Abstract
This study aimed to evaluate the Brucella ovis ΔabcBA strain as a vaccine candidate in the murine model. BALB/c mice were subcutaneously or intraperitoneally immunized with a single dose or three doses of the B. ovis ΔabcBA strain and then were challenged with wild-type B. ovis. Single or multiple immunizations provided only mild protection, with significantly smaller numbers of wild-type B. ovis CFU in the livers of immunized mice but not in the spleens. Encapsulation of B. ovis ΔabcBA significantly improved protection against experimental challenges in both BALB/c and C57BL/6 mice. Furthermore, immunization with encapsulated B. ovis ΔabcBA markedly prevented lesions in the spleens and livers of experimentally challenged mice. These results demonstrated that the encapsulated B. ovis ΔabcBA strain confers protection to mice; therefore, this strain has potential as a vaccine candidate for rams.
INTRODUCTION
Brucella ovis is a facultative, intracellular, Gram-negative coccobacillus (1) that causes economic losses due to its potential to induce reproductive failure in sheep (2, 3). The most important lesions caused by B. ovis in rams are epididymitis and seminal vesiculitis (4), and B. ovis occasionally causes abortion in ewes (5, 6).
In spite of attempts to develop safe, effective vaccines (7–10), there is no available vaccine specifically against B. ovis. The ideal vaccine against B. ovis must prevent infection and clinical signs or at least reduce the risk of infection and transmission (11). Rev-1, a live attenuated Brucella melitensis strain, is the most commonly used vaccine against B. melitensis, and it also protects against B. ovis (12). However, the Rev-1 strain retains pathogenic potential, so it is capable of infecting humans. It may cause abortions in ruminants, it may interfere with serological tests, and it is resistant to streptomycin (13, 14).
Mice have been extensively used as a model for brucellosis, which is very important due to the limitations of experimentation with larger animals (15–18). The murine model has allowed studies for the development of safe, effective brucellosis vaccines (15, 19, 20).
Inactivation of a predicted abcEDCBA-encoded ATP-binding cassette (ABC) transporter of B. ovis (21), which is located in B. ovis pathogenicity island 1 (BOPI-1), results in strong attenuation of this pathogen in the murine model (22). Although the substrate of this ABC transporter is not known, it is required for expression of B. ovis virulence proteins, including Omp31, Sod, and the virB-encoded type IV secretion system (23), which is required for B. ovis intracellular survival and in vivo persistence (17). Furthermore, a B. ovis ΔabcBA strain is capable of triggering humoral and cellular immune responses like the wild-type strain in rams; importantly, however, the mutant strain is not excreted in semen and urine (24). Therefore, this mutant strain has great potential as an attenuated vaccine strain. However, considering that the ΔabcBA mutant strain is attenuated at very early stages during infection, resulting in poor persistence in vivo (22), methods for prolonging release of the vaccine strain in vivo may be highly desirable in this context. Vaccine encapsulation has been used as a useful approach for improving immune responses. Capsules are progressively and slowly eroded, and they function as antigen deposits, extending antigen availability in the organisms (25). Alginate is a widely used compound for encapsulation. It is a natural nontoxic biopolymer that has biocompatibility with live microorganisms (26). The aim of this study was to evaluate the protective potential of an encapsulated live attenuated strain of B. ovis (ΔabcBA strain) against experimental challenge with wild-type B. ovis in mice.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Wild-type B. ovis strain ATCC 25840, B. ovis ΔabcBA (22), and a B. ovis ΔabcBA isogenic strain expressing mCherry fluorescence, with the insertion of pKSoriT-bla-kan-PsojA-mCherry (23), were used in this study. Bacteria were grown for 3 days at 37°C on tryptic soy agar (TSA) plates with 1% hemoglobin (Becton Dickinson), in a humidified incubator with 5% CO2, and then were suspended in sterile phosphate-buffered saline (PBS) (pH 7.4). The mutant strain (B. ovis ΔabcBA) was grown on TSA with 1% hemoglobin and 100 μg/ml kanamycin (Gibco, Invitrogen, Brazil). Inoculum concentrations were estimated using a SmartSpec spectrophotometer (Bio-Rad, Hercules, CA) measuring the optical density at 600 nm (OD600). For bacterial cultures, mouse tissue homogenates were plated on TSA with 1% hemoglobin for CFU counting after 5 to 7 days in culture at 37°C in 5% CO2. For quantification of CFU of the mutant strain in tissues, 100 μg/ml of kanamycin (Gibco, Invitrogen, Brazil) was added to TSA with 1% hemoglobin.
B. ovis ΔabcBA encapsulation.
Encapsulation of the B. ovis ΔabcBA strain was performed as follows: 100 μl of a suspension containing 1 × 1011 CFU of B. ovis ΔabcBA was added to 2 ml of 1% sodium alginate (Sigma-Aldrich), and 0.2 ml of this mixture was placed in a 1-ml syringe and dripped with a needle (0.23 mm by 4 mm) into 10 ml of a 5% CaCl2 solution. After the solution was dripped, capsules were formed and then homogenized for 15 min. Capsules were washed twice in MOPS (morpholinepropanesulfonic acid) buffer (Sigma-Aldrich), followed by 0.5% poly-l-lysine solution (Sigma-Aldrich), for 15 min under agitation and then were washed in MOPS buffer. Particles were added to a 0.03% alginate solution over 5 min and then were suspended in MOPS buffer. Particles were inoculated subcutaneously at a final dose of 108 CFU per mouse. Particle sizes were assessed by light microscopy, and the effectiveness of bacterial encapsulation and density were assessed by encapsulation of mCherry-expressing B. ovis ΔabcBA and fluorescence microscopy (Leica DM4000 B).
Vaccination of mice.
Animal experiments were approved by the Institutional Ethics Committee on Animal Experimentation of the Universidade Federal de Minas Gerais (CETEA/UFMG protocols 204/2012 and 395/2013). This study included three independent experiments, which are detailed below.
Subcutaneous and intraperitoneal routes of immunization were compared. Six- to 7-week-old male BALB/c mice (n = 35) were used. Ten mice were immunized by the intraperitoneal route and 10 mice were immunized by the subcutaneous route with 100 μl of a suspension containing 108 CFU of the B. ovis ΔabcBA strain in PBS. Ten mice were inoculated with sterile PBS (five by the subcutaneous route and five by the intraperitoneal route). Additionally, five mice were not inoculated or challenged. Six weeks after immunization, the mice (n = 30) were challenged with 106 CFU of wild-type B. ovis (ATCC 25840) by the intraperitoneal route. Two weeks later, the mice were euthanized with an overdose of 2% xylazine hydrochloride (0.1 mg/kg) and 1% ketamine hydrochloride (35 mg/kg) by the intraperitoneal route, followed by cervical dislocation.
In order to evaluate repeated subcutaneous vaccinations, ten 6- to 7-week-old male BALB/c mice were immunized three times, by the subcutaneous route, with 100 μl of a suspension containing 108 CFU of B. ovis ΔabcBA in PBS, with intervals of 1 week between the immunizations. Another ten 6- to 7-week-old male BALB/c mice were inoculated three times with 100 μl of PBS and were used as controls. Six weeks after the last immunization, all 20 mice were challenged with 106 CFU of a wild-type B. ovis (ATCC 25840) suspension in PBS by the intraperitoneal route. Two weeks later, the mice were euthanized as described above.
The effects of attenuated vaccine strain encapsulation were also investigated. Six- to 7-week-old BALB/c (n = 20) and C57BL/6 (n = 20) mice were divided into four groups for each mouse strain, namely, a control group (subcutaneous inoculation of sterile PBS), a group with subcutaneous immunization with empty alginate capsules, a group with subcutaneous immunization with the B. ovis ΔabcBA strain (108 CFU per mouse), and a group with subcutaneous immunization with the B. ovis ΔabcBA strain encapsulated with alginate (108 CFU per mouse). After 8 weeks, all mice were challenged with wild-type B. ovis (106 CFU per mouse); 2 weeks later, mice were euthanized as described above.
In vivo persistence of encapsulated B. ovis ΔabcBA.
To evaluate the persistence of alginate-encapsulated B. ovis ΔabcBA, three groups of male BALB/c mice (n = 6) were infected with 106 CFU per mouse of wild-type B. ovis, B. ovis ΔabcBA, or encapsulated B. ovis ΔabcBA by peritoneal injection. At 24 h postinfection, mice were euthanized and peritoneal macrophages were harvested using intraperitoneal injection of 10 ml of cold PBS, which was immediately aspirated from the peritoneal cavity and transferred to a 15-ml tube on ice. The aspirate was centrifuged at 400 × g for 10 min at 4°C. The supernatant was discarded, and macrophages were suspended in a depolymerization solution (50 mM sodium citrate, 0.45% sodium chloride, and 10 mM MOPS) in order to disrupt capsules. After another centrifugation step and washing of the macrophages with PBS, 500 μl of sterile water was added, followed by vigorous vortex-mixing, serial 10-fold dilution in PBS, and plating for CFU counting. To evaluate systemic wild-type B. ovis, B. ovis ΔabcBA, and encapsulated B. ovis ΔabcBA infections in mice, spleens were collected for bacterial isolation at 24 h postinfection and results were compared among these groups.
Bacterial isolation.
Fragments of spleen and liver were weighed and homogenized in tubes with 2 ml of sterile PBS, using a mixer (Ultra Stirrer; Biotech, Brazil). Each sample was prepared in serial 10-fold dilutions and plated for CFU counting in TSA with 1% hemoglobin. Plates were incubated at 37°C in a humidified atmosphere with 5% CO2 and were evaluated for 7 days. Protection index values were calculated by considering the difference in log CFU numbers in the spleens of nonvaccinated versus vaccinated mice at 2 weeks after the experimental challenge (i.e., log CFU unvaccinated − log CFU vaccinated).
Histopathological assessments.
Fragments of spleen, liver, and subcutaneous tissue from the vaccination site were fixed by immersion in 10% buffered formalin for 24 h. After fixation, tissues were processed for paraffin embedding. Four-micrometer sections were stained with hematoxylin and eosin. Occurrences of microgranulomas, necrosis, and thrombosis in the liver or microgranulomas in the spleen were scored as follows: 0, absent; 1, mild; 2, moderate; 3, severe. Therefore, the scores of lesions for the liver ranged from 0 to 9 (combined scores for the three distinct lesions), whereas those for the spleen ranged from 0 to 3 (referring to the intensity of inflammation). Livers and spleens from mice that were neither immunized nor challenged were used as controls.
Statistical analyses.
CFU data were logarithmically transformed and then subjected to analysis of variance (ANOVA). Means were compared by Tukey's test. Histopathological scores were compared using the Kruskal-Wallis nonparametric test (GraphPad Prism version 5.0).
RESULTS
Immunization with B. ovis ΔabcBA induced mild protection against experimental challenge.
In a preliminary evaluation, the subcutaneous and intraperitoneal routes of vaccination were compared and bacterial loads recovered from spleens and livers were measured 2 weeks after challenge with wild-type B. ovis. Bacterial loads in the livers were significantly lower in BALB/c mice immunized with B. ovis ΔabcBA by the subcutaneous (P < 0.001) or intraperitoneal (P < 0.05) route than in nonimmunized mice (Fig. 1A). Surprisingly, there were no significant differences in CFU numbers recovered from spleens from mice immunized by the subcutaneous or intraperitoneal routes, compared to nonimmunized mice (Fig. 1B). These results also indicated that the subcutaneous and intraperitoneal routes provided similar protection in mice.
FIG 1.
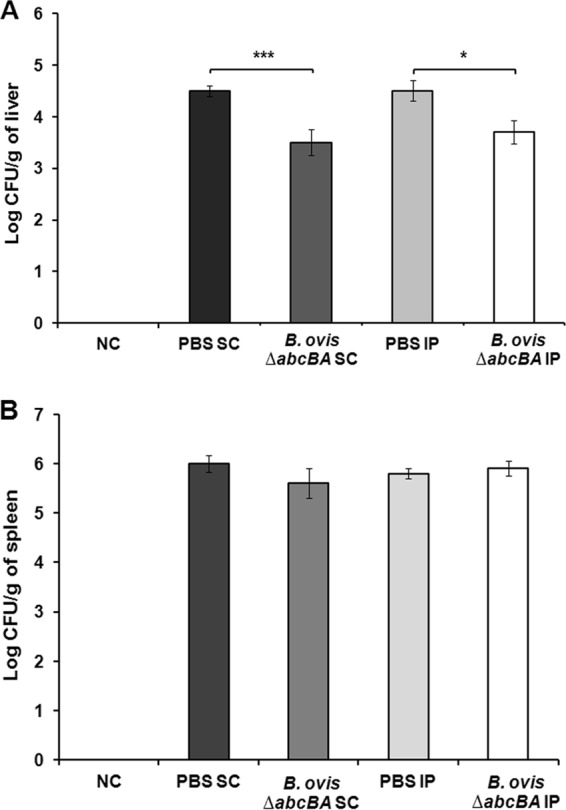
Numbers of CFU of Brucella ovis recovered from livers (A) and spleens (B) of BALB/c mice that were not immunized or were immunized with the B. ovis ΔabcBA strain and were challenged with wild-type B. ovis. Vaccination reduced the challenge inoculum by 90% in the liver, compared to controls, when administered by the subcutaneous (SC) or intraperitoneal (IP) route but had no effect in the spleen, a potential site of persistent infection. Bars represent the mean and standard deviation of results for mice (n = 5) that were neither immunized nor challenged (NC), mice inoculated with PBS by the subcutaneous or intraperitoneal routes, and mice immunized with B. ovis ΔabcBA intraperitoneally or subcutaneously. Raw data were logarithmically transformed prior to ANOVA, and means were compared by Tukey's test. Statistical differences between groups are indicated. *, P < 0.05; ***, P < 0.001.
Repeated subcutaneous vaccination with Brucella ovis ΔabcBA induced mild protection against experimental challenge.
Because a single immunization resulted in only mild protection, a prime immunization by the subcutaneous route followed by two buster immunizations at 1-week intervals was evaluated. Repeated immunizations induced only mild protection (Fig. 2), which was similar to the findings for a single immunization (Fig. 1). Indeed, there were significant reductions in bacterial loads in the livers after immunization (P < 0.001), compared to nonimmunized mice (Fig. 2A). However, there was no statistical difference in bacterial loads recovered from the spleens of immunized mice versus nonimmunized mice (Fig. 2B).
FIG 2.
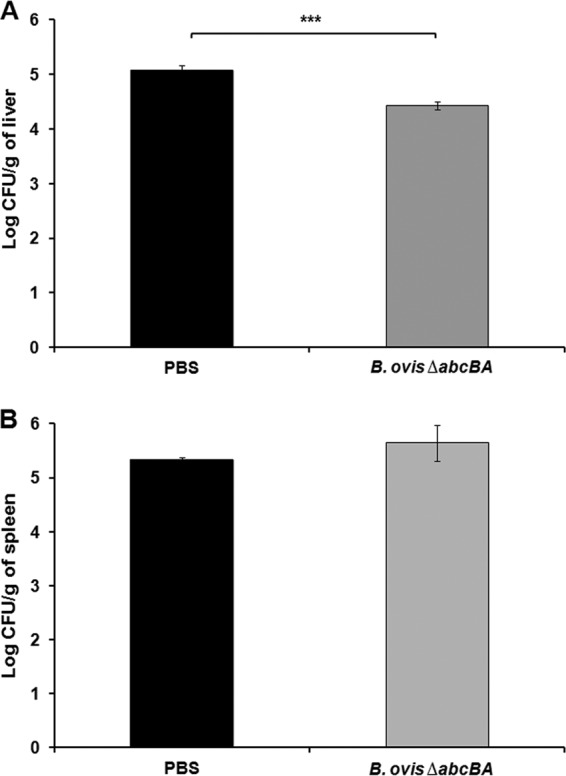
Numbers of CFU of Brucella ovis recovered from livers (A) and spleens (B) of BALB/c mice that were not immunized or were immunized three times with B. ovis ΔabcBA and were challenged with wild-type B. ovis. Bars represent the mean and standard deviation of results for 10 mice that were not immunized and 10 mice that were immunized three times. Raw data were logarithmically transformed prior to ANOVA, and means were compared by Tukey's test. Statistical differences between groups are indicated. ***, P < 0.001.
Encapsulation enhanced protection induced by Brucella ovis ΔabcBA against experimental challenge in mice.
Alginate capsules were evaluated by light microscopy, whereas B. ovis ΔabcBA expressing mCherry was encapsulated with alginate and evaluated by fluorescence microscopy. Empty capsules and capsules containing B. ovis had similar sizes and shapes. These capsules were spherical and ranged from 300 to 800 μm in diameter (Fig. 3A). Abundant red fluorescent mCherry-expressing B. ovis ΔabcBA was observed inside the alginate capsules, confirming that B. ovis was efficiently encapsulated (Fig. 3B).
FIG 3.
Light microscopic image of empty alginate capsules (A) and fluorescence microscopic images of alginate capsules containing mCherry-expressing Brucella ovis ΔabcBA (B). Inset, individual mCherry-expressing Brucella ovis ΔabcBA. Bars = 100 μm.
Considering the strong attenuation and poor persistence of the B. ovis ΔabcBA strain in mice (22), this candidate vaccine strain was encapsulated in alginate microcapsules, to overcome the lack of persistence. BALB/c and C57BL/6 mice were subcutaneously immunized with B. ovis ΔabcBA encapsulated in sterile alginate, thus providing a system for gradual release and uptake of antigens by the host.
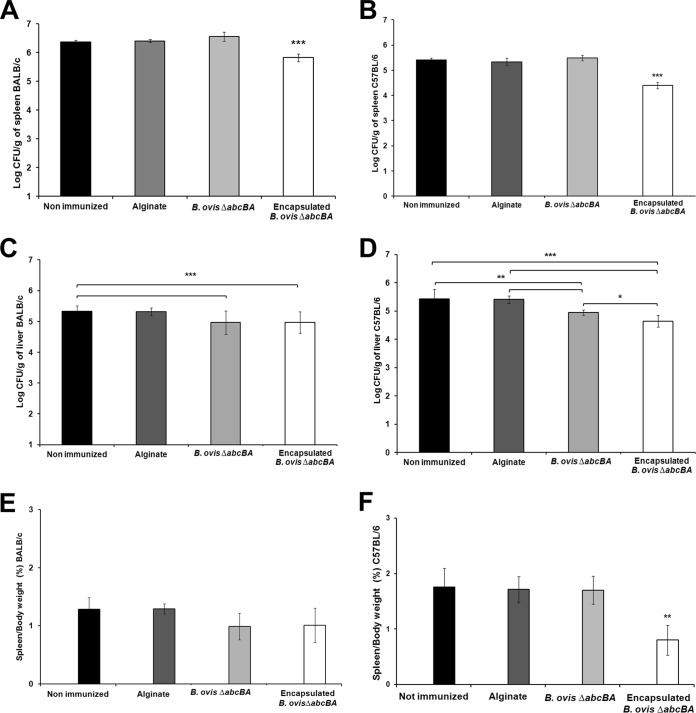
Bacterial loads were significantly reduced (P < 0.001) in spleens from both BALB/c and C57BL/6 mice immunized with encapsulated B. ovis ΔabcBA, compared to mice immunized with nonencapsulated B. ovis ΔabcBA or nonimmunized controls (Fig. 4A and B). Bacterial loads in the livers were significantly reduced in BALB/c and C57BL/6 mice immunized with encapsulated or nonencapsulated B. ovis ΔabcBA (P < 0.001), compared to nonimmunized controls (Fig. 4C and D). Furthermore, spleen weights were significantly lower (P < 0.001) in C57BL/6 mice immunized with encapsulated B. ovis ΔabcBA than in nonimmunized mice or mice immunized with nonencapsulated B. ovis ΔabcBA (Fig. 4E and F). Protection index values for encapsulated B. ovis ΔabcBA in experimentally challenged BALB/c and C57BL/6 mice are described in Table 1.
FIG 4.
(A to D) Numbers of CFU of Brucella ovis recovered from spleens of BALB/c (A) and C57BL/6 (B) mice and livers of BALB/c (C) and C57BL/6 (D) mice subcutaneously inoculated with sterile PBS or sterile alginate capsules or immunized with B. ovis ΔabcBA or encapsulated B. ovis ΔabcBA and then challenged with wild-type B. ovis. (E and F) Spleen/body weight ratios of BALB/c (E) and C57BL/6 (F) mice. Bars represent the mean and standard deviation of results for 5 mice per group. Raw data were logarithmically transformed prior to ANOVA, and means were compared by Tukey's test. Statistical differences between groups are indicated. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
TABLE 1.
Protection induced by Brucella ovis ΔabcBA encapsulated with alginate in BALB/c and C57BL/6 mice experimentally challenged with wild-type B. ovisa
| Mouse strain | Vaccine strain | Route/dose (CFU) | No. of immunizations | Challenge | Protection indexb |
|---|---|---|---|---|---|
| BALB/c | Encapsulated B. ovis ΔabcBA | s.c./1 × 108 | 1 | B. ovis (i.p./1 × 106 CFU) | 0.54 |
| C57BL/6 | Encapsulated B. ovis ΔabcBA | s.c./1 × 108 | 1 | B. ovis (i.p./1 × 106 CFU) | 1.01 |
s.c., subcutaneous; i.p., intraperitoneal.
Protection index was calculated considering the difference in CFU numbers (log) in the spleens of nonvaccinated and vaccinated mice at 2 weeks after experimental challenge, i.e., log CFU unvaccinated − log CFU vaccinated.
Immunization with encapsulated Brucella ovis ΔabcBA prevents lesions in spleens and livers of experimentally challenged mice.
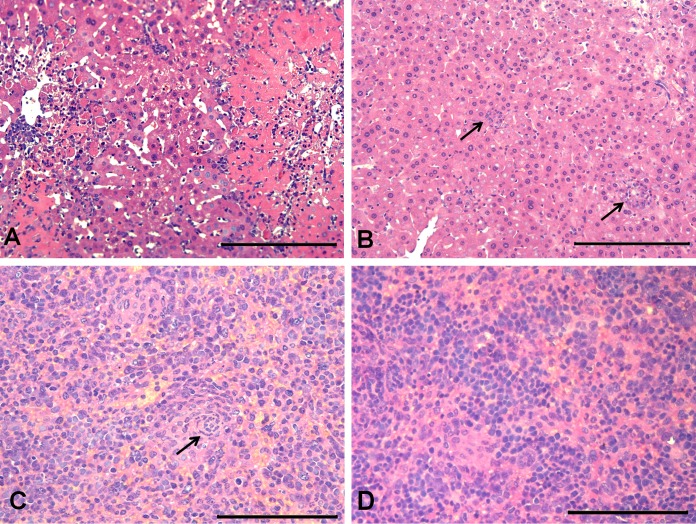
Spleens of C57BL/6 mice immunized with encapsulated B. ovis ΔabcBA did not develop splenomegaly, compared to nonimmunized mice (Fig. 5). In the livers of nonimmunized BALB/c (data not shown) and C57BL/6 mice, there were firm white nodular lesions approximately 0.3 cm in diameter (Fig. 5A), which histologically corresponded to microgranulomas. Interestingly, BALB/c (data not shown) and C57BL/6 mice immunized with encapsulated B. ovis ΔabcBA did not develop lesions in their spleens (Fig. 5B).
FIG 5.
Representative macroscopic lesions in the livers and spleens of C57BL/6 mice not immunized (A) or immunized with encapsulated Brucella ovis ΔabcBA (B) and then challenged with wild-type B. ovis (both images are from challenged mice), showing splenomegaly and numerous hepatic granulomas in nonimmunized C57BL/6 mice (white arrows) and neither hepatic granulomas nor splenomegaly in C57BL/6 mice immunized with encapsulated B. ovis ΔabcBA.
In the livers, there was a multifocal, randomly distributed, inflammatory infiltrate composed of neutrophils, lymphocytes, and macrophages, characterizing microgranulomas associated with moderate multifocal necrosis and thrombosis. A moderate amount of fibrin and numerous neutrophils in the intima and media of portal blood vessels, characterizing vasculitis, were observed in nonimmunized BALB/c (data not shown) and C57BL/6 (Fig. 6A) mice. In BALB/c (data not shown) and C57BL/6 mice immunized with encapsulated B. ovis ΔabcBA, there were only few microgranulomas in the livers (Fig. 6B).
FIG 6.
Representative microscopic changes in the livers and spleens of C57BL/6 mice not immunized or immunized with encapsulated Brucella ovis ΔabcBA and challenged with wild-type B. ovis (all micrographs are from challenged mice). (A and B) Numerous microgranulomas associated with necrosis in the liver of a nonimmunized C57BL/6 mouse (A), compared to only scarce microgranulomas (black arrows) in the liver of a C57BL/6 mouse immunized with encapsulated B. ovis ΔabcBA (B). Hematoxylin and eosin staining. Bars = 100 μm. (C and D) Microgranulomas (black arrow) in the spleen of a nonimmunized C57BL/6 mouse (C) and rare neutrophils in the spleen of a C57BL/6 mouse immunized with encapsulated B. ovis ΔabcBA (D). Hematoxylin and eosin staining. Bars = 50 μm.
There was a moderate, multifocal, inflammatory infiltrate composed of epithelioid macrophages and neutrophils in the marginal zone and red pulp of nonimmunized mice, characterizing moderate, multifocal, pyogranulomatous splenitis (Fig. 6C). In contrast, both mouse strains immunized with encapsulated B. ovis ΔabcBA had just rare neutrophils in the red pulp (Fig. 6D).
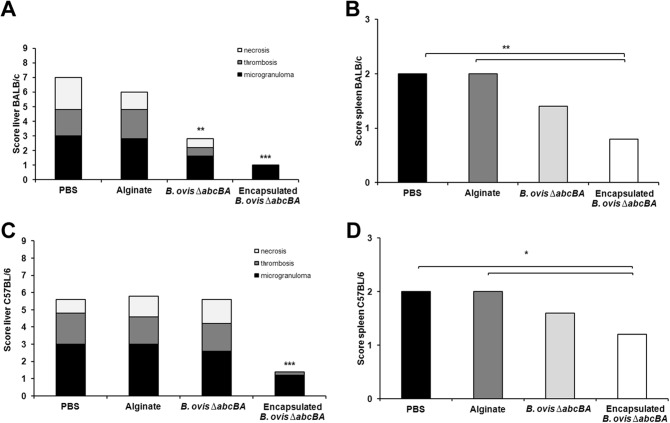
Scores for hepatic and splenic lesions in BALB/c mice immunized with encapsulated B. ovis ΔabcBA were significantly lower than scores for nonimmunized mice (P < 0.01) (Fig. 7A and B). Similar results were obtained for C57BL/6 mice (P < 0.05) (Fig. 7C and D).
FIG 7.
Scores for lesions in the livers of BALB/c (A) and C57BL/6 (C) mice and the spleens of BALB/c (B) and C57BL/6 (D) mice that were not immunized (inoculated with PBS or sterile alginate, as indicated) or were immunized with nonencapsulated or encapsulated Brucella ovis ΔabcBA and were challenged with wild-type B. ovis. Means were compared with the Kruskal-Wallis nonparametric test. Significant differences between nonimmunized controls (PBS and alginate) and immunized groups are indicated. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Encapsulated Brucella ovis ΔabcBA induced granuloma formation in subcutaneous tissue at inoculation site.
Grossly, mice immunized with encapsulated B. ovis ΔabcBA developed swelling at the inoculation site (approximately 3 mm in diameter), which histologically corresponded to an inflammatory infiltrate composed of numerous epithelioid macrophages, lymphocytes, and multinucleated giant cells, surrounded by fibroblasts and fibrocytes (Fig. 8A). Interestingly, mice inoculated with nonencapsulated B. ovis ΔabcBA (not shown) or empty sterile alginate capsules did not develop any lesions (Fig. 8B).
FIG 8.
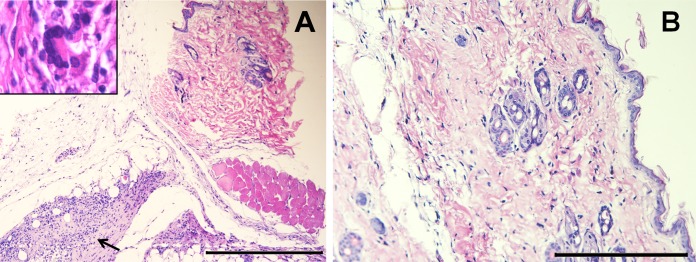
Microscopic changes at the immunization sites of mice, showing a granuloma in the hypodermis of a mouse immunized with encapsulated Brucella ovis ΔabcBA (black arrow) (A) and no histopathological changes in the skin of a mouse inoculated with empty alginate capsules only (B). Inset, multinucleated giant cell associated with epithelioid macrophages. Hematoxylin and eosin staining. Bars = 200 μm.
Alginate capsules enhanced persistence of Brucella ovis ΔabcBA in peritoneal cavities and spleens of BALB/c mice.
Enhanced protection by encapsulated B. ovis ΔabcBA (Fig. 4) is hypothetically due to slow release of the vaccine strain, which overcomes its strong attenuation. In order to compare the persistence of B. ovis ΔabcBA with that of encapsulated B. ovis ΔabcBA, the total numbers of CFU recovered from intraperitoneal washes and spleens of infected BALB/c mice were evaluated at 24 h postinfection. The total numbers of CFU isolated from the intraperitoneal cavities (Fig. 9A) or the spleens (Fig. 9B) of encapsulated B. ovis ΔabcBA-infected mice were significantly higher (P < 0.05 and P < 0.01, respectively) than those from mice inoculated with nonencapsulated B. ovis ΔabcBA.
FIG 9.
Numbers of CFU of wild-type B. ovis, B. ovis ΔabcBA, and encapsulated B. ovis ΔabcBA recovered from intraperitoneal macrophages (A) and spleens (B) of male BALB/c mice at 24 h postinfection. Raw data were logarithmically transformed prior to ANOVA, and means were compared by Tukey's test. Significant differences in CFU numbers between B. ovis ΔabcBA and encapsulated B. ovis ΔabcBA and between wild-type B. ovis and the other groups are indicated. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
Currently there is no specific B. ovis vaccine available, which, considering the significance of B. ovis infection for the sheep industry, makes the development of such a vaccine a relevant scientific and technological challenge. In this study, we employed an encapsulation technology to overcome the poor performance of a candidate vaccine strain that has limited persistence in mice (22). Our results demonstrated that the subcutaneous route of immunization had a performance that was similar to that of the intraperitoneal route. The subcutaneous route is the most frequently used for human and animal vaccinations, although the intraperitoneal route has been used in experimental studies involving B. ovis (8), Brucella abortus (27), and B. melitensis (28) mutants as vaccine candidates in mice. Therefore, both immunization routes were evaluated in this study.
Live attenuated vaccines tend to provide the best results against Brucella infections (29, 30), which prompted us to develop a live attenuated vaccine against B. ovis. Considering the limited protection observed in the first experiment in this study, BALB/c mice were immunized three times by the subcutaneous route, with intervals of 1 week between immunizations, but the results were strikingly similar to those from the first experiment. This limited protection by B. ovis ΔabcBA could be attributed to its low level of persistence in mice (22). Therefore, an encapsulated formulation was used for immunization, which clearly improved the protective potential of B. ovis ΔabcBA. Experimental findings support the hypothesis that bacterial encapsulation modifies antigen uptake and processing (31, 32). For instance, a live attenuated B. abortus S19 vjbR mutant encapsulated with alginate conferred greater protection than nonencapsulated B. abortus S19 in mice (33). Similarly, a live attenuated B. melitensis vjbR mutant strain provided enhanced protection against a B. melitensis challenge in mice (34). These previous studies clearly indicated that prolongation of antigen persistence in the host is beneficial for the development of anti-Brucella immunity (35). Indeed, our results clearly demonstrated that encapsulated B. ovis ΔabcBA persisted longer than the nonencapsulated strain in the peritoneal cavity and spleen. Therefore, the third experiment in this study evaluated the effects of B. ovis ΔabcBA encapsulation with alginate, which resulted in improved protection against an experimental challenge in the mouse model. To the best of our knowledge, this is the first time that a live attenuated B. ovis mutant strain encapsulated with alginate has been tested as a vaccine candidate.
Bacterial loads and lesions in spleens and livers from BALB/c and C57BL/6 mice immunized with encapsulated B. ovis ΔabcBA were significantly reduced, demonstrating that encapsulation improved immunogenic efficacy against B. ovis infections in mice under the conditions of this study. These results are in good agreement with previous reports demonstrating improved protective efficacy of alginate-encapsulated B. abortus S19 ΔvjbR in BALB/c mice (33) and alginate-encapsulated live RB51 in red deer (36).
Although the B. melitensis Rev-1 vaccine strain provides some protection against B. ovis infections in sheep (12), this vaccine cannot be used in areas free of B. melitensis, since Rev-1 retains pathogenic potential for animals and humans (14). Therefore, a specific live attenuated vaccine is highly desirable for controlling B. ovis infections, particularly because a vaccine strain developed on a B. ovis background would not pose any occupational risk for personnel involved in animal vaccination and handling, since, in contrast to B. melitensis, which has the greatest pathogenic potential for humans, B. ovis has no zoonotic potential (3). This study indicates that B. ovis ΔabcBA has potential as a vaccine strain. Although the mouse model has been extensively used to validate live Brucella vaccine-induced protection (37), results cannot be directly extrapolated to other host species, since even different mouse strains influence the protection results (38). Indeed, we have recently demonstrated that the B. ovis ΔabcBA strain induces humoral and cellular immune responses in rams that are indistinguishable from those triggered by the parental wild-type strain (24). Thus, the results of the present study encouraged us to pursue additional studies to demonstrate the potential of B. ovis ΔabcBA as a vaccine candidate in rams, which are the preferential hosts for this pathogen. Such experiments are under way, with highly encouraging unpublished preliminary results.
Immunization with encapsulated B. ovis ΔabcBA is capable of inducing protection, leading to reductions in bacterial loads and lesions in the spleens and livers of experimentally challenged BALB/c and C57BL/6 mice. These results support the notion that this strain has potential as a vaccine candidate for rams.
ACKNOWLEDGMENTS
Work in the laboratory of R.L.S. is supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brazil) and the Fundação de Amparo a Pesquisa do Estado de Minas Gerais (Brazil). R.L.S. has a fellowship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brazil). A.P.C.S. has a scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
REFERENCES
- 1.Garrity GM. 2001. Bergey's manual of systematic bacteriology, 2nd ed, vol 1 Springer, New York, NY. [Google Scholar]
- 2.Burgess GW. 1982. Ovine contagious epididymitis: a review. Vet Microbiol 7:551–575. doi: 10.1016/0378-1135(82)90049-9. [DOI] [PubMed] [Google Scholar]
- 3.Xavier MN, Costa EA, Paixão TA, Santos RL. 2009. The genus Brucella and clinical manifestations of brucellosis. Cienc Rural 39:2252–2260. doi: 10.1590/S0103-84782009005000167. [DOI] [Google Scholar]
- 4.Carvalho Junior CA, Moustacas VS, Xavier MN, Costa EA, Costa LF, Silva TMA, Paixão TA, Borges AM, Santos RL. 2012. Andrological, pathologic, morphometric, and ultrasonographic findings in rams experimentally infected with Brucella ovis. Small Rumin Res 102:213–222. doi: 10.1016/j.smallrumres.2011.08.004. [DOI] [Google Scholar]
- 5.Molello JA, Jensen R, Flint JC. 1963. Placental pathology. I. Placental lesions of sheep experimentally infected with Brucella ovis. Am J Vet Res 24:897–904. [PubMed] [Google Scholar]
- 6.Osbourn BI, Kennedy PC. 1966. Pathologic and immunologic responses of the fetal lamb to Brucella ovis. Vet Pathol 3:110–136. doi: 10.1177/030098586600300202. [DOI] [PubMed] [Google Scholar]
- 7.Soler-Lloréns P, Gil-Ramírez Y, Zabalza-Baranguá A, Iriarte M, Conde-Álvarez R, Zúñiga-Ripa A, San Román B, Zygmunt MS, Vizcaíno N, Cloeckaert A, Grillo MJ, Moriyón I, López-Goñi I. 2014. Mutants in the lipopolysaccharide of Brucella ovis are attenuated and protect against B. ovis infection in mice. Vet Res 45:72. doi: 10.1186/s13567-014-0072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sancho P, Tejedor C, Sidhu-Muñoz R, Lago-Fernández L, Vizcaíno N. 2014. Evaluation in mice of Brucella ovis attenuated mutants for use as live vaccines against B. ovis. Vet Res 45:61–71. doi: 10.1186/1297-9716-45-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Da Costa Martins R, Gamazo C, Sánchez-Martínez M, Barberán M, Peñuelas I, Irache JM. 2012. Conjunctival vaccination against Brucella ovis in mice with mannosylated nanoparticles. J Control Release 162:553–560. doi: 10.1016/j.jconrel.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 10.Cassataro J, Pasquevich KA, Estein SM, Laplagne DA, Velikovsky CA, de la Barrera S, Bowden R, Fossati CA, Giambartolomei GH, Goldbaum FA. 2007. A recombinant subunit vaccine based on the insertion of 27 amino acids from Omp31 to the N-terminus of BLS induced a similar degree of protection against B. ovis than Rev.1 vaccination. Vaccine 25:4437–4446. doi: 10.1016/j.vaccine.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Díaz AG, Clausse M, Paolicchi MAF, Ghersi G, Zylberman V, Goldbaum FA, Estein SM. 2013. Immune response and serum bactericidal activity against Brucella ovis elicited using a short immunization schedule with the polymeric antigen BLOmp31 in rams. Vet Immunol Immunopathol 154:36–41. doi: 10.1016/j.vetimm.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Ridler AL, West DM. 2011. Control of Brucella ovis infection in sheep. Vet Clin North Am Food Anim Pract 27:61–66. doi: 10.1016/j.cvfa.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Blasco JM. 1997. A review of the use of B. melitensis Rev 1 vaccine in adult sheep and goats. Prev Vet Med 31:275–283. doi: 10.1016/S0167-5877(96)01110-5. [DOI] [PubMed] [Google Scholar]
- 14.Grilló MJ, Bosseray N, Blasco JM. 2000. In vitro markers and biological activity in mice of seed lot strains and commercial Brucella melitensis Rev 1 and Brucella abortus B19 vaccines. Biologicals 28:119–127. doi: 10.1006/biol.2000.0249. [DOI] [PubMed] [Google Scholar]
- 15.Ko J, Splitter GA. 2003. Molecular host-pathogen interaction in brucellosis: current understanding and future approaches to vaccine development for mice and humans. Clin Microbiol Rev 16:65–78. doi: 10.1128/CMR.16.1.65-78.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva TMA, Costa EA, Paixao TA, Tsolis RM, Santos RL. 2011. Laboratory animal models for brucellosis research. J Biomed Biotechnol 2011:518323. doi: 10.1155/2011/518323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sá JC, Silva TMA, Costa EA, Silva APC, Tsolis RM, Paixão TA, Carvalho Neta AV, Santos RL. 2012. The virB-encoded type IV secretion system is critical for establishment of infection and persistence of Brucella ovis infection in mice. Vet Microbiol 159:130–140. doi: 10.1016/j.vetmic.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 18.Vieira ALS, Silva TMA, Mol JP, Oliveira SO. 2013. MyD88 and TLR9 are required for early control of Brucella ovis infection in mice. Res Vet Sci 94:399–405. doi: 10.1016/j.rvsc.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 19.Baldwin CL, Parent M. 2002. Fundamentals of host immune response against Brucella abortus: what the mouse model has revealed about control of infection. Vet Microbiol 90:367–382. doi: 10.1016/S0378-1135(02)00222-5. [DOI] [PubMed] [Google Scholar]
- 20.Izadjoo MJ, Mense MG, Bhattacharjee AK, Hadfield TL, Crawford RM, Hoover DL. 2008. A study on the use of male animal models for developing a live vaccine for brucellosis. Transbound Emerg Dis 55:145–151. doi: 10.1111/j.1865-1682.2008.01019.x. [DOI] [PubMed] [Google Scholar]
- 21.Tsolis RM, Sechadri R, Santos RL, Sangari FJ, Lobo JM, de Jong MF, Ren Q, Myers G, Brinkac LM, Nelson WC, Deboy RT, Angiuoli S, Khouri H, Dimitrov G, Robinson JR, Mulligan S, Walker RL, Elzer PE, Hassan KA, Paulsen IT. 2009. Genome degradation in Brucella ovis corresponds with narrowing of its host range and tissue tropism. PLoS One 4:e5519. doi: 10.1371/journal.pone.0005519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva TMA, Paixão TA, Costa EA, Xavier MN, Sá JC, Moustacas VS, Hartigh AB, Neta AVC, Costa SO, Tsolis R, Santos RL. 2011. Putative ATP-binding cassette transporter is essential for Brucella ovis pathogenesis in mice. Infect Immun 79:1706–1717. doi: 10.1128/IAI.01109-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva TMA, Mol JPS, Winter MG, Atruli V, Xavier MN, Pires SF, Paixão TA, Andrade HM, Santos RL, Tsolis RM. 2014. The predicted ABC transporter AbcEDCBA is required for type IV secretion system expression and lysosomal evasion by Brucella ovis. PLoS One 9:e114532. doi: 10.1371/journal.pone.0114532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva APC, Macêdo AA, Costa LF, Turchetti AP, Bull V, Pessoa MS, Araújo MSS, Nascimento EF, Martins Filho OA, Paixão TA, Santos RL. 2013. Brucella ovis lacking a species-specific putative ATP-binding cassette transporter is attenuated but immunogenic in rams. Vet Microbiol 167:546–553. doi: 10.1016/j.vetmic.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Rice-Ficht AC, Arenas-Gamboa AM, Kahl-McDonagh MM, Ficht TA. 2010. Polymeric particles in vaccine delivery. Curr Opin Microbiol 13:106–112. doi: 10.1016/j.mib.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Wee S, Gombotz WR. 1998. Protein release from alginate matrices. Adv Drug Deliv Rev 31:267–285. doi: 10.1016/S0169-409X(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 27.Trant CGMC, Lacerda TLS, Carvalho NB, Azevedo V, Rosinha GMS, Salcedo SP, Gorvel JP, Oliveira SC. 2010. The Brucella abortus phosphoglycerate kinase mutant is highly attenuated and induces protection superior to that of vaccine strain 19 in immunocompromised and immunocompetent mice. Infect Immun 78:2283–2291. doi: 10.1128/IAI.01433-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arenas-Gamboa AM, Rice-Ficht AC, Kahl-McDonagh MM, Ficht TA. 2011. Protective efficacy and safety of Brucella melitensis 16MmucR against intraperitoneal and aerosol challenge in BALB/c mice. Infect Immun 79:3653–3658. doi: 10.1128/IAI.05330-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Godfroid J, Scholz HC, Barbier T, Nicolas C, Wattiau P, Fretin D, Whatmore AM, Cloeckaert A, Blasco JM, Moriyon I, Saegerman C, Muma JB, Al Dahouk S, Neubauer H, Letesson JJ. 2011. Brucellosis at the animal ecosystem human interface at the beginning of the 21st century. Prev Vet Med 102:118–131. doi: 10.1016/j.prevetmed.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Adams G. 1990. Development of live Brucella vaccines, p 250 In Adams G. (ed), Advances in brucellosis research. Texas A&M University Press, College Station, TX. [Google Scholar]
- 31.Eyles JE, Bramwell VW, Williamson ED, Alpar HO. 2001. Microsphere translocation and immunopotentiation in systemic tissues following intranasal administration. Vaccine 19:4732–4742. doi: 10.1016/S0264-410X(01)00220-1. [DOI] [PubMed] [Google Scholar]
- 32.Sun H, Pollock KG, Brewer JM. 2003. Analysis of the role of vaccine adjuvants in modulating dendritic cell activation and antigen presentation in vitro. Vaccine 21:849–855. doi: 10.1016/S0264-410X(02)00531-5. [DOI] [PubMed] [Google Scholar]
- 33.Arenas-Gamboa AM, Ficht TA, Kahl-McDonagh MM, Gomez G, Rice-Ficht AC. 2009. The Brucella abortus S19 ΔvjbR live vaccine candidate is safer than S19 and confers protection against wild-type challenge in BALB/c mice when delivered in a sustained-release vehicle. Infect Immun 77:877–884. doi: 10.1128/IAI.01017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arenas-Gamboa AM, Ficht TA, Kahl-McDonagh MM, Rice-Ficht AC. 2008. Immunization with a single dose of a microencapsulated Brucella melitensis mutant enhances protection against wild-type challenge. Infect Immun 76:2448–2455. doi: 10.1128/IAI.00767-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahl-McDonagh MM, Ficht TA. 2006. Evaluation of protection afforded by Brucella abortus and Brucella melitensis unmarked deletion mutants exhibiting different rates of clearance in BALB/c mice. Infect Immun 74:4048–4057. doi: 10.1128/IAI.01787-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arenas-Gamboa AM, Ficht TA, Davis DS, Elzer PH, Gonzalez AW, Rice-Ficht AC. 2009. Enhanced immune response of red deer (Cervus elaphus) to live RB51 vaccine strain using composite microspheres. J Wildl Dis 45:165–173. doi: 10.7589/0090-3558-45.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Organisation for Animal Health, Biological Standards Commission. 2008. Manual of diagnostic tests and vaccines for terrestrial animals, ed 6. World Organisation for Animal Health, Paris, France. [Google Scholar]
- 38.Todd TE, Tibi O, Lin Y, Sayers S, Bronner DN, Xiang Z, He Y. 2013. Meta-analysis of variables affecting mouse protection efficacy of whole organism Brucella vaccines and vaccine candidates. BMC Bioinformatics 14(Suppl 6):S3. doi: 10.1186/1471-2105-14-S6-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]