Abstract
Background
Diabetes is a risk factor for coronary heart disease (CHD) but CHD does not occur in all diabetic individuals. The goal of this study was to assess the relationship between family history of myocardial infarction (MI) and incident CHD in diabetic postmenopausal women.
Methods
We conducted a prospective cohort study among 2642 diabetic postmenopausal women without CHD at baseline in the Women’s Health Initiative Observational Study. Family history was defined as a proband report of MI in first-degree relatives. Incident CHD was defined as non-fatal MI, coronary revascularization, or CHD death.
Results
During 7.3 (±1.8) years of follow-up, 14.3% of the participants had incident CHD. The risk of incident CHD was 50% higher (HR = 1.50, 95% CI: 1.20–1.87, p = 0.0003) in those with a family history of an MI in at least one first-degree relative, and 79% higher (HR = 1.79, 95% CI: 1.36–2.35, P < 0.0001) if two or more first-degree relatives had an MI, compared to participants without a family history, after adjustment for covariates. The CHD risk increased with elevated systolic blood pressure (SBP) (HR = 1.01, 95% CI: 1.003–1.02, p = 0.001) but decreased with elevated diastolic BP (HR = 0.98, 95% CI: 0.97–0.999, p = 0.005) and with two or more episodes per week of physical activity (HR = 0.70, 95% CI: 0.52–0.93, p = 0.02).
Conclusions
The results suggest that a family history of MI predicts CHD in diabetic postmenopausal women. Close attention should be paid to BP control and physical activity in these women.
Keywords: family history, CHD, diabetes, women’s health
Introduction
Diabetes is the sixth leading cause of death in the United States. The age-adjusted proportion of physician-diagnosed diabetes is increasing among adults aged 20 years or older [1]. Diabetes is associated with serious complications and premature death with coronary heart disease (CHD) as the major cause of death [2]. In the Nurses’ Health Study, diabetic women aged 30–55 years at study entry had a seven-fold higher risk for CHD than that of their non-diabetic counterparts after a 8.5-year follow-up [3]. The population- attributable risk of CHD due to diabetes was 13.8% among women in the Nurses’ Health Study [3], and 7.7% among women in the Framingham cohort [4].
Although diabetes itself is a strong risk factor for CHD, other traditional (age, race, smoking, BMI, LDL and HDL cholesterol, hypertension) and non-traditional (albumin, fibrinogen, von Willebrand factor, factor VIII activity, and leukocyte count) risk factors are also associated with CHD in diabetic individuals [5,6]. In addition, over recent decades, case–control and cohort studies have found that family history of CHD is a risk factor for incident CHD [7–16], and the postmenopausal state is a unique CHD risk factor for women [17–20]. However, whether family history of CHD independently predicts CHD in postmenopausal women with diabetes remains unclear. Identifying women at particular risk based on a positive family history could justify more aggressive attempts at risk-factor management. The aim of this study was to assess the association between family history of myocardial infarction (MI) and incident CHD in a cohort of postmenopausal women who had medication-treated diabetes but not cardiovascular disease (CVD) at baseline in the Women’s Health Initiative Observational Study (WHI-OS).
Methods
Participants
The WHI consists of two components: a cluster of four randomized clinical trials (estrogen plus progestin, estrogen alone, dietary modification, and calcium/vitamin D) and an observational study [21]. The observational study included 93 676 women who had been invited to join the randomized clinical trials but who were either ineligible or were not interested in any trial after initiating screening and who were interested in participating in the observational study [22]. Participants who were postmenopausal and aged 50–79 years were recruited through 40 clinical centers around the United States between September 1994 and December 1998. Postmenopausal status was defined as absence of menses for 1 year if under age 55 years and for 6 months if over age 55. The participants provided written informed consent in a form approved by the clinical centers’ institutional review boards before completing self-administered forms and undergoing an interview, physical examination, and blood sample collection at baseline.
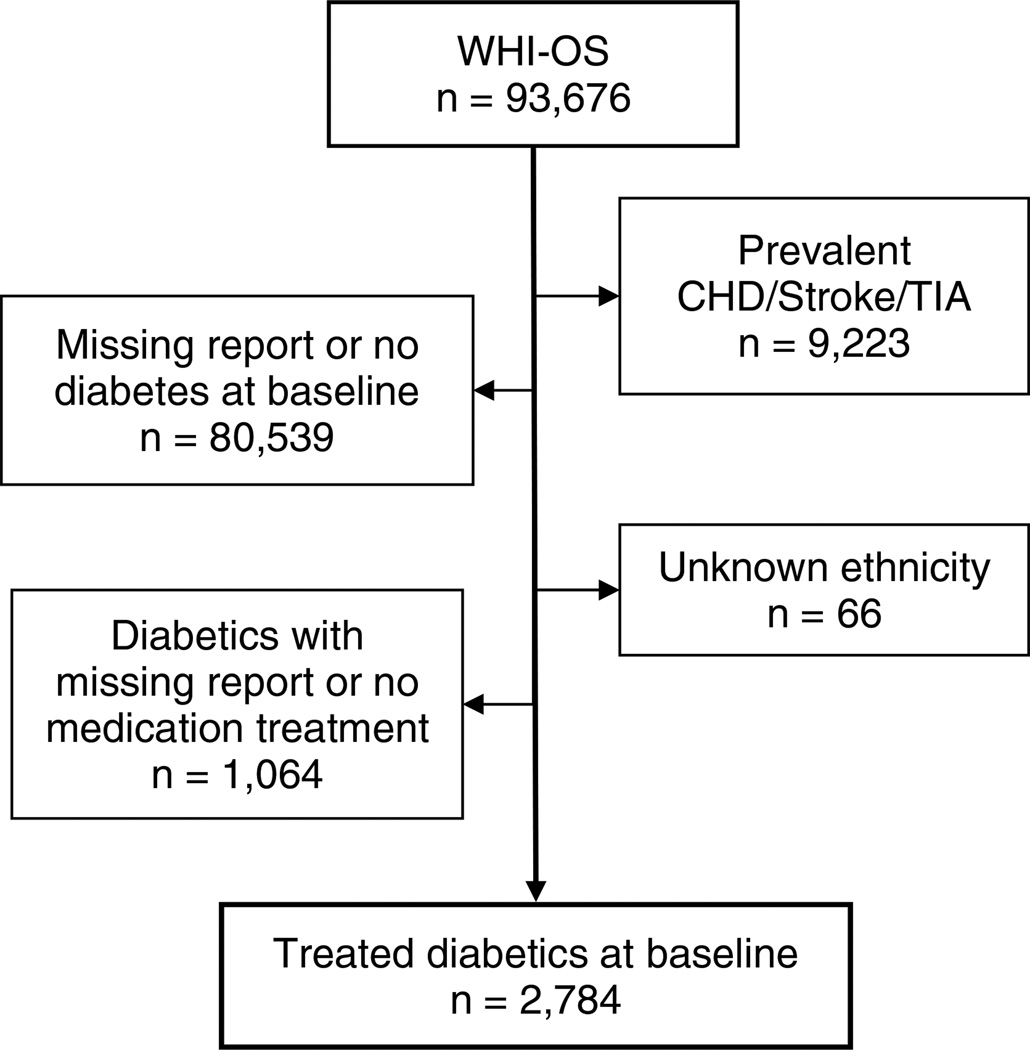
This analysis was conducted in a subset of OS cohort of 2784 women (62.9% of whites, 24.7% of blacks, 7.0% of Hispanics, and 5.4% of others) who had medication-treated diabetes but no history of CHD (angina, MI or cardiac procedures), transient ischemic attack (TIA) or stroke at baseline (Figure 1). Diabetes was identified by answering ‘yes’ to the question ‘Did a doctor ever say that you had sugar diabetes or high blood sugar when you were not pregnant and were using insulin or other diabetic medications at baseline (self-reported use of diabetic medications, presence of diabetic medications on medication inventory in clinic, or both)’. To reduce misclassifications of diabetes at baseline, only treated diabetic patients were included in this analysis. Age at diabetes diagnosis was based on the participant’s answer to the question ‘How old were you when you were first told you had sugar diabetes? (Don’t include diabetes you had only when pregnant.)’ In this sub-cohort of treated diabetic postmenopausal women, the duration of having diabetes ranged from 0.80 to 9.84 years at baseline. Thus, patients with type 1 diabetes were highly unlikely to have been included in this sub-cohort. The cohort was followed up to 10.5 years (mean 7.3 years ± standard deviation 1.8 years). The focus of this analysis was on the association between CHD outcomes and a positive family history of MI; to reduce misclassification of CHD due to misclassification of unstable angina, 142 participants who had unstable angina during the follow-up were excluded from this analysis. The final sample size for this analysis was 2642 participants.
Figure 1.
A sub-cohort of treated diabetic patients selected from the Women’s Health Initiative Observational Study (WHI-OS) at baseline
Ascertainment of CHD outcomes
Details of definitions, classifications of outcomes, and methods for ascertainment and documentation were published [23]. In brief, women were contacted annually to determine whether they had been hospitalized or had undergone a procedure suggestive of a CHD end point. Medical records for all overnight hospitalizations and coronary procedures were retrieved. Adjudication of hospitalizations, including the key cardiovascular outcomes requiring hospitalization, was performed by WHI physician adjudicators who were blinded to the participants’ baseline risks. An incident CHD event during follow-up was defined as the first-time occurrence of either (1) acute MI that required overnight hospitalization, (2) coronary revascularization procedures, which included percutaneous transluminal coronary angioplasty (PTCA), stent placement, and coronary artery bypass graft surgery (CABG), or (3) coronary death. MI was identified if the level of any cardiac enzyme (creatine kinase, lactate dehydrogenase, troponin, or myoglobin) was at least twice the upper limit of normal regardless of electrocardiographic findings while patients had ischemic symptoms, or if enzymes were twice the upper limit of normal with Q-wave or ST-T-wave abnormalities suggestive of an MI while patients did not have ischemic symptoms, or if enzymes were 1–2 times the upper limit of normal with Q waves or ST-T-wave abnormalities suggestive of an MI, or if enzymes were normal or absent, but evolving Q-wave and evolving ST-T abnormalities were documented.
Determination of family history of MI and diabetes
Participants’ family history was collected through a family history questionnaire at baseline. For a positive family history of heart attack, participants answered ‘yes’ to the question ‘Did your mother or father or full-blooded sisters, full-blooded brothers, daughters, or sons ever have a heart attack or myocardial infarction?’ Age at the first heart attack was recorded for father, mother, and three older sisters and/or three older brothers, and two older daughters and/or two older sons. Family history of diabetes was defined by answering ‘yes’ to the question ‘Did your mother or father, or full-blooded sisters, full-blooded brothers, daughters, or sons ever have sugar diabetes or high blood sugar that first appeared as an adult?’ The number of these relatives with diabetes was also recorded.
Inclusions of covariates
The information on covariates in this analysis was collected at baseline. The selected covariates were age; race/ethnicity; socioeconomic status (education and income); smoking (status and length of lifetime smoking); alcohol consumption; physical activity; hysterectomy; hypertension with or without treatment; length of anti-diabetic, anti-hypertensive medication use; hormone replacement therapy (HRT); blood pressure; body mass index (BMI); and waist/hip ratio (WHR). Race/ethnicity was determined by self-report with the following categories: non-Hispanic white, African American/black (non-Hispanic), Hispanic, Asian/Pacific Islander, American Indian/Alaska Native, or unknown (women who indicated ‘other’ ethnicity or did not answer the question). Participants with unknown ethnicity were excluded. In this analysis, Asian/Pacific Islander and American Indian/Alaska Native were combined with the category of ‘others.’ Education and income were ascertained by choice from a range of categories. Smoking status, lifetime smoking status, and physical activity were obtained through a personal habits questionnaire. The information about alcohol consumption was collected by a personal habits questionnaire, a personal history questionnaire, and a food frequency questionnaire (FFQ). Hysterectomy and the use of HRT were recorded according to self-report. Blood pressure was measured with a standard protocol. Subjects sat quietly for 5 min before the measurement, and an appropriately sized cuff was used. The average of two measurements, 30 s apart, was used. Hypertension was defined as a blood pressure greater than or equal to 140/90 mmHg or by self-reported, doctor-diagnosed high blood pressure with or without anti-hypertensive treatment. Medication use was ascertained by a medication inventory in the clinic at baseline. Baseline weight was determined on a calibrated balance beam scale, with the women wearing indoor clothing but no shoes. Height was determined with a calibrated, wall-mounted stadiometer. Waist circumference was measured at the end of normal expiration over non-binding undergarments in a horizontal plane at the natural waist. Hip circumference was measured at the site of maximum extension of the buttocks. Laboratory measures, such as lipids, were only available for a randomly selected 1% subsample of observational study participants, so those measures were not included in this analysis. However, in this analysis, the use of lipid-lowering medication was considered as a surrogate of lipid abnormality.
Statistical analyses
Family history of MI was categorized in four different ways: (1) positive family history of MI in at least one relative, ‘yes’ or ‘no’; (2) number of relatives with MI regardless of age at onset; (3) premature MI, if male relatives had the first onset of MI at age <55 years, or female relatives had the first onset of MI at age <65 years old in at least one relative, ‘yes’ or ‘no,’ and (4) number of relatives with premature MI. The frequency of three or more relatives with MI or premature MI was low in both cases and non-cases (Table 1). Thus, the number of relatives with MI or premature MI was coded as 0, 1, and 2+ in the Cox proportional hazards models.
Table 1.
Participants’ Baseline Characteristics by Incident CHD in the WHI-OS Diabetic Cohor*
| Baseline risk factors | CHD+ (n = 377) | CHD− (n = 2265) | P* |
|---|---|---|---|
| FHx MI in ≥1 relative | 56.6 | 46.7 | 0.0002 |
| 1 relative | 32.6 | 29.8 | <0.0001 |
| 2 relatives | 16.9 | 11.8 | |
| ≥3 relatives | 7.1 | 5.1 | |
| FHx premature MI in ≥1 relative | 33.8 | 25.1 | 0.001 |
| 1 relative | 25.2 | 20.0 | 0.0004 |
| 2 relatives | 8.0 | 4.2 | |
| ≥3 relatives | 0.6 | 0.9 | |
| FHx diabetes in ≥1 relative | 57.6 | 60.1 | 0.34 |
| 1 relative | 31.5 | 28.0 | 0.07 |
| 2 relatives | 10.4 | 15.5 | |
| ≥3 relatives | 14.5 | 16.6 | |
| Education ≤High School | 48.7 | 42.8 | 0.03 |
| Income <$50k | 81.8 | 74.6 | 0.004 |
| White | 74.0 | 61.3 | <0.0001 |
| Anti-hypertensive Med | 64.4 | 58.6 | 0.046 |
| Hormone users | 54.7 | 57.3 | 0.07 |
| Physical activity Noa | 23.4 | 20.1 | 0.08 |
| Some but <2 episodes/week | 46.2 | 45.5 | |
| ≥2 episodes/week | 30.5 | 34.4 | |
| Hypertension | 65.2 | 60.7 | 0.2 |
| Ever smoking | 47.9 | 46.9 | 0.5 |
| Smoking 20+ years | 26.4 | 25.1 | 0.8 |
| <20 years | 18.7 | 19.9 | |
| Alcohol consumption Nob | 17.5 | 19.3 | 0.37 |
| Past | 39.8 | 41.1 | |
| Current <1 drink/week | 29.2 | 27.0 | |
| Current ≥1 drink/week | 13.5 | 12.6 | |
| Hysterectomy | 50.0 | 47.4 | 0.4 |
| Lipid lowering Med | 28.2 | 25.2 | 0.25 |
| Age (years) | 66.4 (0.34) | 64.0 (0.15) | <0.0001 |
| Diabetic Med (years) | 4.7 (0.34) | 3.5 (0.11) | <0.0001 |
| SBP (mmHg) | 137.7 (0.97) | 134.0 (0.39) | 0.0003 |
| DBP (mmHg) | 73.4 (0.53) | 75.0 (0.21) | 0.005 |
| Waist/hip ratio | 0.88 (0.004) | 0.87 (0.002) | 0.045 |
| BMI (kg/m2) | 31.5 (0.37) | 31.7 (0.15) | 0.7 |
% for categorical measures and mean (standard error) for continuous measures.
No walk outside for ≥20 min without stopping.
Never drank ≥12 alcoholic beverages.
One episode of physical activity: ≥20 min duration of physical activity, which included walking fairly fast or very fast, moderate physical activity and strenuous physical activity (expenditure of energy from physical activity in kcal/week/kg ≥4).
CHD, coronary heart disease; FHx, family history; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, bodymass index; Med, medication use. Numbers in parentheses are standard errors.
Smoking was coded as current, past and never, or ≥20 pack-year and <20 pack-year. Physical activity was coded as no activity (no walk outside for ≥20 min without stopping), some activity but less than two episodes per week, and two or more episodes per week. An episode was defined as ≥20 min duration of physical activity, which included walking fairly fast or very fast, moderate physical activity and strenuous physical activity (expenditure of energy from physical activity in kcal/week/kg ≥4). Alcohol intake was categorized as none (never drank ≥12 alcoholic beverages), past, current less than one drink per week or one or more drinks per week by combining questions on past and current use and number of current servings of wine, beer and liquor per week from the food frequency questionnaire (FFQ).
Descriptive analyses were used to estimate participants’ baseline characteristics overall and stratified by CHD status. Proportion and Chi-square statistics were used to describe distributions of categorical variables and to test significance of the distribution by CHD status. Means (standard error) and t-statistics were used for distribution of continuous variables and test for significance by CHD status. A Kaplan-Meier survival distribution function was used to describe proportions of participants free of CHD associated with family history of MI during 10 years of follow-up. Cox proportional hazards models were used to estimate hazard rate ratios and 95% confidence interval of time-to-events associated with family history of MI during follow-up. Multivariable analyses were conducted to include significant CHD risk factors as covariates (confounders/intermediate variables) into regression models. All analyses were performed using the SAS/STAT (SAS, Inc., Cary, North Carolina) for Windows, version 9.2.
Results
The incident CHD rate was significantly higher in whites (16.1%) than in other racial groups (10.1%, P < 0.0001). The higher incident CHD rate in whites was related to a higher rate of coronary revascularization procedures (12.2%) in whites compared to that in other racial groups (6.3%, P < 0.0001).
Participants’ baseline characteristics by incident CHD during follow-up are shown in Table 1. The proportion of a family history of MI in at least one first-degree relative was significantly higher in diabetic women who experienced an incident CHD event (56.6%) compared to participants free of CHD (46.7%, P = 0.0002) during the period of follow-up. Distributions of other CHD risk factors in Table 1 indicated a higher risk profile among women with CHD compared to those without CHD. Women with CHD had lower education (48.7% vs. 42.8% with high school education or less, P = 0.03), lower income (income > $50,000: 81.8% vs. 74.6%, P = 0.004), and were more likely to use anti-hypertensive medication (64.4% vs. 58.6%, P = 0.046), and less likely to be physically active (two or more episodes per week: 30.5% vs. 34.4%, P = 0.08). In addition, women with CHD were older (66.4 vs. 64.0, P < 0.0001) and had higher SBP (137.7 mmHg vs. 134.0 mmHg, P = 0.0003), lower DBP (73.4 mmHg vs. 75.0 mmHg, P = 0.005), and higher waist/hip ratio (0.88 vs. 0.87, P < 0.05) than those without CHD. Other risk factors, such as hypertension, history of smoking, hysterectomy, HRT, lipid-lowering medications, and baseline BMI, were not significantly different between those with and without CHD. Family history of diabetes was not significantly different between the incident CHD group (57.6%) and the non-CHD group (60.1%, P = 0.34). A lipid profile was measured in only 34 of 2642 participants (3 CHD cases, 31 non-cases) at baseline with no significant difference noted in the small number of incident CHD cases and non-cases (data not shown).
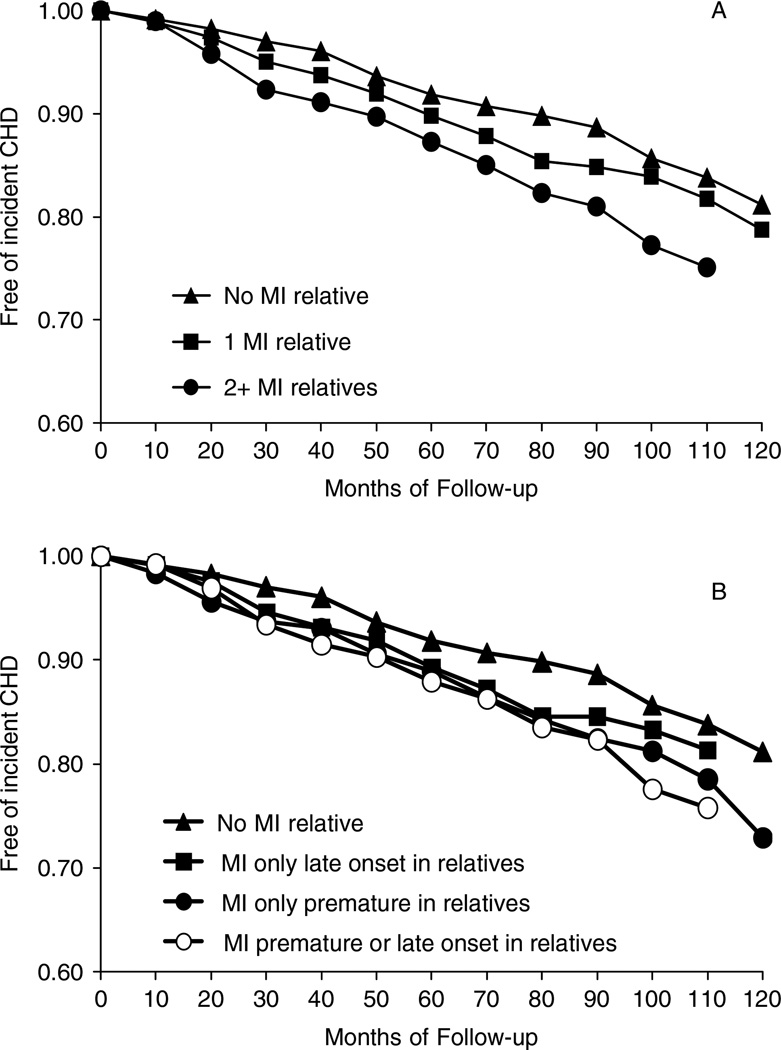
The association between family history of MI and incident CHD in this diabetic cohort is shown in Figure 2, Table 2, and Table 3. Figure 2 illustrates participants remaining free of incident CHD over the follow-up time of the study given a family history of MI. Incident CHD events were more likely to occur in women with a family history of MI, especially in women with two or more relatives who had an MI or a family history of premature MI compared to those without a family history of MI. The difference in survival function by family history of MI was statistically significant at P = 0.0002 when family history was classified as the number of relatives with MI, and P = 0.004 when family history was coded as premature or late onset compared to no MI relatives.
Figure 2.
Survivals function of free of incident coronary heart disease (CHD) in diabetic patients during follow-up stratified by myocardial infarction (MI) in first-degree relatives. Log-rank test for strata homogeneity: P = −0.0002 for Figure 2A and P = −0.004 for Figure 2B
Table 2.
Family History of MI Associated with Incident CHD with or without Adjustment for Covariates in the WHI-OS Diabetic Cohort
| Unadjusted | Adjusted for age and duration of diabetes Med |
Fully adjusteda | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CHD (n = 377) | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P |
| FHx (yes) | 1.43 | 1.16, 1.75 | 0.0003 | 1.44 | 1.18, 1.77 | 0.0004 | 1.50 | 1.20, 1.87 | 0.0003 |
| ≥2 relativesb | 1.69 | 1.31, 2.18 | <0.0001 | 1.67 | 1.30, 2.16 | <0.0001 | 1.79 | 1.35, 2.35 | <0.0001 |
| 1 relativeb | 1.27 | 1.003, 1.61 | 0.047 | 1.31 | 1.03, 1.65 | 0.03 | 1.34 | 1.04, 1.73 | 0.024 |
| Prematurec | 1.38 | 1.11, 1.71 | 0.004 | 1.43 | 1.15, 1.78 | 0.001 | 1.42 | 1.13, 1.79 | 0.003 |
| Late onsetc | 1.22 | 0.99, 1.51 | 0.06 | 1.18 | 0.96, 1.46 | 0.12 | 1.28 | 1.02, 1.60 | 0.03 |
| MI/coronary death (n = 113) | |||||||||
| FHx (yes) | 1.03 | 0.71, 1.49 | 0.9 | 1.04 | 0.72, 1.51 | 0.83 | 1.13 | 0.75, 1.70 | 0.55 |
| ≥2 relativesb | 1.05 | 0.63, 1.74 | 0.86 | 1.00 | 0.60, 1.66 | 0.98 | 1.08 | 0.63, 1.88 | 0.77 |
| 1 relativeb | 1.02 | 0.67, 1.55 | 0.93 | 1.07 | 0.70, 1.64 | 0.75 | 1.16 | 0.73, 1.85 | 0.52 |
| Coronary proceduresd (n = 264) | |||||||||
| FHx (yes) | 1.66 | 1.30, 2.12 | <0.0001 | 1.68 | 1.31, 2.15 | <0.0001 | 1.71 | 1.31, 2.24 | <0.0001 |
| ≥2 relativesb | 2.08 | 1.54, 2.80 | <0.0001 | 2.08 | 1.54, 2.81 | <0.0001 | 2.21 | 1.15, 3.06 | <0.0001 |
| 1 relativeb | 1.41 | 1.06, 1.89 | 0.02 | 1.44 | 1.09, 1.92 | 0.01 | 1.45 | 1.07, 1.97 | 0.017 |
Adjustment for additional covariates: systolic and diastolic blood pressure, years of smoking (20+, 10–20), race, education (≤ high school), income (<$50 000), hypertension medication, lipid lowering medication, hysterectomy, use of HRT, waist/hip ratio, physical activity, and alcohol consumption.
Number of family relatives with MI compared to no family relative with MI
At least one relative with MI first onset at age <55 years for men and/or <65 years for women defined as premature, otherwise, as late onset. The comparison group was family relatives free of MI.
Includes: percutaneous transluminal coronary angioplasty (PTCA), coronary artery bypass angioplasty (CABG), and stent placement. CHD, coronary heart disease; FHx, family history; MI, myocardial infarction; HR, hazard ratio; CI, confidence interval.
Table 3.
Family History of MI and Covariates (in the Same Best Fitting Model) Associated with Incident CHD in the WHI-OS Diabetic Cohort
| All participants | White participants | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | L 95 | U 95 | P | HR | L 95 | U 95 | P | |
| FHx MI in ≥2 relativesa | 1.79 | 1.36 | 2.35 | <0.0001 | 1.60 | 1.16 | 2.20 | 0.004 |
| 1 relativea | 1.38 | 1.08 | 1.78 | 0.011 | 1.42 | 1.07 | 1.90 | 0.016 |
| Age (year) | 1.03 | 1.01 | 1.05 | 0.0005 | 1.03 | 1.01 | 1.05 | 0.005 |
| SBP (mmHg) | 1.01 | 1.003 | 1.02 | 0.001 | 1.01 | 1.002 | 1.02 | 0.018 |
| DBP (mmHg) | 0.98 | 0.97 | 0.999 | 0.005 | 0.98 | 0.97 | 0.998 | 0.026 |
| Diab Meds (years) | 1.03 | 1.02 | 1.05 | <0.0001 | 1.04 | 1.02 | 1.06 | <0.0001 |
| White | 1.49 | 1.16 | 1.93 | 0.002 | ||||
| Physical activity (2) | 0.70 | 0.52 | 0.93 | 0.02 | 0.69 | 0.50 | 0.97 | 0.03 |
| Physical activity (1) | 0.81 | 0.62 | 1.06 | 0.13 | 0.79 | 0.58 | 1.08 | 0.14 |
| Income <$50 000 | 1.42 | 1.07 | 1.89 | 0.01 | 1.46 | 1.07 | 2.00 | 0.02 |
Number of family relatives with MI compared with no family history of MI.
CHD, coronary heart disease; FHx, family history (reference group: no positive family history); MI, myocardial infarction; HR, hazard ratio; CI, confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure; Diab Meds, years of diabetic medication use.
White: compared with all other racial groups; Physical activity: 0 = no walk outside for ≥20 min without stopping (reference group); 1 = <2 episodes/week; 2 = ≥2 episodes/week.
The estimated hazard ratios (HRs) of incident CHD given family history of MI with and without adjustment for the selected covariates are shown in Table 2. Overall, the risk of CHD in participants with a positive family history of MI was 43% higher than in those without such a history. After adjustment for age and years of diabetic medications, the HR was not attenuated. Further adjustment for other CHD risk factors slightly increased the HR (HR = 1.50, 95% CI: 1.20–1.87). Participants who had more relatives with MI were more likely to experience incident CHD (Figure 2A) after adjustment for all covariates (Table 2, HR = 1.79, 95% CI: 1.35–2.35, P < 0.0001 for two or more relatives with MI; HR = 1.34, 95% CI: 1.04–1.72, P = 0.025 for one relative with MI compared to no relative with MI). A family history of premature MI was not better than a family history defined as yes or no in predicting incident CHD among these participants, which can be seen in Table 2 and Figure 2B. In addition, a positive family history of MI was more likely to be associated with coronary procedures (Table 2, HR = 1.71, 95% CI: 1.31, 2.24, P < 0.0001).
Table 3 presents incident CHD associated with a family history of MI and significant covariates (age, duration of anti-diabetic medications, race, physical activity, and low income) of the best-fitting Cox proportional hazards model in all participants or in white participants alone. No interaction between a family history of MI and covariates for the risk of incident CHD was observed in the analysis. In all participants, the risk of incident CHD was 38% higher (HR = 1.38, 95% CI: 1.08–1.78, P = 0.011) if one relative had MI, and was 79% higher (HR = 1.79, 95% CI: 1.36–2.35, P < 0.0001) if two or more relatives had MI, after adjustment for significant covariates. Physical activity decreased the risk of incident CHD, especially having two or more episodes per week (HR = 0.70, 95% CI: 0.52–0.93, P = 0.02, Table 3) after adjustment for covariates. Comparing cases to non-cases, a 1 mmHg increase of SBP was associated with 1% increased risk of incident CHD (HR = 1.01, 95% CI: 1.003–1.02, P = 0.001), whereas a 1 mmHg increase of DBP was associated with 2% decreased risk of incident CHD (HR = 0.98, 95% CI: 0.97–0.99, P = 0.005) after adjustment for other significant covariates. The results from analyses of white participants only were similar to the results from all participants (Table 3). Family history of diabetes, lipid-lowering medication, anti-hypertensive medication, waist/hip ratio, hysterectomy, HRT, smoking, and alcohol consumption were not associated with CHD in the best-fitting model (data not shown). Education was not associated with CHD after accounting for income.
Discussion
This study found a significant association between family history of MI and incident CHD in diabetic postmenopausal women. The extent of CHD associated with a family history of MI in this report is much smaller than that reported by Schumacher et al(RR = 7.6) [16], the only published association study on family history of premature CHD predicting CHD in diabetic individuals. Several factors may cause the differences between the two studies. First of all, the sample size of diabetic women in Schumacher’s study was smaller (n = 948) than in this study (n = 2642). Their small sample size may have made their estimates less precise. Second, also related to the small sample size, the study conducted by Schumacher et al., did not stratify by gender, which may affect the association with CHD. Third, the age distributions in the two studies were different. Our study included 2642 postmenopausal women with diabetes, whereas Schumacher’s study included both pre- and postmenopausal women. The association between family history of MI and incident CHD can be attenuated after accounting for menopausal status, which itself is a risk factor for CHD [19,20]. This may partially explain why our estimated risk of CHD given a positive family history was smaller than that reported by Schumacher et al. Finally, Schumacher’s study was geographically restricted to Utah, whereas WHI was a national sample.
White participants were more likely to have incident CHD in our study because they were more likely to have coronary revascularization procedures, one of the components in the CHD definition. Low socioeconomic status associated with CHD observed in this study was consistent with that observed in many previous studies [24,25]. Some biomarkers such as blood insulin, glucose, and inflammatory markers may confound the association between family history of MI and incident CHD. Unfortunately, those biomarker data were not available at this time.
While an increased SBP was associated with an increased risk of incident CHD, an increased DBP decreased the risk of incident CHD in the same model, suggesting the relative stiffening of the arteries due to diabetes and atherosclerosis as patients age. Hypertension treatment in diabetic and postmenopausal women may need to focus on the reduction of SBP.
Physical activity was associated with a significantly decreased risk of CHD in postmenopausal women with diabetes, which sheds light on CHD prevention strategies for this high-risk group. According to the results shown in Table 3, walking outside for 20 or more min without stopping, for at least twice a week, was associated with a 30% reduction of CHD risk. It should be pointed out that an association study cannot determine the causal factors. Physical inactivity might result from an intermediate influence in the pathway to CHD events. Thus, risk factor management for these high-risk women, especially those with a positive family history of CHD, may include appropriate guidance for physical activity.
Although this study has the strengths of a large sample and a high-risk population, postmenopausal women with diabetes, the study also has some limitations. First, the self-reported family history was not validated in the WHI-OS, which may have led to some misclassification of family history status. However, family history data were collected before the occurrence of CHD events; therefore misclassification, if any, should be non-differential, which causes a bias towards the null. Second, according to the Third Report of the National Cholesterol Education Program [26], diabetic individuals should receive aggressive risk-factor management. Because biomarkers were not available, the effectiveness of risk-factor management on biomarkers such as inflammatory factors and lipid profile was unknown; this may in part explain an association between family history of MI and CHD in diabetic individuals. Third, a positive family history of MI did not significantly predict acute MI/CHD death alone in this report, although the point estimates of the associations were in the expected direction. This was most likely due to insufficient statistical power given the small number of the events (n = 113 of 2642 participants). Fourth, to reduce the misclassification of diabetes at baseline, the study included only participants who had used diabetic medications at baseline and excluded those who had not used diabetic medications. This exclusion may cause a biased estimate of the association. However, the distributions of a positive family history of MI by incident CHD in the included and excluded diabetic women were similar although distributions of some other covariates were slightly different between them. Moreover, we conducted additional analyses in all diabetic participants (with and without diabetic treatment) at baseline (data not shown). Results were similar to the results shown in this report. Finally, because the selection of participants was restricted to postmenopausal women with diabetes, the results may not be generalizable to other populations.
In conclusion, a positive family history of MI in first-degree relatives identifies a group of postmenopausal women under diabetic treatment as being at particularly high risk of CHD. This study suggests that physical activity and SBP control may provide additional benefit to diabetic treatment in the prevention of CHD in these women.
Acknowledgments
We acknowledge all WHI-OS participants and NIH for making the funds available for the study. The WHI program is funded by NIH Contracts: NO1-WH-3-2100, NO1-WH-3-2101, NO1-WH-3-2102, NO1-WH-3-2105, NO1-WH-3-2106, NO1-WH-3-2108, NO1-WH-3-2109, NO1-WH-3-2110, NO1-WH-3-2111, NO1-WH-3-2112, NO1-WH-3-2113, NO1-WH-3-2115, NO1-WH-3-2118, NO1-WH-3-2119, NO1-WH-3-2120, NO1-WH-3-2122, NO1-WH-4-2107, NO1-WH-4-2108, NO1-WH-4-2109, NO1-WH-4-2110, NO1-WH-4-2111, NO1-WH-4-2112, NO1-WH-4-2113, NO1-WH-4-2114, NO1-WH-4-2115, NO1-WH-4-2116, NO1-WH-4-2117, NO1-WH-4-2118, NO1-WH-4-2119, NO1-WH-4-2120, NO1-WH-4-2121, NO1-WH-4-2122, NO1-WH-4-2123, NO1-WH-4-2124, NO1-WH-4-2125, NO1-WH-4-2126, NO1-WH-4-2129, NO1-WH-4-2130, NO1-WH-4-2131, and NO1-WH-4-2132 from the National Heart, Lung, and Blood Institute, NIH.
Footnotes
Conflict of interest
None declared.
References
- 1.CDC. Centers for Disease Control and Prevention. Health, United States, 2007, with chartbook on trends in the health of Americans. CDC, 2007. http://www.cdc.gov/nchs/data/hus/hus07.pdf.
- 2.ADA. Economic consequences of diabetes mellitus in the U.S. in 1997. American Diabetes Association. Diabetes Care. 1998;21:296–309. doi: 10.2337/diacare.21.2.296. [DOI] [PubMed] [Google Scholar]
- 3.Moss SE, Klein R, Klein BE. Cause-specific mortality in a population-based study of diabetes. Am J Public Health. 1991;81:1158–1162. doi: 10.2105/ajph.81.9.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kannel WB, McGee DL. Diabetes and cardiovascular risk factors: the Framingham study. Circulation. 1979;59:8–13. doi: 10.1161/01.cir.59.1.8. [DOI] [PubMed] [Google Scholar]
- 5.Cho E, Manson JE, Stampfer MJ, et al. A prospective study of obesity and risk of coronary heart disease among diabetic women. Diabetes Care. 2002;25:1142–1148. doi: 10.2337/diacare.25.7.1142. [DOI] [PubMed] [Google Scholar]
- 6.Saito I, Folsom AR, Brancati FL, Duncan BB, Chambless LE, McGovern PG. Nontraditional risk factors for coronary heart disease incidence among persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Ann Intern Med. 2000;133:81–91. doi: 10.7326/0003-4819-133-2-200007180-00007. [DOI] [PubMed] [Google Scholar]
- 7.Barrett-Connor E, Khaw K. Family history of heart attack as an independent predictor of death due to cardiovascular disease. Circulation. 1984;69:1065–1069. doi: 10.1161/01.cir.69.6.1065. [DOI] [PubMed] [Google Scholar]
- 8.Colditz GA, Rimm EB, Giovannucci E, Stampfer MJ, Rosner B, Willett WC. A prospective study of parental history of myocardial infarction and coronary artery disease in men. Am J Cardiol. 1991;67:933–938. doi: 10.1016/0002-9149(91)90163-f. [DOI] [PubMed] [Google Scholar]
- 9.Colditz GA, Stampfer MJ, Willett WC, Rosner B, Speizer FE, Hennekens CH. A prospective study of parental history of myocardial infarction and coronary heart disease in women. Am J Epidemiol. 1986;123:48–58. doi: 10.1093/oxfordjournals.aje.a114223. [DOI] [PubMed] [Google Scholar]
- 10.Hopkins PN, Williams RR, Kuida H, et al. Family history as an independent risk factor for incident coronary artery disease in a high-risk cohort in Utah. Am J Cardiol. 1988;62:703–707. doi: 10.1016/0002-9149(88)91206-4. [DOI] [PubMed] [Google Scholar]
- 11.Leander K, Hallqvist J, Reuterwall C, Ahlbom A, de Faire U. Family history of coronary heart disease, a strong risk factor for myocardial infarction interacting with other cardiovascular risk factors: results from the Stockholm Heart Epidemiology Program (SHEEP) Epidemiology. 2001;12:215–221. doi: 10.1097/00001648-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Li R, Bensen JT, Hutchinson RG, et al. Family risk score of coronary heart disease (CHD) as a predictor of CHD: the Atherosclerosis Risk in Communities (ARIC) study and the NHLBI family heart study. Genet Epidemiol. 2000;18:236–250. doi: 10.1002/(SICI)1098-2272(200003)18:3<236::AID-GEPI4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Rissanen AM. Familial occurrence of coronary heart disease: effect of age at diagnosis. Am J Cardiol. 1979;44:60–66. doi: 10.1016/0002-9149(79)90251-0. [DOI] [PubMed] [Google Scholar]
- 14.Rose G. Familial patterns in ischaemic heart disease. Br J Prev Soc Med. 1964;18:75–80. doi: 10.1136/jech.18.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schildkraut JM, Myers RH, Cupples LA, Kiely DK, Kannel WB. Coronary risk associated with age and sex of parental heart disease in the Framingham Study. Am J Cardiol. 1989;64:555–559. doi: 10.1016/0002-9149(89)90477-3. [DOI] [PubMed] [Google Scholar]
- 16.Schumacher MC, Hunt SC, Williams RR. Interactions between diabetes and family history of coronary heart disease and other risk factors for coronary heart disease among adults with diabetes in Utah. Epidemiology. 1990;1:298–304. doi: 10.1097/00001648-199007000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Arias RD. Cardiovascular health and the menopause: the gynecologist as the patients’ interface. Climacteric. 2006;9(Suppl. 1):6–12. doi: 10.1080/13697130600916148. [DOI] [PubMed] [Google Scholar]
- 18.Gorodeski GI. Impact of the menopause on the epidemiology and risk factors of coronary artery heart disease in women. Exp Gerontol. 1994;29:357–375. doi: 10.1016/0531-5565(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 19.Howard BV, Kuller L, Langer R, et al. Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: the Women’s Health Initiative Observational Study. Circulation. 2005;111:1462–1470. doi: 10.1161/01.CIR.0000159344.21672.FD. [DOI] [PubMed] [Google Scholar]
- 20.Kuh D, Langenberg C, Hardy R, et al. Cardiovascular risk at age 53 years in relation to the menopause transition and use of hormone replacement therapy: a prospective British birth cohort study. BJOG. 2005;112:476–485. doi: 10.1111/j.1471-0528.2005.00416.x. [DOI] [PubMed] [Google Scholar]
- 21.WHI, The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 22.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–S121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 23.Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13:S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 24.Loucks EB, Lynch JW, Pilote L, et al. Life-course socioeconomic position and incidence of coronary heart disease: the Framingham Offspring Study. Am J Epidemiol. 2009;169:829–836. doi: 10.1093/aje/kwn403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quatromoni J, Jones R. Inequalities in socio-economic status and invasive procedures for coronary heart disease: a comparison between the USA and the UK. Int J Clin Pract. 2008;62:1910–1919. doi: 10.1111/j.1742-1241.2008.01943.x. [DOI] [PubMed] [Google Scholar]
- 26.NCEP. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]