Abstract
Initiation of protein translation by the 5' mRNA cap is a tightly regulated step in cell growth and proliferation. Aberrant activation of cap-dependent translation is a hallmark of many cancers including non-small cell lung cancer. The canonical signaling mechanisms leading to translation initiation include activation of the Akt/mTOR pathway in response to the presence of nutrients and growth factors. We have previously observed that inhibition of c-jun N-terminal kinase (JNK) leads to inactivation of cap-dependent translation in mesothelioma cells. Since JNK is involved in the genesis of non-small cell lung cancer (NSCLC), we hypothesized that JNK could also be involved in activating cap-dependent translation in NSCLC cells and could represent an alternative pathway regulating translation. In a series of NSCLC cell lines, inhibition of JNK using SP600125 resulted in inhibition of 4E-BP1 phosphorylation and a decrease in formation of the cap-dependent translation complex, eIF4F. Furthermore, we show that JNK-mediated inhibition of translation is independent of mTOR. Our data provide evidence that JNK is involved in the regulation of translation and has potential as a therapeutic target in NSCLC.
Keywords: c-jun N-terminal kinase, non-small cell lung cancer, eIF4E, 4E-BP1, cap-dependent translation
Introduction
Initiation of 5' cap-dependent protein translation is a highly regulated cellular process that is finely tuned to the metabolic needs of a cell. We and others have shown that aberrant activation of the cap-dependent translational machinery is important to the survival of cancer cells, including NSCLC (1–3). Furthermore, in many cancer cells, the negative regulator of cap-dependent translation, eukaryotic initiation factor 4E binding protein 1 (4E-BP1) is found to be hyperphosphorylated and thereby unable to sequester eukaryotic initiation factor 4E (eIF4E) and inhibit the translation of key growth and anti-apoptotic proteins (4,5). The canonical pathway leading to phosphorylation of 4E-BP1 and initiation of cap-dependent translation occurs via activation of receptor tyrosine kinases by growth factor signaling through Akt/mammalian target of rapamycin (mTOR), which phosphorylates 4E-BP1. This induces a conformational change in 4E-BP1 allowing the release of eIF4E, freeing it to associate with eIF4G leading to recruitment of ribosomes and activation of translation (6). Since aberrant activity of translation correlates with poorer outcomes in cancer, there has been increasing interest in understanding this pathway and signaling events regulating translation in cancer cells (1,7).
4E-BP1 contains 6 phosphorylation sites which undergo hierarchical phosphorylation. Each phosphorylation event reduces the affinity of 4E-BP1 for eIF4E. mTOR has been shown to phosphorylate 4E-BP1 at Thr-37 and Thr-46 which has been seen as a priming event for full phosphorylation by as yet unidentified kinases, which is required for release of 4E-BP1 from eIF4E (8). 4E-BP1 can serve as a substrate for several kinases in vitro including c-Jun N-terminal kinase (JNK) and extracellular-regulated kinase (ERK) 2, however, in its physiologic state bound to eIF4E many of the phosphorylation sites are unavailable. Only mTOR has been shown to phosphorylate it in this state (9). Thus, it has been concluded that mTOR phosphorylation causes a conformational shift in 4E-BP1 allowing other kinases to phosphorylate 4E-BP1 on the remaining sites and lead to full activity of the cap-complex (8). We recently published results showing that c-jun N-terminal kinase (JNK) inhibition by SP600125 led to decreased phosphorylation of 4E-BP1 and decreased initiation of cap-dependent translation in mesothelioma cells (10). Interestingly, several publications have demonstrated that JNK plays an important role in lung carcinogenesis (11–13). We were interested to further study the role of JNK activation in regulating cap-dependent translation in NSCLC cells and gain further insight into the signaling pathways involved. Here we show that JNK is activated in several NSCLC cell lines and SP600125, a JNK inhibitor, leads to decreased phosphorylation of 4E-BP1 in a dose-dependent manner. Furthermore, we show that this decrease in phosphorylation results in decreased formation of the eIF4F initiation complex and this effect is independent of mTOR. These data suggest that regulation of the initiation of cap-dependent protein translation is a novel function of JNK signaling.
Materials and methods
Cell lines and reagents
Cell lines were purchased from the ATCC or provided by Frederick Kaye (NIH). H2009, H522, H520, H460, H1299, H2030, H661 NSCLC cells were grown in RPMI-1640 supplemented with 10% fetal calf serum and 1% penicillin/streptomycin/fungicin antibiotic (2). Normal human bronchial epithelial cells (NHBE) were grown in BEGM medium (BEBM supplemented with SingleQuots, Cambrex Biosciences) as described previously (2). For treatment experiments, the JNK inhibitor, SP600125, and rapamycin were purchased from Calbiochem. Both drugs were dissolved in 100% DMSO and stored at −20°C. Cells were treated with various concentrations of drugs as well as controls treated with an equal volume of the drug's vehicle, DMSO.
Kinase assays
JNK activity was determined by in vitro kinase assay kit (Cell Signaling) using the manufacturer's instructions as described (10). Cells were grown to 70% confluence and lysed. Lysate (300 µg) was added to 30 µl of c-Jun sepharose beads and rotated overnight at 4°C to pull-down JNK. Beads were washed with lysis buffer thrice and incubated in kinase buffer supplemented with 200 µM ATP for 30 min at 30°C. Beads were eluted using 3× loading buffer and run on SDS-PAGE gels and subjected to Western blotting for phospho-c-jun.
Immunoblot analyses
Gels were transferred onto PVDF membranes, blocked in 5% milk-Tris-buffered saline with Tween-20 (TBST) for 1–2 h at room temperature. Primary antibodies were diluted in TBST and incubated overnight at 4°C. For phospho-specific antibodies, the blocking and primary antibody dilutions were in 5% BSA-TBST. Anti-JNK, anti-phospho-c-Jun (Ser63) (1:500 dilution), anti-eIF4E, anti-phospho-eIF4E (Ser209), anti-4E-BP1, anti-phospho-4E-BP1 (Ser65), anti-p70S6 kinase, anti-phospho-p70S6 kinase (Thr389), anti-Akt, anti-phospho-Akt (Ser473), anti-poly-ADP-ribose polymerase (PARP), anti-Bcl-2, and anti-c-Myc antibodies were obtained from Cell Signaling. Anti-Bcl-xL was obtained from Santa Cruz Biotechnology. Rabbit anti-eIF4G (diluted at 1:2,000) was provided in kind by Nahum Sonenberg (10,14). All antibodies were diluted 1:1,000 except where indicated. Western blots were analyzed for optical density using NIH Image J software.
Cell proliferation assay
Cells were seeded in triplicate onto 96-well plates with 2,000 cells/well along with controls. Cells were treated with SP600125 24 h after plating. All samples and controls were treated with equal volume of the drug vehicle, dimethyl sulfoxide (DMSO), and grown for 72 h. Cell viability was measured using the Cell Counting Kit-8 (CCK-8, Dojindo Molecular Technologies) following the manufacturer's protocol. The amount of CCK-8 reagent reduced to formazan by cellular dehydrogenase indicating cell viability was assayed by reading the absorbance at 405 nm on a 96-well plate reader. Statistical analysis was performed by the t-test. A p<0.05 was significant.
Cap-binding assay
The cap-binding assay was carried out as previously described (10). Cells grown to 70% confluence were lysed using 1× cell lysis buffer (Cell Signaling). Protein lysate (300 µg) was added to 50 µl of 50% slurry of 7mGTP-sepharose beads to isolate eIF4E and its binding partners 4E-BP1 and eIF4G. Lysates were rotated at 4°C for 2 h followed by 3 washes in lysis buffer. Proteins bound to the beads were eluted by adding 50 µl of 5× Laemmli's buffer and boiling at 95°C for 5 min. The eluate was analyzed by Western blotting for eIF4E, 4E-BP1, eIF4G, and JNK.
Co-immunoprecipitation
Antibodies for immunoprecipitation, anti-4E-BP1 and anti-JNK antibodies, were obtained from Cell Signaling and Millipore, respectively. Protein (300 µg) was diluted in 1× lysis buffer to a concentration of 1 µg/µl and antibody was added to each tube to make a final dilution of 1:50. Samples were rotated at 4°C overnight followed by 5-h incubation with protein-A sepharose beads. Samples were centrifuged at 14,000 rpm for 30 sec and supernatant was removed. Beads were washed thrice with 500 µl of 1× lysis buffer and eluted with 3× Laemmli's buffer and subjected to 10% SDS-PAGE.
Results
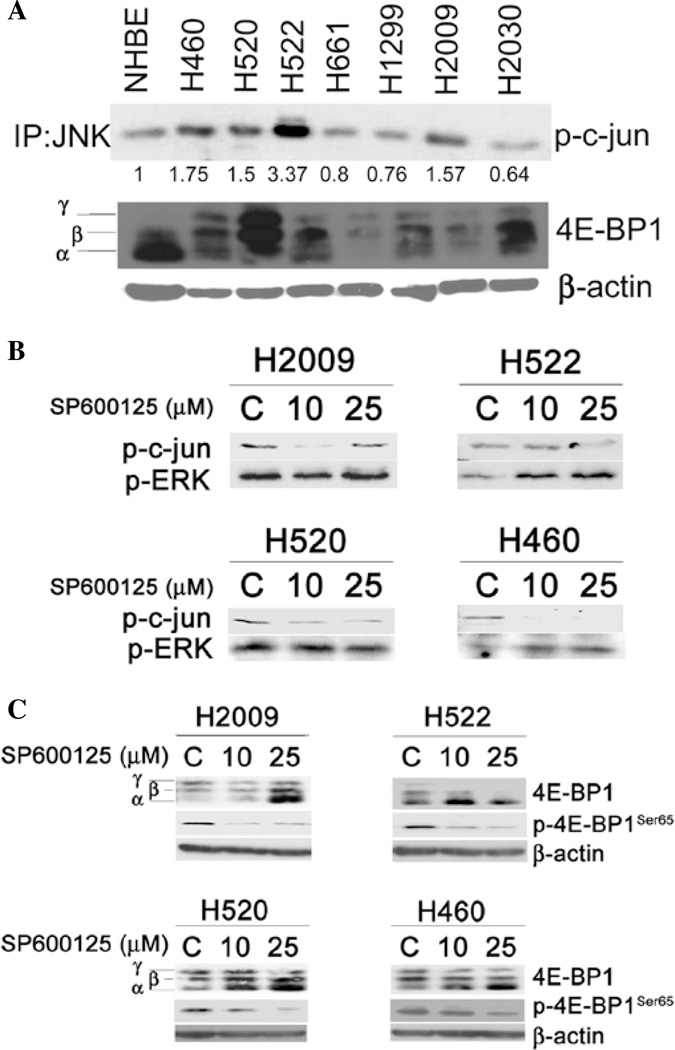
A series of NSCLC cell lines were cultured under standard conditions and lysates from these cells were subjected to in vitro kinase assays to detect activation of JNK. Normal human bronchial epithelial cells (NHBE) were used as a control. As shown in Fig. 1A, JNK activity was at least 1.5-fold greater in 4 of 7 NSCLC cells compared to NHBE cells. 4E-BP1 can be resolved into 3 isoforms based on electrophoretic mobility: α, β, and γ; indicating hypo-, intermediate, and hyper-phosphorylated isoforms, respectively (15). Successive phosphorylation of 4E-BP1 causes a slowing of its electrophoretic mobility such that it is detectable as a separate band on a Western blot. Compared to NHBE cells, 4E-BP1 was predominantly in the hyper-phosphorylated form in the majority of NSCLC cell lines examined, in accord with reported results (2). Since we had previously shown that JNK inhibition leads to decreased phosphorylation of 4E-BP1 in mesothelioma (10), we further studied the effect of JNK inhibition using a specific inhibitor, SP600125, on 4E-BP1 phosphorylation in NSCLC. SP600125 decreased JNK activity as assessed by in vitro kinase assay in all 4 cell lines (Fig. 1B). In H2009 cells, JNK activity returned to basal levels when treated with 25 µM SP600125. Because the kinase assay involves pull-down of c-jun and its binding partners, it is possible that this result reflects pull-down and phosphorylation of c-jun by an alternate kinase (16), however, this is speculative. SP600125 appeared to specifically target JNK at these doses as extracellular-regulated kinase (ERK) phosphorylation was not decreased in any of the cell lines. In the 4 NSCLC cell lines that showed increased JNK activity, JNK inhibition decreased phosphorylation (less γ isoform) of 4E-BP1 in a dose-dependent manner (Fig. 1C), a finding that was confirmed using a phospho-specific antibody to 4E-BP1Ser65.
Figure 1.
JNK activity and effect of JNK inhibition on 4E-BP1 phosphorylation of NSCLC cells. (A) NSCLC cells and NHBE were grown under normal growing conditions. Lysates were subjected to in vitro kinase assay employing c-jun sepharose beads to immunoprecipitate JNK and membranes were then probed for phospho-c-jun. Numbers below the blot indicate fold-change in optical density when NHBE cells are used as the reference control. Also shown is a Western blot for 4E-BP1. β-actin was used as a loading control. (B) The indicated cell lines were treated with JNK inhibitor, SP600125, at the indicated concentrations for 24 h and subjected to in vitro kinase assay. JNK activity is indicated by its ability to phosphorylate c-junSer63. Western blotting for phospho-ERK1/2Thr202/Try204 are shown and β-actin is used as a loading control. (C) Cells treated with SP600125 and control were probed for 4E-BP1 by Western blotting. γ, β and α indicate hyper-, intermediate, and hypophosphorylated isoforms of 4E-BP1 respectively. Phospho-4E-BP1 was assayed using phospho-specific antibody for Ser65. β-actin was used as a loading control (C, vehicle control).
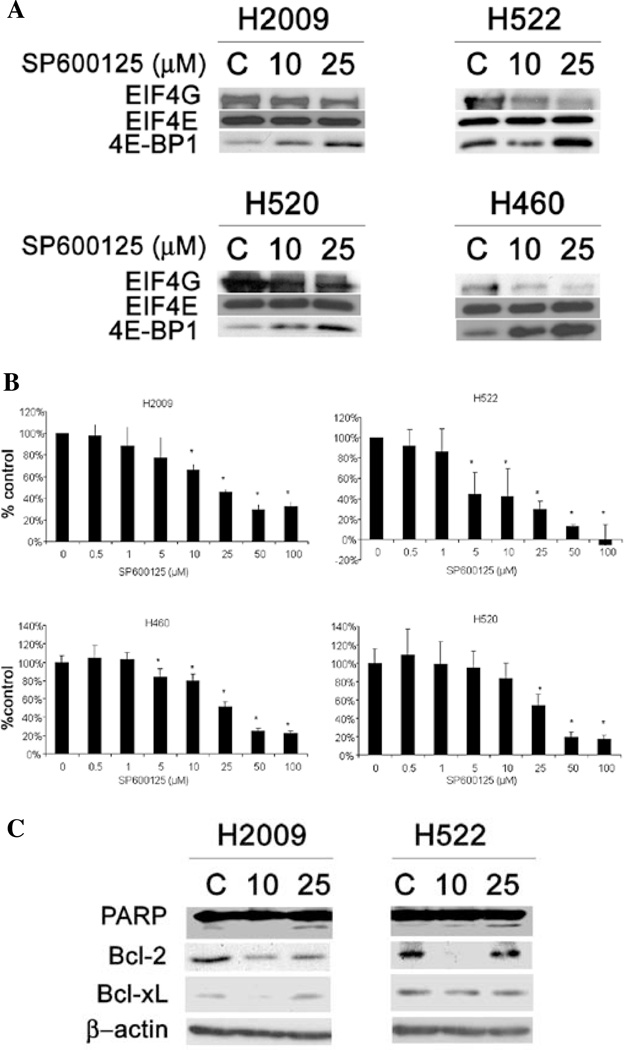
Since hypophosphorylated 4E-BP1 binds eIF4E and prevents eIF4F formation, we examined the effects of JNK inhibition on the cap complex formation using a 5' cap-affinity assay. As shown in Fig. 2A, JNK inhibition did not alter the ability of eIF4E to bind to the 5' cap for any of the cell lines tested. However, 4E-BP1 loading onto eIF4E was enhanced for all cell lines tested. Correspondingly, eIF4G binding was decreased in all cell lines. Taken together, our results show that JNK inhibition leads to increased activity of 4E-BP1 and repression of cap complex formation.
Figure 2.
Effect of JNK inhibition on cap-affinity assay, apoptosis markers, and cell proliferation. (A) Cells treated with SP600125 were subjected to 5'-cap affinity assay using 7mGTP-sepharose beads to detect eIF4E and its binding partners by Western blotting. A relative increase in eIF4G binding to eIF4E indicates translational activation while increased 4E-BP1 binding designates translational repression. Protein (25 µg) was taken from the supernatant and probed for β-actin as a loading control. (B) Proliferation of these cell lines was determined 72 h after treatment with SP600125 by CCK-8 viability assay. Data are presented as percentage of untreated control. (C) H2009 and H522 cells were probed for expression of PARP, Bcl-2, and Bcl-xL by Western blot analysis. β-actin was used as a loading control (C, vehicle control).
We next examined the effect of JNK inhibition on 2 key cancer related functions, proliferation and apoptosis. Fig. 2B shows data from proliferation assays after treatment with various concentrations of SP600125 in the 4 cell lines tested. Each cell line showed a dose-dependent decrease in proliferation. H522 cells were the most sensitive to this treatment with an IC50 of ~5 µM while H520 cells were the least sensitive with an IC50 of >25 µM.
To examine JNK and apoptosis, we quantified 2 translationally-regulated anti-apoptotic proteins, bcl-2 and bcl-xL (5,17) after JNK inhibition. Fig. 2C shows bcl-2 levels decreased in H2009 cells upon JNK inhibition. Bcl-2 levels decreased in H522 cells with 10 µM of SP600125, but the levels returned to basal levels after 25 µM treatment. Bcl-xL levels similarly were decreased at the 10 µM dose for H2009 but not at 25 µM. For H522 cells, Bcl-xL levels were only modestly decreased at both concentrations compared to control. To see if this decrease in anti-apoptotic proteins resulted in induction of apoptosis, we assessed the cleavage of poly (ADP) ribose polymerase, a marker of caspase activation. H2009 showed an increase in PARP cleavage at the 25 µM dose only, however, H522 cells showed a dose-dependent increase in PARP cleavage after JNK inhibition.
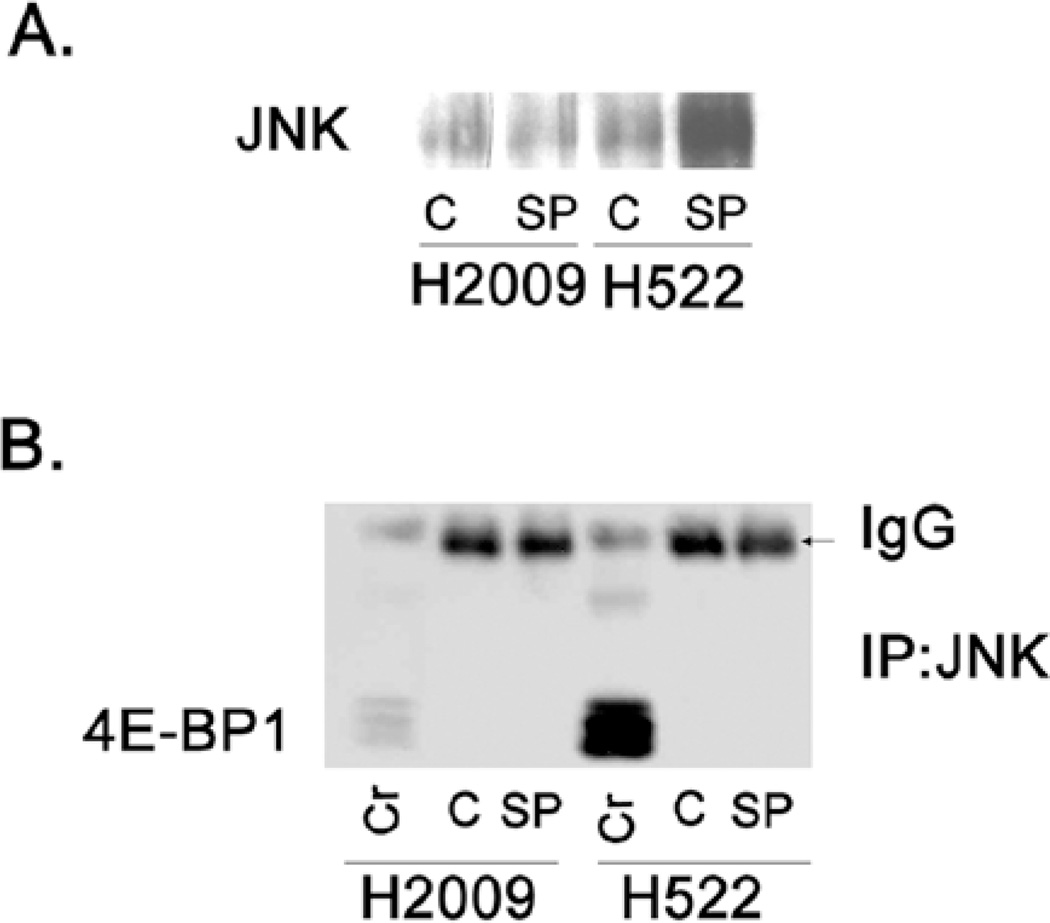
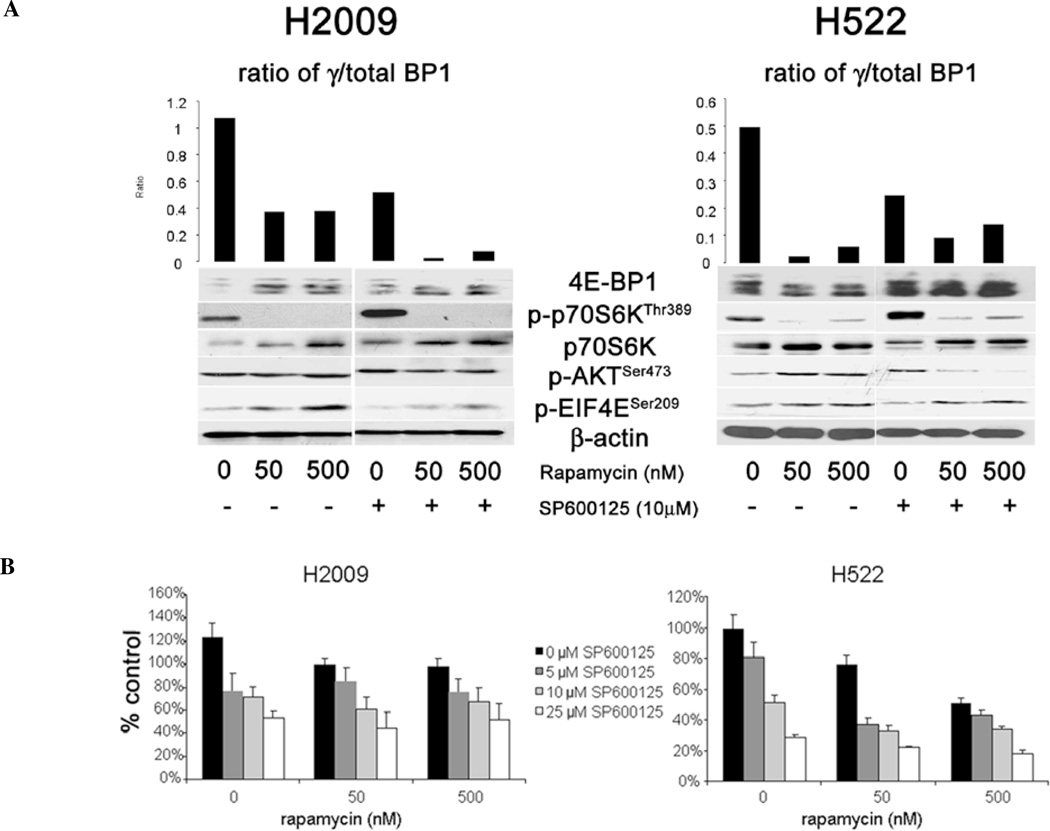
Having established that JNK inhibition decreased 4E-BP1 phosphorylation and thereby repressed formation of the eIF4F complex, we sought to determine the mechanism involved. First, we assessed whether JNK directly associated with the cap-binding complex using the cap-affinity assay. Fig. 3A shows the presence of a band corresponding to JNK in the cap-affinity assay suggesting that JNK physically associates with the cap complex. We next assayed for direct binding of JNK to 4E-BP1 by co-immunoprecipitation (Co-IP). When JNK was immunoprecipitated, we were unable to detect any interaction of 4E-BP1 with JNK (Fig. 3B). Similarly, when 4E-BP1 was immunoprecipitated and probed for JNK, none was bound (data not shown). Therefore, we concluded that the effect of JNK inhibition was not by direct binding to 4E-BP1. Since mTOR regulates 4E-BP1 phosphorylation, we examined if JNK effects on 4E-BP1 phosphorylation were dependent upon mTOR. The major downstream targets of mTOR are p70 S6 kinase (p70S6K) and 4E-BP1 (18). Fig. 4A shows the effects of treatment with rapamycin, an mTOR inhibitor, and SP600125 as well as the combination on 4E-BP1 and p70S6K phosphorylation. Rapamycin treatment alone resulted in a decrease in phosphorylation of 4E-BP1 as well as potent inhibition of phosphorylation of p70S6K, consistent with mTOR inhibition. While JNK inhibition decreased the phosphorylation of 4E-BP1, p70S6K phosphorylation was increased upon JNK inhibition. Since p70S6K activity is dependent upon mTOR activity, we conclude that JNK inhibition decreased 4E-BP1 phosphorylation without affecting mTOR signaling. Combination treatment with rapamycin and SP600125 resulted in a greater reduction in the phosphorylation of 4E-BP1 in H2009 cells compared to rapamycin alone. In H522 cells, however, JNK inhibition did not further decrease 4E-BP1 phosphorylation compared to rapamycin alone. Because rapamycin is known to cause an increase in phosphorylation of Akt and eIF4E due to feedback mechanisms, we also assessed the combination of JNK and mTOR inhibition on the phosphorylation of these proteins (19,20). Our data show that phosphorylation of Akt and eIF4E increased with rapamycin treatment as expected. While SP600125 alone had no effect on Akt phosphorylation, the combination of SP600125 and rapamycin did blunt the feedback increase in p-Akt in H2009 cells. In H522 cells, JNK inhibition alone had no effect on Akt phosphorylation, however, combination treatment abolished any increase mediated by rapamycin. Phosphorylation of eIF4E did not appear to be greatly affected by SP6100125 treatment and the combination did not appear different from treatment with rapamycin alone. To see if the combination of rapamycin and SP600125 had any additional effects on the growth of these cells, we performed proliferation assays employing the combination. Rapamycin alone had minimal effect on the growth of H2009 cells, however, a dose-dependent response was seen in H522 cells (Fig. 4B). Combination with SP600125 did not have a great effect upon H2009 cells, largely representing the efficacy of SP600125 in decreasing proliferation. The combination appeared to be more effective in H522 cells.
Figure 3.
Assays to detect physical association of JNK to cap complex and 4E-BP1. (A) Cell lysates were subjected to cap-affinity assay using 7mGTP-sepharose beads to pull-down eIF4E and binding partners. Eluates were subjected to SDS-PAGE and Western blotting for JNK (C, vehicle control; SP, 10 µM SP600125). (B) Co-immunoprecipitation was performed on cell lysates to assess the potential interaction of JNK and 4E-BP1. JNK was immunoprecipitated using a specific antibody. Eluate was run on SDS-PAGE gel and probed for 4E-BP1 (Cr, crude lysate; C, vehicle control; SP, 10 µM SP600125).
Figure 4.
Effect of combination of mTOR inhibition and JNK inhibition on biochemical pathways and cell proliferation. (A) H2009 and H522 cells were treated with rapamycin alone, SP600125 alone, or in combination and lysates were assessed for phosphorylation of 4E-BP1, p70S6KThr389, AktSer473, and eIF4ESer209. Bar graphs represent optical density of the ratio of hyperphospho-4E-BP1 (γ)/total 4E-BP1 for each treatment condition. β-actin is used as a loading control for these experiments. (B) H2009 and H522 cells were assessed for proliferation after treatment with rapamycin alone, SP600125 alone, or the combination for 72 h. Data are presented as percentage of untreated control. Error bars indicate standard deviation.
Discussion
We have now shown in two different tumor types, that inhibition of JNK using SP600125 represses the formation of cap-dependent initiation complex (10). The data presented add to our previous work by demonstrating that the inhibition of 4E-BP1 phosphorylation by SP600125 is independent of mTOR. Though several authors have postulated mTOR-independent signaling pathways mediating phosphorylation of 4E-BP1, there is scant literature demonstrating that this occurs. It has been observed that RalA signaling can influence S6K signaling in an mTOR-independent manner to decrease the translation of FLIP(S) anti-apoptotic protein (21). In addition, it has been shown that protein phosphatase 2A (PP2A) activation by protein kinase C (PKC) leads to decreased translation of cyclin D1 via activation of (hypophosphorylation) of 4E-BP1 in intestinal epithelial cells. While Akt/PI3K-independent 4E-BP1 phosphorylation has been mediated through AMPK, this phenomenon is mTOR-dependent (22). That JNK regulates cap-mediated translation independent of the classically defined pathway is further evidence of translation initiation as a key regulatory node integrating several oncogenic signals (6,7).
The mechanism by which JNK regulates 4E-BP1 phosphorylation remains unclear. Co-immunoprecipitation experiments failed to reveal any direct interaction between 4E-BP1 and JNK suggesting that there is an intermediary kinase or phosphatase regulated by the JNK pathway. Whether or not the growth inhibitory effects of JNK inhibition are related to activation of 4E-BP1 independent of the known activity of JNK on AP-1 transcriptional events remains to be seen. That JNK can function outside of its classical role in AP-1 activation has been demonstrated in several studies (23). While the oncogenic activity of JNK has been attributed to its activation of AP-1 and transcriptional events, there are at least 10 isoforms of JNK identified by alternative splicing and post-translational modifications (24). Therefore, it is conceivable that one or more of these isoforms are involved in other signaling events aside from transcriptional activation. In fact, JNK has been shown to be both a classic oncogene as well as a pro-apoptosis protein in response to UV light depending on the cell type and context (25). Our data suggest that JNK activity maintains the proliferative and anti-apoptotic phenotype in NSCLC. Therefore, these data suggest that JNK could be an attractive therapeutic target for this disease (13). Finally, JNK inhibition in our experiments was accomplished using SP600125 which has recently been shown to have effects on other kinases, most notably p70S6K (26). However, our data show that p70S6K is not affected by SP600125 (Fig. 3B), and therefore this described effect of SP600125 is not likely relevant to our experiments.
Combination treatment of rapamycin and SP600125 demonstrates the importance of 4E-BP1 phosphorylation and activation of translation as an important survival mechanism of cancer cells. In H2009 cells, rapamycin treatment alone incompletely activates 4E-BP1, which leads to only a minimal decrease in proliferation. However, the addition of SP600125, decreases 4E-BP1 phosphorylation more completely and results in a greater decrease in proliferation with combination treatment. H522 cells are much more sensitive to the effects of rapamycin treatment and 4E-BP1 phosphorylation is potently inhibited by rapamycin alone. While the combination treatment did result in additive inhibition of proliferation, there is no additional effect on 4E-BP1 phosphorylation than rapamycin alone. Rapamycin treatment of cancer cells have been shown to result in feedback phosphorylation of Akt and eIF4E (19), and therefore it was interesting to see the effect of JNK inhibition on this phenomenon. In H522 cells, we did observe that Akt phosphorylation was abrogated by adding SP600125, however, this was not the case in H2009, therefore, we cannot definitively conclude that effects seen with SP600125 are mediated by eliminating this feedback mechanism. There was minimal effect of combination treatment on eIF4E phosphorylation in these cells. There is some published literature indicating that mTOR inhibition may lead to JNK activation in mouse embryonic fibroblasts (27). Therefore, it is tempting to speculate that JNK-mediated 4E-BP1 phosphorylation may mediate rapamycin resistance, however, this requires further investigation.
In conclusion, this work demonstrates that JNK activation in NSCLC acts, at least in part, to regulate the initiation of cap-dependent translation, and that JNK-mediated phosphorylation of 4E-BP1 may be a mechanism of resistance to mTOR inhibition. This work is another example of the importance of cap-dependent translation in enhancing NSCLC survival and that redundant signaling pathways converge upon this regulatory node to ensure the efficient translation of critical oncogenic and anti-apoptotic proteins. As such, these data provide further evidence that targeting translation initiation might be a fruitful approach for treating NSCLC.
References
- 1.Graff JR, Konicek BW, Vincent TM, et al. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J Clin Invest. 2007;117:2638–2648. doi: 10.1172/JCI32044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson BA, Alter MD, Kratzke MG, et al. Repression of cap-dependent translation attenuates the transformed phenotype in non-small cell lung cancer both in vitro and in vivo. Cancer Res. 2006;66:4256–4262. doi: 10.1158/0008-5472.CAN-05-2879. [DOI] [PubMed] [Google Scholar]
- 3.Legrier ME, Yang CP, Yan HG, et al. Targeting protein translation in human non small cell lung cancer via combined MEK and mammalian target of rapamycin suppression. Cancer Res. 2007;67:11300–11308. doi: 10.1158/0008-5472.CAN-07-0702. [DOI] [PubMed] [Google Scholar]
- 4.Avdulov S, Li S, Michalek V, et al. Activation of translation complex eIF4F is essential for the genesis and maintenance of the malignant phenotype in human mammary epithelial cells. Cancer Cell. 2004;5:553–563. doi: 10.1016/j.ccr.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Mamane Y, Petroulakis E, Rong L, Yoshida K, Ler LW, Sonenberg N. eIF4E - from translation to transformation. Oncogene. 2004;23:3172–3179. doi: 10.1038/sj.onc.1207549. [DOI] [PubMed] [Google Scholar]
- 6.Sonenberg N, Pause A. Signal transduction. Protein synthesis and oncogenesis meet again. Science. 2006;314:428–429. doi: 10.1126/science.1134031. [DOI] [PubMed] [Google Scholar]
- 7.Armengol G, Rojo F, Castellvi J, et al. 4E-binding protein 1: a key molecular ‘funnel factor’ in human cancer with clinical implications. Cancer Res. 2007;67:7551–7555. doi: 10.1158/0008-5472.CAN-07-0881. [DOI] [PubMed] [Google Scholar]
- 8.Gingras AC, Gygi SP, Raught B, et al. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawrence JC, Jr, Fadden P, Haystead TA, Lin TA. PHAS proteins as mediators of the actions of insulin, growth factors and cAMP on protein synthesis and cell proliferation. Adv Enzyme Regul. 1997;37:239–267. doi: 10.1016/s0065-2571(96)00016-7. [DOI] [PubMed] [Google Scholar]
- 10.Patel MR, Jacobson BA, De A, et al. Ras pathway activation in malignant mesothelioma. J Thorac Oncol. 2007;2:789–795. doi: 10.1097/JTO.0b013e31811f3aab. [DOI] [PubMed] [Google Scholar]
- 11.Nitta RT, Del Vecchio CA, Chu AH, Mitra SS, Godwin AK, Wong AJ. The role of the c-Jun N-terminal kinase 2-alpha-isoform in non-small cell lung carcinoma tumorigenesis. Oncogene. 2011;30:234–244. doi: 10.1038/onc.2010.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cellurale C, Sabio G, Kennedy NJ, et al. Requirement of c-Jun NH(2)-terminal kinase for Ras-initiated tumor formation. Mol Cell Biol. 2011;31:1565–1576. doi: 10.1128/MCB.01122-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khatlani TS, Wislez M, Sun M, et al. c-Jun N-terminal kinase is activated in non-small-cell lung cancer and promotes neoplastic transformation in human bronchial epithelial cells. Oncogene. 2007;26:2658–2666. doi: 10.1038/sj.onc.1210050. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson BA, De A, Kratzke MG, et al. Activated 4E-BP1 represses tumourigenesis and IGF-I-mediated activation of the eIF4F complex in mesothelioma. Br J Cancer. 2009;101:424–431. doi: 10.1038/sj.bjc.6605184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gingras AC, Kennedy SG, O'Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raivich G. c-Jun expression, activation and function in neural cell death, inflammation and repair. J Neurochem. 2008;107:898–906. doi: 10.1111/j.1471-4159.2008.05684.x. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Takasu T, Perlman DM, et al. Translation factor eIF4E rescues cells from Myc-dependent apoptosis by inhibiting cytochrome c release. J Biol Chem. 2003;278:3015–3022. doi: 10.1074/jbc.M208821200. [DOI] [PubMed] [Google Scholar]
- 18.Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–6422. doi: 10.1038/sj.onc.1209888. [DOI] [PubMed] [Google Scholar]
- 19.Sun SY, Rosenberg LM, Wang X, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 20.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 21.Panner A, Nakamura JL, Parsa AT, et al. mTOR-independent translational control of the extrinsic cell death pathway by RalA. Mol Cell Biol. 2006;26:7345–7357. doi: 10.1128/MCB.00126-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 23.Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev. 2006;70:1061–1095. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manning AM, Davis RJ. Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov. 2003;2:554–565. doi: 10.1038/nrd1132. [DOI] [PubMed] [Google Scholar]
- 25.Karin M, Gallagher E. From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life. 2005;57:283–295. doi: 10.1080/15216540500097111. [DOI] [PubMed] [Google Scholar]
- 26.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang S, Shu L, Dilling MB, et al. Sustained activation of the JNK cascade and rapamycin-induced apoptosis are suppressed by p53/p21(Cip1) Mol Cell. 2003;11:1491–1501. doi: 10.1016/s1097-2765(03)00180-1. [DOI] [PubMed] [Google Scholar]