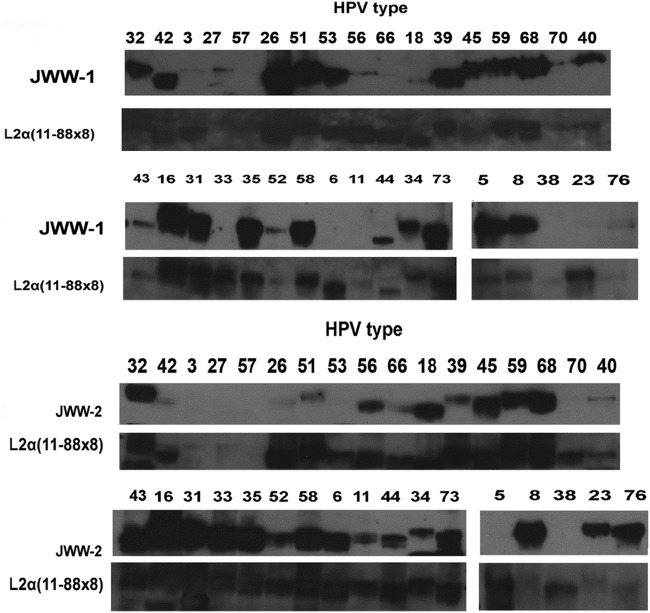
The breadth of JWW-1 and JWW-2 reactivity toward pseudovirion preparations of 34 clinically relevant HPV genotypes was next tested via Western blot (Fig. 3 and summarized in Table 4). As a control, the presence of the L2 in the pseudovirion preparations was confirmed by reprobing the stripped blots with a broadly reactive rabbit antiserum raised against L2α 11-88x5 (Fig. 3). JWW-1 was able to react with L2 of 29/34 HPV types tested and was consistent with its parental rat monoclonal antibody WW-1 in failing to recognize HPV6, -11, -33, -38, and -23 L2 only. In contrast, JWW-2 was able to bind to 25/34 types, and its spectrum of reactivity was distinct from that of JWW-1; for example, JWW-2 failed to bind to HPV26 and HPV51, whereas JWW-1 recognizes them, and JWW-2 reacted with HPV6, -11, -33, and -23, but JWW-1 did not. Thus, together, the two antibodies are complementary with respect to their breadth of reactivity, and they recognize 33/34 clinically relevant HPV types tested (Table 4).
FIG 3.
Reactivity of JWW-1 and JWW-2 human chimeric monoclonal antibodies with L2 of diverse HPV, as determined by Western blotting. Pseudovirion preparations of 34 clinically relevant alpha- and beta-HPV types were separated by SDS-PAGE and transferred to a membrane. The reactivity of JWW-1 or JWW-2 against L2 of each type was determined by probing with peroxidase-linked anti-human IgG for JWW-1 and JWW-2 or peroxidase-linked anti-rabbit IgG for the L2α 11-88x8 antiserum. After washing, these Western blots were developed by chemiluminescence.
TABLE 4.
Summary of JWW-1 and JWW-2 Western blot reactivity against L2 of 34 clinically relevant alpha- and beta-HPV types
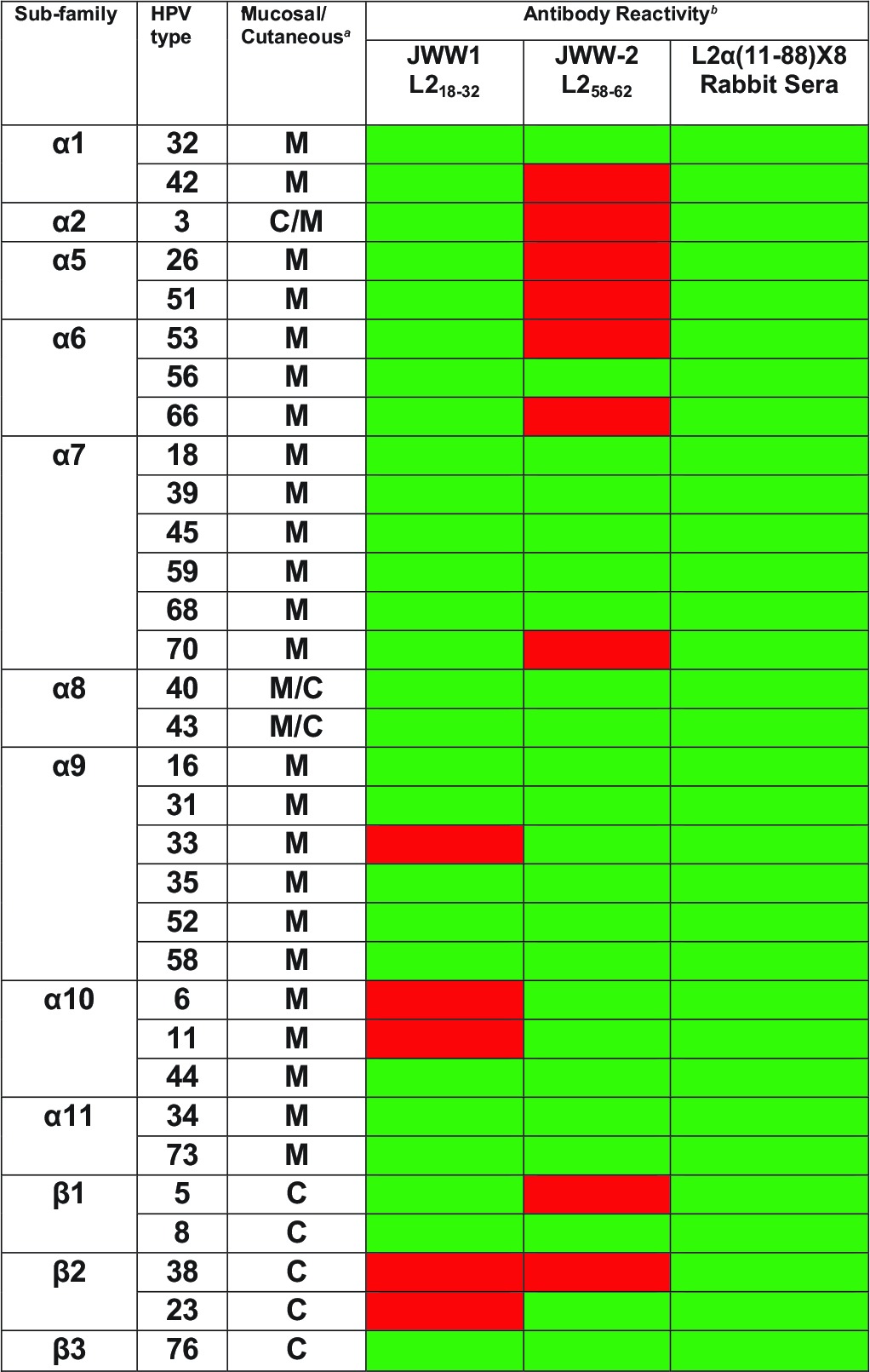
M, mucosal; C, cutaneous.
Green cells, band; red cells, no band detected by Western blotting of HPV pseudovirion preparations. The Western blot data are provided in Fig. 3.
JWW-1 and JWW-2 retain the neutralizing capacity of their parental antibodies.
We also tested whether the JWW-1 and JWW-2 chimeric antibodies retained neutralizing capacity against HPV16. Additionally, we selected a few HPV genotypes to assess if the antibody's spectrum of strong Western blot reactivity against HPV6, -26, -45, and -58 was consistent with its in vitro neutralizing activity. Mouse monoclonal antibody RG-1 was also tested, along with a human IgG control antibody. RG-1 neutralized HPV16 and -26 only, but the EC50 for RG-1 against HPV16 was lower than that of the more broadly reactive JWW-1 and JWW-2 antibodies. JWW-1 neutralized HPV16, -26, -45, and -58 but not HPV6, consistent with prior Western blot data (Table 5) and previous in vitro neutralization data for WW-1 (29). JWW-2 was able to neutralize HPV6, -16, -45, and -58, as described for its parental antibody MAb24 (39), but not HPV26, and this spectrum of neutralization was consistent with its strong reactivity by Western blotting to L2 of these genotypes (Table 2). Thus, JWW-1 and JWW-2 are complementary in terms of their spectrum of neutralizing capability (Table 5) and similarly neutralized HPV16 (Fig. 2D).
TABLE 5.
In vitro neutralization capacity of purified JWW-1 and JWW-2 monoclonal antibodiesa
| HPV luciferase reporter | Monoclonal antibody | IC50 (95% CI) (nM) |
|---|---|---|
| HPV6 | RG-1 | >200 (NA) |
| JWW-1 | >200 (NA) | |
| JWW-2 | 160 (22–1,192) | |
| Human IgG control | >200 (NA) | |
| HPV16 | RG-1 | <0.27 (NA) |
| JWW-1 | 0.4 (0.2–0.88) | |
| JWW-2 | 4.8 (3.3–6.9) | |
| Human IgG control | >200 (NA) | |
| HPV26 | RG-1 | 3.2 (2.1–4.9) |
| JWW-1 | <0.27 (NA) | |
| JWW-2 | >200 (NA) | |
| Human IgG control | >200 (NA) | |
| HPV45 | RG-1 | >200 (NA) |
| JWW-1 | 6.6 (2.3–19) | |
| JWW-2 | 71 (5.2–980) | |
| Human IgG control | >200 (NA) | |
| HPV58 | RG-1 | >200 (NA) |
| JWW-1 | 67 (10–510) | |
| JWW-2 | 75 (12–480) | |
| Rat IgG control | >200 (NA) |
Shown is a summary of the 50% inhibitory concentration (IC50) (95% confidence interval [CI]) of purified monoclonal antibodies RG-1, JWW-1, JWW-2, and a negative-control human IgG determined using the FC-PBNA against HPV6/16/26/45/58 pseudovirions using a starting antibody concentration of 200 nM (30 μg/ml). NA, not applicable.
DISCUSSION
L2-based vaccination is strongly protective against experimental viral challenge in numerous preclinical models (13, 15, 42–45). A limited number of early phase TA-CIN vaccine trials suggest that HPV16 L2 is also immunogenic in patients, although the response is much weaker than that for L1 VLP (35, 46, 47). Nevertheless, the passive transfer of serum of most TA-CIN-vaccinated patients was robustly protective against heterologous-type genital challenge when tested in a murine model (48). This suggests the need for an adjuvant and/or virus display for L2-based vaccines to elicit durable titers. However, the immune response to L2 elicited by infection with HPV and its associated anogenital neoplasia is poorly understood, and the few existing studies vary widely in their estimates of seroprevalence. Here, using a two-step screening approach on serum specimens from a total of 1,078 patients entered in four prior studies, we find that the prevalence of antibodies toward HPV16 L2 is very low (<1%); only 7 out of 1,078 patients (6 from the CATCH study and 1 from the HPV16+ CIN2/3 group) showed reactivity against HPV16 L2 in both ELISA and Western blot (Table 1). We also note that once HPV16+ high-grade neoplasia has been established, neither the topical treatment of high-grade VIN lesions with imiquimod nor lesion ablation with PDT elicited a detectable systemic L2 antibody response (Fig. 1C), suggesting that local inflammation was not a sufficient trigger, although this may also reflect low L2 expression or the absence of L2 expression in VIN2/3. In contrast, when these VIN patients were subsequently vaccinated with TA-CIN, an L2 antibody response was detected in 12/19 patients, even without the use of an adjuvant (Fig. 1D). This suggests that the failure of most HPV-infected patients to generate an L2 antibody response does not reflect an inability to do so, but rather an inadequate presentation of L2 to the immune system.
The use of the Western blot approach was important to verify reactivity, as the HPV16 L2 antigen used in the ELISA was generated by recombinant bacterial expression, and patients may have preexisting antibodies to bacterial proteins. Indeed, 12/18 CATCH patient serum samples were positive in the HPV16 L2 ELISA but failed to react with L2 by Western blotting, suggesting their reactivity with low-level bacterial impurities in the HPV16 L2 preparations (summarized in Table 2, Fig. 1E, and F). This issue may explain in part the wide variation in the seroprevalence of L2 antibodies reported in the literature, as many studies did not use a second complementary assay to verify reactivity.
None of the 6 patients from the CATCH study with HPV16 L2-specific antibodies were positive for high-risk HPV DNA by Hybrid Capture 2 in contemporaneously collected cervical cytologic specimens. These subjects had either normal pap smears or atypical squamous cells of undetermined significance (ASCUS) cytology (Table 1). The single high-grade CIN patient with HPV16 L2-reactive serum was diagnosed at study entry with HPV16+ CIN2 that spontaneously regressed within 15 weeks (Table 3). Importantly, 6/7 patient serum samples were nonreactive to HPV16/18 VLP or the pseudovirion ELISAs, suggesting that these patients lacked L1-specific antibodies to epitopes on the virion surface and that the antibodies potentially recognized buried epitopes on the virion. These data also imply that HPV infection generates a predominantly L1-specific antibody response or rarely an L2-specific antibody response, but typically not both (Table 2). Indeed, animal studies suggest that L2 is subdominant to L1 in the context of a VLP. It is possible that the L1- and L2-specific responses are mutually exclusive, but we noted a discrepancy in one CATCH study patient whose sample is part of the 7 reactive samples out of 1,078 (Table 2, patient 9), whereby the serum reacted with HPV16 L1 VLP and HPV16 L2 by ELISA and Western blotting. However, the serum failed to neutralize HPV16 pseudovirions. One possible explanation is that the serum contains antibodies to either insect proteins or denatured L1 present in the HPV16 L1 VLP preparations that are generated in Sf9 insect cells.
Assuming the HPV16 L2 antibody responses were induced by HPV16 infections, they were detectable only in patients who cleared their infections, since none of the samples from 6 CATCH study patients with HPV16 L2 antibodies was positive by the Hybrid Capture 2 (hc2), and the HPV16+ CIN2 patient cleared her HPV16 infection. Another possible reason why the six positive patients in the CATCH study were currently hc2 negative might be that the HPV16 L2 reactivity reflects the induction of a cross-reactive L2 antibody induced by infection by HPV type(s) other than those detected by the hc2 test.
Although these 7 patient serum samples were reactive toward HPV16 full-length L2 by ELISA and Western blot, none were detectably neutralizing for HPV16 (EC50, <1:50) using the standard in vitro neutralization assay (L1-PBNA). One explanation for this discrepancy is that ELISA using bacterially expressed L2 and Western blots present primarily linear epitopes, while L2 neutralizing responses require a specific conformation. However, the neutralizing epitopes of L2 are typically linear, suggesting this explanation is unlikely. Further, these sera also failed to react detectably with HPV16 L2 residues 11 to 88 (in the L2α 11-88x5 antigen) or HPV16 pseudovirions (which contain full-length L2) by ELISA (Table 2). Taken together, these observations suggest that the L2 antibodies in these serum samples are directed toward nonneutralizing epitopes at the C terminus of the HPV16 L2 protein, a region mostly buried below the virion surface (49). This suggestion is also based on prior serologic studies mapping epitopes of HPV L2 and/or L2 antibody prevalence that have reported immunoreactivity toward L2 amino acids (aa) 110 to 210 and 391 to 402 (20–23). In some studies, L2 antibodies were weakly associated with HPV infection, CIN3, or cervical cancer, while others have reported an L2 antibody seroprevalence of 20 to 30% in genital wart and cervical dysplasia patients (24–26). Here, we observed a much lower HPV16 L2 antibody seroprevalence (<1%). However, we note that in the above-mentioned studies, either Western blot or ELISA with bacterially expressed L2 was solely utilized for screening in several previous studies. In contrast, in this study, while a two-step validation approach was used to minimize potential false positives due to bacterial protein-specific serum antibody, it may compromise assay sensitivity.
The current WHO guidelines for HPV VLP vaccines require a comparison of neutralizing antibodies induced from HPV vaccines to the responses from infection for specific HPV types (50). Although there are WHO international standards for HPV16 and HPV18 antibodies based upon pooled sera from infected patients, they are unsuitable for validation of L2-specific antibody responses, as they failed to react to HPV16 L2 by Western blot (data not shown) or ELISA (Fig. 2C and D). As these WHO standards contain only type-specific anti-L1 antibodies, an alternate serological standard for human serum antibody responses to L2 is still needed.
Despite reactivity by both HPV16 L2 ELISA and Western blot, none of the seven serum samples from infected patients were detectably neutralizing. While the sera of patients vaccinated with TA-CIN could potentially be pooled and utilized as a standard, the limited amount and value of the remaining sera render this approach impractical. An alternate strategy to derive a sustainable and generalizable L2-specific neutralizing antibody standard is to synthesize chimeric human L2-specific monoclonal antibodies based upon well-defined neutralizing rodent monoclonal antibodies. To this end, we created two chimeric human monoclonal antibodies, JWW-1 and JWW-2, which retained, respectively, the L2 aa 18 to 32 and 58 to 64 epitope specificity and neutralizing activity of their parental rodent monoclonal antibodies. Both JWW-1 and JWW-2 were detected using anti-human IgG secondary antibody, demonstrating its potential utility as a standard reagent for immunoassay validation when testing human sera by ELISA. JWW-1 was reactive to many clinically relevant mucosal and cutaneous HPV types (29/34), but, like WW-1, it does not bind to L2 of two common low-risk types, HPV6 and HPV11, or to high-risk HPV33 (Fig. 3 and Table 4). In contrast, JWW-2 is able to bind to these three important types but not certain hrHPV types, such as HPV26 and HPV51, which are recognized by JWW-1 (Fig. 3 and Table 4). However, the avidity of each antibody to L2 of different HPV types varies significantly (Fig. 3), and this also can be seen from the in vitro neutralization data, for example (Table 5). Taken together, the ability of these antibodies to recognize different epitopes on L2 and complement in terms of their spectrum of reactivity suggests the utility of both antibodies as potential reference standards. Importantly, both monoclonal antibodies individually were neutralizing to a variety of clinically relevant HPV types (Table 5), thereby showing their utility for neutralization studies and their functional relevance. The complementary neutralization of diverse HPV types by JWW-1 and JWW-2 suggests the utility of incorporating both of their epitopes in next-generation L2-based vaccines.
In conclusion, patients rarely have L2-specific serum antibodies, even in populations at high risk for HPV infection in which candidate L2-based preventive HPV vaccines would be tested. Second, because spontaneous responses are rare and may contain neutralizing L1-specific antibodies, the creation of an international standard for L2-specific neutralizing antibody based upon finite pools of sera from infected patients would be challenging. Further, the natural L2-specific sera identified in our study were not detectably neutralizing. In contrast, the human chimeric monoclonal antibodies JWW-1 and JWW-2 are broadly reactive, neutralizing, and readily replenished, and thus represent a promising alternative as antibody standards to validate in future L2-specific immunologic assays, including in vitro neutralization, for seroepidemiological or L2 vaccine studies.
ACKNOWLEDGMENTS
Richard B. S. Roden and Subhashini Jagu are inventors of L2-related patents licensed to Shantha Biotechnics Ltd., GlaxoSmithKline, PaxVax, Inc., and Acambis, Inc. Richard B. S. Roden has received research funding from Sanofi Pasteur, Shantha Biotechnics, and GlaxoSmithKline and is a founder of Papivax, LLC and a scientific advisor to Papivax Biotech, Inc. The terms of these arrangements are managed by Johns Hopkins University in accordance with its conflict of interest policies.
The study was funded by Public Health Service grants P50 CA098252 and RO1 CA118790 from the National Cancer Institute, and the V Foundation (http://www.jimmyv.org/). Sai Daayana was funded by Wigan Cancer Research Fund. Henry Kitchener and Peter L. Stern were funded by Cancer Research UK (http://www.cancerresearchuk.org/).
REFERENCES
- 1.zur Hausen H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 2.Walboomers JMM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJF, Peto J, Meijer CJLM, Munoz N. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189:12–19. doi:. [DOI] [PubMed] [Google Scholar]
- 3.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. 2007. Human papillomavirus and cervical cancer. Lancet 370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 4.Schiller JT, Castellsague X, Garland SM. 2012. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine 30(Suppl 5):F123–F138. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, Moreira ED Jr, Ngan Y, Petersen LK, Lazcano-Ponce E, Pitisuttithum P, Restrepo JA, Stuart G, Woelber L, Yang YC, Cuzick J, Garland SM, Huh W, Kjaer SK, Bautista OM, Chan IS, Chen J, Gesser R, Moeller E, Ritter M, Vuocolo S, Luxembourg A, Broad Spectrum HPV Vaccine Study Group. 2015. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 372:711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 6.Schabath MB, Villa LL, Lin HY, Fulp WJ, Akogbe GO, Abrahamsen ME, Papenfuss MR, Lazcano-Ponce E, Salmeron J, Quiterio M, Giuliano AR. 2013. Racial differences in the incidence and clearance of human papilloma virus (HPV): the HPV in men (HIM) study. Cancer Epidemiol Biomarkers Prev 22:1762–1770. doi: 10.1158/1055-9965.EPI-13-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hariri S, Unger ER, Powell SE, Bauer HM, Bennett NM, Bloch KC, Niccolai LM, Schafer S, Steinau M, Markowitz LE, HPV-IMPACT Working Group . 2012. Human papillomavirus genotypes in high-grade cervical lesions in the United States. J Infect Dis 206:1878–1886. doi: 10.1093/infdis/jis627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niccolai LM, Russ C, Julian PJ, Hariri S, Sinard J, Meek JI, McBride V, Markowitz LE, Unger ER, Hadler JL, Sosa LE. 2013. Individual and geographic disparities in human papillomavirus types 16/18 in high-grade cervical lesions: associations with race, ethnicity, and poverty. Cancer 119:3052–3058. doi: 10.1002/cncr.28038. [DOI] [PubMed] [Google Scholar]
- 9.Agosti JM, Goldie SJ. 2007. Introducing HPV vaccine in developing countries–key challenges and issues. N Engl J Med 356:1908–1910. doi: 10.1056/NEJMp078053. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler CM, Castellsague X, Garland SM, Szarewski A, Paavonen J, Naud P, Salmeron J, Chow SN, Apter D, Kitchener H, Teixeira JC, Skinner SR, Jaisamrarn U, Limson G, Romanowski B, Aoki FY, Schwarz TF, Poppe WA, Bosch FX, Harper DM, Huh W, Hardt K, Zahaf T, Descamps D, Struyf F, Dubin G, Lehtinen M, HPV PATRICIA Study Group. 2012. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 13:100–110. doi: 10.1016/S1470-2045(11)70287-X. [DOI] [PubMed] [Google Scholar]
- 11.Malagón T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J, Brisson M. 2012. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis 12:781–789. doi: 10.1016/S1473-3099(12)70187-1. [DOI] [PubMed] [Google Scholar]
- 12.Christensen ND, Kreider JW, Kan NC, DiAngelo SL. 1991. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology 181:572–579. doi: 10.1016/0042-6822(91)90890-N. [DOI] [PubMed] [Google Scholar]
- 13.Gambhira R, Jagu S, Karanam B, Gravitt PE, Culp TD, Christensen ND, Roden RB. 2007. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N terminus of human papillomavirus type 16 minor capsid antigen L2. J Virol 81:11585–11592. doi: 10.1128/JVI.01577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gambhira R, Karanam B, Jagu S, Roberts JN, Buck CB, Bossis I, Alphs H, Culp T, Christensen ND, Roden RBS. 2007. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J Virol 81:13927–13931. doi: 10.1128/JVI.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jagu S, Karanam B, Gambhira R, Chivukula SV, Chaganti RJ, Lowy DR, Schiller JT, Roden RBS. 2009. Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines. J Natl Cancer Inst 101:782–792. doi: 10.1093/jnci/djp106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jagu S, Kwak K, Karanam B, Huh WK, Damotharan V, Chivukula SV, Roden RB. 2013. Optimization of multimeric human papillomavirus L2 vaccines. PLoS One 8:e55538. doi: 10.1371/journal.pone.0055538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jagu S, Kwak K, Schiller JT, Lowy DR, Kleanthous H, Kalnin K, Wang C, Wang HK, Chow LT, Huh WK, Jaganathan KS, Chivukula SV, Roden RB. 2013. Phylogenetic considerations in designing a broadly protective multimeric L2 vaccine. J Virol 87:6127–6136. doi: 10.1128/JVI.03218-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schellenbacher C, Kwak K, Fink D, Shafti-Keramat S, Huber B, Jindra C, Faust H, Dillner J, Roden RBS, Kirnbauer R. 2013. Efficacy of RG1-VLP vaccination against infections with genital and cutaneous human papillomaviruses. J Invest Dermatol 133:2706–2713. doi: 10.1038/jid.2013.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schellenbacher C, Roden R, Kirnbauer R. 2009. Chimeric L1-L2 virus-like particles as potential broad-spectrum human papillomavirus vaccines. J Virol 83:10085–10095. doi: 10.1128/JVI.01088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehtinen M, Niemela J, Dillner J, Parkkonen P, Nummi T, Liski E, Nieminen P, Reunala T, Paavonen J. 1993. Evaluation of serum antibody response to a newly identified B-cell epitope in the minor nucleocapsid protein L2 of human papillomavirus type 16. Clin Diagn Virol 1:153–165. doi: 10.1016/0928-0197(93)90010-3. [DOI] [PubMed] [Google Scholar]
- 21.Jenison SA, Yu XP, Valentine JM, Galloway DA. 1991. Characterization of human antibody-reactive epitopes encoded by human papillomavirus types 16 and 18. J Virol 65:1208–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenison SA, Firzlaff JM, Langenberg A, Galloway DA. 1988. Identification of immunoreactive antigens of human papillomavirus type 6b by using Escherichia coli-expressed fusion proteins. J Virol 62:2115–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yaegashi N, Jenison SA, Batra M, Galloway DA. 1992. Human antibodies recognize multiple distinct type-specific and cross-reactive regions of the minor capsid proteins of human papillomavirus types 6 and 11. J Virol 66:2008–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanda T, Teshima H, Katase K, Umezawa S, Watabe H, Takahashi H, Onda T, Yoshiike K. 1995. Occurrence of the antibody against human papillomavirus type 16 virion protein L2 in patients with cervical cancer and dysplasia. Intervirology 38:187–191. [DOI] [PubMed] [Google Scholar]
- 25.Suchánková A, Ritter O, Hirsch I, Krchnák V, Kalos Z, Hamsíková E, Brichácek B, Vonka V. 1990. Presence of antibody reactive with synthetic peptide derived from L2 open reading frame of human papillomavirus types 6b and 11 in human sera. Acta Virol 34:433–442. [PubMed] [Google Scholar]
- 26.Paez CG, Yaegashi N, Sato S, Yajima A. 1993. Prevalence of serum IgG antibodies for the E7 and L2 proteins of human papillomavirus type 16 in cervical cancer patients and controls. Tohoku J Exp Med 170:113–121. doi: 10.1620/tjem.170.113. [DOI] [PubMed] [Google Scholar]
- 27.Day PM, Pang YY, Kines RC, Thompson CD, Lowy DR, Schiller JT. 2012. A human papillomavirus (HPV) in vitro neutralization assay that recapitulates the in vitro process of infection provides a sensitive measure of HPV L2 infection-inhibiting antibodies. Clin Vaccine Immunol 19:1075–1082. doi: 10.1128/CVI.00139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sehr P, Rubio I, Seitz H, Putzker K, Ribeiro-Müller L, Pawlita M, Müller M. 2013. High-throughput pseudovirion-based neutralization assay for analysis of natural and vaccine-induced antibodies against human papillomaviruses. PLoS One 8:e75677. doi: 10.1371/journal.pone.0075677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang JW, Jagu S, Kwak K, Wang C, Peng S, Kirnbauer R, Roden RB. 2014. Preparation and properties of a papillomavirus infectious intermediate and its utility for neutralization studies. Virology 449:304–316. doi: 10.1016/j.virol.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferguson M, Wilkinson DE, Zhou T. 2009. WHO meeting on the standardization of HPV assays and the role of the WHO HPV Laboratory Network in supporting vaccine introduction held on 24–25 January 2008, Geneva, Switzerland. Vaccine 27:337–347. doi: 10.1016/j.vaccine.2008.10.062. [DOI] [PubMed] [Google Scholar]
- 31.Wilkinson DEH, Heath AB, Faust H, Panicker G, Unger ER, Dillner J, Ngamkham J, Collaborative Study Group. 2012. Collaborative study to evaluate the proposed 1st WHO international standard for antibodies to human papillomavirus type 18 WHO/BS/2012.2191 World Health Organization, Geneva, Switzerland https://extranet.who.int/iris/restricted/bitstream/10665/96608/1/WHO_BS_2012.2191_eng.pdf. [Google Scholar]
- 32.Gravitt PE, Paul P, Katki HA, Vendantham H, Ramakrishna G, Sudula M, Kalpana B, Ronnett BM, Vijayaraghavan K, Shah KV, CATCH Study Team . 2010. Effectiveness of VIA, Pap, and HPV DNA testing in a cervical cancer screening program in a peri-urban community in Andhra Pradesh, India. PLoS One 5:e13711. doi: 10.1371/journal.pone.0013711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trimble CL, Piantadosi S, Gravitt P, Ronnett B, Pizer E, Elko A, Wilgus B, Yutzy W, Daniel R, Shah K, Peng S, Hung C, Roden R, Wu TC, Pardoll D. 2005. Spontaneous regression of high-grade cervical dysplasia: effects of human papillomavirus type and HLA phenotype. Clin Cancer Res 11:4717–4723. doi: 10.1158/1078-0432.CCR-04-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winters U, Daayana S, Lear JT, Tomlinson AE, Elkord E, Stern PL, Kitchener HC. 2008. Clinical and immunologic results of a phase II trial of sequential imiquimod and photodynamic therapy for vulval intraepithelial neoplasia. Clin Cancer Res 14:5292–5299. doi: 10.1158/1078-0432.CCR-07-4760. [DOI] [PubMed] [Google Scholar]
- 35.Daayana S, Elkord E, Winters U, Pawlita M, Roden R, Stern PL, Kitchener HC. 2010. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br J Cancer 102:1129–1136. doi: 10.1038/sj.bjc.6605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viscidi RP, Snyder B, Cu-Uvin S, Hogan JW, Clayman B, Klein RS, Sobel J, Shah KV. 2005. Human papillomavirus capsid antibody response to natural infection and risk of subsequent HPV infection in HIV-positive and HIV-negative women. Cancer Epidemiol Biomarkers Prev 14:283–288. [PubMed] [Google Scholar]
- 37.Roden RB, Yutzy WH IV, Fallon R, Inglis S, Lowy DR, Schiller JT. 2000. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology 270:254–257. doi: 10.1006/viro.2000.0272. [DOI] [PubMed] [Google Scholar]
- 38.Wang JW, Jagu S, Wang C, Kitchener HC, Daayana S, Stern PL, Pang S, Day PM, Huh WK, Roden RB. 2014. Measurement of neutralizing serum antibodies of patients vaccinated with human papillomavirus L1 or L2-based immunogens using furin-cleaved HPV pseudovirions. PLoS One 9:e101576. doi: 10.1371/journal.pone.0101576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakao S, Mori S, Kondo K, Matsumoto K, Yoshikawa H, Kanda T. 2012. Monoclonal antibodies recognizing cross-neutralization epitopes in human papillomavirus 16 minor capsid protein L2. Virology 434:110–117. doi: 10.1016/j.virol.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Pastrana DV, Gambhira R, Buck CB, Pang YY, Thompson CD, Culp TD, Christensen ND, Lowy DR, Schiller JT, Roden RB. 2005. Cross-neutralization of cutaneous and mucosal papillomavirus types with anti-sera to the amino terminus of L2. Virology 337:365–372. doi: 10.1016/j.virol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Kondo K, Ochi H, Matsumoto T, Yoshikawa H, Kanda T. 2008. Modification of human papillomavirus-like particle vaccine by insertion of the cross-reactive L2-epitopes. J Med Virol 80:841–846. doi: 10.1002/jmv.21124. [DOI] [PubMed] [Google Scholar]
- 42.Rubio I, Bolchi A, Moretto N, Canali E, Gissmann L, Torrimasino M, Muller M, Ottonello S. 2009. Potent anti-HPV immune responses induced by tandem repeats of the HPV16 L2 (20–38) peptide displayed on bacterial thioredoxin. Vaccine 27:1949–1956. doi: 10.1016/j.vaccine.2009.01.102. [DOI] [PubMed] [Google Scholar]
- 43.Tumban E, Peabody J, Peabody DS, Chackerian B. 2011. A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2. PLoS One 6:e23310. doi: 10.1371/journal.pone.0023310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Embers ME, Budgeon LR, Pickel M, Christensen ND. 2002. Protective immunity to rabbit oral and cutaneous papillomaviruses by immunization with short peptides of L2, the minor capsid protein. J Virol 76:9798–9805. doi: 10.1128/JVI.76.19.9798-9805.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campo MS, Grindlay GJ, O'Neil BW, Chandrachud LM, McGarvie GM, Jarrett WF. 1993. Prophylactic and therapeutic vaccination against a mucosal papillomavirus. J Gen Virol 74(Pt 6):945–953. [DOI] [PubMed] [Google Scholar]
- 46.Gambhira R, Gravitt PE, Bossis I, Stern PL, Viscidi RP, Roden RBS. 2006. Vaccination of healthy volunteers with human papillomavirus type 16 L2E7E6 fusion protein induces serum antibody that neutralizes across papillomavirus species. Cancer Res 66:11120–11124. doi: 10.1158/0008-5472.CAN-06-2560. [DOI] [PubMed] [Google Scholar]
- 47.Kawana K, Yasugi T, Kanda T, Kino N, Oda K, Okada S, Kawana Y, Nei T, Takada T, Toyoshima S, Tsuchiya A, Kondo K, Yoshikawa H, Tsutsumi O, Taketani Y. 2003. Safety and immunogenicity of a peptide containing the cross-neutralization epitope of HPV16 L2 administered nasally in healthy volunteers. Vaccine 21:4256–4260. doi: 10.1016/S0264-410X(03)00454-7. [DOI] [PubMed] [Google Scholar]
- 48.Wang JW, Jagu S, Wang CG, Kitchener HC, Daayana S, Stern PL, Pang S, Day PM, Huh WK, Roden RBS. 2014. Measurement of neutralizing serum antibodies of patients vaccinated with human papillomavirus L1 or L2-based immunogens using furin-cleaved HPV pseudovirions. PLoS One 9:e101576. doi: 10.1371/journal.pone.0101576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buck CB, Cheng N, Thompson CD, Lowy DR, Steven AC, Schiller JT, Trus BL. 2008. Arrangement of L2 within the papillomavirus capsid. J Virol 82:5190–5197. doi: 10.1128/JVI.02726-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization. 2006. Guidelines to assure the quality, safety and efficacy of recombinant human papillomavirus-like particle vaccines. WHO/BS/06.2050 World Health Organization, Geneva, Switzerland http://www.who.int/immunization/hpv/learn/guidelines_to_assure_the_quality_safety_and_efficacity_ecbs_2006.pdf. [Google Scholar]