Abstract
We evaluated the clinical feasibility of using drug-coated balloon (DCB) angioplasty in patients undergoing primary percutaneous coronary intervention (PPCI). Between January 2010 to September 2014, 89 ST-elevation myocardial infarction patients (83% male, mean age 59 ± 14 years) with a total of 89 coronary lesions were treated with DCB during PPCI. Clinical outcomes are reported at 30 d follow-up. Left anterior descending artery was the most common target vessel for PCI (37%). Twenty-eight percent of the patients had underlying diabetes mellitus. Mean left ventricular ejection fraction was 44% ± 11%. DCB-only PCI was the predominant approach (96%) with the remaining 4% of patients receiving bail-out stenting. Thrombolysis in Myocardial Infarction (TIMI) 3 flow was successfully restored in 98% of patients. An average of 1.2 ± 0.5 DCB were used per patient, with mean DCB diameter of 2.6 ± 0.5 mm and average length of 23.2 ± 10.2 mm. At 30-d follow-up, there were 4 deaths (4.5%). No patients experienced abrupt closure of the infarct-related artery and there was no reported target-lesion failure. Our preliminary experience showed that DCB angioplasty in PPCI was feasible and associated with a high rate of TIMI 3 flow and low 30-d ischaemic event.
Keywords: Acute myocardial infarction, Drug coated balloon, Efficacy, Primary angioplasty, Safety
Core tip: Primary percutaneous coronary intervention (PPCI) is the preferred reperfusion therapy for ST-elevation myocardial infarction (STEMI). Stent implantation is considered as a routine during PPCI as it is associated with reduction of ischaemic end-points. Drug-coated balloon (DCB) has emerged as a new therapeutic option to treat coronary artery disease as stent technology has certain limitations. There is however limited data on the feasibility of using DCB as primary therapy in PPCI. We evaluated the clinical safety and efficacy of using paclitaxel-coated balloon in patients undergoing PPCI for STEMI and report on our 30 d clinical outcomes.
INTRODUCTION
Primary percutaneous coronary intervention (PPCI) is the preferred reperfusion therapy for ST-elevation myocardial infarction (STEMI) if performed in a timely fashion[1]. Stent implantation[2-5] whether with bare metal stent (BMS) or drug-eluting stent (DES) is considered as a routine during PPCI as it is associated with reduction of early ischaemia, restenosis and re-occlusion of culprit artery in comparison with pure old balloon angioplasty (POBA).
Drug-coated balloon (DCB)[6-8] has emerged as a new therapeutic option to treat coronary artery disease (CAD) as stent technology has certain limitations. There is however limited data on the feasibility of using DCB as primary therapy in PPCI. Previous clinical studies[2-3] had shown no difference in the mortality rates between those who received stents or POBA during PPCI with the main difference driven largely by lower rate of target vessel revascularization (TVR) in the stenting group.
It is possible that DCB could close this gap for the POBA group and we therefore evaluated the clinical feasibility (i.e., safety and efficacy) of using paclitaxel-coated balloon in our cohort of South-East Asian patients undergoing PPCI for STEMI.
RESEARCH
Between January 2010 to September 2014, 89 STEMI patients with a total of 89 coronary lesions were treated with SeQuent Please DCB (B.Braun, Melsungen, Germany) as primary therapy during PPCI. The PPCI strategy was to perform thrombus aspiration followed by predilatation of the lesion site before treatment with DCB. Bail-out stenting was performed only when there was significant vessel recoil/coronary dissection. Clinical outcomes are reported at 30 d follow-up.
RESULTS
Table 1 shows the baseline clinical characteristics, angiographic features, procedural data and clinical outcomes of the study patients. The mean age of the patients at presentation was 59 ± 14 years with male preponderance (83%). Twenty-eight percent of the patients had underlying diabetes mellitus. Mean left ventricular ejection fraction was 44% ± 11%. The majority of patients presented with inferior STEMI (55%) with the left anterior descending artery (LAD) being the most common target vessel for PCI (37%) followed by right coronary artery (33%), left circumflex (13%) and others (17%).
Table 1.
Baseline clinical characteristics, angiographic features, procedural data and clinical outcomes of patients n (%)
| n = 89 | |
| Mean age (yr) | 59 ± 14 |
| Male: female | 74:15 (83:17) |
| Ever smokers | 50 (56) |
| Diabetes | 25 (28) |
| Hyperlipidemia | 41 (46) |
| Hypertension | 49 (55) |
| Previous myocardial infarction | 9 (10) |
| LVEF | 44% ± 11% |
| Presentation | |
| Anterior MI | 40 (45) |
| Inferior MI | 49 (55) |
| Target vessel | |
| LAD | 33 (37) |
| CIRC | 12 (13) |
| RCA | 29 (33) |
| Others | 15 (17) |
| Reference diameter, mm | 2.4 ± 0.4 |
| Thrombus aspiration | 50 (56) |
| Predilatation with non-coated balloon | 89 (100) |
| Glycoprotein 2b/3a inhibitors | 71 (80) |
| TIMI flow | |
| Post-procedural TIMI 2 flow | 2 (2) |
| Post-procedural TIMI 3 flow | 87 (98) |
| 30-d clinical outcomes | |
| Mortality | 4 (4.5) |
| Target vessel revascularisation | 0 (0) |
| Target vessel MI | 0 (0) |
| Target lesion thrombosis | 0 (0) |
LVEF: Left ventricular ejection fraction; MI: Myocardial infarction; LAD: Left anterior descending artery; CIRC: Left circumflex artery; RCA: Right coronary artery; TIMI: Thrombolysis in myocardial infarction.
Thrombus aspiration was performed in 50 patients (56%) with glycoprotein 2b/3a inhibitors administered in 71 patients (80%). Pre-procedural Thrombolysis in Myocardial Infarction (TIMI) flow was 0 in 70% of patients. At the end of PPCI, TIMI 3 flow was successfully restored in 98% of patients with residual stenosis of 29%.
DCB-only PCI was the predominant approach (96% of patients) with the remaining 4% of patients receiving bail-out stenting for significant recoil/dissection after treatment with DCB. An average of 1.2 ± 0.5 DCB were used per patient, with mean DCB diameter of 2.6 ± 0.5 mm and average length of 23.2 ± 10.2 mm. The mean inflation pressure for DCB was 10 ± 3 atm and mean inflation time was 54 ± 22 s.
At 30-d follow-up, there were 4 deaths (4.5%). Three patients succumbed due to cardiogenic shock and 1 died of sepsis. No patient experienced abrupt closure of the infarct-related artery (IRA) and there was no reported TVR, target-vessel-MI or target lesion thrombosis.
DISCUSSION
When compared with fibrinolytic therapy, PPCI in STEMI1 reduced the rates of death, reinfarction, and stroke. The use of POBA in PPCI has been superseded by routine stenting[2-3] in the contemporary era as the former approach was associated with recurrent ischemia, restenosis, and reocclusion of the IRA. However, prior studies so far had not shown any difference in the mortality rates between those who received stents or POBA during PPCI. The main difference is consistently a lower rate of TVR in the stenting group and it is possible that DCB could close this gap for selected group of patients in the POBA arm. From our preliminary experiences, we demonstrated that the use of DCB as primary therapy for STEMI patients in PPCI was feasible and associated with a high rate of final TIMI 3 flow and low 30-d major adverse cardiac event (MACE).
In recent years, DCB[6-8] has emerged as a viable therapeutic option for treating CAD as the current DES technology has limitations like late stent thrombosis and prolonged dual anti-platelet therapy. Paclitaxel is the drug of choice for all the commercially available DCBs because of its highly lipophilic properties which allows rapid diffusion into the vessel wall and sustained anti-proliferative effect despite its short contact with the vessel wall. The best long term results with DCB is achieved with a DCB-only approach when compared to DCB plus BMS as the former approach is associated with a lower late lumen loss and lower target vessel revascularization.
To gain the utmost benefit from DCB[9], adequate lesion preparation is necessary so that we can maximize balloon contact area to vessel wall for a minimum of 30 s. Similarly in PPCI, we advocate 2 key steps, i.e., thrombus aspiration (for visible thrombus) and predilatation with a non-coated balloon prior to DCB angioplasty as the final step (Figure 1). Removal of thrombus will enable DCB to have better contact with the vessel wall and initial predilatation of the lesion will also allow us to evaluate whether the patient can tolerate prolonged balloon inflation with DCB.
Figure 1.
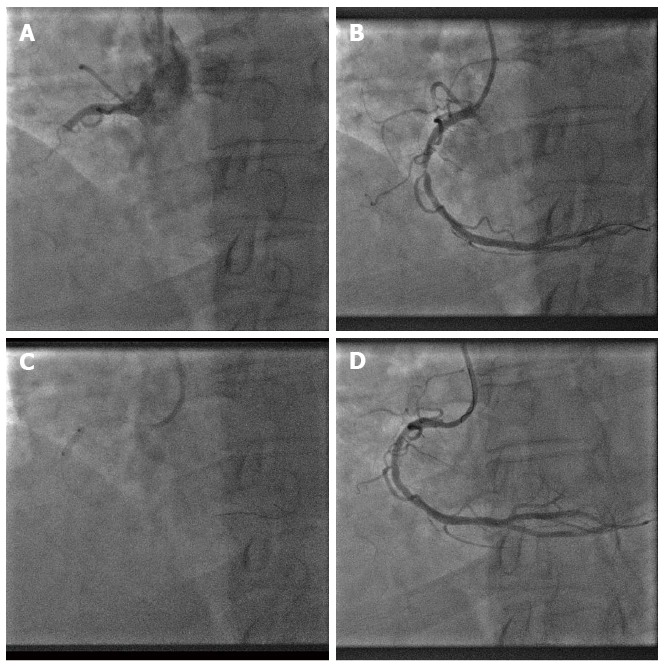
Thrombus aspiration (for visible thrombus) and predilatation with a non-coated balloon prior to drug-coated balloon angioplasty as the final step. A: Baseline coronary angiography showing acute thrombotic occlusion of mid right coronary artery (RCA); B: Mid RCA after thrombus aspiration; C: Predilatation of mid RCA with non-coated balloon; D: Final angiography of mid RCA (after DCB angioplasty).
Coronary dissection (iatrogenic) occurs inevitably as result of POBA and abrupt closure of vessel remains one of the most fearful complications. Having good knowledge on the different grades of coronary dissection according to the National Heart, Lung, and Blood Institute (NHLBI) classification[10], one can carefully select patients for DCB angioplasty during PPCI and in our study, no patients experienced abrupt closure of IRA. Only 4% of our patients required bailout stenting for significant recoil/dissection (> Type B dissection). The incidence of abrupt closure of IRA is also significantly reduced in the current era of more potent anti-platelet agents.
There were several limitations to our study. The number of patients is relatively small and our study was a single-center registry, subject to selection and operator bias. All patients in our study received treatment with SeQuent Please DCB and our results could only be extrapolated to those who had received similar therapy. Whether similar results would be seen with patients receiving other types of DCB is unknown as not all DCBs are equal in terms of clinical efficacy.
In conclusion, the use of DCB as primary therapy in PPCI represents a novel approach in treating STEMI patients. Our preliminary experiences were favourable ie a high rate of final TIMI 3 flow and low 30-d MACE. This approach is possible with appropriate patient selection and by performing 2 key preconditioning steps. Further studies with longer follow-up are necessary to confirm our preliminary findings.
Footnotes
Conflict-of-interest: The authors report no conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 8, 2015
First decision: January 20, 2015
Article in press: April 20, 2015
P- Reviewer: Lymperopoulos A, Ueda H S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
References
- 1.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 2.Grines CL, Cox DA, Stone GW, Garcia E, Mattos LA, Giambartolomei A, Brodie BR, Madonna O, Eijgelshoven M, Lansky AJ, et al. Coronary angioplasty with or without stent implantation for acute myocardial infarction. Stent Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med. 1999;341:1949–1956. doi: 10.1056/NEJM199912233412601. [DOI] [PubMed] [Google Scholar]
- 3.Stone GW, Grines CL, Cox DA, Garcia E, Tcheng JE, Griffin JJ, Guagliumi G, Stuckey T, Turco M, Carroll JD, et al. Comparison of angioplasty with stenting, with or without abciximab, in acute myocardial infarction. N Engl J Med. 2002;346:957–966. doi: 10.1056/NEJMoa013404. [DOI] [PubMed] [Google Scholar]
- 4.Stone GW, Lansky AJ, Pocock SJ, Gersh BJ, Dangas G, Wong SC, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, et al. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med. 2009;360:1946–1959. doi: 10.1056/NEJMoa0810116. [DOI] [PubMed] [Google Scholar]
- 5.Cox DA, Stone GW, Grines CL, Stuckey T, Cohen DJ, Tcheng JE, Garcia E, Guagliumi G, Iwaoka RS, Fahy M, et al. Outcomes of optimal or “stent-like”balloon angioplasty in acutemyocardial infarction: the CADILLAC trial. J Am Coll Cardiol. 2003;42:971–977. doi: 10.1016/s0735-1097(03)00911-2. [DOI] [PubMed] [Google Scholar]
- 6.Scheller B, Speck U, Abramjuk C, Bernhardt U, Böhm M, Nickenig G. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation. 2004;110:810–814. doi: 10.1161/01.CIR.0000138929.71660.E0. [DOI] [PubMed] [Google Scholar]
- 7.Wöhrle J, Zadura M, Möbius-Winkler S, Leschke M, Opitz C, Ahmed W, Barragan P, Simon JP, Cassel G, Scheller B. SeQuentPlease World Wide Registry: clinical results of SeQuent please paclitaxel-coated balloon angioplasty in a large-scale, prospective registry study. J Am Coll Cardiol. 2012;60:1733–1738. doi: 10.1016/j.jacc.2012.07.040. [DOI] [PubMed] [Google Scholar]
- 8.Ho HH, Ooi YW, Loh KK, Tan J, Aung TH, Jafary FH, Ong PJL. Clinical Efficacy and Safety of SeQuent Please Paclitaxel-Eluting Balloon in a Real-World Single-Center Registry of South-East Asian Patients. Int J Cardiol Heart Vessels. 2013;1:37–41. doi: 10.1016/j.ijchv.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleber FX, Mathey DG, Rittger H, Scheller B. How to use the drug-eluting balloon: recommendations by the German consensus group. EuroIntervention. 2011;7 Suppl K:K125–K128. doi: 10.4244/EIJV7SKA21. [DOI] [PubMed] [Google Scholar]
- 10.Huber MS, Mooney JF, Madison J, Mooney MR. Use of a morphologic classification to predict clinical outcome after dissection from coronary angioplasty. Am J Cardiol. 1991;68:467–471. doi: 10.1016/0002-9149(91)90780-o. [DOI] [PubMed] [Google Scholar]


