Abstract
Recent trials and metanalysis even not fully conclusive and still debated, at least suggested that mechanical device-based closure of patent foramen ovale (PFO) is more effective than medical therapy in prevent recurrence of stroke. In a proportion ranging from 20% to nearly 40% of patients in literature, PFO is associated to atrial septal aneurysm (ASA): ASA is a well-known entity often associated with additional fenestration. Additionally small atrial septal defects (“Flat ASD”) can present with signs of paradoxical embolism and cannot be easily detected by transthoracic echocardiography or even by transesophageal echocardiography and are usually discovered by intracardiac echocardiography at the moment of transcatheter closure. This evidence might change potentially the anatomical diagnosis from PFO to fenestrated ASA or as we called it to “hybrid defect”, being a bidirectional flow through a small ASD or/and an additional fenestration, often present. Despite the differences in anatomy, pathophysiology and haemodynamic paradoxical embolism may occur in both entities and also may be the first appearance of fenestrated ASA. Because some overlapping do really exist between PFO and hybrid defects, which are often not clearly differentiable by standard diagnostic tools, it is likely that a proportion of patients evaluated for potential transcatheter closure of PFO had actually a different anatomical substrate. These different anatomical and pathophysiologic entities have not been address in any of the previous trials, potentially having an impact on overall results despite the similar mechanical treatment. Neurologists and general cardiologists in charge of clinical management of PFO-related cryptogenic stroke should be aware of the role of hybrid defects in the pathophysiology of paradoxical embolism - mediated cerebral ischemic events in order to apply the correct decision - making process and avoid downgrading of patients with paradoxical embolism-related interatrial shunt variants different from PFO.
Keywords: Atrial septal defect, Patent foramen ovale, Echocardiography, Anatomy
Core tip: Recent trials and met analysis suggested that mechanical device-based closure of patent foramen ovale (PFO) is more effective than medical therapy in prevent recurrence of stroke. Fenestrated atrial septal aneurysms and small atrial septal defects (hybrid defects) can present with signs of paradoxical embolism and because they are often not clearly differentiable by standard diagnostic tools, it is likely that a proportion of patients evaluated for transcatheter closure of PFO, had actually a different anatomical substrate. These different anatomical entities have not been address in any of the previous trials, potentially having an impact on overall results.
INTRODUCTION
Recent trials and metanalysis and in particular, the RESPECT trial[1], even not fully conclusive and still debated, at least suggested that mechanical device-based closure of patent foramen ovale (PFO) is more effective in prevent recurrence of stroke by near 67%. When looking deeply into the number of different metanalysis of PFO closure for cryptogenic (or better paradoxical embolism mediated) stroke[2-7], it appears clear that in a proportion ranging from 20% to nearly 40% of patients PFO is associated to atrial septal aneurysm (ASA): ASA is a well-known entity often associated with additional fenestration[8]. Additionally small atrial septal defects can present with signs of paradoxical embolism and cannot be easily detected by transthoracic echocardiography or even by transesophageal echocardiography and are usually discovered by intracardiac echocardiography at the moment of transcatheter closure[9], confusing even more the diagnosis of PFO which is at the basis of all the studies about effectiveness of transcatheter closure in cryptogenic stroke. This evidence might change potentially the anatomical diagnosis from PFO to fenestrated ASA (or fenestrated secundum atrial septal defect) or to a so called “hybrid defect”, [a small single atrial septal defects (ASD) associated with paradoxical embolism], being a bidirectional flow through a small ASD or and additional fenestration often present. The aim of this review is to analyse conjunction points between PFOs and hybrid defects and outlined the role of these type of interatrial shunt in the pathophysiology of paradoxical embolism.
PRACTICAL EPIDEMIOLOGY
Isolated ASD represent 7% of all cardiac anomalies and can present at any age[10]. Adolescents and adults with isolated atrial septal defects are more likely to reach adult age without being diagnosed. Secundum ASD is by far the most common type, occurring in 1/1500 live births, with 65% to 75% involving females[10]. On the other hand, PFO represents an endemic variant in the normal population with a prevalence of 25%-27%[11]. These two entities appear so different that is difficult to find a conjunction ring: nevertheless we use the same philosophy for the treatment. Indeed, device - based closure has been proved to be effective[12,13] in both settings.
Anatomy, pathophysiology, and haemodynamic
From an anatomic and pathophysiologic point of view these two entities are absolutely different.
The ostium secundum ASD is a defect of the atrial septum (Figure 1) within the limit of the fossa ovalis and causes usually a left-to-right shunt, being the left atrial pressure higher than the right atrial one. The volume of and direction of flow through an ASD depend on the size of the hole and the relative diastolic filling properties of the left and right chambers. Reduced left ventricle compliance and mitral stenosis increase the left-to-right shunt, whereas reduced right ventricle compliance may decrease the left-to-right shunt or may cause a right-to-left shunt. A Qp/Qs ratio > 1.5:1 or dilation of the right chambers defined a left-to-right shunt as significant[14].
Figure 1.
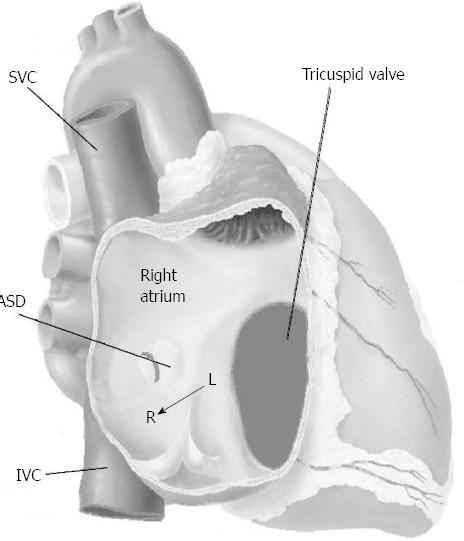
Secundum atrial septal defect is a defect located into the limit of the fossa ovalis. The shunt usually is left-to-right because of the pressure gradient between the left (high pressure) and right (low pressure) atrium. ASD: Atrial septal defect; IVC: Inferior vena cava; L: Left; R: Right; SVC: Superior vena cava.
The PFO is defined as the incompetence of the fossa ovale valve determining a right-to-left shunt (Figure 2). The reason because a right-to-left atrial shunting occurs with normal intracardiac pressures and normal or near-normal pulmonary function through a PFO has still not been completely clarified. An explanation may arise from some few considerations. Firstly, a physiologic transient spontaneous reversal of difference between the left and the right pressure is physiologically present during early diastole and during isovolumetric contraction of the right ventricle of each cardiac cycle; this right-to-left gradient may be sustained by physiologic manoeuvres that increase the right atrial pressure such as posture, inspiration, cough or Valsalva manoeuvre, or by situation in which pulmonary vascular resistances results increased, such as acute pulmonary embolism, hypoxemia due to obstructive sleep apnoea, severe chronic obstructive pulmonary disease, right ventricular infarction and positive end-expiratory pressure during neurosurgical procedures in the sitting position. Secondly, another theory explaining the right-to-left shunting through a PFO, is represented by the “so-called” “flow phenomenon”. It describes a preferential blood flow from the inferior vena cava towards the atrial septum as a part of the ancient foetal circulation pathway[15].
Figure 2.
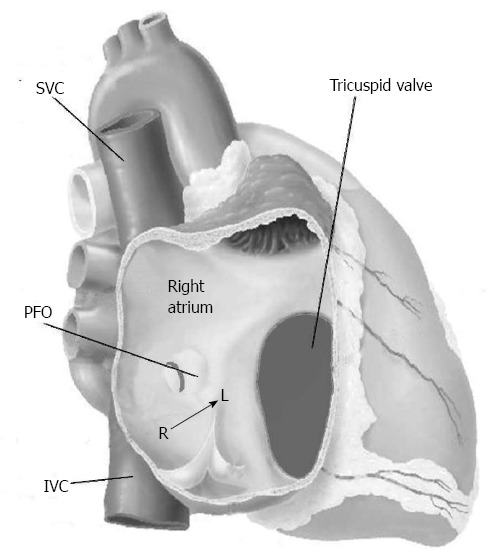
Patent foramen ovale is a communication between the right and left atrium caused by the incompetence of the fossa ovalis valve. The shunt is usually right-to-left despite the gradient pressure between the atria. IVC: Inferior vena cava; L: Left; PFO: Patent foramen ovale; R: Right; SVC: Superior vena cava.
Thirdly, the increasing stiffness of the right chambers compared to the left chambers caused by aging has been postulated. Finally, conditions such due to mediastinal shift or heart counter-clockwise rotation and/or distortion, following an ascending aorta enlargement, right pneumectomy or pericardial effusion may cause an anatomic disarray of the inferior vena cava relationship with the interatrial septum favouring part of the blood flow to enter the left atrium throughout a PFO[16].
Even from a haemodynamic point of view, ASD obviously differs from PFO. ASD are usually associated with pulmonary hypertension of different degree, an increased Qp/Qs ratio and enlarged right chambers, whereas the usual findings in PFO patients is a normal or slightly elevated pulmonary pressure, normal Qp/Qs ratio, and normal right chambers. Sometimes in presence of a PFO associated with large ASA, a mild impairment of the left atrial function can be observed[17].
Usually also fenestrated secundum ASD with or without ASA tends to present less right chambers enlargement and only slightly increase in mean pulmonary pressure compared to secundum ASD.
CONJUNCTION POINTS
Despite the gross differences in anatomy and haemodynamic, when we look to the clinical presentation and patho-physiology, we can find some contact points. Excepted for supraventricular arrhythmias and dyspnoea, usually present only in secundum single and fenestrated ASD, paradoxical embolism may occur in both entities and also may be the first appearance of fenestrated ASD with or without ASA.
Usually paradoxical embolism is associated with PFO but occasionally secundum ASD, pulmonary arterio-venous fistula, and other intracardiac septal defects may act as alternative pathophysiological mechanism. Microemboli from a vein thrombotic location, or as recently postulated[17] microthrombotic stratification on the surface of a huge ASA or in the left atrium itself as a result of a left atrial dysfunction induced by the PFO and ASA itself, may navigate to the left side of the circulation through the PFO causing different ischemic syndromes. Differently, the pathophysiology of paradoxical embolism through a secundum ASD is usually caused by a temporaneous right heart pressure increasing which induce a right-to-left shunting which allows a venous thromboembolus to enter the arterial circulation. As an alternative mechanism, Valsalva manoeuvre, coughing, or straining might increase right-to-left component of a bidirectional shunt inducing a paradoxical embolism in ASD patients, in particular in elderly patients, more prone to rapid change of right chambers pressure because of the increasing stiffness of the chambers.
Recently in an analysis of our institutional database we found 24 (6.2%) with a secundum ASD out of 386 patients evaluated for paradoxical embolism. Defects were multifenestrated in 41.6% (10/24). Single ASD (58.3%) had a “flat” elliptical shape with a major axis of 7.6 ± 2.4 and minimal axis of 2.5 ± 1.6 mm when assessed with intracardiac echocardiography. Patients with ASD-related paradoxical embolism had more frequently a deep venous thrombosis, bigger stroke areas compared to PFO patients, and massive curtain shunt on Valsalva maneuver on transcranial Doppler. When compared to non-emboligenous ASD, they had lower mean pulmonary pressure, lower mean Qp/Qs, and had bidirectional shunt at rest[18]. As matter of fact, flat elliptical shape ASD and fenestrated ASD with or without ASA appear to represent the conjunction ring between ASD and PFO, being a hybrid hemodynamic and clinical profile compared to each of the others (Figure 3).
Figure 3.
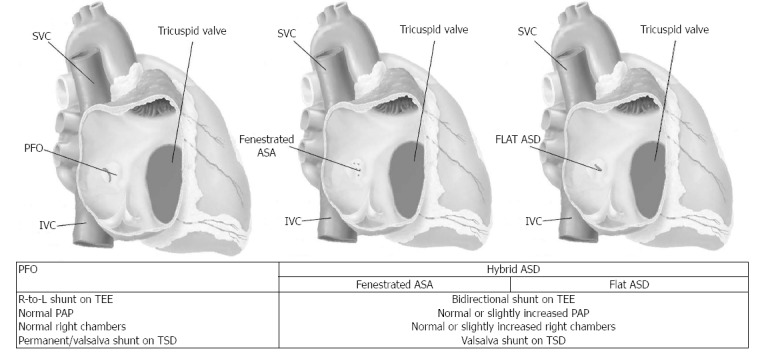
Spectrum of interatrial shunt associated with possible paradoxical embolism and potential related haemodynamic and functional characteristics. ASA: Atrial septal aneurysm; ASD: Atrial septal defect; FO: Fossa ovalis; IVC: Inferior vena cava; PAP: Pulmonary artery pressure; PFO: Patent foramen ovale; R-to-L: Right to left shunt; SVC: Superior vena cava; TCD: Transcranial doppler; TEE: Transesophageal echocardiography.
FINAL CONSIDERATIONS: ARE THE PAST TRIALS REALLY FOCUSED ON THE PROPER ANATOMICAL ENTITY?
Because some overlapping do really exist between PFO, fenestrated ASD and hybrid defects (Figure 1) which are not always clearly differentiable by standard diagnostic tools, it is likely that a proportion of patients evaluated for potential transcatheter closure of PFO had actually a different anatomical substrate. Past trials and case series used Transeophageal guidance in the majority of patients and the severity of the shunt and presence of permanent shunt has not been evaluated systematically by Transcranial Doppler or transesophageal echocardiography in the enrolment process.
Current modern judgement about medical or mechanical closure is suggested to be based, following the only published multidisciplinary consensus[19], on recurrent stroke or ischemic event with positive neuroimaging studies, severe shunt graded by transcranial Doppler and transesophageal echocardiography, presence of permanent shunt, and of additional anatomical features, such as ASA, tunnel-like opening, and Eustachian valve. The large and permanent shunt in particular, as previously suggested[18,20] is one of the most influent parameters, and it appears clear that in presence of an hybrid defect, it doesn’t play the same role as in true PFO, while ASA and Eustachian valve may be more influent facilitating paradoxical shunt through an hybrid defect or a fenestrated ASD when a Valsalva manoeuvre is provoked. These different anatomical and pathophysiologic pictures have not been address in any of the previous trials, potentially having an impact on overall results despite the similar mechanical treatment.
From a practical point of view, patients with deep vein thrombosis and more clinically relevant ischemic syndrome are more likely to have a hybrid defect, whereas patients with no deep vein thrombosis and mild symptomatology are more likely to have a PFO. An ideal screening of a patient with suspected paradoxical embolism should include not only the transthoracic echo, but also the transesophageal echo in order to differentiate between PFO and hybrid defects.
At the light of what we discussed above, neurologists and general cardiologists in charge of clinical management of PFO-related cryptogenic stroke should be aware of the role of hybrid defects and multi-fenestrated ASA in the pathophisiology of paradoxical embolism - mediated cerebral ischemic events in order to apply the correct decision -making process and avoid downgrading of patients with paradoxical embolism-related interatrial shunt variants different from PFO.
Footnotes
Conflict-of-interest: None of the authors has conflict of interest to declare.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 29, 2015
First decision: March 6, 2015
Article in press: April 20, 2015
P- Reviewer: Anan R, Chu D, Jankowski K, Kasai T S- Editor: Tian YL L- Editor: A E- Editor: Zhang DN
References
- 1.Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, Marks DS, Tirschwell DL. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092–1100. doi: 10.1056/NEJMoa1301440. [DOI] [PubMed] [Google Scholar]
- 2.Riaz IB, Dhoble A, Mizyed A, Hsu CH, Husnain M, Lee JZ, Lotun K, Lee KS. Transcatheter patent foramen ovale closure versus medical therapy for cryptogenic stroke: a meta-analysis of randomized clinical trials. BMC Cardiovasc Disord. 2013;13:116. doi: 10.1186/1471-2261-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan AR, Bin Abdulhak AA, Sheikh MA, Khan S, Erwin PJ, Tleyjeh I, Khuder S, Eltahawy EA. Device closure of patent foramen ovale versus medical therapy in cryptogenic stroke: a systematic review and meta-analysis. JACC Cardiovasc Interv. 2013;6:1316–1323. doi: 10.1016/j.jcin.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Udell JA, Opotowsky AR, Khairy P, Silversides CK, Gladstone DJ, O’Gara PT, Landzberg MJ. Patent foramen ovale closure vs medical therapy for stroke prevention: meta-analysis of randomized trials and review of heterogeneity in meta-analyses. Can J Cardiol. 2014;30:1216–1224. doi: 10.1016/j.cjca.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Pandit A, Aryal MR, Pandit AA, Jalota L, Kantharajpur S, Hakim FA, Lee HR. Amplatzer PFO occluder device may prevent recurrent stroke in patients with patent foramen ovale and cryptogenic stroke: a meta-analysis of randomised trials. Heart Lung Circ. 2014;23:303–308. doi: 10.1016/j.hlc.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Capodanno D, Milazzo G, Vitale L, Di Stefano D, Di Salvo M, Grasso C, Tamburino C. Updating the evidence on patent foramen ovale closure versus medical therapy in patients with cryptogenic stroke: a systematic review and comprehensive meta-analysis of 2,303 patients from three randomised trials and 2,231 patients from 11 observational studies. EuroIntervention. 2014;9:1342–1349. doi: 10.4244/EIJV9I11A225. [DOI] [PubMed] [Google Scholar]
- 7.Rigatelli G, Dell’Avvocata F, Vassiliev D, Daggubati R, Buch A, Nanjiundappa A, Giordan M, Oliva L, Adami D, Cardaioli P. Pathophysiology of paradoxical embolism: evaluation of the role of interatrial septum anatomy based on the intracardiac echocardiography assessment of patients with right-to-left shunting. Cardiol Young. 2015;25:47–55. doi: 10.1017/S1047951113001480. [DOI] [PubMed] [Google Scholar]
- 8.Rigatelli G, Dell’Avvocata F, Giordan M, Viceconte N, Osanna RA, Braggion G, Aggio S, Cardaioli P, Chen JP. Usefulness of intracardiac echocardiography with a mechanical probe for catheter-based interventions: a 10-year prospective registry. J Clin Ultrasound. 2014;42:534–543. doi: 10.1002/jcu.22177. [DOI] [PubMed] [Google Scholar]
- 9.Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–172. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 10.Rigatelli G, Rigatelli G. Congenital heart diseases in aged patients: clinical features, diagnosis, and therapeutic indications based on the analysis of a twenty five-year Medline search. Cardiol Rev. 2005;13:293–296. doi: 10.1097/01.crd.0000145928.08280.ef. [DOI] [PubMed] [Google Scholar]
- 11.Pickett CA, Villines TC, Ferguson MA, Hulten EA. Percutaneous closure versus medical therapy alone for cryptogenic stroke patients with a patent foramen ovale: meta-analysis of randomized controlled trials. Tex Heart Inst J. 2014;41:357–367. doi: 10.14503/THIJ-13-3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation. 2006;114:1645–1653. doi: 10.1161/CIRCULATIONAHA.105.592055. [DOI] [PubMed] [Google Scholar]
- 13.Zanchetta M, Rigatelli G, Ho SY. A mystery featuring right-to-left shunting despite normal intracardiac pressure. Chest. 2005;128:998–1002. doi: 10.1378/chest.128.2.998. [DOI] [PubMed] [Google Scholar]
- 14.Kilner PJ, Yang GZ, Wilkes AJ, Mohiaddin RH, Firmin DN, Yacoub MH. Asymmetric redirection of flow through the heart. Nature. 2000;404:759–761. doi: 10.1038/35008075. [DOI] [PubMed] [Google Scholar]
- 15.Rigatelli G, Dell’avvocata F, Cardaioli P, Ronco F, Giordan M, Braggion G, Aggio S, Chinaglia M, Cheng JP, Nanjundappa A. Left atrial dysfunction in patients with patent foramen ovale and atrial septal aneurysm scheduled for transcatheter closure may play a role in aura genesis. J Interv Cardiol. 2010;23:370–376. doi: 10.1111/j.1540-8183.2010.00563.x. [DOI] [PubMed] [Google Scholar]
- 16.Zanchetta M, Rigatelli G, Pedon L, Zennaro M, Carrozza A, Onorato E. Catheter closure of perforated secundum atrial septal defect under intracardiac echocardiographic guidance using a single amplatzer device: feasibility of a new method. J Invasive Cardiol. 2005;17:262–265. [PubMed] [Google Scholar]
- 17.Rigatelli G, Dell’avvocata F, Daggubati R, Dung HT, Nghia NT, Nanjiundappa A, Giordan M, Cardaioli P. Impact of interatrial septum anatomic features on short- and long-term outcomes after transcatheter closure of patent foramen ovale: single device type versus anatomic-driven device selection strategy. J Interv Cardiol. 2013;26:392–398. doi: 10.1111/joic.12048. [DOI] [PubMed] [Google Scholar]
- 18.Rigatelli G, Dell’avvocata F, Tarantini G, Giordan M, Cardaioli P, Nguyen T. Clinical, hemodynamic, and intracardiac echocardiographic characteristics of secundum atrial septal defects-related paradoxical embolism in adulthood. J Interv Cardiol. 2014;27:542–547. doi: 10.1111/joic.12159. [DOI] [PubMed] [Google Scholar]
- 19.Pristipino C, Anzola GP, Ballerini L, Bartorelli A, Cecconi M, Chessa M, Donti A, Gaspardone A, Neri G, Onorato E, et al. Management of patients with patent foramen ovale and cryptogenic stroke: a collaborative, multidisciplinary, position paper: executive summary. Catheter Cardiovasc Interv. 2013;82:122–129. doi: 10.1002/ccd.24693. [DOI] [PubMed] [Google Scholar]
- 20.Xu WH, Xing YQ, Yan ZR, Jiang JD, Gao S. Cardiac right-to-left shunt subtypes in Chinese patients with cryptogenic strokes: a multicenter case-control study. Eur J Neurol. 2014;21:525–528. doi: 10.1111/ene.12351. [DOI] [PubMed] [Google Scholar]


