Abstract
Purpose
Priapism is a vasculopathy occurring in approximately 40% of patients with SCD. Mouse models have suggested that dysregulated NOS and RhoA/ROCK signaling as well as increased oxidative stress may contribute to mechanisms of SCD-associated priapism. We examined changes in protein expressions of NOS and ROCK signaling pathways and a source of oxidative stress, NADPH oxidase, in penile erectile tissue from patients with priapism histories, etiologically related and unrelated to SCD.
Materials and Methods
Human penile erectile tissue was obtained from patients with SCD-associated priapism (SCD, n=5) and priapism of other etiologies (non-SCD, n=6) during non-emergent penile prosthesis surgery for ED or priapism management and urethroplasty, and from control patients without priapism histories (Control, n=5) during penectomy for penile cancer. Samples were collected, immediately placed in cold buffer and then frozen in liquid nitrogen. Expressions of PDE5, eNOS, nNOS, iNOS, RhoA, ROCK1, ROCK2, p47phox, p67phox, gp91phox and β-actin were determined by Western blot analysis and NO amount was measured using the Griess reaction.
Results
In the SCD group, PDE5 (p<0.05), eNOS (p<0.01) and RhoA (p<0.01) expressions were significantly decreased while gp91phox (p<0.05) expression was significantly increased compared to Control group values. In the non-SCD group, eNOS (p<0.05), ROCK1 (p<0.05) and p47phox (p<0.05) expressions were significantly decreased compared to Control group values. Total NO levels were not significantly different across study groups.
Conclusions
The mechanisms of SCD-associated priapism in the human penis may involve dysfunctional NOS and ROCK signaling and increased oxidative stress associated with NADPH oxidase-mediated signaling.
Keywords: Nitric oxide, PDE5, penis, Rho signaling, NADPH oxidase
INTRODUCTION
SCD is a genetic hematologic disorder, in which abnormal sickle hemoglobin causes erythrocytes to form into sickle shapes and become rigid leading to vaso-occlusive manifestations including tissue hypoxia and ischemia 1, 2. SCD also manifests features of vascular dysfunction such as defects in NO bioavailability, unbalanced vasosensitivity, and elevated oxidative stress 3.
Patients with SCD may present with various urological complications including erectile disorders such as priapism. Priapism is a prolonged, commonly painful erection condition, devoid of sexual purpose, with major episodes lasting longer than four hours 4. Ischemic priapism is the most common form of priapism in which blood flow in the corpora cavernosa is absent, and if unresolved, commonly results in irreversible erectile tissue necrosis/fibrosis and ED 4, 5. SCD patients are likely to develop ischemic priapism with prevalence rates of approximately 30–45% 6, 7.
The pathogenesis of SCD-associated priapism has not been completely defined. New science in the field of priapism research has suggested that the etiopathogenesis of priapism represents more than misshapened erythrocytes causing obstruction of penile venous outflow, as previously thought. The major new insight is that the mechanism of priapism involves derangements in the biology of penile erection and its regulatory mechanisms 8. Recent investigations in transgenic SCD mouse models, which display excessive erectile responses to cavernous nerve stimulation that mimic priapic phenotypic changes seen in humans, have demonstrated that priapism arises from chronically impaired NO bioavailability causing a defect in the NO/cGMP/PDE5 signaling pathway in the penis 9, 10. eNOS activity and cGMP production have been shown to be basally decreased in the penis of SCD mice, resulting in local downregulation of PDE5. Therefore, upon psychogenic or reflexogenic neuronal stimulation, prolonged erection occurs because of uncontrolled nNOS-dependent cGMP-induced vasodilation of the corpora cavernosa owing to deficient PDE5-specific cGMP hydrolysis 9. More recently, priapism in SCD mice has also been shown to be associated with dysfunctional signaling of RhoA, and its downstream effector ROCK, which both mediate vasoconstriction of the penile vasculature 11. In addition, dysregulation of opiorphin and adenosine signaling, as well as increased oxidative stress in the penis, are proposed contributory mechanisms based on experimental studies using animal models of priapism 11–16.
Despite the current molecular science of priapism, this science extensively derives from animal model investigations and has not been evaluated at the human level. Therefore, to better understand the molecular mechanisms of priapism in humans with a primary focus on abnormalities associated with this disorder in SCD, we aimed to investigate major signaling pathways governing erection responses which are likely involved in the pathophysiology of priapism using human penile specimens. Specifically, we investigated the presence and extent of NOS (endothelial, neuronal, and inducible) and ROCK (RhoA and ROCK isoforms) signaling pathways and a major biologic source of oxidative stress (NADPH oxidase subunits).
MATERIALS AND METHODS
Tissue Collection and Processing
Penile tissue specimens were obtained from patients with and without priapism histories. The priapism groups were: patients with SCD-associated priapism (SCD, n =5, mean age =35 yr, range 22–52 yr), and patients with priapism of other etiologies and negative hematologic work-ups (non-SCD, n=6, mean age =38 yr, range 24–55 yr) (Table 1). Grossly non-fibrosed penile erectile tissue 17 was retrieved at the time of penile prosthesis surgery for ED or as an option for priapism management (corpus cavernosum) or bulbar anastomotic urethroplasty (corpus spongiosum, patient #5 in SCD group only). A control group (Control, n=5, mean age =63 yr, range 48–77 yr) consisted of patients without priapism histories who underwent penectomy for penile cancer. Penile tissue from this group was unassociated with malignancy. Samples were collected at surgery, immediately placed in cold buffer, and then frozen in liquid nitrogen. Tissue collection and clinical history review were performed with approval by the Institutional Review Board of the Johns Hopkins Medical Institutions (NA_00013784).
Table 1.
Demographic and clinical presentations of patient groups with priapism histories
| Age (yr) |
Comorbidities | Priapism Etiology |
Priapism History (episode frequency; duration range) |
Shunt Surgery |
ED | Time to Surgery after Shun |
|
|---|---|---|---|---|---|---|---|
| SCD group | |||||||
| Patient number | |||||||
| 1 | 40 | Hypertension | Hematologic dyscrasia | 1 episode/2 wk for 24 yr; 4–12 h | Yes | Yes | 12 mo |
| 2 | 25 | Hypertension, Depression, Current smoker | Hematologic dyscrasia | 1 episode/wk for 7 yr; 4–12 h | No | Yes | N/A |
| 3 | 35 | Current smoker | Hematologic dyscrasia | Multiple episodes for 1 yr; 4–12 h | Yes | Yes | 120 mo |
| 4 | 23 | None | Hematologic dyscrasia | 3 episodes/wk for 5 yr; 1–4 h | No | Yes | N/A |
| 5 | 52 | Hypertension, Current smoker | Hematologic dyscrasia | Minor prolonged erection tendencies | No | No | N/A |
| Non-SCD group | |||||||
| Patient number | |||||||
| 1 | 47 | Dyslipidemia, Depression | Idiopathic | Sporadic episodes for 3 yr; 4–144 h | Yes | Yes | 3 mo |
| 2 | 35 | None | Idiopathic | Daily/sporadic episodes for 4 yr ; 4–24 h | No | No | N/A |
| 3 | 32 | Anxiety, Depression | Antidepressant use 1 | 3–5 episodes/wk for 5 mo; 4–12 h | Yes | No | 4 mo |
| 4 | 55 | Hypertension, Dyslipidemia, Current smoker | Intracavernosal injection 2 | Single episode; 36 h | No | Yes | 12 mo |
| 5 | 24 | Current smoker | Idiopathic | Daily episodes for 4 yr; 4–12 h | No | Yes | N/A |
| 6 | 33 | Depression, Bipolar disorder, Current smoker | Idiopathic | Multiple episodes for 2 yr; 4–72 h | Yes | Yes | 90 mo |
Trazodone
Vasoactive pharmacologic erection agents
Western Blot
Tissues were homogenized, centrifuged at 10,000 g for 30 minutes at 4°C, and processed as described previously 18. Supernatants were loaded (30 or 60 µg total protein) on 4–20% Tris HCl gels (Bio-Rad Laboratories, Hercules, CA, USA), transferred to a polyvinylidene fluoride membrane, and incubated overnight at 4°C with the following primary antibodies (dilutions indicated): polyclonal PDE5 (Abcam Inc, Cambridge, MA; 1:1000), monoclonal eNOS (BD Biosciences, San Jose, CA; 1:500), polyclonal nNOS (Cell Signaling Technology, Danvers, MA; 1:1000), monoclonal iNOS (BD Biosciences, 1:250), monoclonal RhoA (Santa Cruz Biotechnology, Santa Cruz, CA; 1:1000), monoclonal ROCK1 (Santa Cruz; 1:1000), monoclonal ROCK2 (BD Biosciences; 1:1000), polyclonal p47phox (Upstate Technology, Lake Placid, NY; 1:1000), monoclonal p67phox (BD Biosciences; 1:500), monoclonal gp91phox (BD Biosciences; 1:600) and monoclonal β-actin (Sigma-Aldrich, St. Louis, MO; 1:10,000). Blots were scanned and quantified using NIH Image J software and standardized to β-actin. Total proteins were also measured in select blots using Ponceau S solution (Sigma-Aldrich) to verify β-actin results. The ratio was determined in terms of arbitrary units and expressed relative to the ratio for the Control group.
NO Assay
The aforementioned penile tissue supernatants were also used to measure total NO production using a commercially available kit (Oxford Biomedical Research, Rochester Hills, MI). This kit employs metallic cadmium for quantitative conversion of nitrate to nitrite before quantitation of nitrite using a Griess reagent, therefore providing for accurate determination of total NO production. Briefly, cadmium pellets were added to supernatants and incubated overnight. The following day, the Griess reaction was performed, as described previously 19. Absorbance was measured at 540 nm, and nitrite concentrations were determined using the nitrite standard provided in the kit.
Statistical Analysis
The data are expressed as the mean ± SEM. Statistical analyses were performed using one-way ANOVA, followed by Newman-Keuls multiple comparison (for the NO Griess assay) or by Student's t-test (for Western blot). P less than 5% was considered significant.
RESULTS
Expression of Proteins Involved in NOS Signaling
Western blot analyses with densitometry were performed to determine expression levels of PDE5, eNOS, nNOS and iNOS in human penile tissue (Figure 1). PDE5 expression was significantly decreased in the SCD group compared to both Control (p<0.05) and non-SCD group values (p<0.05). eNOS expression was significantly decreased in both SCD (p<0.01) and non-SCD (p<0.05) groups compared to the Control group value. nNOS expression was unchanged among treatment groups. However, under the same experimental conditions, iNOS protein expression was undetectable in penile tissue although the signal was confirmed using a positive control (mouse macrophages + IFNy/LPS, BD Biosciences; data not shown). These results suggest dysregulated NO signaling is a potential mechanism of priapism in humans and is also differentially regulated with diverse etiologies of this disorder.
Figure 1.
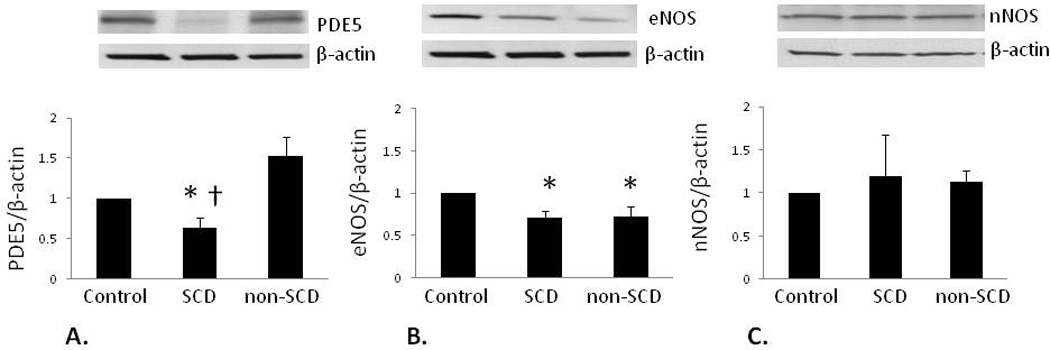
NOS signaling changes in SCD-associated priapism. A.) PDE5 expression (100 kDa) significantly decreased in the SCD group compared to both the Control and non-SCD groups. B.) eNOS expression (140 kDa) significantly decreased in both SCD and non-SCD groups compared to Control. C.) nNOS expression (160 kDa) was similar among all groups. * p<0.05 vs. Control, † p<0.05 vs. non-SCD. Top panels: representative immunoblots; bottom panel: densitometry data; bars represent mean ± SEM of 5–6 patients per group. All protein expressions were normalized to β-actin and expressed as a ratio of Control values. (SCD = patients with sickle cell disease, non-SCD = patients with priapism of other etiologies).
NO Measurement in Penile Tissue
Although the total NO measurement was not significantly different between groups (p = 0.472), the levels were highest in penile tissue from the SCD group (182.3 ± 41.8 µM) compared to the non-SCD (123.3 ± 21.1 µM) and Control groups (144.2 ± 37.8 µM).
Expression of Proteins Involved in ROCK Signaling
Western blot analyses were also performed to determine expression levels of RhoA, ROCK1 and ROCK2 (Figure 2). RhoA expression was significantly decreased in the SCD group compared to the Control group value (p<0.01). In the non-SCD group, RhoA expression was significantly increased compared to the SCD group value (p<0.01). ROCK1 expression in the non-SCD group was significantly lower compared to both SCD (p<0.05) and Control (p<0.05) group values. ROCK2 expression was unchanged among treatment groups. Taken together, these results suggest that decreased expression of RhoA/ROCK occurs in priapism, specifically RhoA, which seems to be differentially regulated in SCD-associated priapism.
Figure 2.
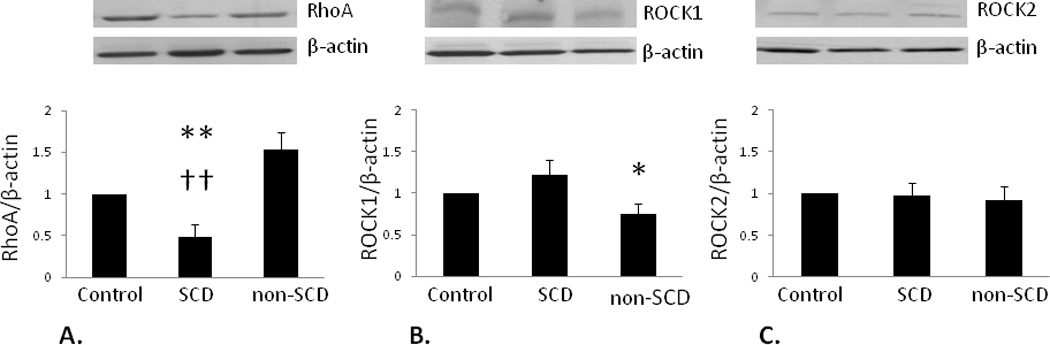
ROCK signaling is attenuated in SCD-associated priapism. A.) RhoA expression (20 kDa) significantly decreased in the SCD group compared to the Control group. B.) ROCK1 expression (160 kDa) did not change in the SCD group while it significantly decreased in the non-SCD group compared to Control. C.) ROCK2 expression (180 kDa) was similar among all groups. * p<0.05, ** p<0.01 vs. Control, †† p<0.01 vs. non-SCD. Top panels: representative immunoblots; bottom panel: densitometry data; bars represent mean ± SEM of 5–6 patients per group. All protein expressions were normalized to β-actin and expressed as a ratio of Control values. Abbreviations are same as in Figure 1.
Protein Expression of NADPH Oxidase Subunits
Among NADPH oxidase subunits, gp91phox expression was significantly increased in the SCD group compared to the Control group value (p<0.05). p47phox expression was significantly decreased in the non-SCD group compared to the Control group value (p<0.05). p67phox expression was unchanged among treatment groups (Figure 3). These results suggest that NADPH oxidase-mediated signaling via increased gp91phox expression in the penis contributes specifically to SCD-associated priapism.
Figure 3.
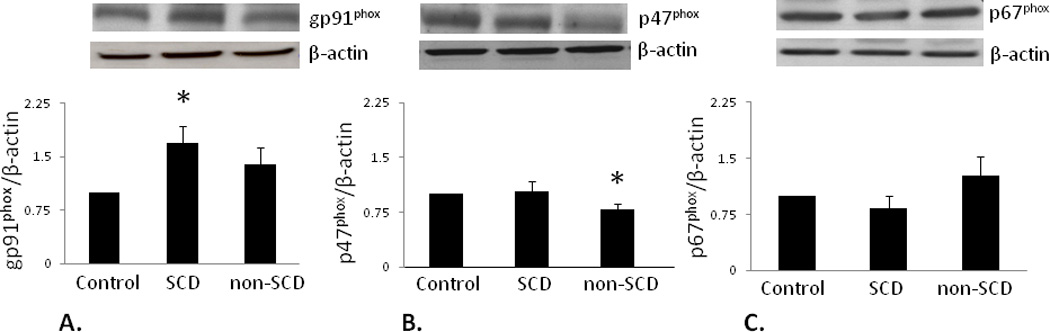
Oxidative stress markers are upregulated in SCD-associated priapism. A.) gp91phox expression (58 kDa) significantly increased in the SCD group compared to the Control group. B.) p47phox expression (47 kDa) significantly decreased in the non-SCD group compared to Control but did not change in the SCD group. C.) p67phox expression (67 kDa) was similar among all groups. * p<0.05 vs. Control. Top panels: representative immunoblots; bottom panel: densitometry data; bars represent mean ± SEM of 5–6 patients per group. All protein expressions were normalized to β-actin and expressed as a ratio of Control values. Abbreviations are same as in Figure 1.
DISCUSSION
This study broadens current knowledge and provides further molecular evidence regarding plausible mechanisms for the pathogenesis of SCD-associated priapism at a clinically relevant level. We characterized a host of molecular factors previously associated with the pathophysiology of priapism in animal models, now employing human penile specimens. Specifically, we showed that PDE5 was downregulated in the SCD group but not in the non-SCD group, whereas eNOS protein expression was downregulated in both groups. Expressions of RhoA and ROCK1 were decreased in SCD and non-SCD groups, respectively. We also showed that the NADPH oxidase subunit gp91phox was increased only in penile tissue from the SCD group. This study establishes several important molecular alterations in erection regulatory factors involved in signaling mechanisms in priapism with human relevance, particularly some that are uniquely related to SCD-associated priapism.
The pathogenesis and specific causes of SCD-associated priapism in men are not completely known. However, using animal models, the diverse mechanisms that are involved have recently begun to be characterized. Decreased PDE5 expression and eNOS activity have been demonstrated to be central in the pathogenesis of SCD-associated priapism as evidenced by the findings in the penis of transgenic mouse models of SCD 10, 12, 20. Similar to mouse models of SCD, earlier studies in eNOS knockout mice demonstrated that these mice exhibit priapic activity and a pronounced erectile response to electrical stimulation of the cavernous nerve and decreased PDE5 activity in the penis 12, 21. Our findings of both reduced PDE5 and constitutive eNOS protein expressions in human penis from SCD patients with priapism histories further establish the major role of this pathway. On the other hand, in priapic penile tissue unrelated to SCD, eNOS protein expression was significantly decreased without a change in PDE5 protein expression. Expression levels of nNOS, another constitutive NO-generating enzyme, remained the same in both types of priapism compared with control measurements. Together, these data indicate that PDE5 dysregulation is a distinct fundamental mechanism of SCD-associated priapism whereas the common eNOS expression loss may be a universal phenomenon of priapism occurring secondarily from endothelial damage and tissue destruction in the penis. We believe that cGMP activity measurements would further corroborate our PDE5 findings, but because these assays produce inconsistent and unreliable results in variably procured human specimens, for the purpose of this study, we specified protein expression studies.
We acknowledge that determinations of NOS function based solely on protein expressions of total eNOS and nNOS in the penis may be problematic. It is now apparent that changes in NOS activity and NO production do not necessarily parallel changes in NOS abundance. Indeed, our recent studies demonstrated decreased basal eNOS phosphorylation on Ser-1177, the main activated form of the enzyme, in the penis of SCD mice compared to WT mice, while total eNOS protein expression remained unchanged 20. However, NOS phosphorylation studies are technically difficult to conduct in human tissue due to inconsistent handling of penile specimens at the time of tissue collection, which may confound the findings.
Multiple studies of SCD strongly suggest impaired NO bioavailability in NO-dependent regulation of the vasculature of the penis and elsewhere 1, 20, 22. Reduction in NO bioavailability can occur in the setting of its decreased production or its increased consumption, both of which have been implicated in SCD 1, 3. In our study, however, we detected a slight but not significant increase in NO, measured as total nitrate and nitrite, in penile tissue from patients with SCD-associated priapism. Although this was an unexpected result since decreased eNOS activity and NO production were observed in animals models of priapism 20, our findings can be explained. It is now recognized that hemolysis plays a significant role in disease pathogenesis leading to free hemoglobin in SCD 1, 3, 23. Reaction of NO with free hemoglobin not only scavenges NO, but also forms nitrate and methemoglobin, and elevated plasma nitrate and nitrite levels have been considered as one of the hallmarks of SCD 24. Since we measured the nitrate/nitrite metabolites of NO and not NO directly, it is possible that our NO assay results reflect an elevation in nitrate despite decreased bioavailable NO in cavernosal tissue. We have also investigated the protein expression of iNOS in human penile tissue as a source of excessive NO production, possibly accounting for an increase in measured nitrate/nitrite. Following numerous attempts, we were not able to detect iNOS protein expression using Western blot analysis in human penile tissue (data not shown). However, the role of iNOS cannot be completely ruled out and may be investigated further in the future as more specialized detection techniques become available.
In addition to pathways involved in vasodilatation, appropriate control of vasoconstriction is also required for a normal erectile response. It has been well established that the Rho pathway, consisting of RhoA and its downstream effector, ROCK (with two isoforms, ROCK1 and ROCK2), plays a major role in maintaining the penis in its flaccid state through vasoconstriction and eNOS regulation 25, 26. In fact, recently, Bivalacqua et al. demonstrated that the RhoA/ROCK pathway is downregulated in the penis of SCD mice and thus contributes to uncontrolled cavernosal vasorelaxation producing priapism 11. Specifically, they showed that the SCD mouse penis has a significant decline in both RhoA and total ROCK activities compared with that of the WT mouse penis. The reduction in ROCK activity was attributed to a decrease in ROCK2 protein expression since ROCK1 protein expression did not change in these mice. On the other hand, it has been previously shown in eNOS knockout mouse, which also display a priapic phenotype, that total ROCK activity in penile tissue is decreased with no change in RhoA activity 27. Despite these indications of slightly differential dysregulation of the Rho pathway in the penis in these two animal models of priapism, the findings are consistent with the overall significance of dysregulated Rho signaling in the pathophysiology of priapism. Similarly, in our study of human penile tissue, we found that RhoA and ROCK1 were significantly decreased in SCD and non-SCD groups, respectively, without a change in ROCK2 in either group compared with control. ROCK isoforms 1 and 2 share 65% homology, have a similar tissue distribution with confirmed expression in the penis 28, and have been implicated in penile vasoconstriction; however, the isoform-specific roles of ROCKs are not well established. We should also note that activity measurements of components of Rho signaling may further be investigated in support of its role.
Recent animal studies also indicate the role of ROS and oxidative stress in priapism. A study by Kanika et al., using two different animal models of priapism (rat opiorphin-induced and SCD mouse models), demonstrated elevated levels of lipid peroxidation, glutathione S-transferase activity, and oxidatively damaged proteins in corporal tissue, suggesting the association of increased oxidative stress with priapism 13. Our research group has recently found that the protein expressions of three NADPH oxidase subunits (gp91phox, p67phox, and p47phox) are increased in the SCD mouse penis 16. These findings specify this enzyme as a major source of ROS generation and subsequent oxidative damage in SCD-associated priapism. Our finding of increased gp91phox subunit expression in human penile tissue from the SCD group, but not from the non-SCD group, supports the role of NADPH oxidase as a mediator of oxidative damage in SCD-associated priapism. In fact, NADPH oxidase has been implicated specifically as a source of ROS-induced vasculopathy in SCD 1, 3, 23. It was interesting that compared to the increase in three subunits of NADPH oxidase in the SCD mouse penis, only gp91phox was increased in the penis in men with SCD without significant changes in the other subunits. Consistent with our results, in the cerebral vasculature of a gp91phox-deficient chimeric mouse model of SCD, which exhibits decreased superoxide generation, leukocyte adhesion is attenuated and vascular flow is restored 29. These data indicate a ROS generating capacity of the gp91phox - containing enzyme and suggest that NADPH oxidase is central in generating ROS and mediating oxidative damage in the SCD penis. Our findings of decreased protein expression of the NADPH oxidase subunit p47phox in the non-SCD penis may indicate a lesser contribution of NADPH-derived oxidative stress to priapism relative to that in SCD. However, in both the SCD and non-SCD priapic penis, the possible roles of other oxidative/antioxidative mechanisms such as xanthine oxidase, eNOS uncoupling, superoxide dismutase and glutathione peroxidase warrant investigation in future studies.
We acknowledge several possible limitations of our study. Since the majority of our tissue specimens were retrieved after major priapism episodes had occurred, it is possible that our molecular findings reflect the pathologic sequelae of priapism to some extent and not exclusively its pathogenesis. Although future studies uniformly applying uninjured SCD penile tissue may resolve the matter, such tissue is usually scarce for these investigations. The widespread differences in age between priapic and control patients may also have influenced our results, and specimens from a younger control population may be useful in further investigations to confirm the absence of confounding aging phenomena. Finally, our study was focused on select signaling pathways associated with priapism and did not include other possible molecular factors associated with the pathophysiology of this disorder. Future studies at the human level may include evaluations of adenosine and opiorphin as well as other yet to be discovered priapism-associated molecular factors and signaling mechanisms 14, 15.
CONCLUSIONS
Priapism has been acknowledged to be a dysregulatory erection disorder based on recent progress in describing its pathogenesis, mainly involving research work done in animal models. This study in human tissue now provides evidence to corroborate basic science preclinical findings suggesting dysfunctional regulatory control of signal transduction systems involved in vasodilation (NO/cGMP/PDE5 signaling) and vasoconstriction (RhoA/ROCK signaling) in interaction with oxidative stress (NADPH oxidase activity) as mechanisms of priapism. Ongoing identification and elucidation of the molecular mechanisms involved in the pathogenesis of priapism, with confirmation at the human level, will conceivably offer new avenues for future treatment of priapism, particularly that associated with SCD.
Supplementary Material
ACKNOWLEDGMENTS
Authors would like to thank Irene Trueheart, RN, for her help in patient data collection. This work was supported by US Public Health Service Grants R01DK067223 and U54HL090515
REFERENCES
- 1.Akinsheye I, Klings ES. Sickle cell anemia and vascular dysfunction: the nitric oxide connection. J Cell Physiol. 2010;224:620. doi: 10.1002/jcp.22195. [DOI] [PubMed] [Google Scholar]
- 2.Kato GJ, Hebbel RP, Steinberg MH, et al. Vasculopathy in sickle cell disease: Biology, pathophysiology, genetics, translational medicine, and new research directions. Am J Hematol. 2009;84:618. doi: 10.1002/ajh.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood KC, Granger DN. Sickle cell disease: role of reactive oxygen and nitrogen metabolites. Clin Exp Pharmacol Physiol. 2007;34:926. doi: 10.1111/j.1440-1681.2007.04639.x. [DOI] [PubMed] [Google Scholar]
- 4.Broderick GA, Kadioglu A, Bivalacqua TJ, et al. Priapism: pathogenesis, epidemiology, and management. J Sex Med. 2010;7:476. doi: 10.1111/j.1743-6109.2009.01625.x. [DOI] [PubMed] [Google Scholar]
- 5.Burnett AL, Allen RP, Tempany CM, et al. Evaluation of erectile function in men with sickle cell disease. Urology. 1995;45:657. doi: 10.1016/s0090-4295(99)80059-4. [DOI] [PubMed] [Google Scholar]
- 6.Burnett AL. Sexual health outcomes improvement in sickle cell disease: a matter of health policy? J Sex Med. 2012;9:104. doi: 10.1111/j.1743-6109.2011.02411.x. [DOI] [PubMed] [Google Scholar]
- 7.Adeyoju AB, Olujohungbe AB, Morris J, et al. Priapism in sickle-cell disease; incidence, risk factors and complications - an international multicentre study. BJU Int. 2002;90:898. doi: 10.1046/j.1464-410x.2002.03022.x. [DOI] [PubMed] [Google Scholar]
- 8.Burnett AL. Pathophysiology of priapism: dysregulatory erection physiology thesis. J Urol. 2003;170:26. doi: 10.1097/01.ju.0000046303.22757.f2. [DOI] [PubMed] [Google Scholar]
- 9.Bivalacqua TJ, Musicki B, Kutlu O, et al. New insights into the pathophysiology of sickle cell disease-associated priapism. J Sex Med. 2012;9:79. doi: 10.1111/j.1743-6109.2011.02288.x. [DOI] [PubMed] [Google Scholar]
- 10.Bivalacqua TJ, Musicki B, Hsu LL, et al. Establishment of a transgenic sickle-cell mouse model to study the pathophysiology of priapism. J Sex Med. 2009;6:2494. doi: 10.1111/j.1743-6109.2009.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bivalacqua TJ, Ross AE, Strong TD, et al. Attenuated RhoA/Rho-kinase signaling in penis of transgenic sickle cell mice. Urology. 2010;76:510, e7–e12. doi: 10.1016/j.urology.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Champion HC, Bivalacqua TJ, Takimoto E, et al. Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc Natl Acad Sci U S A. 2005;102:1661. doi: 10.1073/pnas.0407183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanika ND, Melman A, Davies KP. Experimental priapism is associated with increased oxidative stress and activation of protein degradation pathways in corporal tissue. Int J Impot Res. 2010;22:363. doi: 10.1038/ijir.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanika ND, Tar M, Tong Y, et al. The mechanism of opiorphin-induced experimental priapism in rats involves activation of the polyamine synthetic pathway. Am J Physiol Cell Physiol. 2009;297:C916. doi: 10.1152/ajpcell.00656.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai Y, Zhang Y, Phatarpekar P, et al. Adenosine signaling, priapism and novel therapies. J Sex Med. 2009;6(Suppl 3):292. doi: 10.1111/j.1743-6109.2008.01187.x. [DOI] [PubMed] [Google Scholar]
- 16.Musicki B LT, Sezen SF, Burnett Al. Targeting NADPH oxidase decreases oxidative stress in the transgenic sickle cell mouse penis. J Sex Med. 2012 doi: 10.1111/j.1743-6109.2012.02798.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hedlund P, Ny L, Alm P, et al. Cholinergic nerves in human corpus cavernosum and spongiosum contain nitric oxide synthase and heme oxygenase. J Urol. 2000;164:868. doi: 10.1097/00005392-200009010-00064. [DOI] [PubMed] [Google Scholar]
- 18.Musicki B, Champion HC, Becker RE, et al. Erection capability is potentiated by long-term sildenafil treatment: role of blood flow-induced endothelial nitric-oxide synthase phosphorylation. Mol Pharmacol. 2005;68:226. doi: 10.1124/mol.104.010678. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Teng YH, Hao Y, et al. Preparation and Properties of Polyamines: Part II-Controlled and Sustained Release of Nitric Oxide (NO) from Nitrosated Polymers. Polymer Journal. 2009;41:715. [Google Scholar]
- 20.Musicki B, Champion HC, Hsu LL, et al. Post-translational inactivation of endothelial nitric oxide synthase in the transgenic sickle cell mouse penis. J Sex Med. 2011;8:419. doi: 10.1111/j.1743-6109.2010.02123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burnett AL, Chang AG, Crone JK, et al. Noncholinergic penile erection in mice lacking the gene for endothelial nitric oxide synthase. J Androl. 2002;23:92. doi: 10.1002/j.1939-4640.2002.tb02601.x. [DOI] [PubMed] [Google Scholar]
- 22.Aslan M, Ryan TM, Adler B, et al. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci U S A. 2001;98:15215. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood KC, Hsu LL, Gladwin MT. Sickle cell disease vasculopathy: a state of nitric oxide resistance. Free Radic Biol Med. 2008;44:1506. doi: 10.1016/j.freeradbiomed.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Rees DC, Cervi P, Grimwade D, et al. The metabolites of nitric oxide in sickle-cell disease. Br J Haematol. 1995;91:834. doi: 10.1111/j.1365-2141.1995.tb05397.x. [DOI] [PubMed] [Google Scholar]
- 25.Chitaley K, Wingard CJ, Clinton Webb R, et al. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nat Med. 2001;7:119. doi: 10.1038/83258. [DOI] [PubMed] [Google Scholar]
- 26.Musicki B, Ross AE, Champion HC, et al. Posttranslational modification of constitutive nitric oxide synthase in the penis. J Androl. 2009;30:352. doi: 10.2164/jandrol.108.006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bivalacqua TJ, Liu T, Musicki B, et al. Endothelial nitric oxide synthase keeps erection regulatory function balance in the penis. Eur Urol. 2007;51:1732. doi: 10.1016/j.eururo.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakagawa O, Fujisawa K, Ishizaki T, et al. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392:189. doi: 10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- 29.Wood KC, Hebbel RP, Granger DN. Endothelial cell NADPH oxidase mediates the cerebral microvascular dysfunction in sickle cell transgenic mice. FASEB J. 2005;19:989. doi: 10.1096/fj.04-3218fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


