Abstract
The selector gene apterous (ap) plays a key role during the development of the Drosophila melanogaster wing because it governs the establishment of the dorsal-ventral (D-V) compartment boundary. The D-V compartment boundary is known to serve as an important signaling center that is essential for the growth of the wing. The role of Ap and its downstream effectors have been studied extensively. However, very little is known about the transcriptional regulation of ap during wing disc development. In this study, we present a first characterization of an essential wing-specific ap enhancer. First, we defined an 874-bp fragment about 10 kb upstream of the ap transcription start that faithfully recapitulates the expression pattern of ap in the wing imaginal disc. Analysis of deletions in the ap locus covering this element demonstrated that it is essential for proper regulation of ap and formation of the wing. Moreover, we showed that the mutations apblot and apXasta directly affect the integrity of this enhancer, leading to characteristic wing phenotypes. Furthermore, we engineered an in situ rescue system at the endogenous ap gene locus, allowing us to investigate the role of enhancer fragments in their native environment. Using this system, we were able to demonstrate that the essential wing enhancer alone is not sufficient for normal wing development. The in situ rescue system will allow us to characterize the ap regulatory sequences in great detail at the endogenous locus.
Keywords: Drosophila, apterous, compartment, boundary
The body wall and appendages of the adult fly are generated by specialized clusters of primordial cells in Drosophila larvae called imaginal discs. The patterning of cells in imaginal discs is initiated by establishing cell lineage boundaries, called compartments (Garcia-Bellido et al. 1973; Dahmann and Basler 1999). In the case of the wing imaginal disc, the tissue is subdivided into four different compartments, anterior (A) and posterior (P) as well as dorsal (D) and ventral (V). The A−P compartment is established during the process of segmentation in the embryo. The subdivision into dorsal and ventral compartments takes place later in development during the larval stages when the wing tissue is growing extensively (Wieschaus and Gehring 1976; Lawrence and Morata 1977; Cohen et al. 1992; Williams et al. 1993; Diaz-Benjumea and Cohen 1993). Short-range signaling events between the A−P or D−V compartments specify cells close to the compartment boundaries. These cells, also called organizer, play an important role in patterning the surrounding tissue by secreting long-range signaling molecules, also referred to as morphogens (Struhl and Basler 1993; Diaz-Benjumea and Cohen 1995; Neumann and Cohen 1997; Affolter and Basler 2007).
Compartment specificity is conferred by the cell-autonomous activity of a special class of transcription factors, called selector genes. Selector genes regulate genes important for proper differentiation and genes that control cell−cell interactions at the compartment boundary. apterous (ap), which is expressed in the dorsal compartment of the wing disc, has been shown to act as a selector gene subdividing the wing disc into a D and a V portion (Cohen et al. 1992; Diaz-Benjumea and Cohen 1993; Williams et al. 1994; Blair et al. 1994). Different ap alleles can lead to a wide range of wing phenotypes (Stevens and Bryant 1985). The most striking morphological defect in strong ap alleles is the complete lack of wing and haltere structures (Butterworth and King 1965). Because ap is not essential for the progression through larval and pupal stages, the investigation of adult ap mutant wing phenotypes is possible.
The target genes of Ap and their downstream functions in the patterning of the wing disc are relatively well understood. The activity of Ap initiates a bidirectional Notch signaling cascade at the D−V compartment boundary, which subsequently induces the expression of wingless (wg) in a stripe along the compartment boundary (Diaz-Benjumea and Cohen 1993; Williams et al. 1994; Irvine and Wieschaus 1994; Rulifson and Blair 1995; Kim et al. 1995; Couso et al. 1995). Wg, a ligand of the Wnt family, is responsible for the growth of the wing pouch and patterning along the D−V-axis, although its mode of action as a classical morphogen currently is questioned (Neumann and Cohen 1997; Alexandre et al. 2014).
Despite the rather detailed knowledge about the functions of Ap in wing disc development, our knowledge of the mechanisms regulating ap expression is still limited. It has been shown that activation of the epidermal growth factor receptor by its ligand Vein is necessary and sufficient to activate the expression of ap in the dorsal compartment of the wing disc (Zecca and Struhl 2002a,b). Moreover, early ventral wg expression has been shown to restrict the expression of ap to the dorsal portion of the developing wing disc (Williams et al. 1994).
To identify the wing disc-specific cis-regulatory elements of ap, we used several genetic approaches. First, a classical LacZ enhancer reporter study was performed. Second, deletions with defined breakpoints in the ap genomic locus were generated. Third, we have characterized two classical ap alleles, apblot and apXasta (apXa), at the molecular level and have associated their respective molecular alterations to the minimal wing enhancer. Finally, we engineered a ΦC31-integrase-dependent in situ rescue system, which enabled us to dissect the role of these cis-regulatory elements in their native environment.
Using these assays, we have defined an essential, but not sufficient, minimal 874-bp ap wing enhancer fragment that drives reporter gene expression in the dorsal compartment of the wing imaginal disc.
Material and Methods
Fly stocks and methods
Flies were grown on standard cornmeal agar at 25°, unless otherwise stated. ape01573 (PBac{RB}e01573), apf08090 (PBac{WH}f08090), apf00451 (PBac{WH}f00451), and apf00878 (PBac{WH}f00878) were purchased from the Exelixis stock collection at Harvard Medical School. Df(2R)nap1 (BL#1006), apblot (BL#4190), w*; T(2;3)apXa, apXa/CyO; TM3, Sb1 (BL#2475), P{hsFLP}12, y1 w* (BL#1929), Df(2R)BSC696 (BL# 26548), w*; P{10XUAS-IVS-mCD8::GFP}attP40 (BL#32186), Df(3R)Exel6176 (BL#7655), TM3, ryRK Sb1 Ser1 P{Δ2-3}99B (BL#1808), Bx-Gal4 (w1118 P{GawB}BxMS1096, BL#8860), y1 w*; Mi{y[+mDint2]=MIC}MI00964 (BL#34133), y1 w*; Mi{y[+mDint2]=MIC}MI02330/SM6a (BL#33205), y1 w67c23; P{EPgy2}EY03046 (BL#15619), ptc-Gal4 (P{GawB}ptc559.1; BL#2017) were all obtained from the Bloomington Stock Center. fng-Gal4 (y* w*; P{w+mW.hs = GawB}NP5399 / TM6, P{w-=UAS-lacZ.UW23-1}UW23-1, DGCR#104990) was obtained from Kyoto Drosophila Research Center. Dad4-Gal4 was established in our laboratory as described in the sections to follow. actin-Gal4 (y w1118 ; P{actin5c::Gal4, w-}/CyO) and GMR-Gal4 (w1118 ; P{GMR::Gal4, w-}/CyO) were obtained from Steven Henikoff (Ahmad and Henikoff 2001). salE-Gal4 was obtained from the Basler lab via Fisun Hamarotoglu (Mosimann et al. 2006). dpp-Gal4 is described in Staehling-Hampton et al. (1994). UAS-ap was obtained from Marco Milán (Milán and Cohen 1999). y w M{vas-int.Dm}zh-2A, a stock producing ΦC31-integrase under the control of the vasa promoter, and insertion platform M{3xP3-RFP.attP}zh-86Fb were obtained from Johannes Bischof (Bischof et al. 2007). ap41F/T(2;3)apXa was obtained from John B. Thomas.
According to our genetic and molecular analysis, ap41F should not be listed as an allele of ap. First, and contrary to a previous report (Bourgouin et al. 1992), hemizygous ap41F flies have normal wings and halters. Second, although molecular analysis confirmed the presence of a P-element insertion just proximal to vulcan on the ap41F chromosome, polymerase chain reaction (PCR), and sequencing failed to provide evidence for a ∼200-bp deletion within 1.5 kb of the longest ap cDNA (D. Bieli and M. Müller, unpublished data). The GFP knock-in allele ap::GFP is described in Caussinus et al. (2011) (BL#38423). apMM has been described in Gohl et al. (2008). It contains an insertion ∼400 bp upstream of the longest ap cDNA. Dad4-GFP (P{Dad4::EGFPnuc, w+}) was obtained from Jorgos Pyrowolakis (Vuilleumier et al. 2010). Nuclear enhanced green fluorescence protein (GFP) is expressed under the control of the Dad4 enhancer (Weiss et al. 2010). dadP1883Δ32 / TM3, Sb was obtained from Tetsuya Tabata. This deletion covers at least 24 kb downstream of the DadP1883 insertion, including the complete Dad open-reading frame (ORF) and three neighboring genes (CG3983, CG5184, and CG3962; T . Tabata, personal communication; Tsuneizumi et al. 1997; Henderson et al. 1999). A recombinant between apXa and P{y[+t7.7] w[+mC]=10XUAS-IVS-mCD8::GFP}attP40, inserted on 2R at 25C6 (Pfeiffer et al. 2010), was obtained by meiotic recombination and selection for the dominant Xasta and mini-white markers. The generation of deficiencies apDG1, apDG3, apDG8, and apDG11 is described below in section ΦC31-integrase–mediated transgenesis and generation of deletions.
Adult wings were dissected and mounted in Hoyer’s. Then, wing preparations were baked at 58° for a few hours. Preparations were allowed to harden at room temperature and flattened by applying a 40-g metal cylinder on the cover slip. Pictures were taken with a Nikon Microphot-FXA microscope with a Sony NEX-5RK digital camera. The notums of adult flies were photographed with a Leica M125 binocular equipped with a Leica DFC420C camera.
Introduction of ΦC31-integrase targets into the ap locus by gene conversion at the site of apMM
A method known as direct gene conversion has previously been developed to engineer a desired DNA fragment into the genomic site of a P-element insertion (Gloor et al. 1991; Sipos et al. 2007). Upon exposure of a given P-element insertion to P-element transposase, the transposon is excised and a double strand break is created. It is normally repaired by the cellular machinery using the homologous chromosome as a template. However, the repair process may also use an exogenous plasmid containing the desired DNA fragment flanked by homology arms derived from either side of the P-element insertion site. Such a gene conversion template plasmid containing homology arms flanking the site of apMM insertion, along with hsp70-GFP bracketed by a pair of inverted attB sites was constructed and named pLAPGPRA (see Figure 5A). The construction of this plasmid was a multi-step procedure. Details can be obtained upon request. In brief, left (899 bp long) and right (1981 bp long) ap-homology arms were amplified by PCR. To minimize sequence polymorphism which could decrease the efficiency of gene conversion, apMM genomic DNA (gDNA; isolated as described in Ashburner 1989) was used as the template for PCR. As primers we used apLA-R, apLA-FNotI, apRA-F, and apRA-R (for primer sequences, see Supporting Information, Table S1). Inserts in the proper orientation for subsequent cloning were identified using diagnostic digests and sequencing.
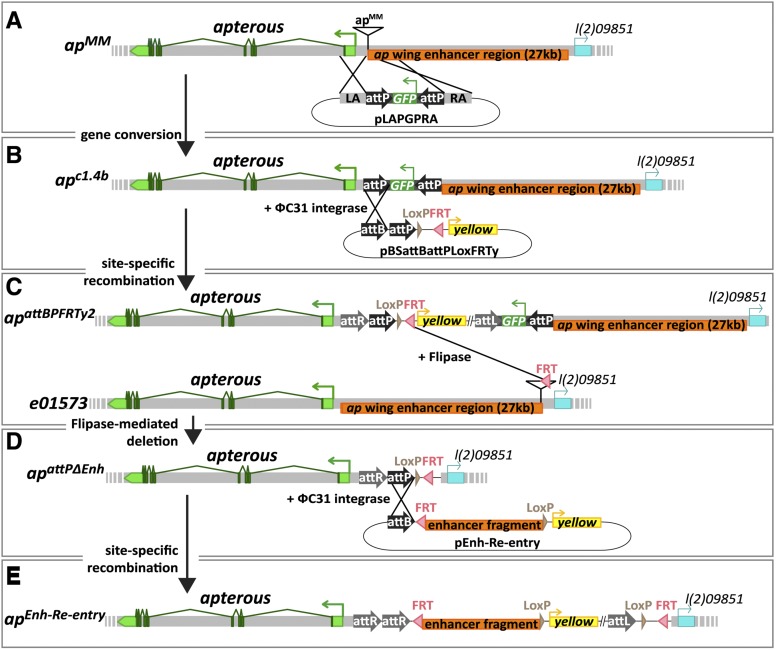
Figure 5.
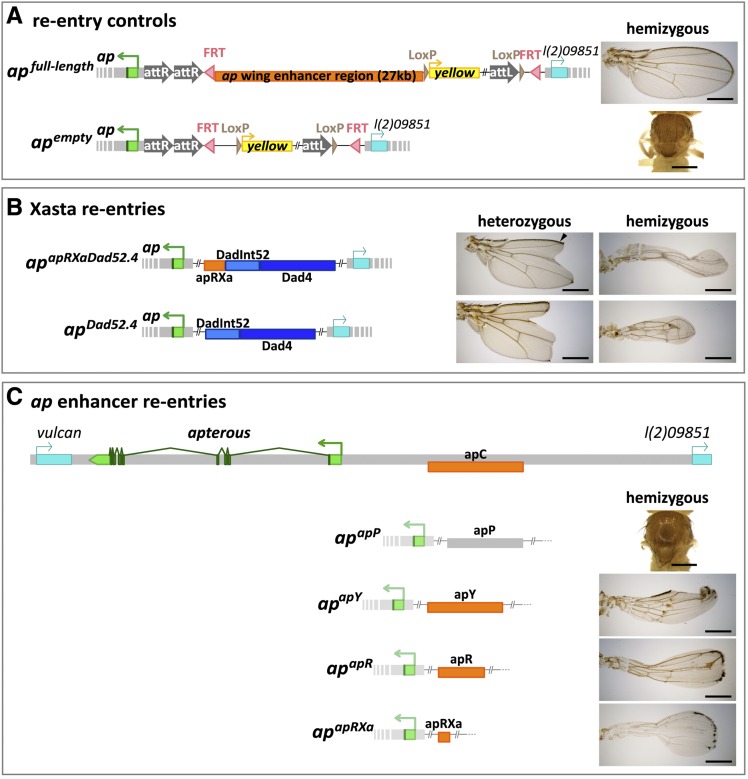
Generation of the in situ rescue system at the endogenous ap locus. (A−B) Direct gene conversion at apterous. P-element insertion apMM located ∼400 bp upstream of the ap TSS was previously isolated. By mobilization of apMM and concomitant injection of plasmid pLAPGPRA, fly line apc1.4b could be isolated. It contains two inverted attP sites flanking a GFP reporter. (B−C) ΦC31-integrase mediated site-specific recombination. By injection of plasmid pBSattBattPLoxFRTy, new attP, LoxP, and FRT sites were introduced into the ap locus. Note that pBSattBattPLoxFRTy can insert in two different attP sites leading to oppositely oriented insertions. apattBPFRTy2 is the appropriate one for our purpose. (C−D) Flipase-mediated deletion. Trans-heterozygous apattBPFRTy2/ape01573 animals were repeatedly treated with Flipase during larval stages. Among the progeny of these flies, apattPΔEnh could be isolated. It lacks the 27kb intergenic spacer but retains a strategically positioned attP site. (D−E) apattPΔEnh serves as a platform to reinsert enhancer fragments. These are cloned into pEnh-Reentry. This plasmid is injected into young embryos and integrates into the ap locus by ΦC31-integrase mediated recombination. Transgenics of the type apEnh-Reentry can be isolated thanks to the yellow marker. If desired, yellow can be removed by Cre-treatment. In addition, the complete insert can be excised by Flipase treatment.
pLAPGPRA was injected (650 ng/μL) along with pTurbo (250 ng/μlL) into embryos derived from a cross of y w; apDG3{w+}/+; TM3, Sb Δ2-3/+ males with y w; apMM{y+} ; + virgins. Surviving injectees were transferred to fresh vials and carefully tended at 18°. Among the hatching adults, males and virgins representing the two desired genotypes (apDG3{w+}/apMM{y+} and apDG3{w+}/apMM{y+}; TM3, Sb Δ2-3/+) were selected for further work. apDG3/apMM flies have normal wings and halteres. The apDG3 chromosome was included because it lacks the DNA corresponding to the homology arms of pLAPGPRA and hence cannot serve as a template for double strand gap repair. A total of 72 fertile crosses involving virgins (in pairs) or single males mated with y w; al b c sp/SM6a flies could be set up. Originally, it was intended to screen the larval progeny of these crosses for GFP expression. Unfortunately, this elegant approach failed in practice. Therefore, the progeny was screened for y− w− males. This phenotype indicates loss of the y marker and therefore most likely also of apMM and was, in the absence of the positive GFP selection, the only selectable marker to identify putative conversion candidates. A total of 105 y− w− males were selected from 32 (out of 72) crosses yielding such males. Balanced lines of potential gene conversion events were established and screened for GFP fluorescence in larval wing discs. Five candidate gene conversion lines with weak GFP expression in wing imaginal discs in an ap-like pattern were obtained from two independent dysgenic crosses (isolation numbers: c1.4a, c1.4b, c1.4d, and c1.4e; c1.13a).
To confirm that the five GFP-positive candidate gene conversion lines had the attP-flanked GFP construct integrated in the ap locus, gDNA was isolated and analyzed by PCR. PCR products were obtained for all five candidates between a primer (SV40out55) within the SV40 trailer sequence (just downstream of GFP) and a primer (apLOF2) in the ap gene outside of the left homology arm. Sequencing of all five lines confirmed the integrity of the ap promoter region and the presence of the ap proximal attP site (data not shown).
On the distal side, PCRs using primers in the hsp70 promoter (just upstream GFP) and several primers outside of the right homology arm initially failed to produce products (data not shown). Later, by use of one of the lines obtained by RMCE (see below), the integrity of the junction between the template plasmid and the right homology arm could be verified by PCR and sequencing using the Mcp-dir-y and ap-dir-3 as primers. Mcp-dir-y primes toward the end of the mini-yellow gene present on our Recombination-Mediated Cassette Exchange (RMCE) insertion cassette. We also tested whether the junction between the right homology arm and the flanking ap sequence is intact by PCR using a primer near the end of the right homology arm (apRAendF), and a primer in the flanking ap sequence (apROR2). A product of the expected size was observed, indicating that the junction is intact.
apc1.4a, apc1.4b, apc1.4d, apc1.4e, and apc1.13a homozygotes all have wild-type wings, indicating that the function of the ap wing enhancer and promoter were not disrupted by the gene conversion event. Gene conversion events apc1.4a, apc1.4b, apc1.4d, apc1.4e also have a rough eye phenotype when homozygous, but not over apDG3. The rough eye phenotype can be separated from the ap locus by meiotic recombination. Finally, only apc1.4b was chosen for further work. One of its applications is the targeted insertion of exogenous DNA into the ap locus by RMCE (Bateman et al. 2006).
ΦC31-integrase−mediated transgenesis and generation of deletions
Constructs for ΦC31-integrase−mediated transgenesis were generated based on plasmid piB-LLFY(BI) [details about the construction of piB-LLFY(BI) can be acquired upon request]. As required for RMCE, it contains two inverted attB sites. Separating them are the following three genetic components: (1) two LoxP sites in direct orientation with a multiple cloning site in between them; (2) the LoxP cassette is followed by a single FRT site; (3) the mini-yellow transformation marker completes piB-LLFY(BI). mini-yellow refers to a yellow reporter gene lacking all of its characterized tissue specific enhancers. It consists of the yellow cDNA fused to ∼330 bp of 5′ genomic DNA, including the yellow promoter and extending up to a KpnI restriction site. The mini-yellow fragment was isolated from plasmid C4yellow (referred to as Dint in Geyer and Corces 1987; Gohl et al. 2008). In the context of the ap gene and in a y background, mini-yellow activity always manifests itself in phenotypically yellow+ wings. Depending on the transgene and orientation of insert, thoracic bristles may also acquire yellow+ pigmentation (D. Gohl and M. Müller, unpublished data).
Constructs were introduced into the ap locus by RMCE into two docking sites, Mi{y[+mDint2]=MIC}MI02330 (Venken et al. 2011) and apc1.4b. DNA was injected at a concentration of 300 ng/µL in 1× phosphate-buffered saline (PBS) into early embryos of the genotype y w M{vas-int.Dm}zh-2A; MI02330/CyO or y w M{vas-int.Dm}zh-2A; apc1.4b/CyO. The relevant transgenic lines obtained in this way are apDD35.34 and apD5f.1, respectively. Their position and the orientation of the FRT are depicted in Figure 1C together with four other FRT containing transposon insertions. Five of the six stocks are homozygous and hemizygous viable. Their wings and halteres are of wild-type appearance. This is not the case for apf08090. The lethality of this chromosome cannot be reverted by excision of the PBac{WH}, indicating that it is associated with a second site lethal. Rare homozygous revertant escapers as well as frequent hemizygous revertants have normal wings. Therefore, the PBac{WH} insert is responsible for the strong phenotype in hemizygous apf08090 flies. However, this phenotype is not dependent on the gypsy insulator present in apf08090 because the wing phenotype is not suppressed in a su(Hw)− background (M. Müller, unpublished data).
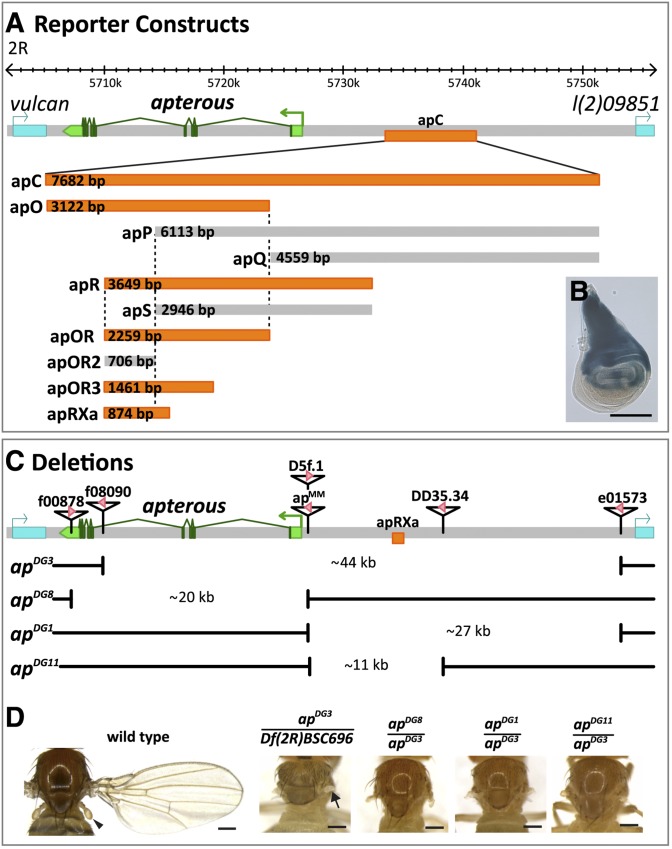
Figure 1.
LacZ reporter assay and deletion analysis at the apterous locus. (A) Diagrammatic representation of the ap locus. As drawn at the top of the panel, it extends over roughly 50 kb. Its transcribed part is shown in green. ap is flanked by two genes indicated in blue: vulcan on the proximal and l(2)09851 on its distal side. Arrows above the genomic interval specify the direction of transcription of the three genes. Fragment apC, indicated in orange, has been reported to drive reporter expression in the dorsal compartment of the pouch, the hinge and the notum of the wing imaginal disc, where ap is normally expressed. Below, the relative positions and dimensions of nine fragments tested with our LacZ reporter assay are depicted. Fragments colored in orange (apO, apR, apOR, apOR3, and apRXa) elicit the same expression pattern as apC. Fragments depicted in gray (apP, apQ, apS, apOR2) do not drive reporter gene expression in the wing disc. (B) X-Gal staining in the wing disc of an apC-LacZ transgenic fly. Scale bar: 100 µm. (C) Deletions generated at the endogenous ap locus with FRT-containing inserts. At the top of the panel, triangles along the ap locus indicate the position of six different inserts. Pink arrowheads within them mark the orientation of the FRT sites according to the definition of Thibault et al. (2004). The location of the apRXa fragment is shown in orange. apDG3 deletes approximately 44 kb between inserts apf08090 to ape01573, thereby removing most of ap ORF and upstream sequences. apDG8 is a 20-kb deficiency that deletes the complete ap ORF from apf00878 to apD5f.1. apDG1 removes the complete intergenic spacer between apMM to ape01573. apDG11 deletes an 11-kb fragment from apMM to apDD35.34. Note that apD5f.1 and apMM have exactly the same insertion site. (D) Notum pictures of a wild-type fly and trans-heterozygous ap mutants. In the wild type, the wing and the haltere (arrowhead) are well formed and clearly visible. Df(2R)BSC696 is a large deletion at the base of 2R, deleting approximately 360 kb, including the whole ap locus. When Df(2R)BSC696 is crossed to apDG3 all wing and haltere structures are lost. Only small stumps of amorphic tissue remain at the actual attachment site of the wing (see arrow).Very similar phenotypes are observed in apDG8/apDG3, apDG1/apDG3 and apDG11/apDG3 flies. Scale bar: 25 µm.
We have noted that in the Drosophila literature, two divergent definitions for FRT orientation are in use! In this study, FRT orientation is indicated according to Thibault et al. (2004).
In Drosophila, the production of deletions by Flipase-catalyzed recombination between two FRT sites either in cis or in trans has enabled the community to obtain a huge collection of tailor-made deficiencies (Golic and Golic 1996; Ryder et al. 2007). We have previously applied this technology to generate a ∼27-kb deletion named Df(2R)apDG between two FRT sites in apMM and ape01573 (Gohl et al. 2008). Note that in this study, Df(2R)apDG is referred to as apDG1. Applying analogous genetic crossing schemes, we have generated three further deletions:
Df(2R)apDG3:
An ∼44-kb deletion between two FRT sites located in apf08090 and ape01573. It is referred to as apDG3. In this deficiency, a large part of the ap transcription unit is lost together with ∼27 kb of intergenic DNA separating ap from l(2)09851. Although a considerable part of the ap ORF located proximal to the break in apDG3 remains in place, genetic observations are consistent with it being a true null allele with respect to ap function in wing and haltere tissue. Flanking the new FRT junction are three genetic elements: gypsy insulator and mini-white (of apf08090) and a splice acceptor (of ape01573). Over several kilobases, the region of the new fusion is identical to PBac{RB}e01573 and, hence, no adequate apDG3-specific PCR primers could be designed. Thus, four PCR primer pairs distributed evenly over the ∼44-kb interval missing on the apDG3 chromosome were tested on w1 and on apDG3/Df(2R)nap1 flies (Df(2R)nap1 being a cytologically visible deletion also uncovering ap). The absence of the corresponding DNA in the latter could unambiguously be demonstrated (data not shown).
Df(2R)apDG8{w+}:
An ∼20-kb deletion between two FRT sites located in apf00878 and apD5f.1. It is referred to as apDG8. It corresponds to a rather clean deletion of the complete ap transcription unit. Its phenotypes are indistinguishable from those observed for apDG3. Flanking the new FRT junction are two genetic elements: a UAS-inducible promoter (of apf00878) and mini-white (of apD5f.1). It was verified by PCR and sequencing. Aprec-LA-AscI-F and WARIout#1 primers were used for PCR. For sequencing, we used primers apEnhDel-Seq-PBrev and WARIout#2.
Df(2R)apDG11, al:
An ∼11-kb deletion between 2 FRT sites in al apMM sp and apDD35.34. It is referred to as apDG11. Because apDG1, homozygous apDG11 flies have no wings. Both deficiencies share the same proximal break point. Previous transvection studies have suggested that the ap promoter immediately proximal to apDG1 (and hence also of apDG11) remains intact (Gohl et al. 2008). Flanking the new FRT junction are two genetic elements: a LoxP site (of apMM) and mini-yellow (of apDD35.34). The new junction was verified by sequencing. For PCR amplification of the region, the apMM-200for and yellow5′out primer pair was used. Part of the fragment was sequenced with yellow5′out and Inverseappromfor.
Generation of a ΦC31-integrase based in situ rescue system at ap
Construction of pBSattBattPLoxFRTy:
Two complementary oligos (attBPfor and attBPrev) containing attB and attP sites in tandem were purchased from Sigma-Aldrich. These oligos were annealed and cloned between the XhoI and KpnI sites of pBSIIKS. The new plasmid’s name is pBSIIKSattBattP. A XhoI-ClaI fragment containing LoxP, FRT, and mini-yellow was isolated from piB-LLFY(BI) and subcloned into pBSIIKSattBattP, thereby generating the pBSattBattPLoxFRTy vector used for ΦC31 integrase mediated transgenesis (see Figure 5B). The attB and attP sites on this vector are separated by only 6 bp. It was assumed that therefore the two elements are too close for efficient intramolecular recombination. The fact the two desired insertions (one in each attP site present in apc1.4b, see Figure 5B) could be isolated seems to support this assumption.
Generation of apattPΔEnh, a platform for insertion of ap enhancer fragments:
pBSattBattPLoxFRTy DNA was injected into y w M{vas-int.Dm}zh-2A; apc1.4b/CyO embryos. Yellow+ marked flies could be isolated and mated. The desired insert orientation could be identified by PCR using the apdown-forN and aptransch_yw_rev primers. A stock with isolation number 6.1 was selected for correct orientation of insert pBSattBattPLoxFRTy. It is referred to as apattBPFRTy2 (see Figure 5C; the other insert orientation was also isolated and called apattBPFRTy1). We wished to further modify this stock by introducing the same ∼27-kb deletion as in apDG1. Therefore, y w; apattBPFRTy2 males were mated with y w hsFlp; PBac{RB}e01573 virgins. Progeny was heat-shocked at three subsequent days during its larval development for 1 hr in a 37° water bath. Hatchlings were individually crossed to y w M{vas-int.Dm}zh-2A ; Sp Pin/CyO flies. A total of 7 of 80 single crosses produced phenotypically yellow− and white− flies, indicating the loss of all DNA between the FRT sites of apattBPFRTy2 and PBac{RB}e01573, including mini-yellow and mini-white. The newly established deletion was named apattPΔEnh (see Figure 4D) and kept as a y w M{vas-int.Dm}zh-2A ; apattPΔEnh/CyO stock. The deletion was confirmed by PCR and sequencing with the primer pair apEnhDel_seq_dwnst_for and apEnhDel_seq_PB_rev.
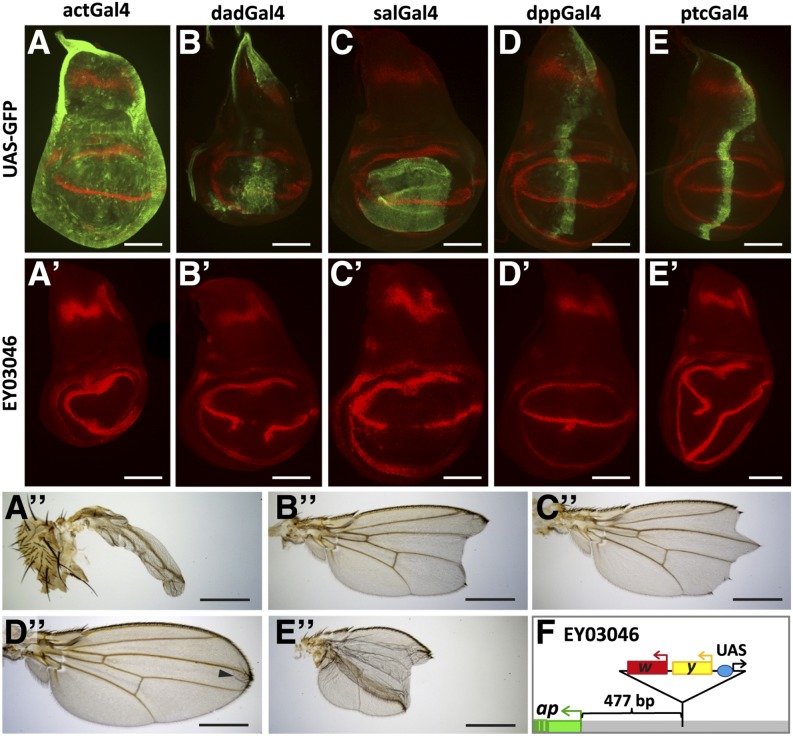
Figure 4.
Margin formation in adult wings depends on well-defined On-Off Apterous expression levels during larval development. All discs are shown anterior to the left and dorsal side up. (A−E) 3rd instar imaginal wing discs showing UAS-GFP patterns (in green) elicited by the five Gal4 drivers indicated at the top of the panel. α-Wg antibody staining (in red) outlines the pouch and the position of the D−V compartment boundary. (A´−E´) α-Wg antibody staining. The effect of ectopic Ap production as a consequence of Gal4 > EY03046 on D-V boundary formation is shown. (A´´−E´´) Adult wings as obtained after ectopic Ap expression in (A´´) actin > EY03046, (B´´) Dad4 > EY03046, (C´´) salE > EY03046, (D´´) dpp > EY03046, and (E´´) ptc > EY03046 animals. In (D´´), the arrowhead points to a small lesion near the tip of the wing. Scale bars in (A−F) and (A´−F´) are 100 µm. Scale bars in (A´´−F´´) are 50 µm. (F) Insertion site of P{EPgy2}EY03046 relative to the ap TSS is shown. The triangle depicts the structure of the transgene. The red box corresponds to the mini-white marker, the yellow box to the yellow marker and the blue oval to an array of UAS sites. Arrows specify the transcriptional direction of mini-white, yellow, and the UAS-driven promoter. P{EPgy2} transgenes are intended for regulated expression of genes proximate to the site of the insertion: genes in direct orientation with respect to the UAS-controlled promoter can be conditionally expressed via transgene-derived Gal4 activity (Bellen et al. 2004). Note that at apterous, the UAS-driven promoter is at a considerable distance from and in opposite orientation to the apterous promoter (shown in green). We propose that in Gal4 > EY03046 flies, Gal4 activates ap transcription in much the same way as the eye-specific GMR-Gal4{w-} driver boosts mini white expression in GMR-Gal4{w-}/EY03046 flies. These have red eyes while the eye pigmentation in EY03046/+ flies is faint yellow (M. Müller, unpublished data). Drawing not to scale.
The apattPΔEnh chromosome contains a single functional attP site ready for ΦC31-integrase catalyzed insertion of pEnh-Reentry derived plasmids (see Figure 5D). Insertion events can be further modified by suitably placed LoxP and FRT sites, allowing for the deletion of the yellow marker or the enhancer fragment-yellow+ marker cassette, respectively (see Figure 5E).
Generation of pEnh-Reentry constructs:
yellow+ coding sequence and body cuticle enhancer were subcloned into pBSIIKS as a BglII fragment from C4yellow, thereby generating plasmid pBSIIKS-yellow. Please note that the yellow wing enhancer is not part of the BglII fragment! attB and FRT LoxP fragments were cloned by first annealing and phosphorylating oligos attBtop and attBbottom as well as FRTLoxPtop and FRTLoxPbottom followed by three fragment ligation with pBSIIKS-yellow vector cut with SacI and XbaI. The resulting plasmid was called pEnh-Reentry and served as the backbone for all constructs described below.
The 27-kb full-length enhancer was recombineered in pEnh-Reentry from BACR45O18 (purchased from the Berkeley Drosophila Genome Project). The left homology arm was amplified with PCR with primers containing NotI and XhoI sites (primer pair: apenhrecLA_Not_for and apenhrecLA_XhoI_rev). The right homology arm was amplified with primers containing XhoI and BglII sites (primer pair: apenhrecRA_XhoI_for and apenhrecRA_BglII_rev). Homology arms were cloned in pEnh-Reentry cut with NotI/BglII as 3 fragment ligation. Recombineering was performed according to Thomason et al. 2007. In brief, the pEnh-Reentry-homologyarms vector was linearized with XhoI and transformed into bacterial strain DY380 (purchased from NCI at Frederick) pretransformed with BAC45O18 (purchased from BDGP), and pre-induced at 42° for 15 min. Recombinants were selected on ampicillin and screened by PCR. The correct recombineering product’s name is pEnh-Reentry-Full-length.
Dad enhancer fragments and apRXa were amplified from apXa gDNA. First, fragments apRXaDadInt2, DadInt52, and Dad4 were cloned into a pBluescript II KS(+) vector, where the XbaI site was mutated previously into a AvrII site. For apRXaDadInt52, primers apR_AvrII_for and dadint52_XmaI_SpeI_rev were used. For DadInt52, primers dadint52_XmaI_SpeI_rev andXa_brkpnt_AvrII_for were used. To clone Dad4, we used the primer pair dad4_AvrII_for and dad4_XmaI_SpeI_rev. These fragments were combined via the respective SpeI or AvrII sites to produce apRXaDadInt52Dad4 and DadInt52Dad4 fusion fragments. These were subcloned from pBluescript II KS(+) via AvrII and XmaI sites into pEnh-Re-entry cut with AvrII and AgeI. apR, apRXa, apP, and apY were amplified from pEnh-Reentry-Full-length plasmid and cloned into pEnh-Re-entry via NotI, AvrII or AgeI sites. To clone apR, primers apR_AvrII_for and apR_XmaI_SpeI_rev were used. For apRXa, primer pair apR_AvrII_for and apRXa_AgeI_rev was used. To amplify apP, primers apP_NotI_for and apP_AvrII_rev were used. apY was amplified using primer pair apY_NotI_for and apY_AgeI_rev.
All pEnh-Reentry derived constructs were brought into the ap locus by ΦC31-integrase mediated recombination (see Figure 4, D and E). DNAs were injected at a concentration of 300 ng/µL in 1×PBS into y w M{vas-int.Dm}zh-2A ; apattPΔEnh/CyO embryos. Transgenic flies were selected with the help of the yellow+ marker and balanced stocks were generated according to standard genetic procedure.
Generation of LacZ-reporter lines
ap regulatory DNA were amplified via PCR from y1 w67c23 gDNA with primers containing restriction enzyme sites as overhangs, and subsequently cloned into plasmid pAttBLaZ (Weiss et al. 2010) using the respective enzymes. apC was amplified with the primer pair apC_AscI_for and apC_BglII_rev. The apC fragment was defined by Lundgren et al. 1995. The apO fragment was cloned with the primers apC_AscI_for and apO_BglII_rev. For apP, primers apC_BglII_rev and apP_AscI_for were used. To clone apQ, primer pair apC_ BglII_rev and apQ_AscI_for was used. For apR, the primers apR_AscI_for and apR_BglII_rev were used. apS was cloned with the primers apS_AscI_for and apS_BglII_rev. apOR was amplified with apR_AscI_for and apO_BglII_rev. For apOR2, primers apR_AscI_for and apOR2_XbaI_rev were used. apOR3 was amplified with apR_AscI_for and apOR3_XbaI_rev. apRXa was cloned with apR_AscI_for and apRXa_XbaI_rev.
All the reporter transgenes were generated with the ΦC31-based integration system using the landing platform M{3xP3-RFP.attP}zh-86Fb (Bischof et al. 2007).
Molecular characterization of apblot
Complementation crosses with apblot over a set of overlapping ap deletions mapped the mutation to am ∼11-kb interval upstream of apMM. Therefore, a set of PCR primer pairs was designed to screen for a lesion in that region of apblot gDNA. y1 w67c23 gDNA served as positive control. With one primer pair, a discontinuity could be identified on the apblot chromosome. It could be best reconciled with the presence of a larger insertion of DNA of unknown origin. Inverse PCR (iPCR) was subsequently used to obtain sequence information about the ends of the putative insertion. Toward that end, apblot gDNA was digested with BsaWI and ligated with T4 Ligase under conditions as previously described (Ochman et al. 1988). Primer pairs used for iPCR on the proximal side of the insertion were iPCR_for and iPCR_rev. Primer pairs used for iPCR on the distal side of the insertion were K_for and L_rev. Following this strategy, sequence information could be obtained for both ends of the inserted DNA. Sequence comparison identified them as LTRs of the blood retrotransposon (Bingham and Chapman 1986). To verify the insertion, primers out of blood 3′ and 5′ LTR (blood3prime and blood5prime, respectively) were used with primers binding in adjacent ap regions (iPCR_for and L_rev, respectively). Sequencing was performed by Microsynth AG, Switzerland.
Molecular characterization of apXa
The dominant Xasta allele was originally induced by X-ray mutagenesis in a stock already containing two large inversions on 2R and 3R (Serebrovsky and Dubinin 1930; Waddington 1940; Lewis 1951; Hetherington et al. 1968). The new rearrangement was classified as a reciprocal translocation with breakpoints 41F9-41F11;89E8-89F1. Allelism with ap was inferred from noncomplementation with known ap alleles (Butterworth and King 1965; Stevens and Bryant 1985). Complementation crosses with a set of small overlapping ap deletions failed to narrow down the location of apXa. Hence, the whole ap locus was screened by overlapping primer pairs. PCR products obtained from amplification of apXa/+ and y1 w67c23 gDNA were compared. The analysis of these reactions identified a difference close to the insertion break point found in apblot. Again, this region was probed by iPCR. apXa/+ gDNA was cut with NlaIII and religated under diluted conditions. For iPCR, the primer pair iPCR_Xa_rev and 19_for was used. Sequencing of the iPCR product revealed that the reciprocal translocation had fused DNA originating from dad locus on 3R to ap-specific sequences. The fusion was confirmed by PCR and sequencing with 19_for and a primer in the dad region (primer dadint52out). The breakpoint associated with Xasta in ap was found to be identical in the two stocks ap41F/T(2;3)apXa and w*; T(2;3)apXa, apXa/CyO; TM3, Sb1.
Generation of Dad4-Gal4 fly line
The minimal hsp70 promoter was amplified from the pUAST vector with the primer pair hsp70_XbaI_for and hsp70_BamHI_rev, then cloned into pBluescript II KS(+) via the XbaI and BamHI sites. The Dad4 fragment was amplified from gDNA with the primers dad_NotI_for and dad_NheI_rev, followed by the insertion of the fragment into the NotI and XbaI digested pBS-hsp70 plasmid. Gal4 was amplified from a pCaSpeR4-Gal4 plasmid, obtained from the lab of Konrad Basler, with the primer pair Gal4_BglII_for and Gal4_HindIII_rev. The Gal4 fragment was subsequently cloned into the BamHI and HindIII digested pBS-Dad4-hsp70 plasmid. We amplified the SV40-PA terminator sequence from the pUAST vector, using the primer pair SV40_HindIII_for and SV40_BamHI_ApaI. The SV40_PA was subsequently inserted into the pBS-Dad4-hsp70-Gal4 plasmid using the HindIII and ApaI restriction sites. Finally, the Dad4-hsp70-Gal4-SV40_PA sequence was subcloned into the pCaSpeR4 vector, using the NotI and BamHI restriction sites. Transgenic flies were selected in a y1 w67c23 background with the help of the mini-white marker. The Dad4-Gal4 insert used in this study is linked to the X chromosome.
X-Gal staining of imaginal discs
Third instar larvae were cut in half, and the anterior part was inverted and subsequently fixed in 1% glutaraldehyde (Fluka) in PBS for 15 min on ice. After fixation, the fixative was removed and the larvae were washed twice with PBST (0.1% Tween 20 in PBS). The tissue was then stained as previously described (Ashburner 1989). Afterward, the imaginal discs were dissected and mounted in 80% glycerol. Discs were analyzed under the Zeiss Axiophot microscope and photographed with a Sony NEX-5RK digital camera.
In situ hybridization
A 1.5-kb fragment from the 3′ end of the ap cDNA was amplified from the cDNA clone HL02012 (purchased from DGRC) with primers insitu_SacI_for and insitu_KpnI_rev. The fragment was cloned between SacI and KpnI sites of pBluescript II KS(+) vector. Then, the resulting plasmid was linearized with Acc65I and digoxigenin-(DIG)-labeled RNA was produced from T7 promoter according to the manufacturer’s protocol (Roche, Switzerland). In situ hybridizations were performed as described in Tautz and Pfeifle (1989). Wing imaginal discs were dissected and mounted in 80% glycerol and photographed under a Nikon Microphot-FXA microscope with a Sony NEX-5RK digital camera.
Immunostaining
The anterior part of third instar larvae was inverted and fixed with 4% paraformaldehyde in PBS for 25 min at room temperature. Standard protocols were used to perform immunostaining. As primary antibodies, rabbit α-GFP (1:1000; Abcam) and mouse α-Wg (1:120, DSHB, University of Iowa) were used. α-rabbit AlexaFlour488 and α-mouse AlexaFlour568 (Molecular Probes) were used at a 1:750 dilution. Samples were mounted in Vectashield (Vector Laboratories, Inc.). Confocal imaging was performed using a Leica SP5 microscope with a vertical step size of 1 µm. Image processing was done with the ImageJ software.
Results
Defining a short wing-specific enhancer element in apC
At apterous, four different transcripts starting from three different promoters have been annotated (see www.flybase.org). In this study, the transcription start site for transcripts ap-RA and ap-RC will be referred to as ap TSS.
An ∼8-kb DNA fragment named apC located several kilobases upstream of the ap TSS had been shown to drive reporter gene expression in an ap-specific pattern in the wing disc (Lundgren et al. 1995). We used a LacZ reporter assay to analyze the cis-regulatory elements in apC in more detail. apC was first sub-divided into four overlapping fragments, apO, apR, apP and apQ (Figure 1A). Of these, only the two promoter proximal fragments, apO and apR were found to drive reporter gene expression in the wing disc. To further pinpoint the wing disc enhancers, we generated five subfragments that span the DNA sequences covered by apO and apR. As shown in Figure 1A, this analysis defines a minimal 874-bp fragment, apRXa. Because apP and apOR2 together cover the minimal apRXa element, but neither showed any expression in the wing disc, key ap wing enhancer elements are likely to be on both sides of the breakpoint that divides these two fragments.
To determine whether the wing enhancer element identified in the LacZ reporter assay is necessary for the proper regulation of the endogenous ap gene, we generated several deletions with defined breakpoints (Figure 1C; for details see the section Materials and Methods). The largest of these, apDG3, removes almost the entire ap locus, from the 4th intron to a site located about 500 bp upstream of the flanking distal gene, l(2)09851. Previous observations suggested that l(2)09851 activity is not affected by the proximity of apDG3’s distal break (Gohl et al. 2008). As a homozygote or when in trans to a large deficiency, Df(2R)BSC696, that includes the entire ap locus, apDG3 flies displayed a complete loss of all wing and haltere structures (Figure 1D). This result suggests that apDG3 represents an amorphic allele of ap, at least with respect to Ap function during wing development. Furthermore, we generated a deletion, called apDG8, which removes the whole ORF from the end of the 3′UTR to ∼400 bp upstream of the ap TSS. Trans-heterozygous apDG8/apDG3 flies again showed a typical ap-null mutant phenotype. Finally, two deficiencies affecting only the 5′ regulatory region were generated, namely apDG1 and apDG11. They share the same proximal break located ∼400 bp upstream of the ap TSS. Previous transvection studies suggested that the activity of the ap promoter is not affected by this breakpoint (Gohl et al. 2008). apDG1 extends ∼27 kb distally to the same position as apDG3. apDG11 removes only ∼11 kb of upstream DNA, including the whole apC fragment. apDG1/apDG3 as well as apDG11/apDG3 flies lacked all wing and haltere structures (Figure 1D). In these two deletions, the minimal wing enhancer element defined by apRXa is removed, suggesting that elements within apRXa are indeed necessary for the regulation of ap in the endogenous locus (see Figure 1C).
Although ap is expressed in the presumptive notum of the developing wing disc, the phenotypic appearance of the adult dorsal thorax is only mildly affected in flies lacking any ap activity (e.g., apDG8/apDG3 or homozygous apDG8). Apart from a few missing macro- and microchaetae in the vicinity of the wing appendage, it appears largely normal (Figure 1D and data not shown). The reduced size of the dorsal thorax and the aberrant bristle pattern in apDG3/Df(2R)BSC696 flies can probably be attributed to other genetic loci deleted in Df(2R)BSC696.
Apart from the dominant apXa allele, lesions in the ap gene have been reported as recessive in genetic character. Careful inspection of wings obtained from flies heterozygous for any of the 4 deletions presented in Figure 1C corroborated this fact. However, ∼2% of them had small margin defects, indicating a mild dominance of strong ap loss-of-function alleles (data not shown).
Mutations in the apR region result in wing phenotypes
In the course of investigating the cis-regulatory region of ap, we identified two classical ap alleles, apblot and apXa, that map to the apR region. apblot was isolated as a spontaneous, hypomorphic mutation that causes notching mostly of the posterior wing margin in homozygous mutant flies, while the anterior wing margin remains largely unaffected (Figure 2A; Butterworth and King 1965; Whittle 1979). To narrow down the genomic site affected by the mutation, intragenic complementation crosses with the aforementioned deletions were analyzed. They showed that apDG11 was the smallest deletion that failed to complement apblot. This observation suggested that apblot maps to the ∼11-kb interval defined by apDG11. Consequently, this region was screened with a set of overlapping PCR primer pairs. One primer pair did not yield a PCR product and thus identified the site of the putative lesion on the apblot chromosome. Using iPCR, we identified the insertion of a retrotransposable element of the blood family in the apRXa sequence (Figure 2B, see the section Materials and Methods for details). This event caused the typical 4-bp duplication at the insertion site characteristic for blood family transposons (Figure 2C; Bingham and Chapman 1986; Wilanowski et al. 1995).
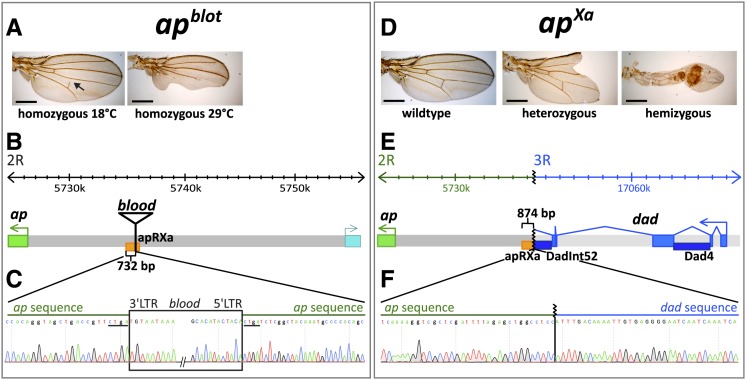
Figure 2.
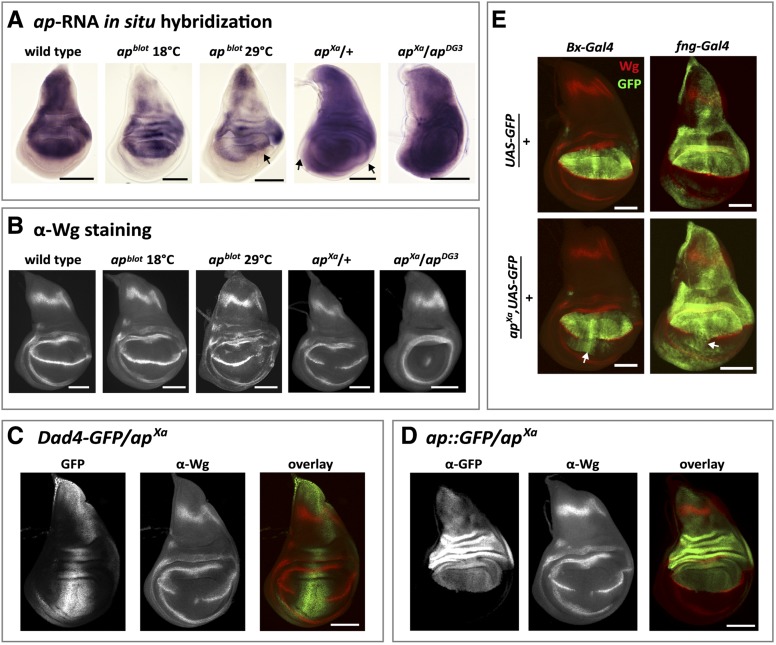
The mutations apblot and apXa affect the ap wing enhancer region. (A) Temperature-sensitive wing phenotypes obtained for the homozygous apblot allele. At 18°, less than 30% of the wings are affected and most of them only show a disruption of the posterior crossvein (arrow). At 29°, ∼70% of the wings have a phenotype. In many of them, the posterior compartment is severely affected. (B) At the top of the panel, the coordinates of the apterous locus are indicated. The insertion site of blood, a retrotransposable element, within the apRXa wing enhancer is depicted. (C) Sequence data close to the insertion site of the blood element in apblot. The insertion causes a four bp duplication (CTGA, underlined). Exact coordinates of the 4 bp duplication: 2R:5735176.0.5735179 (Flybase Release FB2014_06). (D) Preparations of wild type and apXa mutant wings. All apXa/+ flies show a dominant phenotype: the distal part of the wing blade is lost and the characteristic mitten phenotype is formed. In hemizygous condition, the wing tissue of apXa/apDG3 flies forms a short tube-like structure. Margin bristles are absent except for sometimes a few at the tip. All scale bars are 50 µm. (E) Molecular characteristics of the apXa mutation. A reciprocal translocation involving the right arms of the second and third chromosome causes a breakpoint just upstream of the apRXa wing enhancer (indicated in orange) and juxtaposes the daughters against dpp (dad) locus (indicated in blue) next to the ap gene. The dark blue rectangles represent the well-studied cis-regulatory elements Dadint52 and Dad4 which are active in the wing disc (Weiss et al. 2010). (F) Chromatograph of the apXa sequence across the rearrangement break point. The coordinates of the breakpoints are: 2R:5375319 and 3R:17065902 (Flybase Release FB2014_06).
Phenotypes caused by blood insertions at other loci are sometimes temperature-sensitive (Bingham and Chapman 1986). To test this possibility, we raised homozygous apblot flies at different temperatures and scored their wing phenotypes (Table 1). At 18°, only 28% of the wings displayed minor defects. In most of these, the posterior cross vein failed to connect with the 4th wing vein (Figure 2A). At greater temperatures, more severe wing phenotypes were detected with a higher penetrance. At 25° and 29°, 52% and 70%, respectively, of the wings showed extensive notching within the posterior compartment and reduced wing size (Figure 2A).
Table 1. Temperature sensitivity of apblot.
| Temperature | Total Wings Scored | Normal Wings | Wings with Phenotypes |
|---|---|---|---|
| 18° | 294 | 72% | 28% |
| 25° | 284 | 48% | 52% |
| 29° | 242 | 30% | 70% |
The dominant apXa allele was generated by X-ray mutagenesis and is associated with a reciprocal translocation between chromosome arms 2R and 3R. The breakpoints were mapped to 41F and 89EF, respectively (Serebrovsky and Dubinin 1930; Waddington 1940; Lewis 1951; Hetherington et al. 1968). When heterozygous, apXa flies show the characteristic dominant mitten-shaped wing phenotype, in which the distal tip of the wing is missing leading to a deep notching of the wing blade. In hemizygous apXa flies, only long wing stumps with little or no wing margin and unstructured vein patterns are formed (Figure 2D). The break on 2R has long been known to affect the ap locus (Butterworth and King 1965; Stevens and Bryant 1985). However, our attempt to map apXa by intragenic complementation was not successful, suggesting that the lesion in apterous prevents this type of genetic analysis (see also Figure 3D). Thus, we screened the entire ap locus with overlapping PCR primer pairs. We identified a discontinuity in the apR region and determined the molecular nature of the breakpoint (Figures 2, E and F; for details see the section Materials and Methods). It localized right at the edge of the apRXa fragment, 142 bp distal to the insertion site of the blood transposon in apblot. Only the proximal 874 bp of apR remain associated with the ap transcription unit (see Figure 2E). The DNA on the other side of the breakpoint is from the daughters against dpp (dad) locus located at 89E on 3R. As predicted from the cytological mapping of the rearranged apXa chromosomes, the dad locus is inverted compared to its wild-type orientation on 3R (for a comprehensive drawing of the apXa polytene chromosomes, see Hetherington et al. 1968). We were not able to determine the breakpoint at the reciprocal site of the translocation. Nevertheless, based on its reciprocal nature, it is conceivable that the dad locus is split within its 4th intron and hence destroyed. Because dad is expressed in the imaginal wing disc, it is formally possible that the Xasta phenotype is due to the loss of Dad activity. This possibility was addressed by crossing apXa with 2 known dad deletions, Df(3R)Exel6176 and dadP1883Δ32. The wings of trans-heterozygous animals displayed the characteristic mitten phenotype seen in apXa heterozygous flies, suggesting that an amorphic dad background does not modify the Xasta phenotype. Hence Dad function is not relevant for the production of the Xasta phenotype (data not shown). This is not unexpected, since dad mutants show no visible phenotype in the adult wing (Ogiso et al. 2011).
Figure 3.
Wing disc phenotypes in apblot and apXa. All discs are shown anterior to the left and dorsal side up. (A) in situ hybridization against ap mRNA in late 3rd instar larval wing discs. In wild type, the dorsal compartment of the wing pouch is filled and outlined by the ap transcript. apblot discs show reduced ap mRNA levels. At 18°, the ap expression pattern remains very similar as that in wild type. At 29°, expression of ap in the posterior compartment is disturbed and the tissue is deformed (see arrow). In heterozygous apXa discs, ectopic ap expression is seen in the ventral part of the wing disc, with the strongest signal in median regions. The black arrows point to the edges of the disc where ap transcript is absent. In hemizygous apXa/apDG3 larvae, a similar pattern is observed. Note the change in shape of the wing disc. (B) α-Wg antibody staining of 3rd instar wing discs. In wild type, a characteristic thin stripe of Wg traverses the wing pouch along the D-V compartment boundary. In apblot, Wg expression is normal at 18°C. At 29°C, the Wg stripe is much weaker and less well defined in posterior regions of the wing pouch. In apXa/+ discs, the Wg stripe is interrupted in the median pouch region. In hemizygous apXa discs, the Wg stripe is lost and only a dot of Wg expression in the middle of the pouch is visible. In addition, the size of the pouch is reduced. (C) GFP expression driven by the Dad4 enhancer is detected in the central part of an apXa/+ wing disc. Note that absence of Wg stripe correlates well with higher GFP levels. Therefore, stripe formation is more affected in the anterior than in the posterior compartment. (D) α-GFP and α-Wg antibody staining of an ap::GFP/apXa wing disc. GFP expression is restricted to the dorsal compartment of the wing pouch. In particular, Ap-GFP fusion protein does not spread ventrally where the Wg stripe is interrupted. This indicates that dad enhancers on the apXasta chromosome are unable to activate ap::GFP located on the homologous chromosome. (E) Expression of Beadex- and fringe-Gal4 enhancer trap lines in wild type and apXa/+ discs. Note that ectopic expression (white arrows) of these two validated Ap targets in the ventral compartment is only detected where the Wg stripe is interrupted. All scale bars are 100 µm.
The proximity of dad enhancers to the ap transcription unit in the apXa chromosome suggests a plausible explanation for the Xasta wing phenotype. Two cis-regulatory elements, Dad4 and DadInt52, are located in the dad introns (Figure 2E) and are known to drive reporter gene expression in the wing disc in a stripe along the A−P compartment boundary in response to Dpp signaling (Weiss et al. 2010). Because dad territory encompasses not only the dorsal but also the ventral compartment of the presumptive wing pouch, a likely scenario is that the ap promoter responds to these two dad enhancers, leading to ectopic Ap expression in the ventral compartment of the pouch.
Ectopic expression of ap in apXa leads to the ectopic expression of Ap target genes
To further characterize the effect of the apblot and apXa mutations on wing development, we examined ap mRNA and Wingless protein (Wg) expression in 3rd instar larval wing discs (Figure 3, A and B). In wild-type discs, ap mRNA is restricted to the dorsal compartment of the wing pouch, the hinge and the notum (Figure 3A). In the pouch, Ap activity is required to direct the expression of Wg in a stripe along the D−V compartment boundary (Figure 3B). This Wg stripe is essential for the proper formation of the wing margin (Couso et al. 1994).
The temperature sensitivity of apblot was faithfully recapitulated by the expression patterns in 3rd instar wing discs. Although ap mRNA levels were reduced at 18° as well as at 29°, an obvious deviation of the ap mRNA pattern was only observed at 29° in the posterior compartment of the pouch. This change correlated with a size reduction of the posterior compartment and the appearance of additional tissue folding in this region (arrow in Figure 3A). Consistent with the sharp boundary of the ap mRNA expression pattern at 18°, the Wg stripe along the D-V compartment boundary remained unchanged (Figure 3B). In contrast, at 29°, the fuzzy appearance of the ap mRNA pattern in the posterior compartment correlated with the disruption of the Wg stripe. In summary, these results are consistent with the adult wing phenotypes and provide an explanation for the abnormalities in the posterior wing margin as well as for the reduced size of the posterior compartment in apblot flies raised at elevated temperature.
In apXa heterozygotes, a strong ectopic misexpression of the ap transcript was detected in the ventral compartment of the wing disc, with the highest signal along the medial part of the disc (Figure 3A). As a consequence, the Wg stripe was disrupted in the medial region of the wing pouch (Figure 3B). Remarkably, the disruption of the Wg stripe correlated well with the expression domain of the Dad4-GFP reporter construct (Figure 3C). Wherever GFP was detected, the expression of Wg was either very low or absent. Wing discs of hemizygous apXa/apDG3 flies showed strong ap expression in the entire pouch region. The characteristic Wg stripe in the wing pouch was lost, leaving behind only a small dot of Wg expression in the middle of the pouch. Moreover, the dimension of the wing pouch was reduced to about half the size of a wild-type pouch.
In Drosophila, the somatic pairing of the two homologous chromosomes can lead to a special situation of gene regulation called transvection (Lewis 1954; Sipos et al. 1998; Morris et al. 1999; Coulthard et al. 2005). In this case the regulatory elements of a gene can regulate the expression of its homolog in trans. Transvection has been described for many gene loci (for reviews see Wu and Morris 1999; Duncan 2002) including the ap locus (Gohl et al. 2008). Therefore, we decided to test the transvection ability of apXa by crossing it with ap::GFP. In this combination only the gene in trans is labeled with GFP, allowing for the independent detection of the gene product from this chromosome. Trans-heterozygous ap::GFP/apXa flies displayed no ectopic expression of Ap-GFP in the ventral wing pouch (Figure 3D). This result demonstrates that the misexpression of ap is limited to the chromosome affected by the rearrangement.
As a selector gene, ap is known to regulate multiple downstream genes (Bronstein et al. 2010). We wished to know whether the ectopic Ap expression observed in heterozygous apXa flies was sufficient to induce its targets also in the ventral compartment of the pouch. Toward that end, we analyzed Gal4-enhancer trap lines of two validated Ap target genes, Beadex (Bx) and fringe (fng) (Irvine and Wieschaus 1994; Milán et al. 2004). Their activity was monitored with the help of a UAS-CD8-GFP transgene. Under wild-type conditions, Beadex > GFP expression was detected exclusively in the dorsal wing pouch, whereas fng > GFP was observed predominantly in the whole dorsal compartment with weak ventral GFP outside the wing pouch (Figure 3E). When analyzed in a heterozygous apXa background, the expression of both reporters extended to the medial-ventral region of the wing discs (Figure 3E). In summary, the data presented in Figure 3 strongly suggest that in apXa, ap is ectopically expressed due to the juxtaposition of dad wing enhancer elements and the ap promoter. As a consequence, Ap target genes are up-regulated in the ventral compartment of the wing pouch. These molecular events correlate well with the disruption of the Wg stripe in median parts of the wing disc and, finally, the altered adult wing morpholgy.
Requirements for ectopic wing margin induction
A puzzling observation is that Ap expression does not induce Wg in the ventral part of the pouch, ultimately leading to extra margin formation in adult wings. One explanation for this finding is that compartment and compartment boundaries must be defined by a clear on-off state of selector gene activity. However, the dad gene and its enhancers are regulated by a Dpp concentration gradient (high Dpp in medial parts, low Dpp in lateral parts of the wing disc; for a review see Affolter and Basler 2007). As a consequence, in apXa, Ap is expressed in the ventral part of the wing pouch in response to the dad enhancers in a gradient-like manner. Thus, no clear selector gene on-off state between neighboring cells would be generated. In this case, the initiation of the signaling cascade that usually induces the wg gene at the compartment boundary fails to be activated.To explore this possibility in more detail, we used the Gal4/UAS-system (brand and perrimon 1993). Preliminary test crosses indicated that upon Gal4 activation, a UAS-ap transgene leads to lethality or pleiotropic phenotypes with all Gal4 drivers tested except dpp-Gal4. For this reason, we tested an insertion into ap, EY03046, which contains a UAS-driven promoter located several 100 bp upstream of the ap TSS (Figure 4F). In contrast to Gal4 > UAS-ap combinations, Gal4 > EY03046 flies were viable and obvious phenotypes were restricted to the dorsal thoracic appendages. One possible explanation for the difference is that the activation of EY03046 by Gal4 is reduced or eliminated by the ap PRE (Schwartz et al. 2006; Tolhuis et al. 2006; Oktaba et al. 2008; D. Bieli et al., unpublished data) in most tissues outside of the wing disc. To activate EY03046 expression in the wing pouch, we used the following Gal4 drivers: actin-Gal4, dad4-Gal4, salE-Gal4, dpp-Gal4, and ptc-Gal4. Their expression domains are depicted in Figure 4, A−E. For our purposes, they can be grouped into three classes: (1) actin > GFP is found in all cells of the pouch; (2) Dad4 > GFP and salE > GFP expression domains are rather broad with a rather ill-defined edge and centered on the A-P axis; and (3) dpp > GFP and ptc > GFP form a narrow stripe along the A-P axis.
To analyze the effects of ectopic Ap expression, we examined Wg stripe formation along the D-V compartment boundary (see Figure 4, A´−E´) and adult wing morphology (Figure 4, A´´−E´´). Ubiquitous Ap expression in the pouch using actin::Gal4 prevents Wg activation. As a consequence, margin formation in the tiny adult wings was abolished. As expected from the data in Figure 3, Dad4 and spalt-mediated Ap expressions led to Xasta phenocopies. Wg stripe formation was abolished in the center of the pouch. Occasionally, small ectopic Wg stripes extended into the ventral compartment in Dad4 > EY03046 wing discs. Nevertheless, both Gal4 drivers elicited similar moderate Xasta-like phenotypes in adult wings. Finally, although the expression patterns mediated by the dpp-Gal4 and ptc-Gal4 drivers were remarkably similar, their phenotypic consequences were dramatically different. dpp > EY03046 caused the appearance of a faint ectopic Wg patch on the A-P axis in the ventral compartment. A tuft of ectopic bristles was observed on the ventral side at the intersection of the A-P axis and the wing margin in less than 10% of the adult wings (black arrowhead in Figure 4D´´). ptc > EY03046, on the other hand, interrupted the Wg stripe in the center of the pouch and frequently induced a well-defined Wg stripe which traversed the whole ventral compartment. A Wg stripe of variable length also is formed in the anterior compartment. Adult wings of this genotype often formed three-dimensional, balloon-like structures with an oval-shaped posterior margin extending from the proximal edge of the wing appendage to its distal end and back to proximal. In addition, anterior and posterior margins were not continuous at the tip of the wing.
These observations corroborate our expectations. First, the presence of Ap in the ventral compartment at sufficiently high levels impedes the activation of the signaling cascade that induces Wg expression along the D−V compartment boundary. Second, an ectopic compartment boundary can only be formed between cells with sharp on-off levels of Ap. This prerequisite is only satisfied by the ptc-Gal4 driver. In the wing disc, Ptc is expressed in a straight line immediately abutting the posterior compartment where it serves as a receptor for the Hedgehog ligand (Capdevila et al. 1994; Alexandre et al. 1996). Its anterior limit of expression is more graded and less well defined and ectopic anterior margin in the adult wing can only rarely be observed. The question remains why dpp > EY03046 is only marginally active in this experiment. It is possible that ectopic Ap levels remain below a certain threshold because the levels of Gal4 do not suffice. Alternatively, the onset of Gal4 activity in this driver line might be delayed. However, very similar observations were made with a UAS-ap transgene in place of EY03046 and also with a different dpp-Gal4 driver line. In addition, Klein et al. have reported a similar phenotype for ectopic Ser expression by dpp-Gal4 (Klein et al. 1998).
The in situ rescue system
To extend our analysis of the cis-regulatory elements directing ap expression, we decided to characterize and manipulate possible regulatory sequences directly at the endogenous locus. For this purpose, we engineered an in situ rescue system. The establishment of this system was a multistep procedure and is described in detail in the section Materials and Methods. A diagrammatic summary is presented in Figure 5. In brief, we deleted the 27-kb intergenic spacer between the ap and l(2)09851 loci and replaced it with an attP site located 400 bp upstream of the ap TSS (Figure 5, A−D). This ap allele is referred to as apattPΔEnh (Figure 5D). The deleted region is identical to that of apDG1. Therefore, homo- or hemi-zygous apattPΔEnh flies have no wings (data not shown). The attP site of apattPΔEnh serves as docking site for ΦC31-mediated integration of any desired DNA located on a plasmid containing an attB site and the yellow selection marker (Figure 5, D and E).
As proof of principle, two control plasmids were first introduced into apattPΔEnh: (1) the empty pEnh-Reentry vector gave rise to a fly line called apempty; (2) pEnh-Reentry-full-length contained the complete 27-kb intergenic spacer and the corresponding transgenic line was called apfull-length (Figure 6A). The “wing-forming” activity of these two controls as well as all subsequent transgenic lines analyzed in this study was determined in hemizygous condition. Therefore, balanced apempty and apfull-length males were crossed with apDG3/SM6 virgins and the wings of trans-heterozygous progeny were carefully inspected. As expected, apempty/apDG3 flies generated no detectable wing material. In contrast, the reconstituted ap locus produced wild-type wings in apfull-length/apDG3 flies. Taken together, these observations demonstrate the feasibility of our in situ rescue system and suggest that the backbone of the pEnh-Reentry plasmid does not cause any disturbances.
Figure 6.
Testing dad and ap enhancers in the endogenous apterous locus. (A) Positive control: the whole 27-kb ap wing enhancer region was re-inserted and apfull-length flies obtained. In apfull-length/apDG3 animals, perfectly wild-type wings are formed. Negative control: the empty pEnh-Reentry plasmid gave rise to apempty flies. No wing tissue is formed in apempty/apDG3 adults. (B) Xasta phenocopies are obtained with apapRXaDad52.4 and apDad52.4 alleles. apapRXaDad52.4 contains three enhancer elements: apRXa, DadInt52, and Dad4. Heterozygous flies only produce rather weak phenocopies. The junction between wing vein L2 and the margin (see arrow head) is present in almost 100% of the wings. apDad52.4 contains only the 2 dad enhancers. Faithful phenocopies of apXa/+ wings where the junction between vein L2 and the margin is missing are often observed. The wing phenotypes of hemizygous apapRXaDad52.4, apDad52.4 and apXa are similar: tube-like wing stumps of variable length are formed. Margin bristles are absent except for sometimes a few at the tip of the wing. (C) Testing the wing enhancer activity of four apC derivatives. At the top of the panel, the ap locus is depicted. Below, the positions of fragments apP, apY, apR and apRXa are shown relative to apC. The respective wing phenotypes in hemizygous condition are shown to the right of the corresponding fragments. Flies transgenic for the gray apP fragment behave like a true null allele: no wings are formed. Fragments drawn in orange have partial rescue activity: inflated wings are formed, where most of the margin and the alula are missing. The hinge is poorly formed. Note that in B and C, for space reasons, parts of the reentry plasmid have been omitted. All scale bars are 50 µm.
DadInt52 and Dad4 enhancers contribute significantly to the Xasta phenotype
Our model for the Xasta wing phenotype posits that the wing specific dad enhancers Dad4 and DadInt52 are responsible for ectopic Ap expression in the ventral pouch compartment. We wished to test this hypothesis with the in situ rescue system. Two fly lines were established: apapRXaDad52.4 and apDad52.4 (Figure 6B). The former combined the three identified wing specific enhancers apRXa, DadInt52 and Dad4. The latter contained only the two dad regulatory elements. When the 2 transgenics were initially isolated, it was immediately apparent that both phenocopied the dominant Xasta allele. However, a semi quantitative analysis also showed that the severity of their phenotypes was weaker than observed for apXa/+ wings (Table 2). Although roughly 50% of the apDad52.4/+ wings were as strongly affected as those of apXa/+ flies, hardly any such wings appeared in apapRXaDad52.4/+ flies. These observations indicate that apart from DadInt52 and Dad4, other factors contribute to the production of a full blown Xasta wing phenotype.
Table 2. Penetrance of the dominant apXa wing phenotype.
| Genotype | Number of Wings Scoreda | L2 Junction Present | L2 Junction Absent |
|---|---|---|---|
| apXa/+ | 262 | 6.5% | 93.5% |
| apapRXaDad52.4/+ | 160 | 98.1% | 1.9% |
| apDad52.4/+ | 546 | 58.6% | 41.4% |
Wings were scored for the presence or absence of the junction between wing vein L2 and the wing margin.
The wings of apapRXaDad52.4 and apDad52.4 were also analyzed in hemizygous condition. The phenotypes were comparable to the one seen in apXa/ apDG3 flies: only tube-like wing stumps were formed which lacked wing margin completely except for the occasional occurrence of a few margin hairs at the very tip. It is conceivable that the latter arise due to the Wg spot seen in the center of the pouch of apXa/apDG3 wing discs (see Figure 3B). We have never seen homozygous apXa flies but did inspect adult wings of apXa/apDad52.4 animals. They appeared as even smaller versions of those observed in hemizygous apXa flies (data not shown).
The apRXA enhancer is required but not sufficient for wing formation
In Figure 1 of this paper, we have presented evidence that the ∼8 kb apC fragment harbors an 874-bp wing specific enhancer that is essential for wing formation. However, the experimental approaches we used are not adequate to test whether the enhancer is also sufficient for the formation of a wild-type wing. Therefore, four overlapping fragments covering the whole apC were introduced into the ap locus and the corresponding transgenic lines were obtained: apapP, apapY, apapR, and apapRXa. Their wing enhancer activity was tested in a hemizygous genetic background (Figure 6C). apapP/apDG3 flies, which contained the apP fragment that did not yield any LacZ reporter activity (see Figure 1A), also did not develop any wing or haltere tissue and phenotypically resembled ap null alleles. When apY, a fragment which is shifted by 2 kb toward the ap TSS, was tested in apapY/apDG3 flies, wing development was partially restored. However, most of the margin, the alula and the hinge region were poorly formed. Similar phenotypes as for apapY were observed in apapR/apDG3 and apapRXa/apDG3 flies. Note that these three apC derivatives were sufficient to drive ap-specific LacZ expression in our reporter assay (see Figure 1A). “Homozygotes” obtained by pairwise combinations of apapY, apapR or apapRXa were also studied. Such wings looked improved compared to the phenotypes observed in hemizygotes, because the margins, particularly along the anterior but also along the posterior edges of the wing, were formed to a large degree (data not shown). Somewhat unexpectedly, heterozygous apapY, apapR and apapRXa flies showed a weak dominant wing phenotype, associated with a small notch in the tip region in 10–20% of the cases. This phenotype was not observed in apapY/+ or apfull-length/apDG3 flies (data not shown).
These results demonstrate that the 874 bp apRXa wing enhancer element is required but not sufficient in the endogenous context to correctly regulate ap expression. Our observations imply the existence of further unidentified wing enhancer elements elsewhere in the ap region.
Discussion
In the past, cis-regulatory elements were mainly investigated using reporter-based assay systems, in which putative regulatory DNA fragments were tested for their ability to drive reporter gene expression when present on a transgene inserted randomly in the genome (Simon et al. 1985; Hiromi and Gehring 1987). Although this method proved to be a highly useful and valuable approach, it has some shortcomings. Enhancer fragments are tested in a genomic environment that may differ considerably from their native position. Additionally, the results of such studies yield little or no information about whether the investigated elements are sufficient, permissive or even dispensable for the regulation of gene expression at their original location. Recently, some improvements were achieved by using bacterial artificial chromosomes to investigate cis-regulatory elements in a broader genomic context (Dunipace et al. 2013).
To circumvent the problem of positional effects, we performed our classical reporter assay at a single ΦC31-system docking site located on 3R. Our laboratory has successfully used this insertion site for the analysis of wing specific enhancer elements (Weiss et al. 2010). Furthermore, we investigated the relevance of the reporter data with two powerful genetic approaches. We used methods from the Drosophila genetic tool kit and generated useful materials for the in situ dissection of regulatory elements directly at the ap locus. First, a set of small overlapping deletions within the ap region was isolated with the help of different transposable elements carrying FRT sites. Second, the in situ rescue system was established. The novel fly strain generated greatly facilitates the introduction of any DNA fragment by means of integrase-mediated recombination into the apterous locus. It has and will serve us as a tool to dissect important ap regulatory sequences in great detail.
In this study, the combined application of reporter assay, deletion analysis and in situ rescue system has allowed us to firmly establish the 874 bp apRXa fragment as an essential wing-specific regulatory element for apterous transcription. We show that apRXa is sufficient to drive reporter gene expression within the dorsal compartment of the wing pouch. Flies hemizygous for an 11-kb deletion encompassing the apRXa element develop no wing structures. This observation proves that this larger DNA interval including apRXa is essential for ap function. Finally, when tested in the context of the endogenous ap locus, we document that apRXa is required but not sufficient to form wild-type wings.
The importance of the apRXa enhancer element is further highlighted through the molecular characterization of 2 classical ap alleles, apblot and apXa. apblot contains an insertion of a retrotransposon from the blood family. This insertion is located within the apRXa enhancer. We have not attempted to prove the presence of the full length 7.4-kb blood element in apblot, but we have completely sequenced both LTRs. So far, all blood elements detected in the Drosophila genome are full-length insertions. None of them was found to be truncated (Kaminker et al. 2002). Hence, it appears likely that apblot also contains an intact, full length blood element and that it is accountable for the mutagenic effect. For example, it is possible that the insertion destroys an important transcription factor binding site within the apRXa wing enhancer. Alternatively, the inserted DNA might separate important transcription factor binding sites.
The other ap allele we investigated is apXa. In this mutant, a reciprocal translocation event between the right arms of the second and third chromosomes caused a breakpoint immediately upstream of the apRXa wing enhancer. This rearrangement juxtaposes the dad locus next to apterous. Our experimental evidence strongly indicates that in this mutant, ap transcription falls under the control of dad wing specific enhancers Dad4 and DadInt52. As a consequence, ap and its target genes are ectopically expressed in the medial section of the ventral part of the wing disc, conferring ventral cells with a dorsal cell fate identity. This, in turn, likely interferes with signaling at the D-V compartment boundary and causes the disruption of the Wg stripe in the center of the wing pouch. dad-controlled ap expression also provides an explanation why the anterior compartment is more strongly affected than the posterior one in adult wings. As evidenced by asymmetric Dad4-GFP expression along the A-P compartment boundary, a wider domain with higher levels of GFP is produced in the anterior compartment (see Figure 3C). We propose that a similar asymmetrical distribution of Ap causes differential Wg stripe expression in the two compartments.
Our observations also suggest that dad-mediated transcriptional activation of ap is not the sole cause for the explanation of the Xasta phenotype. The dominant phenotypes of apapRXaDad52.4/+ and apDad52.4/+ flies are clearly less pronounced than that documented for apXa/+. Why should this be the case? It is known that the apterous locus is a target of the repressive Polycomb Group (PcG) system. Scm−/− clones reaching into the ventral compartment elicit ectopic Ap expression (Oktaba et al. 2008). In addition, it is well documented that the silencing activity of isolated Polycomb Response Elements is pairing dependent (Kassis et al. 1991; Fauvarque and Dura 1993; Chan et al. 1994; Gindhart and Kaufman 1995; Muller et al. 1999; reviewed in Kassis 2002). It is therefore conceivable that in apXa/+ flies, the chromosomal rearrangement prevents efficient homologous chromosome pairing and thus reduced PcG-mediated silencing. This hypothesis is supported by the fact that the mini-white markers of transgenes inserted in the ap locus are partially derepressed in a Xasta heterozygous background (M. Müller, unpublished data). An alternative explanation could be that as yet-uncharacterized wing-specific enhancers are present in the genomic dad locus which lack in apapRXaDad52.4/+ and apDad52.4/+ flies. It thus might be that the stronger phenotype observed in apXa/+ flies is a consequence of stronger Ap misexpression due to the combined effect of more than two enhancers.
Our findings imply the existence of other, as yet unidentified wing-specific regulatory elements within the realms of the apterous locus. A hint about the possible location of such sequences has previously been obtained through the genetic analysis of insertion PBac{WH}f00451. This transposon is located about 3 kb distal to apRXa. The PBac{WH} element contains an array of Su(Hw) binding sites at its 3′ end (Thibault et al. 2004). It is well established that on transgenes, a cluster of Su(Hw) binding sites acts as enhancer blocker. It interferes with enhancer-promoter interaction when placed in between two such regulatory elements (Holdridge and Dorsett 1991; Hagstrom et al. 1996; Scott et al. 1999). Furthermore, the mutagenic effect of an array of Su(Hw) binding sites located on the gypsy mobile genetic element can often be attributed to a similar mechanism (Geyer et al. 1986; Peifer and Bender 1988; Dorsett 1993; Hogga et al. 2001). Homozygous as well as hemizygous apf00451 flies have been reported to cause a rather strong wing phenotype. Importantly, the phenotype is completely suppressed in a su(Hw)- background (Gohl et al. 2008). This observation suggests the presence of other ap wing enhancer elements distally to PBac{WH}f00451. We are currently exploring this possibility with analogous experimental approaches as outlined above, in particular through the use of the in situ rescue system established and described in this study (D. Bieli et al., unpublished results).
Supplementary Material
Acknowledgments
We thank Johannes Bischof, John B. Thomas, Jorgos Pyrowolakis, Marco Milán, Steven Henikoff, and Fisun Hamarotoglu and the Bloomington and Kyoto stock centers for sending stocks and Jack Bateman for important DNA clones. Stefan Thor is acknowledged for helpful discussions at initial stages of this work. Our direct gene conversion approach benefitted a lot from Laci Sipos’ suggestions. Alex Weiss and Emmanuel Caussinus helped greatly by sharing their injection skills during the screen for gene conversion events at apterous. Thanks are also due to Mario Metzler for verifying the distal foot of gene convertant apc1.4b. Tetsuya Tabata kindly provided us with unpublished information about DadP1883Δ32. This work would not have been possible without the recent efforts of the following labs: the Basler and Karch labs adopted the ΦC31 system for Drosophila and sent us important stocks prior to publication; Hugo Bellen et al. developed the MiMIC tool and shared with us useful insertions in apterous. We would like to thank the Biozentrum Imaging Core Facility for unceasing support. Last but not least, a big thank you to Bernadette Bruno, Gina Evora, and Karin Mauro for constant and reliable supply with world’s best fly food. This study was supported by grants from the Kantons Basel-Stadt and Basel-Land, and the Swiss National Science Foundation.
Footnotes
Supporting information is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.115.017707/-/DC1
Communicating editor: H. D. Lipshitz
Literature Cited
- Affolter M., Basler K., 2007. The decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nat. Rev. Genet. 8: 663–674. [DOI] [PubMed] [Google Scholar]
- Ahmad K., Henikoff S., 2001. Modulation of a transcription factor counteracts heterochromatic gene silencing in Drosophila. Cell 104: 839–847. [DOI] [PubMed] [Google Scholar]
- Alexandre C., Jacinto A., Ingham P. W., 1996. Transcriptional activation of hedgehog target genes in Drosophila is mediated directly by the cubitus interruptus protein, a member of the GLI family of zinc finger DNA-binding proteins. Genes Dev. 10: 2003–2013. [DOI] [PubMed] [Google Scholar]
- Alexandre C., Baena-Lopez A., Vincent J.-P., 2014. Patterning and growth control by membrane-tethered Wingless. Nature 505: 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M., 1989. Drosophila: A Laboratory Handbook and Manual (two volumes) Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. [Google Scholar]
- Bateman J. R., Lee A. M., Wu C. T., 2006. Site-specific transformation of Drosophila via phiC31 integrase-mediated cassette exchange. Genetics 173: 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen H. J., Levis R. W., Liao G., He Y., Carlson J. W., et al. , 2004. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics 167: 761–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham P. M., Chapman C. H., 1986. Evidence that white-blood is a novel type of temperature-sensitive mutation resulting from temperature-dependent effects of a transposon insertion on formation of white transcripts. EMBO J. 5: 3343–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof J., Maeda R. K., Hediger M., Karch F., Basler K., 2007. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA 104: 3312–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair S. S., Brower D. L., Thomas J. B., Zavortink M., 1994. The role of apterous in the control of dorsoventral compartmentalization and PS integrin gene expression in the developing wing of Drosophila. Development 120: 1805–1815. [DOI] [PubMed] [Google Scholar]
- Bourgouin C., Lundgren S. E., Thomas J. B., 1992. apterous is a drosophila LIM domain gene required for the development of a subset of embryonic muscles. Neuron 9: 549–561. [DOI] [PubMed] [Google Scholar]
- Bronstein R., Levkovitz L., Yosef N., Yanku M., Ruppin E., et al. , 2010. Transcriptional regulation by CHIP/LDB complexes. PLoS Genet. 6: e1001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth F. M., King R. C., 1965. The developmental genetics of apterous mutants of Drosophila melanogaster. Genetics 52: 1153–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila J., Estrada M. P., Sánchez-Herrero E., Guerrero I., 1994. The Drosophila segment polarity gene patched interacts with decapentaplegic in wing development. EMBO J. 13: 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caussinus E., Kanca O., Affolter M., 2011. Fluorescent fusion protein knockout mediated by anti-GFP nanobody. Nat. Struct. Mol. Biol. 19: 117–121. [DOI] [PubMed] [Google Scholar]
- Chan C. S., Rastelli L., Pirrotta V., 1994. A Polycomb response element in the Ubx gene that determines an epigenetically inherited state of repression. EMBO J. 13: 2553–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B., McGuffin M. E., Pfeifle C., Segal D., Cohen S. M., 1992. apterous, a gene required for imaginal disc development in Drosophila encodes a member of the LIM family of developmental regulatory proteins. Genes Dev. 6: 715–729. [DOI] [PubMed] [Google Scholar]
- Coulthard A. B., Nolan N., Bell J. B., Hilliker A. J., 2005. Transvection at the vestigial locus of Drosophila melanogaster. Genetics 170: 1711–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couso J. P., Bishop S. A., Martinez Arias A., 1994. The wingless signalling pathway and the patterning of the wing margin in Drosophila. Development 120: 621–636. [DOI] [PubMed] [Google Scholar]
- Couso J. P., Knust E., Martinez Arias A., 1995. Serrate and wingless cooperate to induce vestigial gene expression and wing formation in Drosophila. Curr. Biol. 5: 1437–1448. [DOI] [PubMed] [Google Scholar]
- Dahmann C., Basler K., 1999. Compartment boundaries: at the edge of development. Trends Genet. 15: 320–326. [DOI] [PubMed] [Google Scholar]
- Diaz-Benjumea F. J., Cohen S. M., 1993. Interaction between dorsal and ventral cells in the imaginal disc directs wing development in Drosophila. Cell 75: 741–752. [DOI] [PubMed] [Google Scholar]
- Diaz-Benjumea F. J., Cohen S. M., 1995. Serrate signals through Notch to establish a Wingless-dependent organizer at the dorsal/ventral compartment boundary of the Drosophila wing. Development 121: 4215–4225. [DOI] [PubMed] [Google Scholar]
- Dorsett D., 1993. Distance-independent inactivation of an enhancer by the suppressor of Hairy-wing DNA-binding protein of Drosophila. Genetics 134: 1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan I. W., 2002. Transvection effects in Drosophila. Annu. Rev. Genet. 36: 521–556. [DOI] [PubMed] [Google Scholar]
- Dunipace L., Saunders A., Ashe H., Stathopoulos A., 2013. Autoregulatory feedback controls sequential action of cis-regulatory modules at the brinker locus. Dev. Cell 26: 536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauvarque M. O., Dura J. M., 1993. polyhomeotic regulatory sequences induce developmental regulator-dependent variegation and targeted P-element insertions in Drosophila. Genes Dev. 7: 1508–1520. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A., Ripoll P., Morata G., 1973. Developmental compartmentalisation of the wing disk of Drosophila. Nat. New Biol. 245: 251–253. [DOI] [PubMed] [Google Scholar]
- Geyer P. K., Corces V. G., 1987. Separate regulatory elements are responsible for the complex pattern of tissue-specific and developmental transcription of the yellow locus in Drosophila melanogaster. Genes Dev. 1: 996–1004. [DOI] [PubMed] [Google Scholar]
- Geyer P. K., Spana C., Corces V. G., 1986. On the molecular mechanism of gypsy-induced mutations at the yellow locus of Drosophila melanogaster. EMBO J. 5: 2657–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gindhart J. G., Kaufman T. C., 1995. Identification of Polycomb and trithorax group responsive elements in the regulatory region of the Drosophila homeotic gene Sex combs reduced. Genetics 139: 797–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor G. B., Nassif N. A., Johnson-Schlitz D. M., Preston C. R., Engels W. R., 1991. Targeted gene replacement in Drosophila via P element-induced gap repair. Science 253: 1110–1117. [DOI] [PubMed] [Google Scholar]
- Gohl D., Müller M., Pirrotta V., Affolter M., Schedl P., 2008. Enhancer blocking and transvection at the Drosophila apterous locus. Genetics 178: 127–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golic K. G., Golic M. M., 1996. Engineering the Drosophila genome: chromosome rearrangements by design. Genetics 144: 1693–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstrom K., Muller M., Schedl P., 1996. Fab-7 functions as a chromatin domain boundary to ensure proper segment specification by the Drosophila bithorax complex. Genes Dev. 10: 3202–3215. [DOI] [PubMed] [Google Scholar]
- Henderson K. D., Isaac D. D., Andrew D. J., 1999. Cell fate specification in the Drosophila salivary gland: the integration of homeotic gene function with the DPP signaling cascade. Dev. Biol. 205: 10–21. [DOI] [PubMed] [Google Scholar]
- Hetherington C. M., Whittington W. J., Hossain M. A., Peat W. E., 1968. The genetics of the Xasta mutant of Drosophila melanogaster. Genet. Res. 12: 285–294. [DOI] [PubMed] [Google Scholar]
- Hiromi Y., Gehring W. J., 1987. Regulation and function of the Drosophila segmentation gene fushi tarazu. Cell 50: 963–974. [DOI] [PubMed] [Google Scholar]
- Hogga I., Mihaly J., Barges S., Karch F., 2001. Replacement of Fab-7 by the gypsy or scs insulator disrupts long-distance regulatory interactions in the Abd-B gene of the bithorax complex. Mol. Cell 8: 1145–1151. [DOI] [PubMed] [Google Scholar]
- Holdridge C., Dorsett D., 1991. Repression of hsp70 heat shock gene transcription by the suppressor of hairy-wing protein of Drosophila melanogaster. Mol. Cell. Biol. 11: 1894–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine K. D., Wieschaus E., 1994. fringe, a Boundary-specific signaling molecule, mediates interactions between dorsal and ventral cells during Drosophila wing development. Cell 79: 595–606. [DOI] [PubMed] [Google Scholar]
- Kaminker J. S., Bergman C. M., Kronmiller B., Carlson J., Svirskas R., et al. , 2002. The transposable elements of the Drosophila melanogaster euchromatin: a genomics perspective. Genome Biol. 3: RESEARCH0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis J. A., 2002. Pairing-sensitive silencing, polycomb group response elements, and transposon homing in Drosophila. Adv. Genet. 46: 421–438. [DOI] [PubMed] [Google Scholar]
- Kassis J. A., VanSickle E. P., Sensabaugh S. M., 1991. A fragment of engrailed regulatory DNA can mediate transvection of the white gene in Drosophila. Genetics 128: 751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Irvine K. D., Carroll S. B., 1995. Cell recognition, signal induction, and symmetrical gene activation at the dorsal-ventral boundary of the developing Drosophila wing. Cell 82: 795–802. [DOI] [PubMed] [Google Scholar]
- Klein T., Couso J. P., Martinez Arias A., 1998. Wing development and specification of dorsal cell fates in the absence of apterous in Drosophila. Curr. Biol. 8: 417–420. [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Morata G., 1977. The early development of mesothoracic compartments in Drosophila. An analysis of cell lineage and fate mapping and an assessment of methods. Dev. Biol. 56: 40–51. [DOI] [PubMed] [Google Scholar]
- Lewis E. B., 1951. Additions and corrections to the cytology of rearrangements. Drosoph. Inf. Serv. 25: 108–109. [Google Scholar]
- Lewis E. B., 1954. The theory and application of a new method of detecting chromosomal rearrangements in Drosophila melanogaster. Am. Nat. 88: 225–239. [Google Scholar]
- Lundgren S. E., Callahan C. A, Thor S, Thomas J. B., 1995. Control of neuronal pathway selection by the Drosophila LIM homeodomain gene apterous. Development 121: 1769–1773. [DOI] [PubMed] [Google Scholar]
- Milán M., Cohen S. M., 1999. Regulation of LIM homeodomain activity in vivo: a tetramer of dLDB and apterous confers activity and capacity for regulation by dLMO. Mol. Cell 4: 267–273. [DOI] [PubMed] [Google Scholar]
- Milán M., Pham T. T., Cohen S. M., 2004. Osa modulates the expression of Apterous target genes in the Drosophila wing. Mech. Dev. 121: 491–497. [DOI] [PubMed] [Google Scholar]
- Morris J. R., Chen J., Filandrinos S. T., Dunn R. C., Fisk R., et al. , 1999. An analysis of transvection at the yellow locus of Drosophila melanogaster. Genetics 151: 633–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosimann C., Hausmann G., Basler K., 2006. Parafibromin/Hyrax activates Wnt/Wg target gene transcription by direct association with beta-catenin/Armadillo. Cell 125: 327–341. [DOI] [PubMed] [Google Scholar]
- Muller M., Hagstrom K., Gyurkovics H., Pirrotta V., Schedl P., 1999. The mcp element from the Drosophila melanogaster bithorax complex mediates long-distance regulatory interactions. Genetics 153: 1333–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann C. J., Cohen S. M., 1997. Long-range action of Wingless organizes the dorsal-ventral axis of the Drosophila wing. Development 124: 871–880. [DOI] [PubMed] [Google Scholar]
- Ochman H., Gerber A. S., Hartl D. L., 1988. Genetic applications of an inverse polymerase chain reaction. Genetics 120: 621–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiso Y., Tsuneizumi K., Masuda N., Sato M., Tabata T., 2011. Robustness of the Dpp morphogen activity gradient depends on negative feedback regulation by the inhibitory Smad, Dad. Dev. Growth Differ. 53: 668–678. [DOI] [PubMed] [Google Scholar]
- Oktaba K., Gutiérrez L., Gagneur J., Girardot C., Sengupta A. K., et al. , 2008. Dynamic regulation by polycomb group protein complexes controls pattern formation and the cell cycle in Drosophila. Dev. Cell 15: 877–889. [DOI] [PubMed] [Google Scholar]
- Peifer M., Bender W., 1988. Sequences of the gypsy transposon of Drosophila necessary for its effects on adjacent genes. Proc. Natl. Acad. Sci. USA 85: 9650–9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer B. D., Ngo T.-T. B., Hibbard K. L., Murphy C., Jenett A., et al. , 2010. Refinement of tools for targeted gene expression in Drosophila. Genetics 186: 735–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson E. J., Blair S. S., 1995. Notch regulates wingless expression and is not required for reception of the paracrine wingless signal during wing margin neurogenesis in Drosophila. Development 121: 2813–2824. [DOI] [PubMed] [Google Scholar]
- Ryder E., Ashburner M., Bautista-Llacer R., Drummond J., Webster J., et al. , 2007. The DrosDel deletion collection: a Drosophila genomewide chromosomal deficiency resource. Genetics 177: 615–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz Y. B., Kahn T. G., Nix D. A., Li X. Y., Bourgon R, et al. , 2006. Genome-wide analysis of Polycomb targets in Drosophila melanogaster. Nat. Genet. 38: 700–705. [DOI] [PubMed] [Google Scholar]
- Scott K. C., Taubman A. D., Geyer P. K., 1999. Enhancer blocking by the Drosophila gypsy insulator depends upon insulator anatomy and enhancer strength. Genetics 153: 787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serebrovsky A. S., Dubinin N. P., 1930. X-ray experiments with Drosophila. J. Hered. 21: 259–265. [Google Scholar]
- Simon J. A., Sutton C. A., Lobell R. B., Glaser R. L., Lis J. T., 1985. Determinants of heat shock-induced chromosome puffing. Cell 40: 805–817. [DOI] [PubMed] [Google Scholar]
- Sipos L., Mihály J., Karch F., Schedl P., Gausz J., et al. , 1998. Transvection in the Drosophila Abd-B domain: extensive upstream sequences are involved in anchoring distant cis-regulatory regions to the promoter. Genetics 149: 1031–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipos L., Kozma G., Molnár E., Bender W., 2007. In situ dissection of a Polycomb response element in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 104: 12416–12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehling-Hampton K., Jackson P. D., Clark M. J., Brand A. H., Hoffmann F. M., 1994. Specificity of bone morphogenetic protein-related factors: cell fate and gene expression changes in Drosophila embryos induced by decapentaplegic but not 60A. Cell Growth Differ. 5: 585–593. [PubMed] [Google Scholar]
- Stevens M. E., Bryant P. J., 1985. Apparent genetic complexity generated by developmental thresholds: the apterous locus in Drosophila melanogaster. Genetics 110: 281–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G., Basler K., 1993. Organizing activity of wingless protein in Drosophila. Cell 72: 527–540. [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C., 1989. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma 98: 81–85. [DOI] [PubMed] [Google Scholar]
- Thibault S. T., Singer M. A., Miyazaki W. Y., Milash B., Dompe N. A., et al. , 2004. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat. Genet. 36: 283–287. [DOI] [PubMed] [Google Scholar]
- Thomason L., Court D. L., Bubunenko M., Costantino N., Wilson H., Datta S., Oppenheim A., 2007. Recombineering: Genetic engineering in bacteria using homologous recombination. Curr. Protoc. Mol. Biol. Chapter 1: Unit 1.16. [DOI] [PubMed] [Google Scholar]
- Tolhuis B., de Wit E., Muijrers I., Teunissen H., Talhout W., et al. , 2006. Genome-wide profiling of PRC1 and PRC2 Polycomb chromatin binding in Drosophila melanogaster. Nat. Genet. 38: 694–699. [DOI] [PubMed] [Google Scholar]
- Tsuneizumi K., Nakayama T., Christian J. L., Tabata T., 1997. Daughters against dpp modulates dpp organizing activity in Drosophila wing development. Nature 389: 627–631. [DOI] [PubMed] [Google Scholar]
- Venken K. J. T., Schulze K. L., Haelterman N. A., Pan H., He Y., et al. , 2011. MiMIC: a highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat. Methods 8: 737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier R., Springhorn A., Patterson L., Koidl S., Hammerschmidt M., et al. , 2010. Control of Dpp morphogen signalling by a secreted feedback regulator. Nat. Cell Biol. 12: 611–617. [DOI] [PubMed] [Google Scholar]
- Waddington C. H., 1940. The genetic control of wing development in Drosophila. J. Genet. 41: 75–139. [Google Scholar]
- Weiss A., Charbonnier E., Ellertsdóttir E., Tsirigos A., Wolf C., et al. , 2010. A conserved activation element in BMP signaling during Drosophila development. Nat. Struct. Mol. Biol. 17: 69–76. [DOI] [PubMed] [Google Scholar]
- Whittle J. R., 1979. Replacement of posterior by anterior structures in the Drosophila wing caused by the mutation apterous-blot. J. Embryol. Exp. Morphol. 53: 291–303. [PubMed] [Google Scholar]
- Wieschaus E., Gehring W., 1976. Clonal analysis of primordial disc cells in the early embryo of Drosophila melanogaster. Dev. Biol. 50: 249–263. [DOI] [PubMed] [Google Scholar]
- Wilanowski T. M., Gibson J. B., Symonds J. E., 1995. Retrotransposon insertion induces an isozyme of sn-glycerol-3-phosphate dehydrogenase in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 92: 12065–12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. A., Paddock S. W., Carroll S. B., 1993. Pattern formation in a secondary field: a hierarchy of regulatory genes subdivides the developing Drosophila wing disc into discrete subregions. Development 117: 571–584. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Paddock S. W., Vorwerk K., Carroll S. B., 1994. Organization of wing formation and induction of a wing-patterning gene at the dorsal/ventral compartment boundary. Nature 368: 299–305. [DOI] [PubMed] [Google Scholar]
- Wu C. T., Morris J. R., 1999. Transvection and other homology effects. Curr. Opin. Genet. Dev. 9: 237–246. [DOI] [PubMed] [Google Scholar]
- Zecca M., Struhl G., 2002a Subdivision of the Drosophila wing imaginal disc by EGFR-mediated signaling. Development 129: 1357–1368. [DOI] [PubMed] [Google Scholar]
- Zecca M., Struhl G., 2002b Control of growth and patterning of the Drosophila wing imaginal disc by EGFR-mediated signaling. Development 129: 1369–1376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.