Abstract
Diabetes mellitus (DM) is a highly prevalent condition affecting about 347 million people worldwide. In addition to its numerous clinical implications, DM also exerts a negative effect on patient’s sleep quality. Impaired sleep quality disrupts the adequate glycemic control regarded as corner stone in DM management and also lead to many deleterious effects causing a profound impact on health related quality of life. This article outlines various factors leading to impaired sleep quality among diabetics and delineates how individual factor influences sleep. The article also discusses potential interventions and lifestyle changes to promote healthy sleep among diabetics.
Keywords: Diabetes mellitus, Sleep quality, Quality of life, Obstructive sleep apnea, Nocturnal hypoglycemia
Core tip: Diabetes mellitus (DM) is one of the common chronic medical conditions affecting approximately 347 million people worldwide. Studies have shown that up to one third of patients with DM suffer from concomitant sleep disorder. Factors associated with disrupted sleep among diabetic patient include nocturia, nocturnal hypoglycemia, peripheral neuropathy, restless leg syndrome and sleep disordered breathing. These conditions, when associated with diabetes can cause fragmented sleep and poor quality of life. It is imperative for the primary care physicians and health care providers to address this important issue among the diabetic patients during their visit.
INTRODUCTION
Sleep is a prerequisite for healthy functioning of human mind and body. It is generated based on a circadian rhythm and homeostatic pressure that follows a period of wakefulness. Poor or impaired sleep has not only being associated with various diseases and conditions but also led to poor performance and occupational accidents. Medical literature regarding sleep sciences reveals numerous studies that characterize sleep quality and determine various factors leading to impaired sleep among different patient populations[1-6]. Based on findings from these studies, it is inferred that in addition to many behavioral factors including poor sleep habits and hygiene, various pathological conditions have been recognized that adversely affect the sleep. Most notable among these conditions are obstructive sleep apnea, chronic insomnia and restless legs syndrome.
Diabetes mellitus (DM) is being increasingly recognized as a significant health burden. Based on recent data published by World Health Organization (WHO), it is estimated that approximately 347 million people are suffering from diabetes worldwide, 90% of who have type 2 diabetes. Patients with DM, by virtue of its numerous clinical and associated implications, suffer a poor quality of life[7,8]. It is not surprising that sleep quality among these patients are significantly impaired. Patients with DM can experience challenges to their sleep and wakefulness due to physiological imbalance and co-morbid sleep pathologies. In this review we attempted to present several factors, which directly affect the sleep quality among patients with DM (Figure 1).
Figure 1.
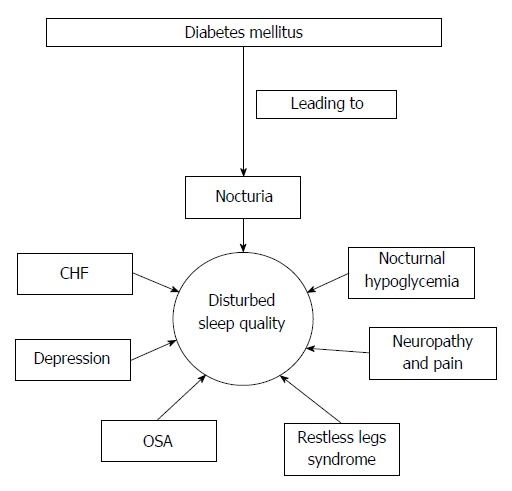
Illustration how different conditions associated with diabetes mellitus affects the sleep quality. OSA: Obstructive sleep apnea; CHF: Congestive heart failure.
DIABETES AND SLEEP
The significance of good sleep cannot be over emphasized when it comes to chronic medicinal condition like DM. Poor sleep quality, apart from its usual effect of daytime sleepiness, has ramifications that affect every aspects of life. The pertinent ones are: exacerbation of seizures, short-term memory deficits, long-term cognitive effects and headache. These, when combine to “already worsened” quality of life in patients with chronic diseases, can have several deleterious consequences in an individual’s life.
Clinical research has shown that up to one third of patients with DM suffered from concomitant sleep disorders, as compared with 8.2% of controls without DM[9]. In another study, more than half of the patients with type 2 DM are likely to report being “poor sleepers”, according to a research poll conducted at University of Pittsburgh. The patients with type 2 DM were more likely to have low Pittsburgh Sleep Quality index (PSQI)[10]. (PSQI is a validated tool which measures sleep quality and pattern in older adults. It discriminate poor sleepers from normal by assessing seven components of sleep over a one month time interval)[10]. Part of the components of these index’s metrics are common insomnia variables, such as sleep latency and efficiency. The same study also showed that the sleep quality correlated well with other diabetic quality of life scores[11]. In general, patients with other chronic medical conditions are also more likely to experience insomnia. In a large community-base sample of 3282 adults, polysomnographic data was analyzed along with self-reported sleep habits and current health. The adjusted odds ratio for insomnia was 1.4 in patients with DM, compared with people without the disorder. Even when data obtained on polysomnography was adjusted for age, gender and apnea-hypopnea index, patients with diabetes mellitus and insomnia had lower sleep efficiency than those without the disease[12]. Sleep deprivation and sleep fragmentation has been shown to correlate with insulin resistance in obese individuals[13]. Poor sleep quality has implications on diabetes self-management. Adult diabetic patients, who reported poor quality sleep underwent completion of questionnaires measuring diabetes control, sleep quality and daytime sleepiness. The patients with poor sleep were found to have difficulty with diabetes control, other demographic factors such as education, age, gender, were measured and these were not found to be associated with diabetes control problems[14]. Studies have linked poor sleep and insomnia to be associated with decrease in gamma-aminobutyric acid (GABA). A lower level of GABA is also seen in the patients with depression[15]. GABA is produced in significant levels in pancreas. It has also been shown to be inhibiting apoptosis of the rodent beta cells. The glutamate decarboxylase the primary enzyme (GAD) involved in the synthesis of GABA has been linked with type 1 DM[16,17]. It is possible that GABA is one of the neurotransmitter involved in the sleep quality among diabetics, when in low levels. Orexins also has been associated with sleep, arousal, energy balance and feeding. Orexins have been implicated in the metabolism of glucose. Its expression is inhibited by obesity, obstructive sleep apnea and depression[18]. The pathogenetic link between sleep deprivation and DM has been further elucidated in a recent cross over study involving fourteen healthy subjects who underwent 4 h sleep restriction. This sub-chronic sleep restriction led to a reduction in peripheral insulin sensitivity but interestingly the hepatic insulin sensitivity did not decrease. There was no significant reduction in slow wave sleep associated with it. There was a modest increase in cortisol and catecholamines. This increases lipolysis. Fasting non-esterified fatty acids and β-OH butyrate levels were elevated indicating an increase in lipolysis and hepatic fatty acid oxidation. The resting energy expenditure was unchanged but the respiratory quotient decreased consistent with fat oxidation. The elevated non-esterified fatty acids may modulate hepatic metabolism. Thus the insulin resistance is driven by extrahepatic tissue[19].
NOCTURIA
Nocturia is defined by waking up at night to void and is considered clinically meaningful if it occurs two or more times per night. Patients report nocturia as a leading cause of sleep disturbance, affecting both sleep onset and maintenance[20]. Polyuria, defined as 24-h urine output of more than 2800 mL (or above 40 mL/kg per 24 h)[21]. Polyuria may cause nocturia through generally increased urine production wherein nocturnal urine output exceeds functional bladder capacity (FBC). However, polyuria and nocturnal polyuria are not mutually inclusive.
Nocturia in persons with DM can occur in association with polyuria, and the mechanism is solute diuresis, but can also be associated with frequently co-morbid sleep-breathing disorder, such as obstructive sleep apnea. It has been postulated that in patients with obstructive sleep apnea, the negative intra-thoracic pressure and stretching of the myocardium releases atrial natriuretic peptide (ANP). This, in turn, causes vasodilatation and inhibits aldosterone resulting in excess sodium and water excretion. When a sleep disorder is suspected, for example in patients with complaints of snoring, gasping for breath when sleeping, or with daytime sleepiness, a nocturnal polysomnography should be performed[22]. In a study conducted in Australia with 74 patients with type 2 DM, nocturia was correlated with sleep maintenance difficulties[23].
NOCTURNAL HYPOGLYCEMIA
Nocturnal hypoglycemia can lead to sleep disruption and can be regarded as one of the many factors leading to poor sleep quality among diabetics. Longer intervals between self-monitoring glycemia are generally seen during the night and this period is associated with the highest sensitivity to insulin. Studies observing continuous glucose monitoring have shown that in patients with diabetes mellitus type 1 spent on average of 2.3 h per day with glucose levels below 70 mg/dL, and most of the hypoglycemic values occur at night[24]. These findings were corroborated by another study with same principles of continuous monitoring, showing that in more than half of the nights studied, nocturnal hypoglycemia occurred[25]. Moreover, various insulin regimens can also predispose a diabetic patient to nocturnal hypoglycemia. In a study comparing bedtime insulin NPH (neutral protamine hagedorn) and bedtime insulin glargine, the incidence of nocturnal hypoglycemia was statistically significant with bedtime insulin NPH (24% vs 9.9%)[26].
RESTLESS LEGS SYNDROME
Restless legs syndrome is a sensorimotor-related sleep disorder and can be either primary or secondary, when related to other conditions such as anemia, pregnancy, or end-stage renal disease, among others. The International Restless Syndrome Study Group (IRLSSG) has published four essential criteria to diagnose patient with restless leg syndrome. It includes: Presence of an urge to move the legs the legs accompanied by an unpleasant sensation (creeping, crawling, painful, itching or jittering feeling in the legs), relieved by movement, aggravated by rest (i.e., when patients lie down to attempt to fall asleep) and worsening of symptoms in the evening or at night[27]. Other body parts such as the upper extremities can also be involved. Females suffer from RLS twice as much as their male counterparts across all different populations and ages[28]. There is evidence linking an increased risk for RLS in patients with diabetes mellitus[29,30]. Several diabetic patients with RLS also suffer from peripheral neuropathy[29]. Diabetic patients who suffer from RLS are more likely to report worse sleep quality, have longer sleep latency and worse sleep efficiency, and experience more daytime dysfunction compared with diabetic controls without RLS[31]. RLS can be treated with non-pharmacologic methods which include implementing lifestyle changes such as regular physical activity, restriction on all caffeinated food and drinks, using pneumatic compression devices applied to the thigh and leg during sleep, massage therapy, soaking the legs in warm water[32]. Pharmacotherapy can be achieved with dopamine agonists, such as pramipexole and ropinirole, anticonvulsants, such as gabapentin, opiates, benzodiazepines, minerals and vitamin supplementation, particularly oral iron in patients with low serum ferritin levels below 50 μg/L[32]. Several pharmacological agents have been implicated in worsening symptoms of RLS. Selective serotonin receptor inhibitors, antihistamines, dopamine agonists and sympathomimetic agents are common drug associated with RLS[33]. These include antihistamines, dopamine antagonist, sympathomimetic agents, caffeine, nicotine and alcohol[33]. Attempt should be made in avoiding the medications, which can worsen the RLS, and in turn impair the sleep quality in those patients.
SLEEP DISORDERED BREATHING
Obstructive sleep apnea (OSA) is a complex disorder characterized by recurrent episodes of pharyngeal obstruction during sleep, leading to repeated episodes of intermittent hypoxia, arousals from sleep, which in turn can be lead to sleep fragmentation, decreased total sleep time and daytime hypersomnolence[34]. Obesity is a risk factor for both obstructive sleep apnea and insulin resistance. Foster at al found that in a population comprising patients with obesity and type 2 DM in whom the impact of lifestyle changes were being studied, obstructive sleep apnea was highly prevalent, with only 13.4% of patients not having some degree of OSA on a home-based portable monitoring sleep test. Among obese adults with type 2 diabetes, half the patients had moderate or severe OSA (apnea-hypopnea index above 15 events per hour)[35]. The interactions between obstructive sleep apnea and insulin resistance and glucose intolerance mellitus are being investigated. It has been postulated that there could be a causal effect for the development of insulin resistance in patients with chronic intermittent hypoxia (as it occurs during episodes of obstructive apneas and hypopneas). Possible mechanisms implicated include higher levels of cytokines in patients with OSA (plasma IL-6 and TNF-α), increase in sympathetic neural traffic and the release of gluco-regulatory neuroendocrine hormones such as cortisol[36]. In a murine model of obese animals, chronic intermittent hypoxemia exacerbated fasting hyperglycemia, glucose intolerance and insulin resistance[37]. A more recent study of patients with poorly controlled DM (HbA1C ≥ 7%) revealed that intermittent hypoxia which is a consequence of sleep apnea, is frequent in these patients (37.2%). Furthermore, it was strongly associated with poor glycemic control (OR: 2.31, 95%CI: 1.06-5.04)[38]. However, it is still unclear whether OSA may lead to the development of DM over time and more studies are needed to evaluate this possible causal relationship.
DIABETES AND HEART FAILURE
The lifetime risk of cardiovascular diseases in individuals with DM is high, ranging from 55%-79%. The risk is further accentuated with increasing adiposity[39]. In a study conducted by Nicholas GA and colleagues, it was estimated that 11.8% subjects with baseline diabetes developed heart failure compared 7.7% without DM[40]. Diabetic patients developing heart failure experiences disturbed sleep by several mechanisms including: Sleep disordered breathing Cheyne Stokes breathing and Obstructive sleep apnea, effects of medicines, symptom of heart failure (dyspnea, orthopnea and paroxysmal nocturnal dyspnea) and health problems including pain and depression[41].
The treatment of impaired sleep quality and sleep fragmentation should involve the education of the patients regarding the sleep hygiene techniques, behavioral modification, cognitive behavioral therapy and pharmacological therapies. The detail discussion of which is beyond the scope of this manuscript. In addition the specific etiologies, which are specific for the sleep fragmentation, poor sleep quality and insomnia among diabetics as nocturia, pain related to diabetic neuropathy, restless leg syndrome, obstructive sleep apnea, heart failure and nocturnal hypoglycemia should be addressed and treated.
CONCLUSION
DM is one of the most common diseases worldwide. DM, in addition to causing direct sleep disturbances as a result of nocturia, polyuria, diabetic neuropathy and neuropathy pain, has also been associated with several chronic illness as obstructive sleep apnea, cardiovascular complications, hypertension, cerebrovascular accidents and depression which can impair sleep and quality of life. The patient may not bring the sleep issues during their visit to healthcare providers, with acute issues taking precedence during their visit. It is important for the health care providers treating the patient with DM to address their sleep issues and the impaired quality of life due to inadequate and fragmented sleep, as it may be severely affect their recovery, control of diabetes as well as quality of life. Sleep education should also be considered an essential part in the diabetic management armamentarium.
Footnotes
Conflict-of-interest: None of the authors have any conflict to disclose.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 2, 2014
First decision: February 7, 2015
Article in press: May 18, 2015
P- Reviewer: Cheng JT S- Editor: Tian YL L- Editor: A E- Editor: Wang CH
References
- 1.Bonsen T, Wijnstok NJ, Hoekstra T, Eringa EC, Serné EH, Smulders YM, Twisk JW. Sleep quality and duration are related to microvascular function: the Amsterdam Growth and Health Longitudinal Study. J Sleep Res. 2015;24:140–147. doi: 10.1111/jsr.12256. [DOI] [PubMed] [Google Scholar]
- 2.Lauderdale DS, Philip Schumm L, Kurina LM, McClintock M, Thisted RA, Chen JH, Waite L. Assessment of sleep in the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2014;69 Suppl 2:S125–S133. doi: 10.1093/geronb/gbu092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Sullivan M, Rahim M, Hall C. The prevalence and management of poor sleep quality in a secondary care mental health population. J Clin Sleep Med. 2015;11:111–116. doi: 10.5664/jcsm.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Surani S, Murphy J, Shah A. Sleepy nurses: are we willing to accept the challenge today? Nurs Adm Q. 2007;31:146–151. doi: 10.1097/01.NAQ.0000264863.94958.40. [DOI] [PubMed] [Google Scholar]
- 5.Surani S, Subramanian S, Aguillar R, Ahmed M, Varon J. Sleepiness in medical residents: impact of mandated reduction in work hours. Sleep Med. 2007;8:90–93. doi: 10.1016/j.sleep.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Surani S, Subramanian S, Babbar H, Murphy J, Aguillar R. Sleepiness in critical care nurses: results of a pilot study. J Hosp Med. 2008;3:200–205. doi: 10.1002/jhm.307. [DOI] [PubMed] [Google Scholar]
- 7.Kara B, Kılıç Ö. Predictors of poor sleep quality and excessive daytime sleepiness in Turkish adults with type 2 diabetes. J Clin Nurs. 2015;24:1436–1439. doi: 10.1111/jocn.12710. [DOI] [PubMed] [Google Scholar]
- 8.Ozturk ZA, Yesil Y, Kuyumcu ME, Savas E, Uygun O, Sayiner ZA, Kepekci Y. Association of depression and sleep quality with complications of type 2 diabetes in geriatric patients. Aging Clin Exp Res. 2014:Epub ahead of print. doi: 10.1007/s40520-014-0293-0. [DOI] [PubMed] [Google Scholar]
- 9.Sridhar GR, Madhu K. Prevalence of sleep disturbances in diabetes mellitus. Diabetes Res Clin Pract. 1994;23:183–186. doi: 10.1016/0168-8227(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 10.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 11.Luyster FS, Dunbar-Jacob J. Sleep quality and quality of life in adults with type 2 diabetes. Diabetes Educ. 2011;37:347–355. doi: 10.1177/0145721711400663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budhiraja R, Roth T, Hudgel DW, Budhiraja P, Drake CL. Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders. Sleep. 2011;34:859–867. doi: 10.5665/SLEEP.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cizza G, Piaggi P, Lucassen EA, de Jonge L, Walter M, Mattingly MS, Kalish H, Csako G, Rother KI. Obstructive sleep apnea is a predictor of abnormal glucose metabolism in chronically sleep deprived obese adults. PLoS One. 2013;8:e65400. doi: 10.1371/journal.pone.0065400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chasens ER, Korytkowski M, Sereika SM, Burke LE. Effect of poor sleep quality and excessive daytime sleepiness on factors associated with diabetes self-management. Diabetes Educ. 2013;39:74–82. doi: 10.1177/0145721712467683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amihăesei IC, Mungiu OC. Main neuroendocrine features and therapy in primary sleep troubles. Rev Med Chir Soc Med Nat Iasi. 2012;116:862–866. [PubMed] [Google Scholar]
- 16.Sorenson RL, Garry DG, Brelje TC. Structural and functional considerations of GABA in islets of Langerhans. Beta-cells and nerves. Diabetes. 1991;40:1365–1374. doi: 10.2337/diab.40.11.1365. [DOI] [PubMed] [Google Scholar]
- 17.Tian J, Dang H, Chen Z, Guan A, Jin Y, Atkinson MA, Kaufman DL. γ-Aminobutyric acid regulates both the survival and replication of human β-cells. Diabetes. 2013;62:3760–3765. doi: 10.2337/db13-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adeghate E. Orexins: tissue localization, functions, and its relation to insulin secretion and diabetes mellitus. Vitam Horm. 2012;89:111–133. doi: 10.1016/B978-0-12-394623-2.00007-X. [DOI] [PubMed] [Google Scholar]
- 19.Rao MN, Neylan TC, Grunfeld C, Mulligan K, Schambelan M, Schwarz JM. Subchronic sleep restriction causes tissue-specific insulin resistance. J Clin Endocrinol Metab. 2015;100:1664–1671. doi: 10.1210/jc.2014-3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Middelkoop HA, Smilde-van den Doel DA, Neven AK, Kamphuisen HA, Springer CP. Subjective sleep characteristics of 1,485 males and females aged 50-93: effects of sex and age, and factors related to self-evaluated quality of sleep. J Gerontol A Biol Sci Med Sci. 1996;51:M108–M115. doi: 10.1093/gerona/51a.3.m108. [DOI] [PubMed] [Google Scholar]
- 21.Van Kerrebroeck P. Standardization of terminology in nocturia: commentary on the ICS report. BJU Int. 2002;90 Suppl 3:16–17. doi: 10.1046/j.1464-410x.90.s3.4.x. [DOI] [PubMed] [Google Scholar]
- 22.Weiss JP, Blaivas JG. Nocturnal polyuria versus overactive bladder in nocturia. Urology. 2002;60:28–32; discussion 32. doi: 10.1016/s0090-4295(02)01789-2. [DOI] [PubMed] [Google Scholar]
- 23.Lamond N, Tiggemann M, Dawson D. Factors predicting sleep disruption in Type II diabetes. Sleep. 2000;23:415–416. [PubMed] [Google Scholar]
- 24.Bode BW, Schwartz S, Stubbs HA, Block JE. Glycemic characteristics in continuously monitored patients with type 1 and type 2 diabetes: normative values. Diabetes Care. 2005;28:2361–2366. doi: 10.2337/diacare.28.10.2361. [DOI] [PubMed] [Google Scholar]
- 25.Raju B, Arbelaez AM, Breckenridge SM, Cryer PE. Nocturnal hypoglycemia in type 1 diabetes: an assessment of preventive bedtime treatments. J Clin Endocrinol Metab. 2006;91:2087–2092. doi: 10.1210/jc.2005-2798. [DOI] [PubMed] [Google Scholar]
- 26.Yki-Järvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130–1136. doi: 10.2337/diacare.23.8.1130. [DOI] [PubMed] [Google Scholar]
- 27.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 28.Benes H, Walters AS, Allen RP, Hening WA, Kohnen R. Definition of restless legs syndrome, how to diagnose it, and how to differentiate it from RLS mimics. Mov Disord. 2007;22 Suppl 18:S401–S408. doi: 10.1002/mds.21604. [DOI] [PubMed] [Google Scholar]
- 29.Lopes LA, Lins Cde M, Adeodato VG, Quental DP, de Bruin PF, Montenegro RM, de Bruin VM. Restless legs syndrome and quality of sleep in type 2 diabetes. Diabetes Care. 2005;28:2633–2636. doi: 10.2337/diacare.28.11.2633. [DOI] [PubMed] [Google Scholar]
- 30.Greco D, Gambina F, Pisciotta M, Abrignani M, Maggio F. Clinical characteristics and associated comorbidities in diabetic patients with restless legs syndrome. Exp Clin Endocrinol Diabetes. 2009;117:496–499. doi: 10.1055/s-0029-1220739. [DOI] [PubMed] [Google Scholar]
- 31.Cuellar NG, Ratcliffe SJ. A comparison of glycemic control, sleep, fatigue, and depression in type 2 diabetes with and without restless legs syndrome. J Clin Sleep Med. 2008;4:50–56. [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh P, Walters AS, Tsuang JW. Restless legs syndrome: a comprehensive overview on its epidemiology, risk factors, and treatment. Sleep Breath. 2012;16:987–1007. doi: 10.1007/s11325-011-0606-x. [DOI] [PubMed] [Google Scholar]
- 33.Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16:283–295. doi: 10.1016/j.smrv.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balachandran JS, Patel SR. In the clinic. Obstructive sleep apnea. Ann Intern Med. 2014;161:ITC1–I15; quiz ITC16. doi: 10.7326/0003-4819-161-9-201411040-01005. [DOI] [PubMed] [Google Scholar]
- 35.Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB, Wadden TA, Kelley D, Wing RR, Sunyer FX, et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32:1017–1019. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stamatakis K, Sanders MH, Caffo B, Resnick HE, Gottlieb DJ, Mehra R, Punjabi NM. Fasting glycemia in sleep disordered breathing: lowering the threshold on oxyhemoglobin desaturation. Sleep. 2008;31:1018–1024. [PMC free article] [PubMed] [Google Scholar]
- 37.Drager LF, Li J, Reinke C, Bevans-Fonti S, Jun JC, Polotsky VY. Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity. Obesity (Silver Spring) 2011;19:2167–2174. doi: 10.1038/oby.2011.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torrella M, Castells I, Gimenez-Perez G, Recasens A, Miquel M, Simo O, Barbeta E, Sampol G. Intermittent hypoxia is an independent marker of poorer glycaemic control in patients with uncontrolled type 2 diabetes. Diabetes Metab. 2015;pii:S1262–3636(15)00004-X. doi: 10.1016/j.diabet.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Fox CS, Pencina MJ, Wilson PW, Paynter NP, Vasan RS, D’Agostino RB. Lifetime risk of cardiovascular disease among individuals with and without diabetes stratified by obesity status in the Framingham heart study. Diabetes Care. 2008;31:1582–1584. doi: 10.2337/dc08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nichols GA, Hillier TA, Erbey JR, Brown JB. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care. 2001;24:1614–1619. doi: 10.2337/diacare.24.9.1614. [DOI] [PubMed] [Google Scholar]
- 41.Redeker NS. Sleep disturbance in people with heart failure: implications for self-care. J Cardiovasc Nurs. 2008;23:231–238. doi: 10.1097/01.JCN.0000305094.20012.76. [DOI] [PubMed] [Google Scholar]


