Abstract
Mitochondrial dysfunction in various tissues has been associated with numerous diseases and conditions including aging. In testes, aging induces atrophy and a decline in male reproductive function but the involvement of mitochondria is not clear. The purpose of this study was to examine whether the mitochondrial profile differed with 1) aging, and 2) 10-weeks of treadmill exercise training, in the testes of young (6 month) and old (24 month) Fischer-344 (F344) animals. Old animals exhibited significant atrophy (30% decline; P<0.05) in testes compared to young animals. However, relative mitochondrial content (cytochrome c oxidase activity and cytochrome c levels) was not altered with age and this was consistent with the lack of change in the mitochondrial biogenesis regulator protein, PGC-1α (peroxisome proliferator-activated receptor gamma coactivator 1-alpha) and its downstream targets NRF-1 (nuclear respiratory factor-1) and Tfam (mitochondrial transcription factor A). No effect was observed in the pro- or anti-apoptotic proteins, Bax and Bcl-2, respectively, but age increased AIF (apoptosis inducing factor; P<0.05) levels. Endurance training induced beneficial mitochondrial adaptations that were more prominent in old animals including greater increases in relative mitochondrial content, biogenesis/remodeling (mitofusin 2; Mfn-2), and antioxidant capacity (MnSOD-mitochondrial superoxide dismutase; P<0.05). Importantly, these exercise-induced changes were associated with an attenuation of testes atrophy in older sedentary animals (P<0.05). Our results indicate that aging-induced atrophy in testes may not be associated with changes in relative mitochondrial content and key regulatory proteins and that exercise started in late-life elicits beneficial changes in mitochondria that may protect against age-induced testicular atrophy.
Keywords: Aging, Exercise, Mitochondria, Biogenesis, Apoptosis
Introduction
Aging is associated with the degeneration and functional decline of numerous tissues and/or organs including the testis, and age-induced testicular atrophy (hypogonadism) affects millions of men each year in the United States (Rhoden and Morgentaler 2004). Age-induced testicular atrophy leads to abnormalities in sperm production and significant reductions in testosterone production, and this occurs across all mammalian species (Bethea and Walker 1979; Ferrini and Barrett-Connor 1998; Kirschner and Coffman 1968; Pirke et al. 1979; Pirke et al. 1980). Notably, reductions in testosterone levels with aging in humans is associated with increased body mass index and waist circumference, and elevated incidence of metabolic syndrome which increases the risk for cardiovascular disease and diabetes (Kupelian et al. 2006; Maggio et al. 2006; Muller et al. 2005; Svartberg et al. 2008). Thus, understanding the underlying cellular and molecular mechanisms responsible for testicular atrophy is a highly relevant health issue. There are several mechanisms that could contribute towards testicular atrophy with age including alterations in the testes composition, impairments in hormone responsiveness, microvascular changes, and mitochondrial dysfunction (Dominguez et al. 2011; Perheentupa and Huhtaniemi 2009; Pirke et al. 1979; Sasano and Ichijo 1969; Mulligan et al. 2001). Although some evidence suggests mitochondrial abnormalities may play a role in testicular dysfunction and/or atrophy, the detailed molecular mechanisms involved have yet to be fully established.
Mitochondria are the primary intracellular source of ATP for the majority of cells in the body and their dysfunction is implicated in the age-related decline of highly metabolic tissues such as muscle, heart, and neurons (Calvani et al. 2012; Trifunovic and Larsson 2008; Wallace 2005). Age-induced mitochondrial dysregulation includes but is not limited to 1) impaired electron transport chain (ETC) function and/or lowered mitochondrial content causing reduced ATP production, 2) ETC overproduction of damaging reactive oxygen species (ROS), and 3) induction of cellular death pathways (apoptosis). Interestingly, exercise is a physiological perturbation that leads to an upregulation of mitochondrial content and function in numerous tissues by activating metabolically sensitive intracellular signaling pathways (i.e. AMPK and p38MAPK) that upregulate a defined set of mitochondrial associated factors/genes. Mitochondrial biogenesis is an intricate process involving the coordinated induction of both nuclear and mitochondrial genomes, the assembly of proteins into an expanding reticulum, and involves dynamic fusion and fission of mitochondria (Hood 2001; Seo et al. 2010). Mitochondrial biogenesis is highly regulated by peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) which coactivates a variety of important transcription factors (i.e. nuclear respiratory factor 1, NRF-1 and mitochondrial transcription factor A, Tfam) involved in mitochondrial regulation (Burgess et al. 2006; Espinoza et al. 2010; Hood 2001; Lin et al. 2005; Scarpulla 2006; Summermatter et al. 2011; Wu et al. 1999). Thus, exercise is a robust physiological perturbation capable of evoking significant improvements in oxidative capacity, physical function, and attenuating age-induced functional decrements in numerous tissues in both young and old individuals, and these beneficial adaptations are partially attributed to the induction of mitochondrial biogenesis pathways (Lanza and Nair 2009; Safdar et al. 2011; Short et al. 2003). However, at present, it is unclear whether exercise induces similar beneficial adaptations and/or alters mitochondrial content within young and old testicular tissue.
Paradoxically, mitochondria are not only responsible for ATP production and energy for “life” of cells, but they also contain pro-apoptotic factors that upon release can lead to cellular “death” through apoptosis (Rigoulet et al. 2011). Mitochondrial apoptotic susceptibility is largely dependent upon the ratio of pro- and anti-apoptotic proteins (Bcl-2 family members; i.e. pro-apoptotic Bax and anti-apoptotic Bcl-2), and ROS that trigger pro-apoptotic release through a specialized mitochondrial permeability transition pore (mtPTP;Primeau et al. 2002). Aging increases mitochondrial apoptotic susceptibility in multiple tissues and exercise has been shown to be efficacious in evoking mitochondrial adaptations that suppress age-induced apoptosis in numerous tissues (Chabi et al. 2008; Dirks and Leeuwenburgh 2002; Dirks and Leeuwenburgh 2004; Ljubicic and Hood 2009; Pistilli et al. 2006). Whether exercise causes adaptive responses in mitochondria and alters mitochondrial apoptotic susceptibility in peripheral tissue such as testis with age has yet to be determined.
Mitochondrial energy metabolism/biogenesis is critical for the normal physiological functioning of all tissues including the testis which is a highly metabolic tissue and heavily dependent on mitochondrial energy production. For example,, mitochondrial function is important for normal spermatogenesis and mitochondrial impairments in the testes are implicated in both testicular atrophy and the decline of male reproductive function but the cellular and molecular mechanisms are not well understood (Amaral et al. 2008; Bajpai et al. 1998; Cummins et al. 1994; Erkkila et al. 2006; Frank and Hurst 1996; Nakada et al. 2006; St John et al. 1997; Vazquez-Memije et al. 2008). Thus, the goal of this study was to investigate whether age-induced testicular atrophy was associated with mitochondrial protein profile changes in aged animals and whether 10-weeks of exercise could confer protection against testicular atrophy and/or potential deleterious mitochondrial protein profile alterations with age. We hypothesized that the testicular mitochondrial protein profile in aged animals would be compromised compared to young, to reflect increased apoptotic susceptibility and reduced mitochondrial content, and that exercise would reduce testicular atrophy and suppress these age-induced changes in key mitochondrial pathways.
Experimental Procedures
Animals
Young (6-mo-old) and aged (24-mo-old) male Fischer-344 rats were obtained from the National Institute on Aging. All animals were housed in a temperature-controlled room with a 12-hr light/dark cycle. Animals were given water and food ad-libitum. The care of the animals in this study was in accordance with NIH guidelines and approved by the University of Florida Institutional Animal Care and Use Committee.
Exercise protocol
Animals from both age groups were randomly assigned to either a sedentary group (Young Sedentary, Y-S; Old Sedentary, O-S) or an exercise-trained group (Young exercise, Y-Ex; Old Exercise, O-Ex). The endurance-exercise protocol was described previously (Dominguez et al. 2011). Briefly, young and old exercise rats were habituated to treadmill exercise, during which each rat walked on a motor-driven treadmill at 15 m/min (0-degree incline), 5 min/day for 3 days. After the habituation period, the incline was raised to 15-degrees for the duration of the training period, while the 15 m/min speed was maintained. During the first 5 wk of training, the time of exercise was increased by 10 min/wk, until 60 min duration was reached by the 6th wk. The trained rats continued to exercise 5-days/wk for 60 min/day for the remainder of the 10 wk training period. Following training, the animals were sacrificed and both of the testes were removed and immediately frozen in liquid N2 and stored for future analyses. It is important to note that while this strain of rodents has a high incidence of developing testicular tumors as they age, animals exhibiting any abnormalities in the phenotype of the testis tissue were excluded from the study.
Cytochrome c oxidase (COX) enzyme activity
COX activity was performed as outlined previously (Adhihetty et al. 2009). Briefly, whole testes homogenates were diluted in buffer (0.1 M KH2PO4 and 2 mM EDTA, pH 7.2) and sonicated (3 × 5 s) on ice. Enzyme activity was determined by the maximal oxidation rate of completely reduced cytochrome c, evaluated as a change in absorbance at 550 nm using a multi-detection microplate reader (Synergy HT, Biotek Instruments, Winooski, VT).
Immunoblotting
Whole testes extracts were prepared as previously described (Joseph et al. 2013a) and separated by 4-15% SDS-PAGE. Gels were subsequently transferred to nitrocellulose membranes using a semi-dry electrotransfer apparatus. Nitrocellulose membranes were then blocked (1 hr) with 5% skim milk in 1x TBST solution [Tris-buffered saline with Tween 20: 25 mM Tris·HCl (pH 7.5), 1 mM NaCl, and 0.1% Tween 20]. Membranes were incubated overnight at 4°C with primary antibodies at a dilution of 1:250 (T-ACC), 1:500 (Bax, Bcl-2, P-AMPK, T-AMPK, P-ACC, PGC-1α, NRF-1, Tfam, Mfn2, 4-HNE), 1:1000 (Hsp70, Fis1), 1:1500 (cytochrome c), 1:2000 (MnSOD) and 1:3500 (AIF). Membranes were washed (3 × 5 min) using TBST and incubated with the corresponding secondary antibody at room temperature (45 min). Antibodies were obtained from Santa Cruz (AIF, sc-9416; Bax, sc-493; Bcl-2, sc-7382; cytochrome c, sc-8385; MnSOD, sc-30080), Abcam (4-HNE, ab46545), Alexis Biochemicals (Fis1, 210-907-R100), Sigma (Mfn2, M6444), Cell Signaling (P-AMPK, 2135S; T-AMPK, 2532S; P-ACC, 3661S; TACC, 3662S), Calbiochem (PGC-1α, 516557; Hsp70, HSP-01; Tfam, DR1071) and Rockland Immunochemicals (NRF-1, 200-401-869). Membranes were washed (3 × 5 min) in TBST and detected using the enhanced chemiluminescence method (ECL, Santa Cruz). Films were scanned and analyzed using the Kodak 1D Imaging Software (v.3.6). Target bands were normalized to the amount of protein loaded in each lane, as determined by densitometric analysis of the corresponding Ponceau S-stained (Joseph et al. 2013b).
Detection of Protein Oxidation
To assess oxidative stress, protein carbonylation was measured by immunoblot analyses utilizing the Oxyblot Protein Oxidation Kit (S7150; Millipore). Briefly, protein extracts (20 μg) were derivatized with 2,4-dinitrophenylhydrazine (DNPH) and subsequently separated by 12% SDS-PAGE and transferred to nitrocellulose membranes. Membranes were probed with antibodies directed toward DNP (1:150) and detected using enhanced chemiluminescence (ECL; Santa Cruz) and exposed to film and analyzed using the Kodak 1D Imaging Software.
Data Interpretation and Analysis
Two-way analyses of variance (ANOVA) were performed to determine the effect of age and exercise in the young and old groups followed by a Bonferroni post-test to determine statistical significance between groups. Statistical differences were considered significant if P < 0.05. Data are expressed as means ± SEM.
Results
Testicular mass is reduced with aging and attenuated with exercise
Testes mass was reduced by 30% (P<0.05) in aged testes when compared to young (Table 1) and this difference was more pronounced (~45% decrease) when corrected for body weight (P<0.05). Exercise training increased absolute testicular weight (and testes weight/body weight) in both age groups (P<0.05) and this effect was more pronounced in older exercised animals than young (1.6- compared to 1.1-fold, respectively) when compared to their age-matched sedentary groups (P<0.05; Table 1). It is well known that Fischer-344 rats are prone to developing testicular tumors as they age and some animals in our colony did display such tumors but were excluded from this study.
Table 1.
Descriptive characteristics of animals
| Young | Old | |||
|---|---|---|---|---|
| Sedentary | Exercise | Sedentary | Exercise | |
| Number of animals | 7 | 7 | 7 | 7 |
| Testis weight, g | 1.57±0.04 | 1.74±0.09# | 1.11±0.12* | 1.79±0.06# |
| Testis weight/Body weight, g/g | 0.0043±0.0001 | 0.0047±0.0002# | 0.0024±0.0002* | 0.0041±0.0001*# |
Animals were weighed at the end of the intervention and body weights represented in grams (g). Testis weights were taken immediately following excision and data represented as a ratio to body weight (g/g). Young (6 mo) and Old (24 mo) animals were randomly assigned to a sedentary or an exercise group.
Significantly different than young (P<0.05).
Significantly different than age-matched sedentary (P±0.05). All data are represented as mean ± SE.
Effect of aging and exercise on mitochondrial content
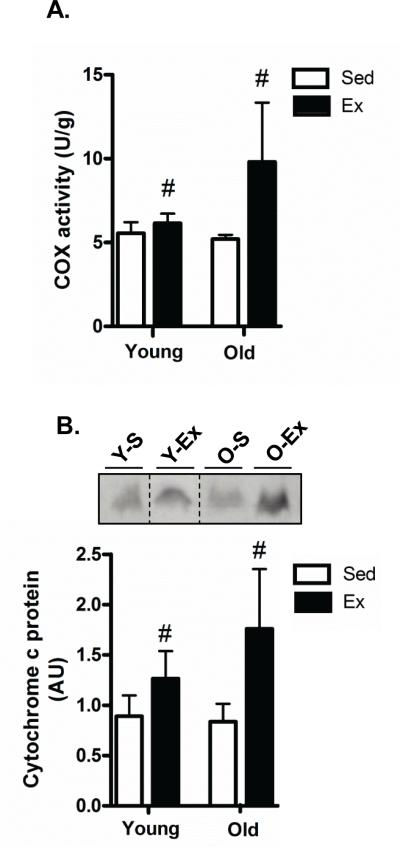
In order to determine whether aging altered mitochondrial content in testes of aged animals, we first measured cytochrome c oxidase (COX) activity, a well-established and commonly used marker of mitochondrial content (Adhihetty et al., 2009; Joseph et al., 2013b). COX activity is a holoenzyme complex comprised of subunits from both the mitochondrial and nuclear genome and thus an increase in its activity reflects a coordinated upregulation of nuclear- and mitochondrial-encoded proteins. The efficacy of our aerobic exercise program was confirmed in a previous report using the same animals where citrate synthase activity in the soleus muscle in both young and old animals was elevated following the training paradigm (Dominguez et al. 2011). While there was no difference in COX activity with age (Fig. 1A), a main effect of training (P<0.05) was observed when compared to age-matched sedentary animals (Fig. 1A). Our increased COX activity with training in young and old testes was confirmed with significant elevations in cytochrome c, another mitochondrial content marker, in both young and old animals (~2.1-fold; P<0.05; Fig. 1B).
Fig. 1. Effect of age and exercise on mitochondrial content.
A) Mitochondrial content was assessed by cytochrome c oxidase (COX) activity in testis tissue isolated from young sedentary (Y-S) and exercise-trained (Y-Ex) animals, and old sedentary (O-S) and exercise-trained (O-Ex) animals. COX activity is expressed per gram of tissue. B) Cytochrome c protein content measured by western blotting. Western blots are shown above with dashed lines indicating that lanes from the gel have been excised and the lanes from a single gel reordered to show a representative image. A graphical summary of the data below (n=7/group). Significance was set at P<0.05 and all data are represented as mean ± SE. Data are expressed as arbitrary units (AU). # P<0.05 vs. age-matched sedentary.
Alterations in mitochondrial signaling molecules in testis
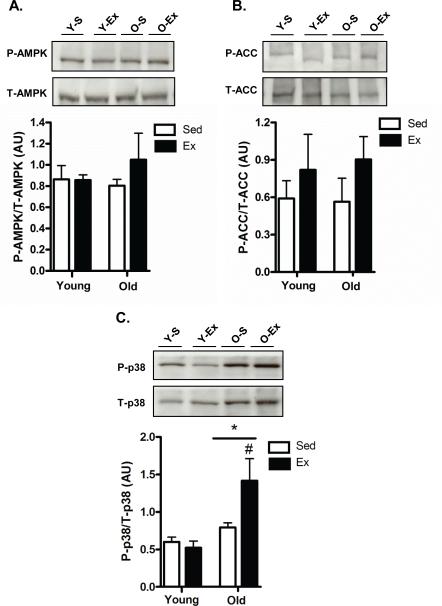
Since our COX activity and cytochrome c data indicated that exercise training increased relative mitochondrial content in the testes of both young and old animals, we next assessed the activity of key proteins involved in upstream mitochondrial biogenesis signaling. No significant changes were detected in 5′ adenosine monophosphate-activated protein kinase (AMPK) or its downstream target acetyl-CoA carboxylase (ACC) with age or exercise (Fig. 2A and 2B, respectively). However, older animals had significantly higher p38 mitogen-activated protein kinase (MAPK) activation (P<0.05) when compared to young (Fig. 2C). Additionally, p38 activation was increased (P<0.05) with exercise in older animals when compared to age-matched sedentary animals (Fig. 2C).
Fig. 2. Mitochondrial biogenesis signaling molecules in testes.
AMPK (A), Acetyl-CoA-carboxylase (ACC; B) and p38 (C) activation in the testes of young sedentary (Y-S) and exercise-trained (Y-Ex) animals and old sedentary (O-S), and exercise-trained (O-Ex) animals. Activation of these proteins was determined by expressing the phosphorylated form of the kinase over total content. Representative blots are shown above with a graphical summary of the data below (n=7/group). Significance was set at P<0.05 and all data are represented as mean ± SE. Data are expressed as arbitrary units (AU).*P<0.05 vs. young, #P<0.05 vs. age-matched sedentary.
Mitochondrial regulators and biogenesis
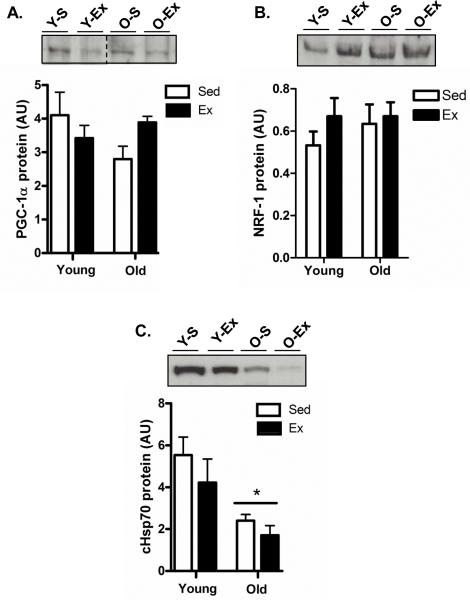
PGC-1α and NRF-1 expression levels have been shown to be important mitochondrial biogenesis regulators in numerous tissues with aging and exercise. Since our data indicated that mitochondrial content was altered with training, we decided to measure these key proteins in testes tissue in young and old tissues following training. PGC-1α and NRF-1 protein content were not altered with age in testis tissue (Fig. 3A and B, respectively), which is consistent with our COX activity and cytochrome c measurements. Interestingly, exercise did not have a significant effect on the levels of these mitochondrial regulatory proteins as anticipated based on the exercise-induced elevations in cytochrome c and COX activity in both young and old animals (Fig. 3A and B, respectively). Additionally, no changes were detected in the mitochondrial transcription factor A (Tfam; data not shown). In contrast, cytosolic heat shock protein 70 (cHSP70), a stress response marker and an important factor for mitochondrial protein import, was markedly reduced in aged animals (P<0.05; Fig. 3C).
Fig. 3. Mitochondrial biogenesis regulators are not altered with age and exercise in testis.
Protein expression levels of the important mitochondrial transcriptional co-activator PGC-1α (A), NRF-1 (B), and the cytosolic mitochondrial chaperone protein, cHSP70 (C) in the testes of young sedentary (Y-S) and exercise-trained (Y-Ex) animals, and old sedentary (O-S) and exercise-trained (O-Ex) animals. Top: Western blots are shown with dashed lines indicating that lanes from the gel have been excised and the lanes from a single gel reordered to show a representative image. Bottom: A summary of data (n=7/group). Significance was set at P<0.05 and all data are represented as mean ± SE. Data are expressed as arbitrary units (AU). *P<0.05 vs. young.
Mitochondrial ultrastructure remodeling in aged testes with exercise
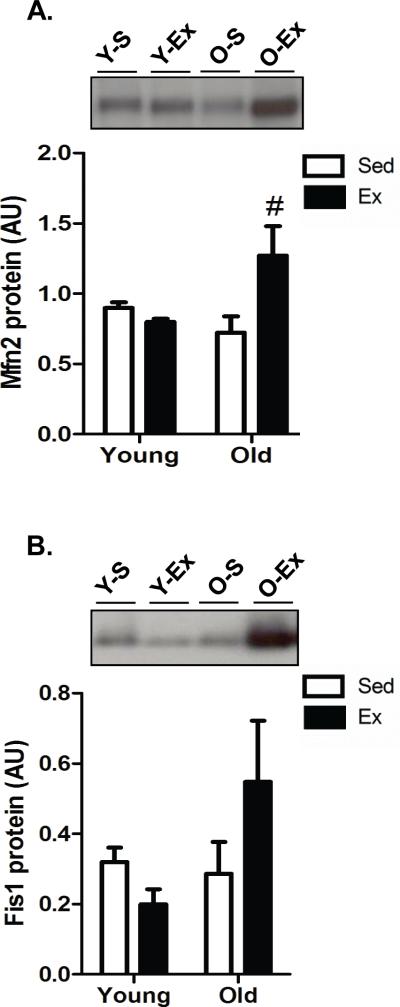
The biogenesis of mitochondria is not only dependent on the upregulation of mitochondrial- and nuclear-encoded proteins to be inserted into the expanding mitochondrial reticulum but is also regulated by fission and fusion processes referred to as mitochondrial dynamics. We therefore measured the key mammalian mitochondrial fusion protein, Mfn2, and the fission protein, Fis1, to determine whether age, or exercise, altered these processes in testis tissue. Indeed, the level of Mfn2 was significantly higher (P<0.05) with exercise in older animals while no change was detected in Fis1 (Fig. 4A and Fig. 4B, respectively).
Fig. 4. Exercise-induced mitochondrial remodeling in testicular tissue.
Protein content of mitochondrial fusion protein Mfn2 (A) and mitochondrial fission protein, Fis1 (B) in the testes of young sedentary (Y-S) and exercise-trained (Y-Ex) animals, and old sedentary (O-S) and exercise-trained (O-Ex) animals. Representative western blots are shown above with a summary of data below. (n=7/group). Significance was set at P<0.05 and all data are represented as mean ± SE. Data are expressed as arbitrary units (AU). #P<0.05 vs. age-matched sedentary.
Effect of aging and exercise on apoptosis pathways and antioxidant capacity in testes
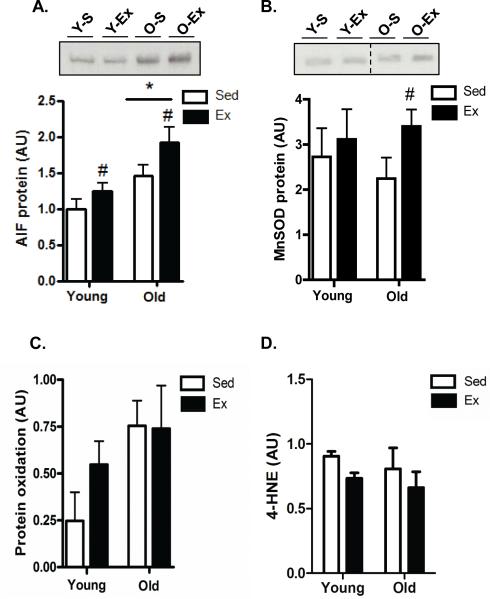
Given the effect of age (decrease-atrophy) and exercise (increase-biogenesis) on testes mass (Table 1), we wanted to determine whether an increase or decrease, respectively, in apoptotic susceptibility might have contributed to these alterations. We found that exercise significantly decreased (P<0.05) pro-apoptotic Bax in the young group while significantly increasing anti-apoptotic Bcl-2 levels (Fig. 5A+5B, respectively). There were no changes in Bax or Bcl-2 levels when comparing young and old testis and exercise did not alter Bax or Bcl-2 in the aged animals. Thus, the Bax-to-Bcl-2 ratio, an indicator of apoptotic susceptibility, was significantly reduced with exercise in the young testis but was unaffected in the old testis (data not shown), and was not different when comparing young and old testis tissue. Levels of apoptosis inducing factor (AIF) were significantly higher in aged testes when compared to young, and exercise caused a significant increase in both young and old testis tissue (p<0.05; Fig. 6A). The antioxidant manganese superoxide dismutase (MnSOD) was not different between young and old testis (Fig. 6B). Interestingly, exercise significantly increased MnSOD in testes tissue from old animals but was unaltered in young testis tissue (P<0.05; Fig. 6B). Lastly, protein carbonylation (Fig. 6C) and lipid peroxidation levels (4-hydroxynonenal, 4-HNE; Fig. 6D) were measured and used as an indirect measure of oxidative stress and/or damage. However, no significant differences were observed with either age or exercise in old or young testes tissue.
Fig. 5. Alterations in key regulators of apoptotic susceptibility in testes.
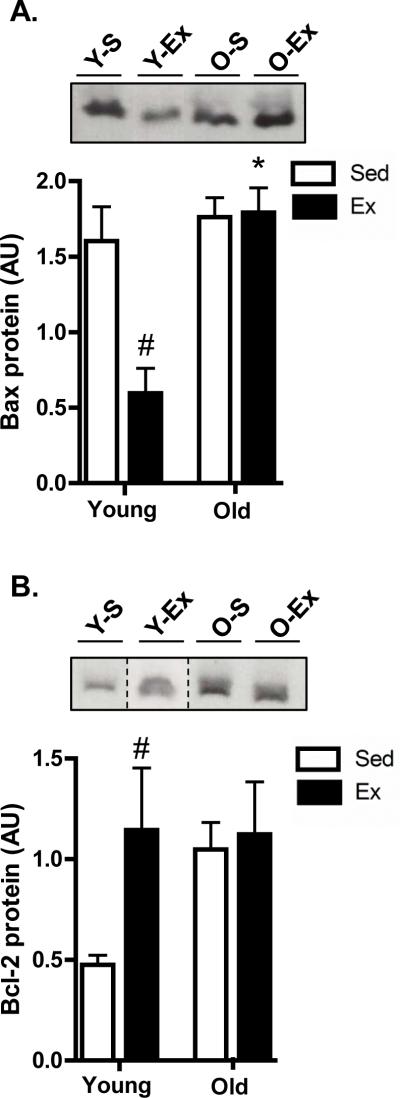
Immunoblotting of the pro-apoptotic protein Bax (A) and the anti-apoptotic protein Bcl-2 (B) in the testes of young sedentary (Y-S) and exercise-trained (Y-Ex) animals, and old sedentary (O-S) and exercise-trained (O-Ex) animals. Western blots are shown above with a graphical summary of data below (n=7/group). Dashed lines indicate that lanes from the gel have been excised and the lanes from a single gel reordered to show a representative image. Significance was set at P<0.05 and all data are represented as mean ± SE. Data are expressed as arbitrary units (AU). *P<0.05 vs. corresponding young group, #P<0.05 vs. age-matched sedentary.
Fig. 6. Exercise training induces greater expression of antioxidant proteins.
Levels of apoptosis inducing factor (AIF; A) and manganese superoxide dismutase (MnSOD; B) in the testes of young sedentary (Y-S) and exercise-trained (Y-Ex) animals, and old sedentary (O-S) and exercise-trained (O-Ex) animals. Western blots are shown above with dashed lines indicating that lanes from the gel have been excised and the lanes from a single gel reordered to show a representative image. A graphical summary of data below (n=7/group). (C) Protein oxidation levels were detected by measuring the amount of carbonylated proteins using Oxyblot analysis and (D) lipid peroxidation by measuring the levels of 4-HNE (4-hydroxynonenal) products. A summary of all experiments is indicated (n=7/group). Significance was set at P<0.05 and all data are represented as mean ± SE. Data are expressed as arbitrary units (AU). *P<0.05 vs. young group, #P<0.05 vs. age-matched sedentary group.
Discussion
Age-induced testicular atrophy affects millions of males within the United States each year and testicular atrophy and/or dysfunction is associated with a significant reduction in circulating testosterone levels. This represents a significant health issue since low testosterone is associated with the development of metabolic syndrome and consequently increases the risk of developing cardiovascular disease and/or diabetes (Kupelian et al. 2006; Maggio et al. 2006; Muller et al. 2005; Svartberg et al. 2008). The etiology of age-induced testicular atrophy is proposed to be multifaceted and may include impairments in hormone responsiveness, microvascular alterations, and mitochondrial dysfunction (Dominguez et al. 2011; Perheentupa and Huhtaniemi 2009; Pirke et al. 1979; Sasano and Ichijo 1969; Mulligan et al. 2001). The purpose of our study was to provide insight into the role of mitochondria in age-induced testicular atrophy. To achieve our objectives, we examined whether mitochondrial biogenesis, mitochondrial dynamics, and mitochondrial apoptotic susceptibility are altered in testicular tissue with aging. Additionally, we investigated whether endurance exercise training, a well-established intervention known to bolster mitochondrial content in muscle, and other peripheral tissues, is capable of altering the mitochondrial profile within testicular tissue when commenced late in life. To our knowledge, this is the first study to investigate whether aging alters the mitochondrial profile in testicular tissue and whether exercise offers protection against age-associated testicular atrophy.
Previous studies have shown that normal testicular function (i.e. spermatogenesis) is dependent upon mitochondria that are capable of modifying their number, shape, and location (De Martino et al. 1979; Fawcett 1970; Hecht 1995; Kaya and Harrison 1976; Machado de Domenech et al. 1972; Meinhardt et al. 1999; Miki et al. 2004; Ruiz-Pesini et al. 1998). Aging has been reported to cause degeneration of testis tissue leading to atrophy and impairments in testicular function (reduced testosterone and sperm production), and mitochondrial dysfunction appears to contribute to these processes (Barnes et al. 1998; Cummins et al. 1994; Frank and Hurst 1996; Nakada et al. 2006; St John et al. 1997; Matsumoto 2002; Vermeulen 1991). To further underscore the importance of mitochondria in testis tissue, patients with mitochondrial disease typically exhibit significant abnormalities in testicular function (Folgero et al. 1993; Ruiz-Pesini et al. 1998; Spiropoulos et al. 2002). Taken together, this evidence suggests mitochondrial integrity and mitochondrial function are critical for maintaining normal testicular function and that aging appears to impair mitochondrial processes which may partially contribute to atrophy and dysfunction within testicular tissue.
In our study, we found that aging caused significant testicular atrophy but were surprised that this was not associated with alterations in mitochondrial content (COX activity and cytochrome c), mitochondrial biogenesis regulators (PGC-1α, NRF-1, or Tfam), or mitochondrial apoptotic susceptibility. Thus, our data indicate age-induced testicular atrophy is not attributable to decrements in mitochondrial content and/or key mitochondrial regulatory proteins or enhanced mitochondrial apoptosis. This was in contrast to our hypothesis and is in opposition to the age-related decline in mitochondrial content typically found in other tissues. However, our data does not preclude the possibility that age may impair mitochondrial function without altering overall mitochondrial content. In fact, the most profound evidence and support for this hypothesis occurs within tumors in cancer where blood flow and microvascular oxygen supply (PO2) is limited, and tumorous cells exhibit a metabolic shift from oxidative to glycolytic metabolism, termed the Warburg effect (Lopez-Lazaro 2008). In tumors, mitochondrial content remains constant but the function of these organelles is significantly impaired and the cells are dependent upon glycolytic pathways for energy production. Interestingly, similar to the suppressed oxygenation within tumors, a previous report by our collaborators showed a 50% reduction in PO2 within the aged testes microvasculature of the animals reported in our study (Dominguez et al. 2011). We speculate that the age-induced reduction in PO2 may lead to suppressed mitochondrial function and increased reliance on glycolytic pathways for energy provision due to the reduced oxygen supply, similar to that of the Warburg effect. Unfortunately, due to the logistics of our experiments, we were unable to directly assess mitochondrial function (i.e. oxygen consumption), and therefore a more detailed investigation of these metabolic pathways is required to confirm this hypothesis.
Although relative mitochondrial content and biogenesis markers were unaltered with age, we did find significant differences in the important mitochondrial chaperone protein, cHSP70. cHSP70 is intimately involved in mitochondrial regulation since it is responsible for the folding, targeting, and translocation of newly synthesized nuclear-encoded precursor proteins from the cytosolic fraction into mitochondria (Hood and Joseph 2004). Moreover, cHSP70 also protects against denaturation and/or formation of mitochondrial protein aggregates under stressful cellular conditions (Parsell and Lindquist 1993; Sarge and Cullen 1997). Thus, reduced cHSP70 in aged testicular tissue suggests possible downstream impairments in the folding and translocation of precursor proteins to mitochondria which could affect the functional assembly of ETC complexes to ultimately reduce mitochondrial function (Morimoto 2008). As previously mentioned, further work is necessary to determine whether intrinsic mitochondrial function is altered in the testes with age.
Mechanistically, the mitochondrial theory of aging suggests that age-related mitochondrial dysfunction would eventually cause an accumulation of ROS-induced damage. In support of this, studies have shown elevations in ROS, and increased markers of oxidative damage in the testes with age which occur coincident with reductions in mitochondrial number and suppressed ETC activity (Amaral et al. 2008; Vazquez-Memije et al. 2008). Thus, we anticipated that oxidative damage markers would be elevated in our study but there were no significant differences when comparing young and old testes tissue. We did detect higher levels of AIF in the old testis, a mitochondrially located flavoprotein with NAD(P)H-dependent redox activity capable of acting as a mitochondrially-located anti-oxidant (Baker and Hepple 2006; Dirks and Leeuwenburgh 2004; Ljubicic et al. 2009). It is possible that age-induced elevations in AIF may represent an antioxidant compensatory upregulation to protect mitochondrial macromolecules against ROS-induced modification with age. Interestingly, we also found that exercise training enhanced the mitochondrial antioxidant MnSOD in testicular tissue from old but not young animals. We speculate that this may be an adaptation to thwart exercise-induced elevations in ROS that would potentially exceed a cellular threshold level in the old but not the young testes tissue.
It is well established that exercise training improves the oxidative capacity of a number of tissues through the upregulation of mitochondrial biogenesis pathways in both young and old animals (Adhihetty et al. 2003; Boveris and Navarro 2008). Exercise-induced elevations in mitochondrial biogenesis have been shown to decrease ROS production, suppress mitochondrial apoptotic susceptibility, and reduce myonuclear DNA fragmentation in numerous tissues of both young and old animals (Kwak et al. 2006; Ljubicic et al. 2009; Siu et al. 2004; Siu et al. 2005; Song et al. 2006). However, only a few studies have investigated whether exercise causes beneficial adaptations in testicular tissue (Chigurupati et al. 2008; Safdar et al. 2011; Zhao et al. 2013). For example, Safdar et al. (2011) demonstrated that endurance exercise confers protection against progeroid aging by preventing decrements in mitochondrial biogenesis/function, suppressing apoptosis, and attenuating the loss in tissue mass in multiple tissues, including testicular tissue. In the present study, we observed a number of key exercise-induced beneficial mitochondrial adaptations in the testes of both young and old animals. However, the magnitude of the exercise-evoked mitochondrial changes tended to be greater in the aged animals (COX activity and cytochrome c), and some of the exercise-mediated mitochondrial adaptations and apparent trends appeared to be exclusive to the old testis (AMPK, ACC, p38 PGC-1, Mfn-2, Fis1) indicating an age-specific mitochondrial adaptive response to exercise in testicular tissue. Additionally, in contrast to findings in muscle and other tissues, the exercise-induced mitochondrial adaptations were not associated with elevations in PGC-1α suggesting that PGC-1α may play a lesser role in mitochondrial adaptations in testis as compared to other tissues (i.e. muscle). It is important to note that the activity of PGC-1α is regulated by a number of post-translational processes (i.e. phosphorylation and acetylation) and without measuring these cellular modifications, the extent of the involvement of this protein cannot be entirely determined (Baur et al. 2006; Nemoto et al. 2005; Rodgers et al. 2005). Mitochondrial biogenesis depends upon key proteins involved in altering the mitochondrial morphology to accommodate compositional changes of the organelle during biogenesis (Seo et al. 2010). Indeed, similar to previous studies (Cartoni et al. 2005; Iqbal et al. 2013), we found exercise-induced alterations in mitochondrial remodeling proteins but these responses only occurred in the aged testicular tissue, and are consistent with much of our data indicating a more robust mitochondrial adaptive response within the aged compared to the young testicular tissue. Taken together, our study represents one of the first to demonstrate that exercise is a physiological perturbation capable of evoking mitochondrial biogenesis in the testicular tissue of both young and old animals. In this study, we were unable to conclude whether the aforementioned exercise-induced alterations in mitochondria and testicular mass were associated with improvements in function since we did not measure testosterone levels or reproductive function in these animals. In fact, previous studies demonstrate that exercise training reduces testosterone levels (Hu et al. 1998; MacKelvie et al. 2000; Tyndall et al. 1996; Webb et al. 1984) and negatively impacts sperm motility and number (Vaamonde et al. 2006; Vaamonde et al. 2009).
In this study we have attempted to offer detailed insight into the mitochondrial alterations that occur in, 1) the age-associated decline in function and/or atrophy of testicular tissue and, 2) the response to exercise training within young and old testis. Our data suggest that relative mitochondrial content and apoptotic susceptibility are not associated with age-induced testicular atrophy. However, we speculate mitochondrial function may be impaired with age and partially contribute towards testicular atrophy but more work is necessary to confirm this hypothesis. Our study confirms exercise training is capable of evoking mitochondrial biogenesis in both young and old testicular tissue but interestingly our data also indicates the novel finding that exercise evokes a more robust mitochondrial biogenesis program in the aged testicular tissue. Moreover, perhaps the most interesting and clinically relevant finding of the current study was that exercise training started in late-life either protects against age-induced testicular atrophy and/or restores testicular mass, and mitochondrial adaptations appear to partially contribute toward this improvement. This study demonstrates the need to pursue more research investigating the role of mitochondria in testicular dysfunction with aging and the potential to improve these age-related decrements in the testes by targeting mitochondria.
Acknowledgements
We are grateful to John Stabley for his assistance in sample preparation and organization. Additionally, we are thankful for the undergraduate research assistants Jason Silvestre, Sean Carey-Love, Daniel Tuckerman, and Nick Wawrzyniak who helped with the pulverization and preparation of samples. This work was supported by start-up funds allocated to Dr. Peter Adhihetty from the College of Health and Human Performance and the Department of Applied Physiology and Kinesiology at the University of Florida (UF). This work was also supported from grants from the National Institutes of Health awarded to Dr. Bradley Behnke (AG-032327 and 1R36-AG-036816-01) and the Florida Biomedical Research Program (1BN-02; Dr. Adhihetty-Co-I).
References
- Adhihetty PJ, Irrcher I, Joseph AM, Ljubicic V, Hood DA. Plasticity of skeletal muscle mitochondria in response to contractile activity. Exp Physiol. 2003;88(1):99–107. doi: 10.1113/eph8802505. [DOI] [PubMed] [Google Scholar]
- Adhihetty PJ, Uguccioni G, Leick L, Hidalgo J, Pilegaard H, Hood DA. The role of PGC-1alpha on mitochondrial function and apoptotic susceptibility in muscle. AmJPhysiol Cell Physiol. 2009;297(1):C217–C225. doi: 10.1152/ajpcell.00070.2009. doi:00070.2009 [pii];10.1152/ajpcell.00070.2009 [doi] [DOI] [PubMed] [Google Scholar]
- Amaral S, Mota P, Rodrigues AS, Martins L, Oliveira PJ, Ramalho-Santos J. Testicular aging involves mitochondrial dysfunction as well as an increase in UCP2 levels and proton leak. FEBS Lett. 2008;582(30):4191–4196. doi: 10.1016/j.febslet.2008.11.020. doi:10.1016/j.febslet.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Bajpai M, Gupta G, Setty BS. Changes in carbohydrate metabolism of testicular germ cells during meiosis in the rat. Eur J Endocrinol. 1998;138(3):322–327. doi: 10.1530/eje.0.1380322. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Hepple RT. Elevated caspase and AIF gene expression correlate with progression of sarcopenia during aging in male F344BN rats. Exp Gerontol. 2006;41(11):1149–1156. doi: 10.1016/j.exger.2006.08.007. doi:10.1016/j.exger.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Barnes CJ, Covington BWt, Cameron IL, Lee M. Effect of aging on spontaneous and induced mouse testicular germ cell apoptosis. Aging (Milano) 1998;10(6):497–501. doi: 10.1007/BF03340164. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le CD, Shaw RJ, Navas P, Puigserver P, Ingram DK, de CR, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. doi:nature05354 [pii];10.1038/nature05354 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Walker RF. Age-related changes in reproductive hormones and in Leydig cell responsivity in the male Fischer 344 rat. J Gerontol. 1979;34(1):21–27. doi: 10.1093/geronj/34.1.21. [DOI] [PubMed] [Google Scholar]
- Boveris A, Navarro A. Systemic and mitochondrial adaptive responses to moderate exercise in rodents. Free radical biology & medicine. 2008;44(2):224–229. doi: 10.1016/j.freeradbiomed.2007.08.015. doi:10.1016/j.freeradbiomed.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Burgess SC, Leone TC, Wende AR, Croce MA, Chen Z, Sherry AD, Malloy CR, Finck BN. Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha)-deficient mice. J Biol Chem. 2006;281(28):19000–19008. doi: 10.1074/jbc.M600050200. doi:10.1074/jbc.M600050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvani R, Joseph AM, Adhihetty PJ, Miccheli A, Bossola M, Leeuwenburgh C, Bernabei R, Marzetti E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. BiolChem. 2012 doi: 10.1515/hsz-2012-0247. doi:10.1515/hsz-2012-0247 [doi];/j/bchm.just-accepted/hsz-2012-0247/hsz-2012-0247.xml [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartoni R, Leger B, Hock MB, Praz M, Crettenand A, Pich S, Ziltener JL, Luthi F, Deriaz O, Zorzano A, Gobelet C, Kralli A, Russell AP. Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J Physiol. 2005;567(Pt 1):349–358. doi: 10.1113/jphysiol.2005.092031. doi:10.1113/jphysiol.2005.092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell. 2008;7(1):2–12. doi: 10.1111/j.1474-9726.2007.00347.x. doi:10.1111/j.1474-9726.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Chigurupati S, Son TG, Hyun DH, Lathia JD, Mughal MR, Savell J, Li SC, Nagaraju GP, Chan SL, Arumugam TV, Mattson MP. Lifelong running reduces oxidative stress and degenerative changes in the testes of mice. J Endocrinol. 2008;199(2):333–341. doi: 10.1677/JOE-08-0306. doi:10.1677/JOE-08-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins JM, Jequier AM, Kan R. Molecular biology of human male infertility: links with aging, mitochondrial genetics, and oxidative stress? Mol Reprod Dev. 1994;37(3):345–362. doi: 10.1002/mrd.1080370314. doi:10.1002/mrd.1080370314. [DOI] [PubMed] [Google Scholar]
- De Martino C, Floridi A, Marcante ML, Malorni W, Scorza Barcellona P, Bellocci M, Silvestrini B. Morphological, histochemical and biochemical studies on germ cell mitochondria of normal rats. Cell and tissue research. 1979;196(1):1–22. doi: 10.1007/BF00236345. [DOI] [PubMed] [Google Scholar]
- Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. AmJPhysiol RegulIntegrComp Physiol. 2002;282(2):R519–R527. doi: 10.1152/ajpregu.00458.2001. doi:10.1152/ajpregu.00458.2001 [doi] [DOI] [PubMed] [Google Scholar]
- Dirks AJ, Leeuwenburgh C. Aging and lifelong calorie restriction result in adaptations of skeletal muscle apoptosis repressor, apoptosis-inducing factor, X-linked inhibitor of apoptosis, caspase-3, and caspase-12. Free RadicBiolMed. 2004;36(1):27–39. doi: 10.1016/j.freeradbiomed.2003.10.003. doi:10.1016/j.freeradbiomed.2003.10.003 [doi];S0891584903006750 [pii] [DOI] [PubMed] [Google Scholar]
- Dominguez JM, 2nd, Davis RT, 3rd, McCullough DJ, Stabley JN, Behnke BJ. Aging and exercise training reduce testes microvascular PO2 and alter vasoconstrictor responsiveness in testicular arterioles. Am J Physiol Regul Integr Comp Physiol. 2011;301(3):R801–810. doi: 10.1152/ajpregu.00203.2011. doi:10.1152/ajpregu.00203.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkkila K, Kyttanen S, Wikstrom M, Taari K, Hikim AP, Swerdloff RS, Dunkel L. Regulation of human male germ cell death by modulators of ATP production. Am J Physiol Endocrinol Metab. 2006;290(6):E1145–1154. doi: 10.1152/ajpendo.00142.2005. doi:10.1152/ajpendo.00142.2005. [DOI] [PubMed] [Google Scholar]
- Espinoza DO, Boros LG, Crunkhorn S, Gami H, Patti ME. Dual modulation of both lipid oxidation and synthesis by peroxisome proliferator-activated receptor-gamma coactivator-1alpha and -1beta in cultured myotubes. Faseb J. 2010;24(4):1003–1014. doi: 10.1096/fj.09-133728. doi:10.1096/fj.09-133728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett DW. A comparative view of sperm ultrastructure. Biology of reproduction Supplement. 1970;2:90–127. [PubMed] [Google Scholar]
- Ferrini RL, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. American journal of epidemiology. 1998;147(8):750–754. doi: 10.1093/oxfordjournals.aje.a009519. [DOI] [PubMed] [Google Scholar]
- Folgero T, Bertheussen K, Lindal S, Torbergsen T, Oian P. Mitochondrial disease and reduced sperm motility. Human reproduction. 1993;8(11):1863–1868. doi: 10.1093/oxfordjournals.humrep.a137950. [DOI] [PubMed] [Google Scholar]
- Frank SA, Hurst LD. Mitochondria and male disease. Nature. 1996;383(6597):224. doi: 10.1038/383224a0. doi:10.1038/383224a0. [DOI] [PubMed] [Google Scholar]
- Hecht NB. The making of a spermatozoon: a molecular perspective. Developmental genetics. 1995;16(2):95–103. doi: 10.1002/dvg.1020160202. doi:10.1002/dvg.1020160202. [DOI] [PubMed] [Google Scholar]
- Hood DA. Invited Review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. JApplPhysiol. 2001;90(3):1137–1157. doi: 10.1152/jappl.2001.90.3.1137. [DOI] [PubMed] [Google Scholar]
- Hood DA, Joseph AM. Mitochondrial assembly: protein import. The Proceedings of the Nutrition Society. 2004;63(2):293–300. doi: 10.1079/PNS2004342. doi:10.1079/PNS2004342. [DOI] [PubMed] [Google Scholar]
- Hu Y, Asano K, Mizuno K, Usuki S, Kawakura Y. Comparisons of serum testosterone and corticosterone between exercise training during normoxia and hypobaric hypoxia in rats. Eur J Appl Physiol Occup Physiol. 1998;78(5):417–421. doi: 10.1007/s004210050440. doi:10.1007/s004210050440. [DOI] [PubMed] [Google Scholar]
- Iqbal S, Ostojic O, Singh K, Joseph AM, Hood DA. Expression of mitochondrial fission and fusion regulatory proteins in skeletal muscle during chronic use and disuse. Muscle Nerve. 2013 doi: 10.1002/mus.23838. doi:10.1002/mus.23838. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Adhihetty PJ, Wawrzyniak NR, Wohlgemuth SE, Picca A, Kujoth GC, Prolla TA, Leeuwenburgh C. Dysregulation of mitochondrial quality control processes contribute to sarcopenia in a mouse model of premature aging. PloS one. 2013a;8(7):e69327. doi: 10.1371/journal.pone.0069327. doi:10.1371/journal.pone.0069327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph AM, Malamo AG, Silvestre J, Wawrzyniak N, Carey-Love S, Nguyen LM, Dutta D, Xu J, Leeuwenburgh C, Adhihetty PJ. Short-term caloric restriction, resveratrol, or combined treatment regimens initiated in late-life alter mitochondrial protein expression profiles in a fiber-type specific manner in aged animals. Exp Gerontol. 2013b;48(9):858–868. doi: 10.1016/j.exger.2013.05.061. doi:10.1016/j.exger.2013.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya M, Harrison RG. The ultrastructural relationships between Sertoli cells and spermatogenic cells in the rat. Journal of anatomy. 1976;121(Pt 2):279–290. [PMC free article] [PubMed] [Google Scholar]
- Kirschner MA, Coffman GD. Measurement of plasma testosterone and “delta-4”-androstenedione using electron capture gas-liquid chromatography. The Journal of clinical endocrinology and metabolism. 1968;28(9):1347–1355. doi: 10.1210/jcem-28-9-1347. [DOI] [PubMed] [Google Scholar]
- Kupelian V, Shabsigh R, Araujo AB, O'Donnell AB, McKinlay JB. Erectile dysfunction as a predictor of the metabolic syndrome in aging men: results from the Massachusetts Male Aging Study. The Journal of urology. 2006;176(1):222–226. doi: 10.1016/S0022-5347(06)00503-9. doi:10.1016/S0022-5347(06)00503-9. [DOI] [PubMed] [Google Scholar]
- Kwak HB, Song W, Lawler JM. Exercise training attenuates age-induced elevation in Bax/Bcl-2 ratio, apoptosis, and remodeling in the rat heart. Faseb J. 2006;20(6):791–793. doi: 10.1096/fj.05-5116fje. doi:10.1096/fj.05-5116fje. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Nair KS. Muscle mitochondrial changes with aging and exercise. Am J Clin Nutr. 2009;89(1):467S–471S. doi: 10.3945/ajcn.2008.26717D. doi:10.3945/ajcn.2008.26717D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1(6):361–370. doi: 10.1016/j.cmet.2005.05.004. doi:10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Ljubicic V, Hood DA. Diminished contraction-induced intracellular signaling towards mitochondrial biogenesis in aged skeletal muscle. Aging Cell. 2009;8(4):394–404. doi: 10.1111/j.1474-9726.2009.00483.x. doi:10.1111/j.1474-9726.2009.00483.x. [DOI] [PubMed] [Google Scholar]
- Ljubicic V, Joseph AM, Adhihetty PJ, Huang JH, Saleem A, Uguccioni G, Hood DA. Molecular basis for an attenuated mitochondrial adaptive plasticity in aged skeletal muscle. Aging (Albany NY) 2009;1(9):818–830. doi: 10.18632/aging.100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lazaro M. The warburg effect: why and how do cancer cells activate glycolysis in the presence of oxygen? Anti-cancer agents in medicinal chemistry. 2008;8(3):305–312. doi: 10.2174/187152008783961932. [DOI] [PubMed] [Google Scholar]
- Machado de Domenech E, Domenech CE, Aoki A, Blanco A. Association of the testicular lactate dehydrogenase isozyme with a special type of mitochondria. Biology of reproduction. 1972;6(1):136–147. doi: 10.1093/biolreprod/6.1.136. [DOI] [PubMed] [Google Scholar]
- MacKelvie KJ, Taunton JE, McKay HA, Khan KM. Bone mineral density and serum testosterone in chronically trained, high mileage 40-55 year old male runners. Br J Sports Med. 2000;34(4):273–278. doi: 10.1136/bjsm.34.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio M, Lauretani F, Ceda GP, Bandinelli S, Basaria S, Ble A, Egan J, Paolisso G, Najjar S, Jeffrey Metter E, Valenti G, Guralnik JM, Ferrucci L. Association between hormones and metabolic syndrome in older Italian men. Journal of the American Geriatrics Society. 2006;54(12):1832–1838. doi: 10.1111/j.1532-5415.2006.00963.x. doi:10.1111/j.1532-5415.2006.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci. 2002;57(2):M76–99. doi: 10.1093/gerona/57.2.m76. [DOI] [PubMed] [Google Scholar]
- Meinhardt A, Wilhelm B, Seitz J. Expression of mitochondrial marker proteins during spermatogenesis. Human reproduction update. 1999;5(2):108–119. doi: 10.1093/humupd/5.2.108. [DOI] [PubMed] [Google Scholar]
- Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O'Brien DA. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci U S A. 2004;101(47):16501–16506. doi: 10.1073/pnas.0407708101. doi:10.1073/pnas.0407708101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22(11):1427–1438. doi: 10.1101/gad.1657108. doi:10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Grobbee DE, den Tonkelaar I, Lamberts SW, van der Schouw YT. Endogenous sex hormones and metabolic syndrome in aging men. The Journal of clinical endocrinology and metabolism. 2005;90(5):2618–2623. doi: 10.1210/jc.2004-1158. doi:10.1210/jc.2004-1158. [DOI] [PubMed] [Google Scholar]
- Mulligan T, Iranmanesh A, Veldhuis JD. Pulsatile iv infusion of recombinant human LH in leuprolide-suppressed men unmasks impoverished Leydig-cell secretory responsiveness to midphysiological LH drive in the aging male. The Journal of clinical endocrinology and metabolism. 2001;86(11):5547–5553. doi: 10.1210/jcem.86.11.8004. [DOI] [PubMed] [Google Scholar]
- Nakada K, Sato A, Yoshida K, Morita T, Tanaka H, Inoue S, Yonekawa H, Hayashi J. Mitochondria-related male infertility. Proc Natl Acad Sci U S A. 2006;103(41):15148–15153. doi: 10.1073/pnas.0604641103. doi:10.1073/pnas.0604641103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha}. JBiolChem. 2005;280(16):16456–16460. doi: 10.1074/jbc.M501485200. doi:M501485200 [pii];10.1074/jbc.M501485200 [doi] [DOI] [PubMed] [Google Scholar]
- Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. doi:10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Perheentupa A, Huhtaniemi I. Aging of the human ovary and testis. Molecular and cellular endocrinology. 2009;299(1):2–13. doi: 10.1016/j.mce.2008.11.004. doi:10.1016/j.mce.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Pirke KM, Bofilias I, Sintermann R, Langhammer H, Wolf I, Pabst HW. Relative capillary blood flow and Leydig cell function in old rats. Endocrinology. 1979;105(3):842–845. doi: 10.1210/endo-105-3-842. [DOI] [PubMed] [Google Scholar]
- Pirke KM, Sintermann R, Vogt HJ. Testosterone and testosterone precursors in the spermatic vein and in the testicular tissue of old men. Reduced oxygen supply may explain the relative increase of testicular progesterone and 17 alpha-hydroxyprogesterone content and production in old age. Gerontology. 1980;26(4):221–230. doi: 10.1159/000212418. [DOI] [PubMed] [Google Scholar]
- Pistilli EE, Jackson JR, Alway SE. Death receptor-associated pro-apoptotic signaling in aged skeletal muscle. Apoptosis : an international journal on programmed cell death. 2006;11(12):2115–2126. doi: 10.1007/s10495-006-0194-6. doi:10.1007/s10495-006-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primeau AJ, Adhihetty PJ, Hood DA. Apoptosis in heart and skeletal muscle. Canadian journal of applied physiology = Revue canadienne de physiologie appliquee. 2002;27(4):349–395. doi: 10.1139/h02-020. [DOI] [PubMed] [Google Scholar]
- Rhoden EL, Morgentaler A. Treatment of testosterone-induced gynecomastia with the aromatase inhibitor, anastrozole. International journal of impotence research. 2004;16(1):95–97. doi: 10.1038/sj.ijir.3901154. doi:10.1038/sj.ijir.3901154. [DOI] [PubMed] [Google Scholar]
- Rigoulet M, Yoboue ED, Devin A. Mitochondrial ROS generation and its regulation: mechanisms involved in H(2)O(2) signaling. Antioxid Redox Signal. 2011;14(3):459–468. doi: 10.1089/ars.2010.3363. doi:10.1089/ars.2010.3363. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434(7029):113–118. doi: 10.1038/nature03354. doi:nature03354 [pii];10.1038/nature03354 [doi] [DOI] [PubMed] [Google Scholar]
- Ruiz-Pesini E, Diez C, Lapena AC, Perez-Martos A, Montoya J, Alvarez E, Arenas J, Lopez-Perez MJ. Correlation of sperm motility with mitochondrial enzymatic activities. Clinical chemistry. 1998;44(8 Pt 1):1616–1620. [PubMed] [Google Scholar]
- Safdar A, Bourgeois JM, Ogborn DI, Little JP, Hettinga BP, Akhtar M, Thompson JE, Melov S, Mocellin NJ, Kujoth GC, Prolla TA, Tarnopolsky MA. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci U S A. 2011;108(10):4135–4140. doi: 10.1073/pnas.1019581108. doi:10.1073/pnas.1019581108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarge KD, Cullen KE. Regulation of hsp expression during rodent spermatogenesis. Cell Mol Life Sci. 1997;53(2):191–197. doi: 10.1007/PL00000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasano N, Ichijo S. Vascular patterns of the human testis with special reference to its senile changes. The Tohoku journal of experimental medicine. 1969;99(3):269–280. doi: 10.1620/tjem.99.269. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC. Nuclear control of respiratory gene expression in mammalian cells. J Cell Biochem. 2006;97(4):673–683. doi: 10.1002/jcb.20743. doi:10.1002/jcb.20743. [DOI] [PubMed] [Google Scholar]
- Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci. 2010;123(Pt 15):2533–2542. doi: 10.1242/jcs.070490. doi:10.1242/jcs.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52(8):1888–1896. doi: 10.2337/diabetes.52.8.1888. [DOI] [PubMed] [Google Scholar]
- Siu PM, Bryner RW, Martyn JK, Alway SE. Apoptotic adaptations from exercise training in skeletal and cardiac muscles. Faseb J. 2004;18(10):1150–1152. doi: 10.1096/fj.03-1291fje. doi:10.1096/fj.03-1291fje. [DOI] [PubMed] [Google Scholar]
- Siu PM, Bryner RW, Murlasits Z, Alway SE. Response of XIAP, ARC, and FLIP apoptotic suppressors to 8 wk of treadmill running in rat heart and skeletal muscle. J Appl Physiol (1985) 2005;99(1):204–209. doi: 10.1152/japplphysiol.00084.2005. doi:10.1152/japplphysiol.00084.2005. [DOI] [PubMed] [Google Scholar]
- Song W, Kwak HB, Lawler JM. Exercise training attenuates age-induced changes in apoptotic signaling in rat skeletal muscle. Antioxid Redox Signal. 2006;8(3-4):517–528. doi: 10.1089/ars.2006.8.517. doi:10.1089/ars.2006.8.517. [DOI] [PubMed] [Google Scholar]
- Spiropoulos J, Turnbull DM, Chinnery PF. Can mitochondrial DNA mutations cause sperm dysfunction? Molecular human reproduction. 2002;8(8):719–721. doi: 10.1093/molehr/8.8.719. [DOI] [PubMed] [Google Scholar]
- St John JC, Cooke ID, Barratt CL. Mitochondrial mutations and male infertility. Nat Med. 1997;3(2):124–125. doi: 10.1038/nm0297-124c. [DOI] [PubMed] [Google Scholar]
- Summermatter S, Troxler H, Santos G, Handschin C. Coordinated balancing of muscle oxidative metabolism through PGC-1alpha increases metabolic flexibility and preserves insulin sensitivity. Biochem Biophys Res Commun. 2011;408(1):180–185. doi: 10.1016/j.bbrc.2011.04.012. doi:10.1016/j.bbrc.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Svartberg J, Agledahl I, Figenschau Y, Sildnes T, Waterloo K, Jorde R. Testosterone treatment in elderly men with subnormal testosterone levels improves body composition and BMD in the hip. International journal of impotence research. 2008;20(4):378–387. doi: 10.1038/ijir.2008.19. doi:10.1038/ijir.2008.19. [DOI] [PubMed] [Google Scholar]
- Trifunovic A, Larsson NG. Mitochondrial dysfunction as a cause of ageing. J Intern Med. 2008;263(2):167–178. doi: 10.1111/j.1365-2796.2007.01905.x. doi:10.1111/j.1365-2796.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- Tyndall GL, Kobe RW, Houmard JA. Cortisol, testosterone, and insulin action during intense swimming training in humans. Eur J Appl Physiol Occup Physiol. 1996;73(1-2):61–65. doi: 10.1007/BF00262810. [DOI] [PubMed] [Google Scholar]
- Vaamonde D, Da Silva-Grigoletto ME, Garcia-Manso JM, Vaamonde-Lemos R, Swanson RJ, Oehninger SC. Response of semen parameters to three training modalities. Fertil Steril. 2009;92(6):1941–1946. doi: 10.1016/j.fertnstert.2008.09.010. doi:10.1016/j.fertnstert.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Vaamonde D, Da Silva ME, Poblador MS, Lancho JL. Reproductive profile of physically active men after exhaustive endurance exercise. Int J Sports Med. 2006;27(9):680–689. doi: 10.1055/s-2005-872906. doi:10.1055/s-2005-872906. [DOI] [PubMed] [Google Scholar]
- Vazquez-Memije ME, Capin R, Tolosa A, El-Hafidi M. Analysis of age-associated changes in mitochondrial free radical generation by rat testis. Mol Cell Biochem. 2008;307(1-2):23–30. doi: 10.1007/s11010-007-9580-9. doi:10.1007/s11010-007-9580-9. [DOI] [PubMed] [Google Scholar]
- Vermeulen A. Clinical review 24: Androgens in the aging male. The Journal of clinical endocrinology and metabolism. 1991;73(2):221–224. doi: 10.1210/jcem-73-2-221. [DOI] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. doi:10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb ML, Wallace JP, Hamill C, Hodgson JL, Mashaly MM. Serum testosterone concentration during two hours of moderate intensity treadmill running in trained men and women. Endocr Res. 1984;10(1):27–38. doi: 10.1080/07435808409046763. [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–124. doi: 10.1016/S0092-8674(00)80611-X. doi:10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Zhao X, Bian Y, Sun Y, Li L, Wang L, Zhao C, Shen Y, Song Q, Qu Y, Niu S, Wu W, Gao F. Effects of moderate exercise over different phases on age-related physiological dysfunction in testes of SAMP8 mice. Exp Gerontol. 2013;48(9):869–880. doi: 10.1016/j.exger.2013.05.063. doi:10.1016/j.exger.2013.05.063. [DOI] [PubMed] [Google Scholar]