Abstract
Objective
Morbidity and mortality in children with cardiac arrest (CA) largely result from neurologic injury. Serum biomarkers of brain injury can potentially measure injury to neurons (neuron specific enolase [NSE]), astrocytes (S100b), and axons (myelin basic protein [MBP]). We hypothesized that serum biomarkers can be used to predict outcome from pediatric CA.
Design
Prospective observational study.
Setting
Single tertiary pediatric hospital.
Patients
Forty-three children with cardiac arrest.
Interventions
None.
Measurements and Main Results
We measured serum NSE, S100b, and MBP on days 1–4 and 7 after CA. We recorded demographics, details of the CA and resuscitation, and Pediatric Cerebral Performance Category (PCPC) at hospital discharge and 6 months. We analyzed the association of biomarker levels at 24, 48, and 72 hours with good (PCPC 1–3) or poor (PCPC 4–6) outcome and mortality. Forty-three children (49% female; mean age of 5.9 ± 6.3 were enrolled and 17 (40%) died. Serum concentration at 24 hours for all 3 biomarkers predicted mortality (all p<0.05). Additionally, serum NSE and S100b concentrations were increased in the poor outcome vs. good outcome group and in subjects who died at all time points (all p<0.05). Receiver operator curves for serum S100b and NSE to predict good vs. poor outcome at 6 months was superior to clinical predictors. There was no association between serum biomarker concentrations and subject temperature.
Conclusions
Preliminary data show that serum S100b, NSE, and MBP may aid in outcome prediction of children surviving CA.
Keywords: Biomarker; heart arrest; hypoxia-ischemia, brain; child; outcome assessment (health care); resuscitation
INTRODUCTION
Children with cardiac arrest (CA) have mortality rates of about 50% for in-hospital CA and 80% for out-of-hospital CA, with many survivors having neurological disability1–8. These poor outcomes are partly attributable to the fact that unlike in adults, most pediatric CAs begin with respiratory arrest or shock, followed by hypoxemia, bradycardia, and finally, global ischemia. Furthermore, unlike adult CA or neonatal asphyxia, there are no novel treatment strategies proven to be effective in improving outcomes in pediatric CA.
Current clinical and laboratory tests performed early in the hospital course in pediatric CA are not robustly associated with long term outcomes. Uncertainty of prognosis can be excruciating for families faced with making medical decisions. Early recognition of brain injury on physical examination can be obscured by medications and developmental stage, and under-recognized brain injury may prevent implementation of neuroprotective therapies and monitoring. In addition, the use of broad entry criteria without stratification for severity of injury in RCTs runs the risk of a heterogeneous sample which can obscure subgroups that may derive benefit from the intervention being evaluated despite an overall negative study9.
Serum brain-specific biomarkers may help predict outcome after various pediatric brain insults. Serum biomarkers of brain injury such as neuron specific enolase (NSE), S100b, and myelin basic protein (MBP), from neurons, astrocytes, and axons respectively, have the potential to assist in the early detection and quantification of the severity of brain injury, response to therapeutic interventions, and prediction of outcome after CA10–13. Brain biomarkers have different patterns of release and limitations to their use14,15. As a result, clinical applicability may be limited by type of brain injury (i.e., trauma, stroke, hypoxia-ischemia, cardiopulmonary bypass)16–20. A prospective pilot study involving pediatric subjects who received 24 hours of therapeutic hypothermia found that serum NSE and S100b had excellent specificity and fair sensitivity at 48 hours or later to predict outcome at hospital discharge10. White matter injury seen after hypoxia-ischemia suggests a potential role for MBP but clinical studies have been limited21,22.
In this single center prospective study, we examined the time course of serum NSE, S100b, and MBP levels in children during the first week after CA and tested the accuracy of serum and clinical biomarkers to predict good vs. poor outcome at 6 months. We hypothesized that serum biomarkers of brain injury targeting various cell types (neurons, astrocytes, axons) would predict subject outcome at clinically relevant time points (24 hours).
MATERIALS AND METHODS
Design and Setting
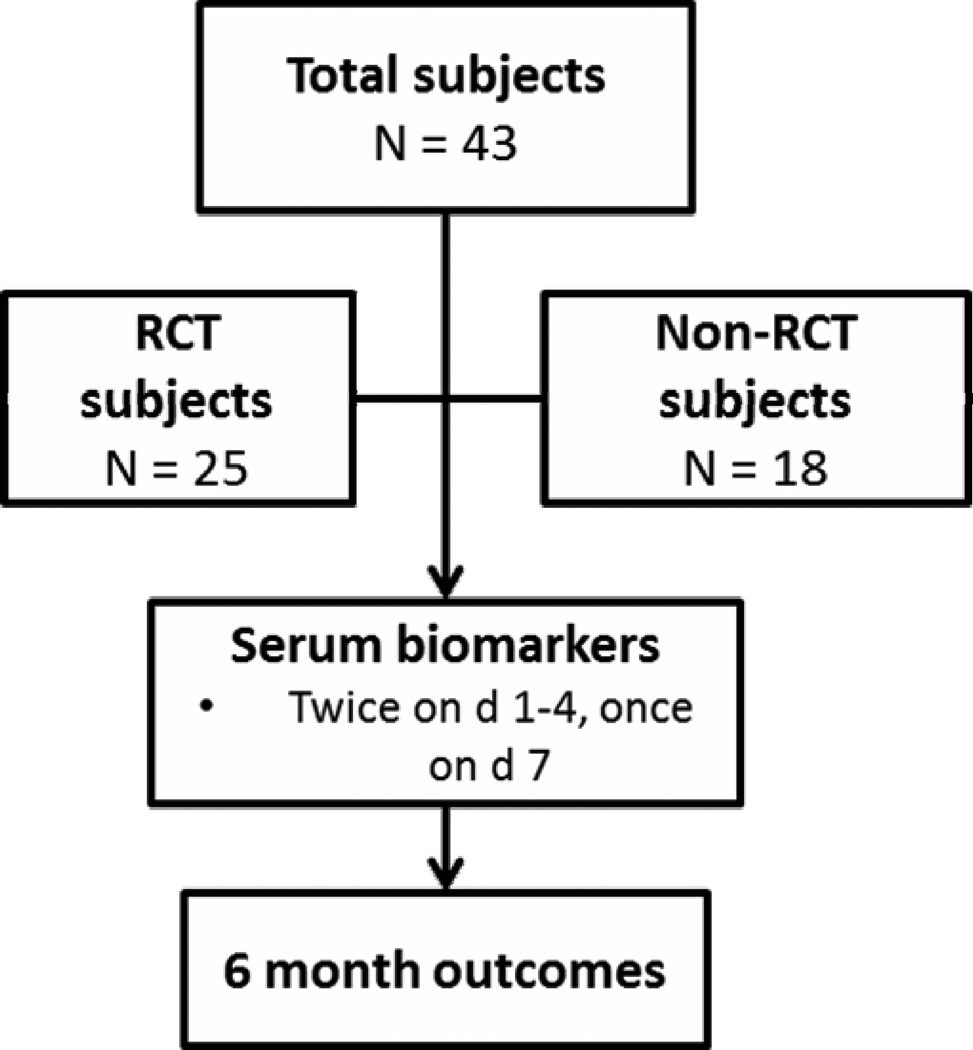
Between November 2009 and September 2011, 43 subjects with CA were enrolled in one of 2 studies at the Children’s Hospital of Pittsburgh (Figure 1). Twenty-five subjects were enrolled in an RCT (NCT00797680) and were randomized to either 24 or 72 hours of therapeutic hypothermia (target temperature 33 ± 1°C). Eighteen subjects who did not meet criteria for the RCT were enrolled in an observational study, and the pediatric ICU physician made decisions about post-resuscitation temperature management. Duration of hypothermia is not disclosed in this manuscript because both studies remain open to enrollment. Both studies were approved by the University of Pittsburgh Institutional Review Board and informed consent was obtained from the subject’s parent or guardian.
Figure 1.
Study flowchart.
Inclusion and Exclusion Criteria
We studied children between the ages 1 week and 17 years who were admitted to the ICU with ROSC after in- or out-of-hospital CA. CA was defined as receipt of chest compressions for pulselessness by a healthcare worker. Subjects were included if they had an indwelling arterial or venous catheter for blood draws. Subjects were excluded if they had a do not resuscitate status, were pregnant, had any contraindication for MRI, had another simultaneous acute brain disease, were undergoing brain death evaluation, or had a metabolic disease affecting the brain. Subjects were also excluded from the RCT if they had active hemorrhage or a pre-existing coagulation defect or if they had a long bone fracture (because of the potential for false positive S100b concentrations). Additionally, specifically for the RCT, subjects were included if Glasgow Coma Scale (GCS) score was ≤ 8 after ROSC and if they had therapeutic hypothermia initiated by their ICU attending.
Post-Resuscitation Care
Post-resuscitation care in the PICU consisted of prevention of secondary neurologic insults, treatment of organ dysfunction, and investigation of cause of CA if unknown23. Standards of care for most children post-CA included intubation and mechanical ventilation and placement of central venous and arterial catheters. Targets for PaO2 and PaCO2 were ~100 mmHg and 40 mmHg, respectively, as well as maintenance of normal mean arterial blood pressure for age. Pain and sedation medications were used at the discretion of the attending physician and were often withheld initially to obtain an accurate neurological examination. Electroencephalography (EEG) and brain CT were commonly obtained in the first 24–48 hours after ROSC, but continuous EEG was not routinely used. Prophylactic anti-epileptic medications were not used as standard of care. Rectal and/or esophageal temperature probes were used for continuous temperature reading. Fever (>38 °C) was treated aggressively in all subjects with methods similar to those used for cooling induction as well as with anti-pyretics24.
Data Collection
Data were collected from medical charts using the Utstein template for CA, including subject demographics, details about the CA and resuscitation, post-resuscitation care, and outcomes25. Inotrope score was calculated using the highest concentrations within the first 24 hours post-arrest26. Initial and subsequent GCS score, GCS motor score, and pupillary reactivity were recorded from nurse charting. EEG result was designated as ‘good’ if it was read by the attending neurologist as a continuous background without diffuse slowing or ‘poor’ if the background was discontinuous, demonstrated diffuse slowing, burst suppression, or was markedly attenuated27. Brain CT scan was recorded as ‘acute brain lesion’ if the attending neuroradiologist identified brain edema, loss of gray-white differentiation, or herniation was present or ‘no acute lesion’ in the absence of those findings.
Serum Biomarkers
Three ml of blood were collected twice daily (days 1–4) and once on day 7 after ROSC. For the first 4 subjects, the initial blood sample was obtained only after informed consent was acquired. Subsequently, the IRB granted permission for the initial sample to be drawn prior to consent. If consent was not obtained, the sample was discarded. Samples were centrifuged, aliquoted, frozen at −70°C, and analyzed in batches. Serum NSE, S100b, and MBP were measured in duplicate using commercially available ELISAs (International Point of Care, Toronto, Ontario, Canada). NSE concentration was corrected for hemolysis14. The sensitivity of the assays was 0.1 ng/mL for NSE and MBP and 0.01 ng/mL for S100b. The coefficient of variation for each assay was < 10%. An experienced technician blinded to subject treatment and outcome performed all biomarker measurements. Clinical team members were unaware of the biomarker results. Serum biomarker time points were measured from time of ROSC as documented in the chart. Samples closest to but not greater than 24, 48, 72, 96, and 120 hours after ROSC were used in the analysis.
Outcome Measures
Subjects were followed until 6 months post-CA. The primary outcome was the accuracy of serum brain biomarker concentrations to predict good (Pediatric Cerebral Performance Category (PCPC) score 1–3) or poor (PCPC 4–6) outcome28. The PI, who assigned the pre-arrest, hospital discharge, and 6 month PCPC scores, was blinded to biomarker results but not to clinical course. Six month outcomes were performed in surviving children either over the telephone or during in-person interview with the parent or guardian during a scheduled outpatient visit. Secondary outcomes included mortality at hospital discharge and 6 months. Additional analyses included effect of temperature on serum biomarker concentration and clinical variable assessment for prediction of primary and secondary outcomes.
Data analysis
Biomarker data are presented as median (interquartile range [IQR]) since data were skewed. Other continuous variables are presented as mean and standard deviation (SD). The data were analyzed for outcome group differences with Fisher’s exact tests for categorical variables. Median serum biomarkers were represented graphically by outcome group. The Wilcoxon rank sum was used to compare serum biomarker concentration and outcome. Spearman’s rank correlation was used to test correlation between serum biomarker concentration and temperature and age. Kruskal-Wallis test was used to detect if there were any differences between categorical temperature groups (< 34°C, 34–36°C, > 36°C). Mann-Whitney U test was then used to determine where differences existed between groups. Receiver operating characteristic (ROC) curves were used to evaluate the probability that a serum or clinical biomarker would correctly classify an outcome. Clinical biomarkers chosen for ROC curves were continuous variables that predicted outcome with p < 0.05 in univariate analysis. ROC curves were generated using raw data from all time points. Sensitivity analysis was performed for serum biomarkers at clinically relevant time points. All p-values were two-sided. Data analysis was performed using SPSS.
RESULTS
Forty-three subjects were enrolled and ranged in age from infancy to adolescence (Table 1). The sample was evenly split by sex and a strong majority of the participants had asphyxia or shock as the etiology of CA. Seventeen (40%) subjects died and 19 (60%) overall had poor outcome at 6 months post-CA (Table 2). Subjects with poor outcome were more likely to have had an unwitnessed CA and asystole as the first documented rhythm. Motor and pupillary examination findings, first lactate and blood pH, and EEG early post-CA were associated with good vs. poor outcome. Thirty-five (81%) subjects received therapeutic hypothermia for ≤ 72 hours while the remaining 8 subjects were maintained normothermic.
Table 1.
Subject demographics and details of cardiac arrest overall and by 6 month outcome post-arrest.
| Mean±SD [25th, 75th %] or n (%) | All (n=43) | Good outcome (n=17) | Poor outcome (n=26) | p-value |
|---|---|---|---|---|
| Age, (y) | 5.87±6.30 [0.33,11.52] | 6.53±6.25 [0.32,13.14] | 5.43±6.42 [0.48,10.21] | 0.785 |
| Sex, n (% male) | 22 (51) | 9 (53) | 13 (50) | 0.549 |
| Race/ethnicity | 0.929 | |||
| White | 34 (79) | 13 (77) | 21 (81) | |
| Black | 7 (16) | 3 (18) | 4 (15) | |
| Other | 2 (5) | 1 (6) | 1 (4) | |
| History of chronic illness | 18 (42) | 8 (47) | 10 (39) | 0.403 |
| Primary etiology | 0.155 | |||
| Asphyxia/shock | 37 (86) | 13 (76) | 24 (92) | |
| Cardiac | 6 (14) | 4 (24) | 2 (8) | |
| Location | 0.205 | |||
| Out-of-hospital | 32 (74) | 11 (65) | 21 (81) | |
| In-hospital | 11 (26) | 6 (35) | 5 (19) | |
| Interval of CPR to ROSC (min) | 26.6±28.3 [9,30] | 18.0±16.3 [7.5,25] | 32.4±33.2 [11,40] | 0.120 |
| Epinephrine boluses | 2.9±2.9 [1,3] | 2.8±2.8 [0,4.5] | 2.9±3.0 [1,3] | 0.811 |
| Defibrillated | 7 (16) | 5 (29) | 2 (8) | 0.073 |
| 1st rhythm | 0.066 | |||
| Pulseless electrical activity | 21 (49) | 10 (59) | 11 (42) | |
| Asystole | 16 (37) | 3 (18) | 13 (50) | |
| Sinus | 3 (7) | 3 (18) | 1 (4) | |
| VT/VF | 1 (2) | 1 (6) | 1 (4) | |
| Witnessed event | 20 (47) | 14 (82) | 6 (23) | <0.001 |
| Bystander CPR | 35 (81) | 13 (77) | 22 (85) | 0.388 |
SD, standard deviation; ROSC, return of spontaneous circulation; CPR, cardiopulmonary resuscitation; VT/VF, ventricular tachycardia/ventricular fibrillation
Table 2.
Clinical, laboratory, radiological results, and outcomes.
| Mean±SD [25th,75th %] or n (%) | All (n=43) |
Good outcome (n=17) |
Poor outcome (n=26) |
p-value |
|---|---|---|---|---|
| Pupillary reaction d1 (% reactive bilaterally) | 24 (56) | 15 (88) | 9 (35) | <0.001* |
| Pupillary reaction d3 (% reactive bilaterally)1 | 31 (72) | 17 (100) | 14 (54) | 0.021* |
| Motor GCS d12 | 2.6±1.7 [1,4] | 3.8±1.4 [3,5] | 1.8±1.4 [1,3] | 0.001** |
| Motor GCS d33 | 3.1±2.1 [1,5] | 5.1±0.9 [4,6] | 1.7±1.4 [1,2] | <0.001** |
| GCS d14 | 5.2±2.7 [3,6.5] | 6.6±2.4 [5,8] | 4.4±2.5 [3,5.5] | 0.001** |
| GCS d34 | 6.6±3.9 [3,9] | 10.4±2.6 [9,11] | 4.2±2.2 [3,4.5] | <0.001** |
| First lactate (mMol/L) | 7.7±6.2 [2.9,12.7] | 4.4±3.0 [2.0,5.8] | 9.9±6.9 [4.5,15.1] | 0.006* |
| First metered glucose (mg/dL) | 271±237 [157,321] | 315±355 [175,340] | 242±108 [150,309] | 0.882 |
| First blood pH | 7.02±0.28 [6.8,7.2] | 7.16±0.22 [7.02,7.27] | 6.92±0.27 [6.7,7.1] | 0.006* |
| Inotrope score 1st 24h | 31.4±342.6 [0,48] | 10.1±20.2 [0,15] | 45.3±47.8 [3,100] | 0.003* |
| First brain CT | 0.330 | |||
| No acute lesion | 20 (47) | 10 (59) | 10 (38) | |
| Acute lesion mild-moderate | 3 (7) | 1 (6) | 2 (8) | |
| Acute lesion severe | 8 (19) | 1 (6) | 7 (27) | |
| Not performed | 12 (28) | 5 (29) | 7 (27) | |
| Day CT performed after CA5 | 1.8±1.4 [1,2] | 2.1±1.7 [1,3.5] | 1.6±1.2 [1,2] | 0.765 |
| EEG pattern | 0.005* | |||
| Continuous | 5 (12) | 5 (29) | 0 (0) | |
| Diffuse slowing or discontinuous | 33 (77) | 9 (53) | 24 (92) | |
| Not performed | 5 (12) | 3 (18) | 2 (8) | |
| Day EEG performed after CA | 1.5±0.5[1,2] | 1.7±0.5 [1,2] | 1.5±0.6 [1,2] | 0.121 |
| Hospital LOS (d) | 20.3±26.4 [3,30] | 26.2±36.1 [7.5,28] | 16.5±17.3 [2,33] | 0.268 |
| ICU LOS (d) | 17.5±24.7 [2,25] | 18.6±32.8 [4,17.5] | 16.9±18.3 [2,30] | 0.823 |
| Survival at HD | 26 (60) | 17 (100) | 9 (35) | <0.001** |
| Good outcome HD | 17 (40) | 15 (100) | 0 (0) | NA |
p < 0.05
N = 42, 17, 25 for all, good outcome, and poor outcome columns
N = 41, 16, 25 for all, good outcome, and poor outcome columns
N = 39, 16, 23 for all, good outcome, and poor outcome columns
N = 41, 16, 25 for all, good outcome, and poor outcome columns
N = 31, 12, 19 for all, good outcome, and poor outcome columns
HD, hospital discharge; LOS, length of stay; ICU, intensive care unit; GCS, Glasgow coma scale; CT, computed tomography; CA, cardiac arrest; EEG, electroencephalography
Serum biomarker patterns after pediatric cardiac arrest
Serum S100b concentration peaked the earliest after ROSC at median of 19 hours followed by NSE at 37.5 hours, and lastly MBP at 57.5 hours (Figures 2 a–b). NSE peaked earlier in subjects with good outcome vs. poor outcome (30 vs. 47.3 hours, p=.043) but there were no differences in time to peak by mortality.
Figures 2.
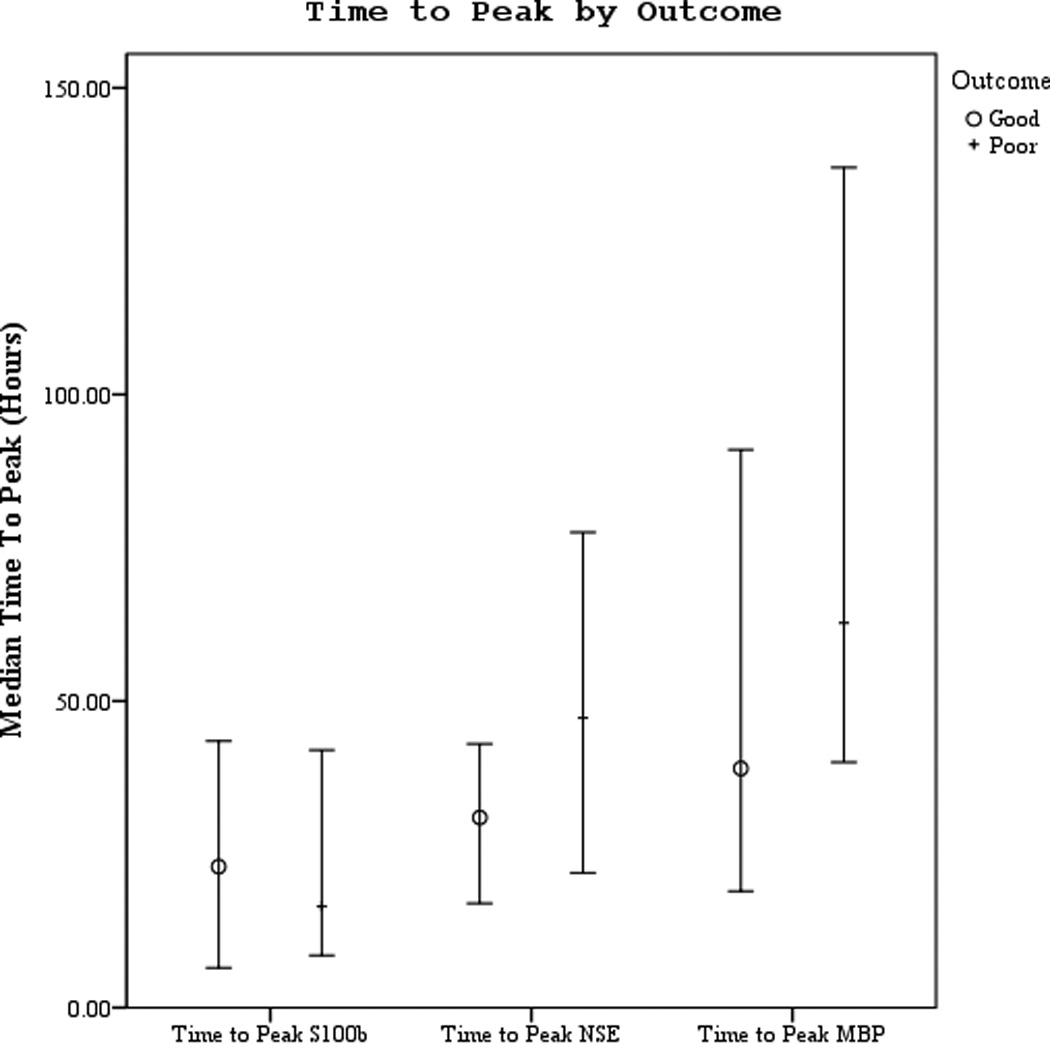
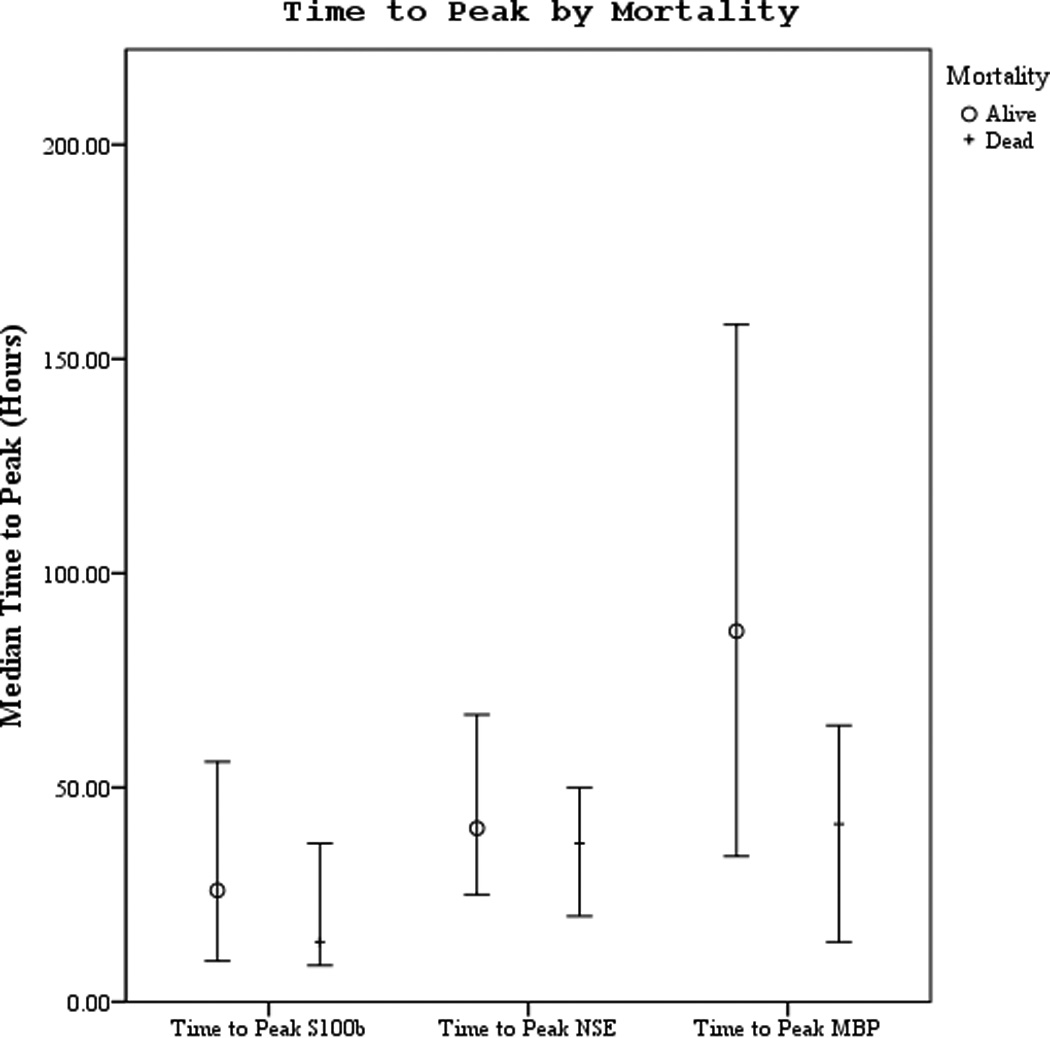
a–b. Time to peak serum NSE, S100, and MBP concentration by good vs. poor outcome and mortality at 6 months.
Serum biomarker concentrations measured between 0–120 hours post-ROSC [median (± 95% CI)] were plotted by both good vs. poor outcome and mortality (Figures 3 a–f). Subjects with good outcome had values within the normal range for all 3 biomarkers when compared to historical controls12. Serum S100b increased and decreased rapidly in subjects with poor outcome or who died. Serum NSE remained increased at 120 hours in most subjects with poor outcome or who died. Serum MBP showed a delayed, sustained increase in subjects with poor outcome or who died.
Figures 3.
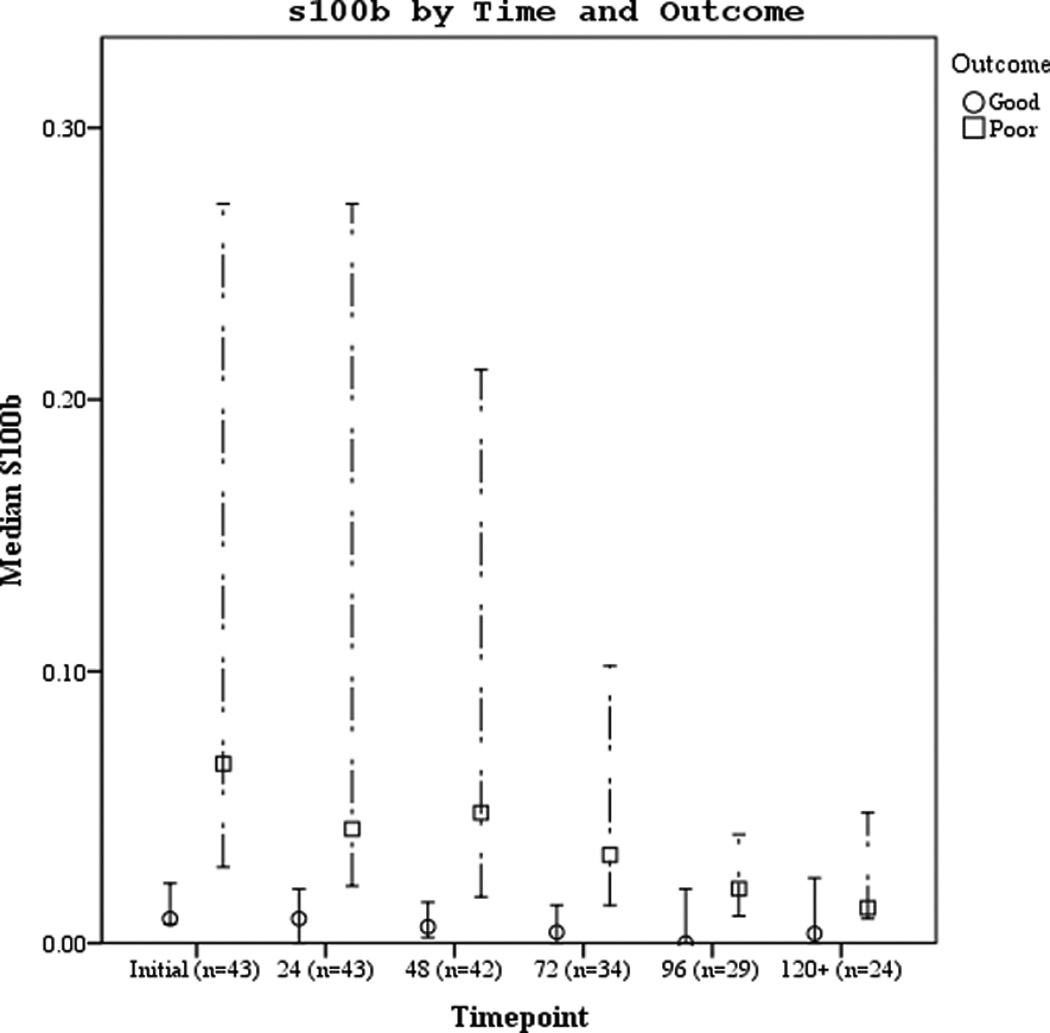
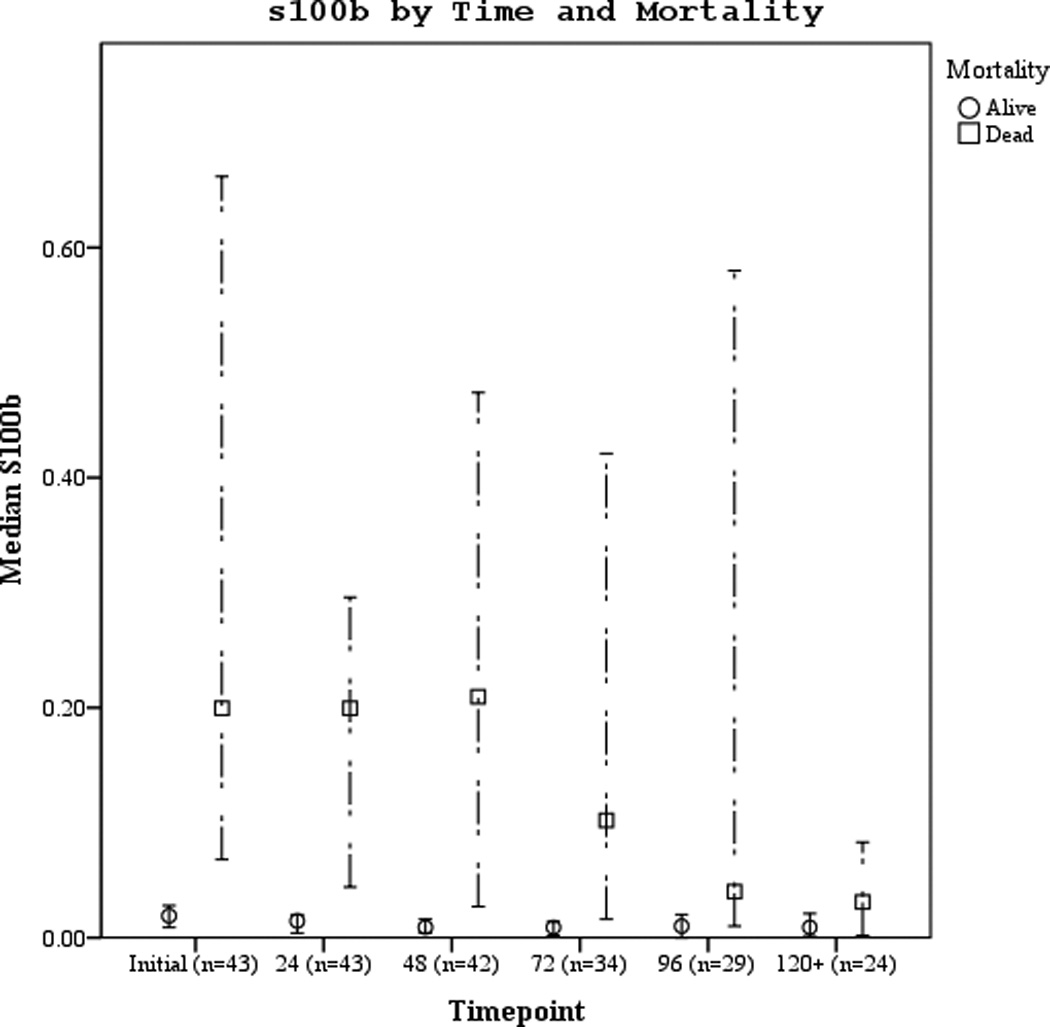
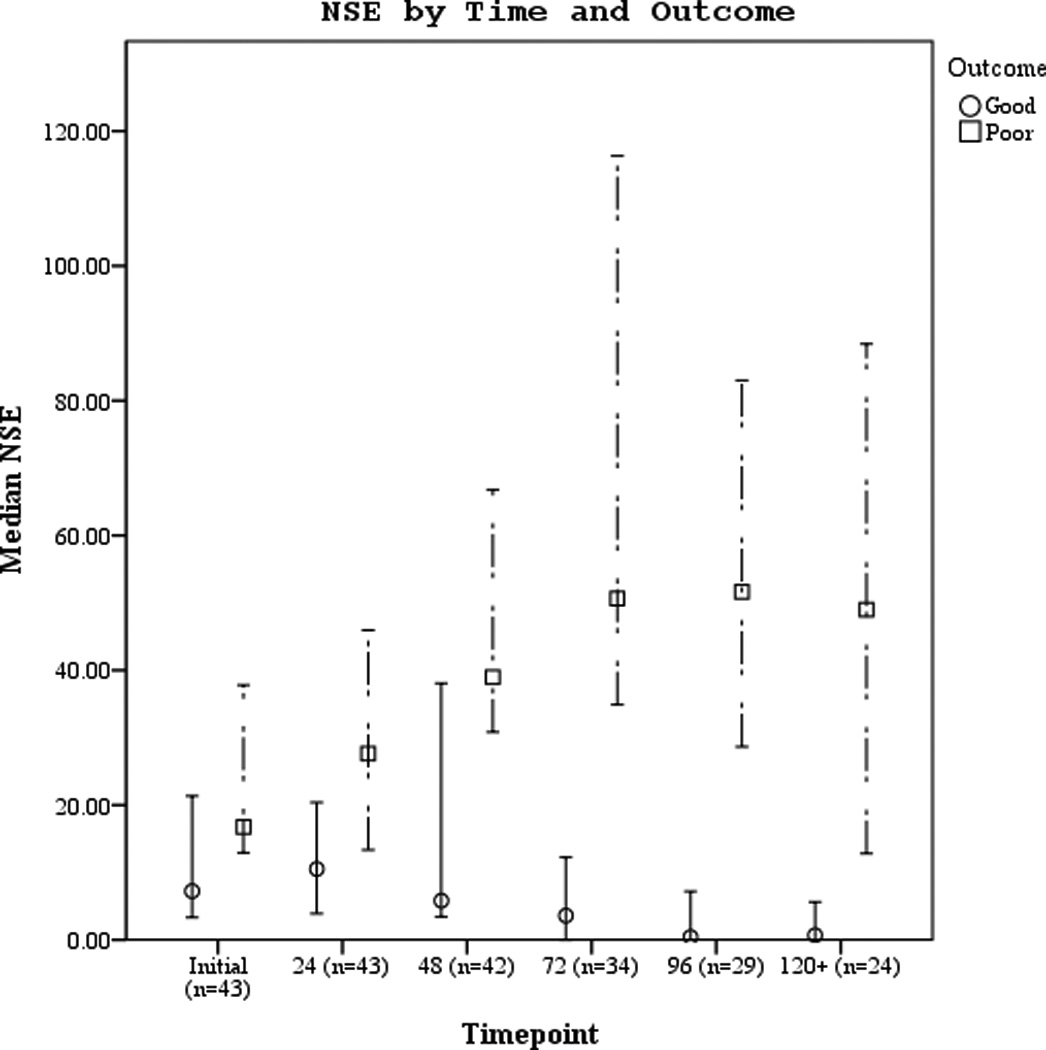
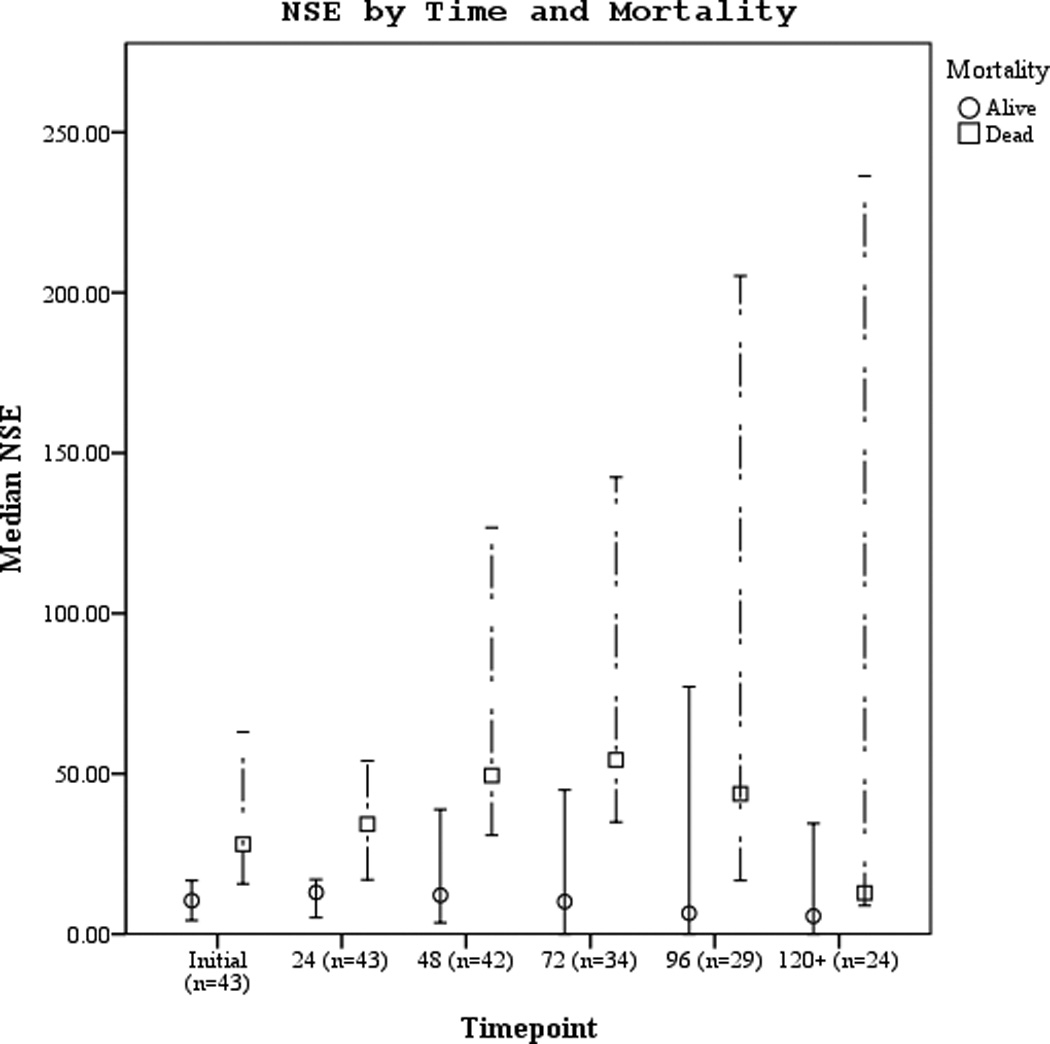
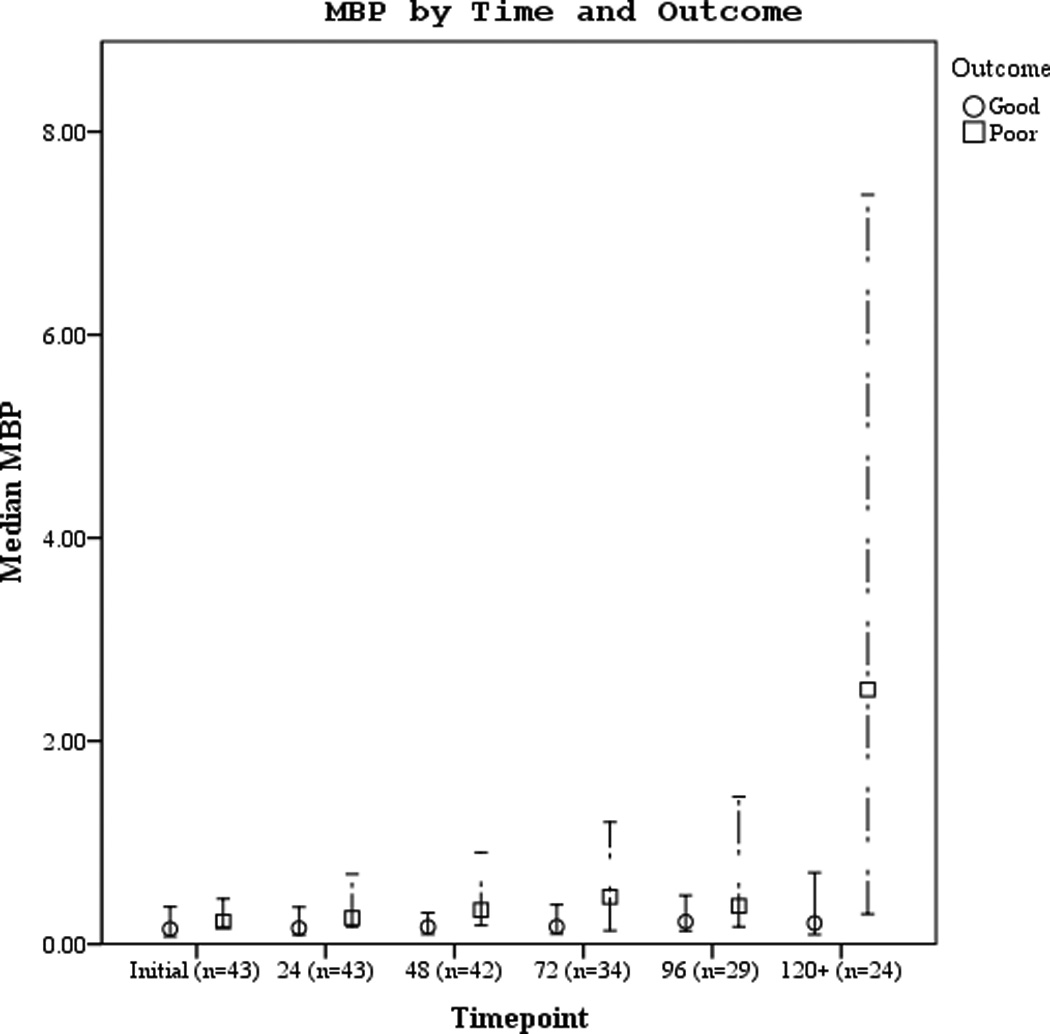
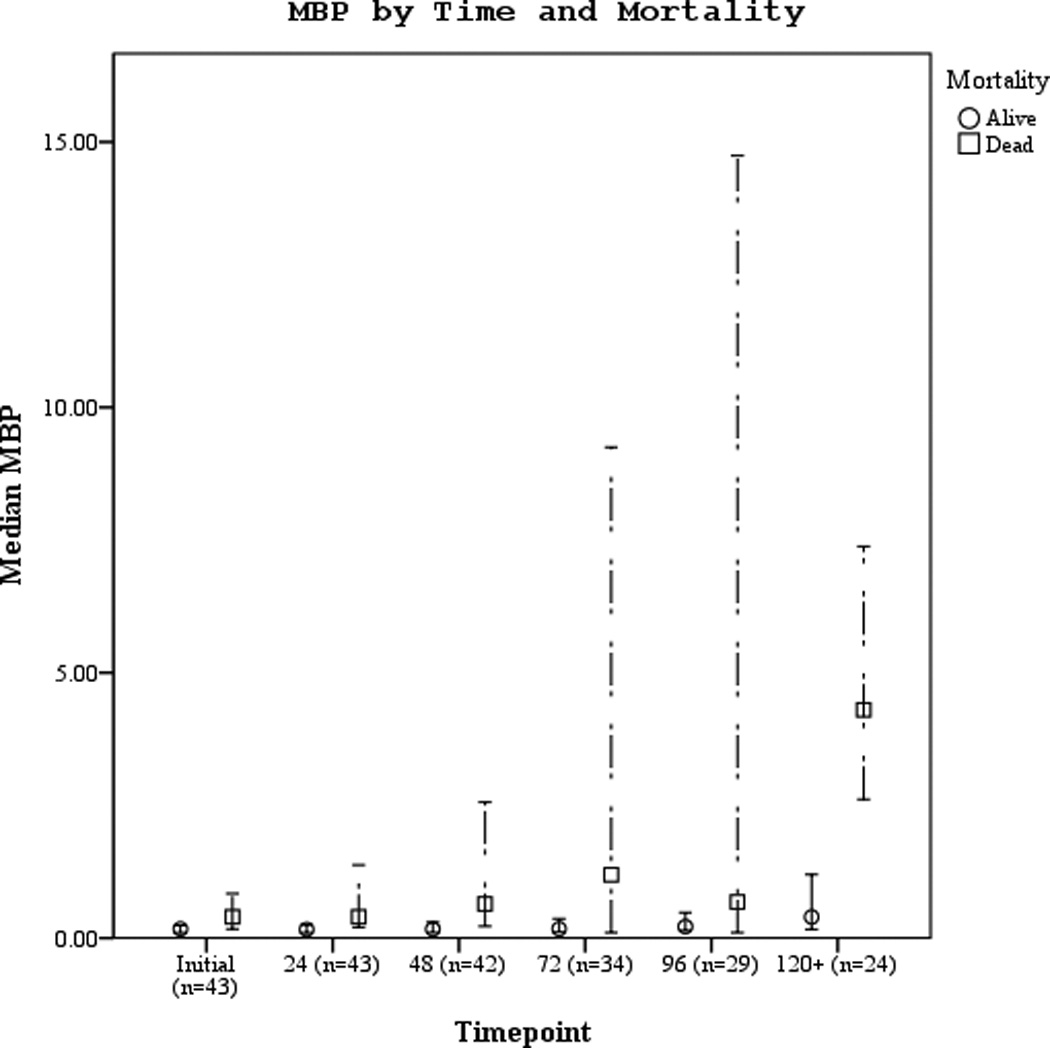
a–f. Serum NSE, S100, and MBP concentrations over the study period by good vs. poor outcome and mortality at 6 months.
Serum NSE, S100b, and MBP concentrations and outcome
Participants who died had significantly higher 24 hour NSE, S100b, and MBP levels when compared to survivors (Table 3). Serum NSE and S100b concentrations were increased in the poor outcome vs. good outcome group and in subjects who died vs. survived at 48, and 72 hours post-ROSC, all p<0.05. Mean, median, and peak serum MBP concentrations were increased in subjects with poor vs. good outcome. Additionally, serum MBP significantly predicted good vs. poor outcome and mortality at 72 hours.
Table 3.
Serum NSE, S100b, MBP concentrations by good vs. poor outcome and mortality at 6 months.
| Mean±SD [25th,75th %] |
Median | All (n=43) |
Good outcome (n=17) |
Poor outcome (n=26) |
p-value Good vs. Poor |
Survived (n=26) |
Died (n=17) |
p-value Mortality |
|---|---|---|---|---|---|---|---|---|
| NSE (ng/ml) | ||||||||
| Initial | 15.40 | 26.9±33.4 | 16.1±20.6 | 33.9±38.4 | 0.047 | 14.5±16.9 | 45.7±43.1 | 0.003 |
| [5.8,34.0] | [3.0,25.2] | [10.8,46.3] | [3.5,17.7] | [14.3, 71.5] | ||||
| Mean | 29.92 | 38.2±38.2 | 14.6±18.9 | 53.6±39.9 | <0.001 | 23.4±23.8 | 60.8±45.2 | 0.001 |
| [8.8,59.0] | [2.5,17.6] | [32.4,66.2] | [3.7,49.8] | [35.1, 75.1] | ||||
| Median | 28.00 | 34.4±37.3 | 13.7±19.4 | 47.8±40.3 | <0.001 | 20.4±23.8 | 55.6±44.4 | 0.001 |
| [6.1,52.0] | [2.1,14.3] | 2[29.3,55.2] | [3.1, 35.3] | [30.7, 60.4] | ||||
| Peak | 49.74 | 67.4±60.1 | 32.6±37.8 | 90.1±61.5 | <0.001 | 49.4±45.6 | 94.8±70.0 | 0.015 |
| [16.7,101.0] | [5.8,38.6] | [48.2,117.5] | [9.5, 91.7] | [51.5, 52.1] | ||||
| 24 h | 16.70 | 27.9±32.2 | 15.1±14.7 | 36.3±37.6 | 0.019 | 15.6±13.9 | 46.8±42.3 | 0.003 |
| [5.8,38.0] | [3.8,24.2] | [9.7,50.6] | [3.9, 22.3] | [15.1, 59.2] | ||||
| 48 h | 34.18 | 44.4±52.7 | 20.4±24.6 | 60.8±60.5 | 0.011 | 25.2±28.6 | 75.8±67.4 | 0.007 |
| [4.0,60.0] | [2.9,38.4] | [22.9,74.8] | [3.2, 39.0] | [31.9, 115.7] | ||||
| 72 h | 30.59 | 46.5±53.6 | 17.2±38.8 | 67.0±53.6 | 0.001 | 29.8±42.8 | 81.5±58.7 | 0.004 |
| [5.4,55.0] | [0.0,11.6] | [28.4,120.0] | [0.0, 49.6] | [42.8, 142.0] | ||||
| S100b (ng/ml) | ||||||||
| Initial | 0.031 | 0.150±0.250 | 0.041±0.075 | 0.221±0.297 | 0.001 | 0.035±0.061 | 0.325±0.324 | <0.001 |
| [0.009,0.190] | [0.005, 0.380] | [0.020, 0.290] | [0.007, 0.030] | [0.060, 0.660] | ||||
| Mean | 0.020 | 0.109±0.159 | 0.027±0.059 | 0.163±0.180 | <0.001 | 0.024±0.047 | 0.239±0.181 | <0.001 |
| [0.010,0.170] | [0.001, 0.019] | [0.010, 0.310] | [0.003, 0.020] | [0.070, 0.370] | ||||
| Median | 0.018 | 0.094±0.140 | 0.024±0.064 | 0.140±0.157 | <0.001 | 0.021±0.052 | 0.260±0.158 | <0.001 |
| [0.007,0.130] | [0.000, 0.010] | [0.010, 0.270] | [0.001, 0.010] | [0.060, 0.300] | ||||
| Peak | 0.048 | 0.196±0.277 | 0.064±0.099 | 0.283±0.321 | 0.001 | 0.055±0.081 | 0.412±0.331 | <0.001 |
| [0.020,0.250] | [0.006, 0.060] | [0.030, 0.590] | [0.008, 0.050] | [0.110, 0.750] | ||||
| 24 h | 0.021 | 0.107±0.181 | 0.023±0.038 | 0.162±0.215 | <0.001 | 0.021±0.031 | 0.239±0.232 | <0.001 |
| [0.008, 0.120] | [0.000, 0.020] | [0.010, 0.270] | [0.001, 0.210] | [0.040, 0.310] | ||||
| 48 h | 0.019 | 0.113±0.191 | 0.034±0.075 | 0.167±0.227 | 0.001 | 0.029±0.061 | 0.251±0.248 | <0.001 |
| [0.006, 0.200] | [0.001, 0.018] | [0.010, 0.210] | [0.002, 0.210] | [0.030, 0.430] | ||||
| 72 h | 0.014 | 0.070±0.125 | 0.028±0.074 | 0.099±0.146 | 0.002 | 0.024±0.058 | 0.167±0.171 | <0.001 |
| [0.003, 0.060] | [0.000, 0.010] | [0.010, 0.150] | [0.000, 0.020] | [0.010, 0.250] | ||||
| MBP(ng/ml) | ||||||||
| Initial | 0.20 | 0.75±1.48 | 0.74±1.54 | 0.76±1.47 | 0.153 | 0.81±1.82 | 0.65±0.75 | 0.094 |
| [0.09,.44.00] | [0.07, 0.40] | [0.15, 0.72] | [0.08, 0.28] | [0.15, 1.11] | ||||
| Mean | 0.36 | 1.11±1.78 | 0.61±1.18 | 1.44±2.03 | 0.010 | 0.83±1.57 | 1.54±2.03 | 0.021 |
| [0.15,1.37] | [0.09,.40.00] | [0.22,1.79] | [0.11, 0.49] | [0.25, 1.82] | ||||
| Median | 0.29 | 0.97±1.75 | 0.62±1.28 | 1.20±2.00 | 0.030 | 0.71±1.51 | 1.36±2.06 | 0.006 |
| [0.11,68.00] | [0.09,.31.00] | [0.19,1.41] | [0.09, 0.33] | [0.22, 1.79] | ||||
| Peak | 0.69 | 2.46±3.85 | 0.92±1.50 | 3.46±4.6 | 0.007 | 1.86±3.17 | 3.36±4.67 | 0.074 |
| [0.29,2.61] | [0.16, 0.95] | [0.42,4.38] | [0.19, 1.68] | [0.47, 3.56] | ||||
| 24 h | 0.20 | 0.82±1.47 | 0.59±1.13 | 0.98±1.65 | 0.053 | 0.73±1.67 | 0.97±1.11 | 0.017 |
| [0.09, 0.68] | [0.08,.36.00] | [0.15,1.12] | [0.09, 0.36] | [0.19, 1.67] | ||||
| 48 h | 0.27 | 1.17±2.23 | 0.62±1.23 | 1.55±2.67 | 0.055 | 0.72±1.54 | 1.91±2.96 | 0.060 |
| [0.11, 0.74] | [0.09,.32.00] | [0.16,1.38] | [0.09, 0.31] | [0.24, 2.30] | ||||
| 72 h | 0.27 | 1.37±2.90 | 0.54±1.24 | 1.95±3.57 | 0.080 | 0.70±1.52 | 2.78±4.42 | 0.020 |
| [0.11,1.08] | [0.09,.36.00] | [0.13,1.56] | [0.10, 0.38] | [0.26, 2.31] |
IQR, interquartile range; SD, standard deviation; NSE, neuron specific enolase; MBP, myelin basic protein
Serum biomarker correlation with temperature and age
Core temperatures were noted at the time of biomarker sampling. MBP had a positive correlation with subject temperature (r=0.241, p < .001) while NSE and S100b concentrations were not correlated with temperature. Subject temperature was categorized as < 34°C, 34–36°C, and > 36°C. There were no differences between temperature groups for NSE. S100b was increased in the 34–36°C group vs. <34°C and > 36 °C and MBP concentrations were increased in the > 36°C group compared to both <34°C and 34–36°C groups (all p<0.05).
Age was inversely correlated with initial S100b (r=−0.40, p=0.008), peak S100b (r=−0.31, p=0.042) and at the 24 hour S100b (r=−0.30, p=0.05). Initial NSE was inversely correlated with age (r=−0.41, p=0.006) while MBP concentration was not correlated with age.
Serum biomarker receiver operating characteristics (ROC) and sensitivity analysis
The area under the curve (AUC) [95% CI] for serum S100b to predict poor 6 month outcome and mortality were 0.955 [0.922–0.987] and 0.908 [0.866–0.950], respectively (Figures 4 a–f). Similarly, serum NSE AUC was 0.859 [0.796–0.922] and 0.787 [0.721–0.852] and serum MBP was 0.732 [0.647–0.817] and 0.727 [0.654–0.799] (all p<0.05). AUC for CPR-ROSC time, first lactate and blood pH are shown in Figures 4 g–l. AUC for S100b and NSE for both outcomes were superior to all clinical variables tested (Table 4).
Figure 4.
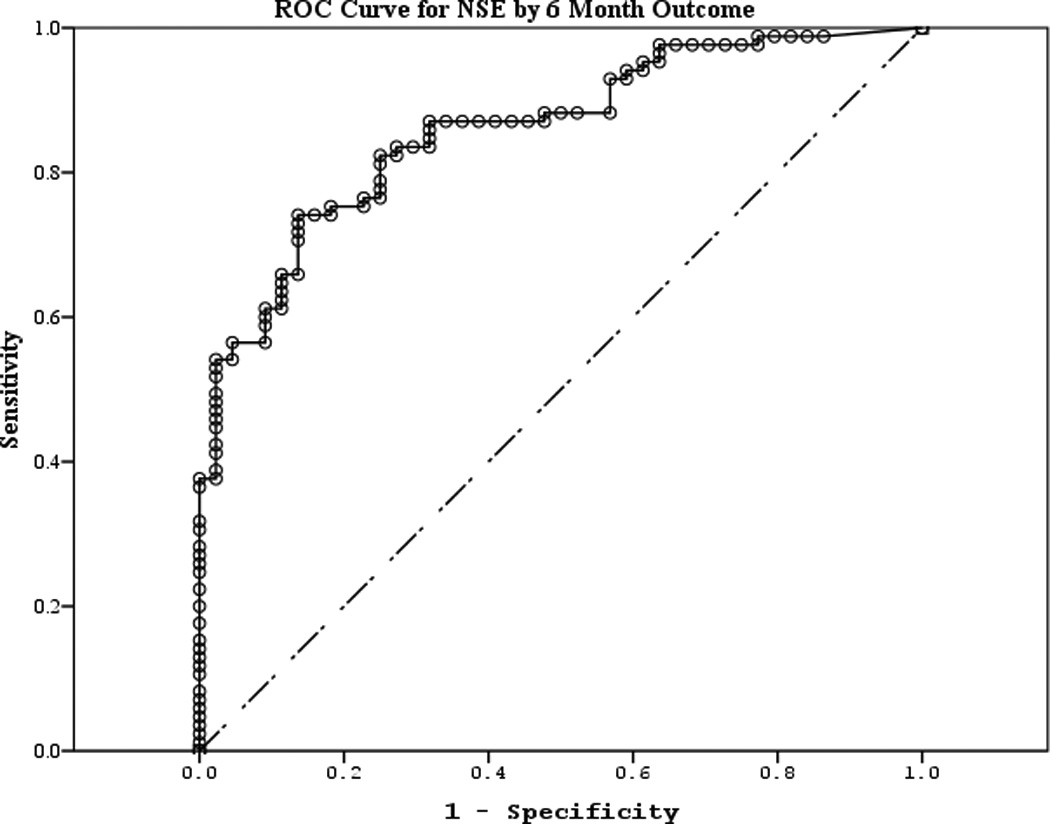
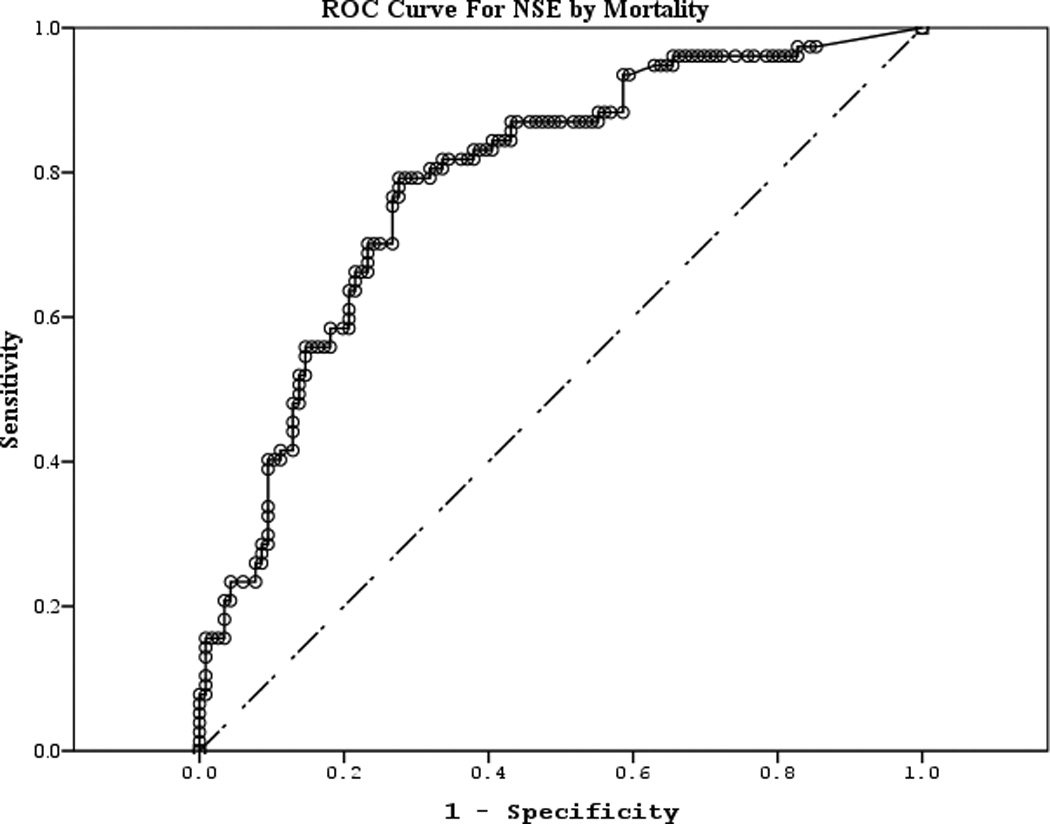
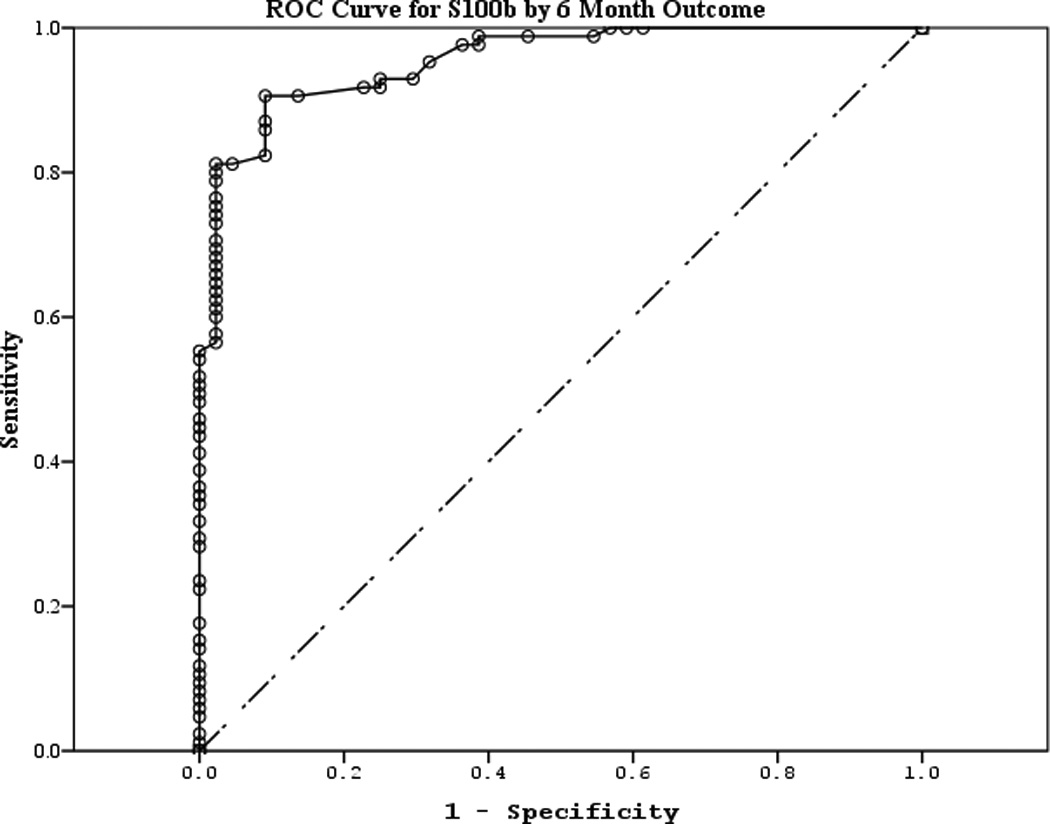
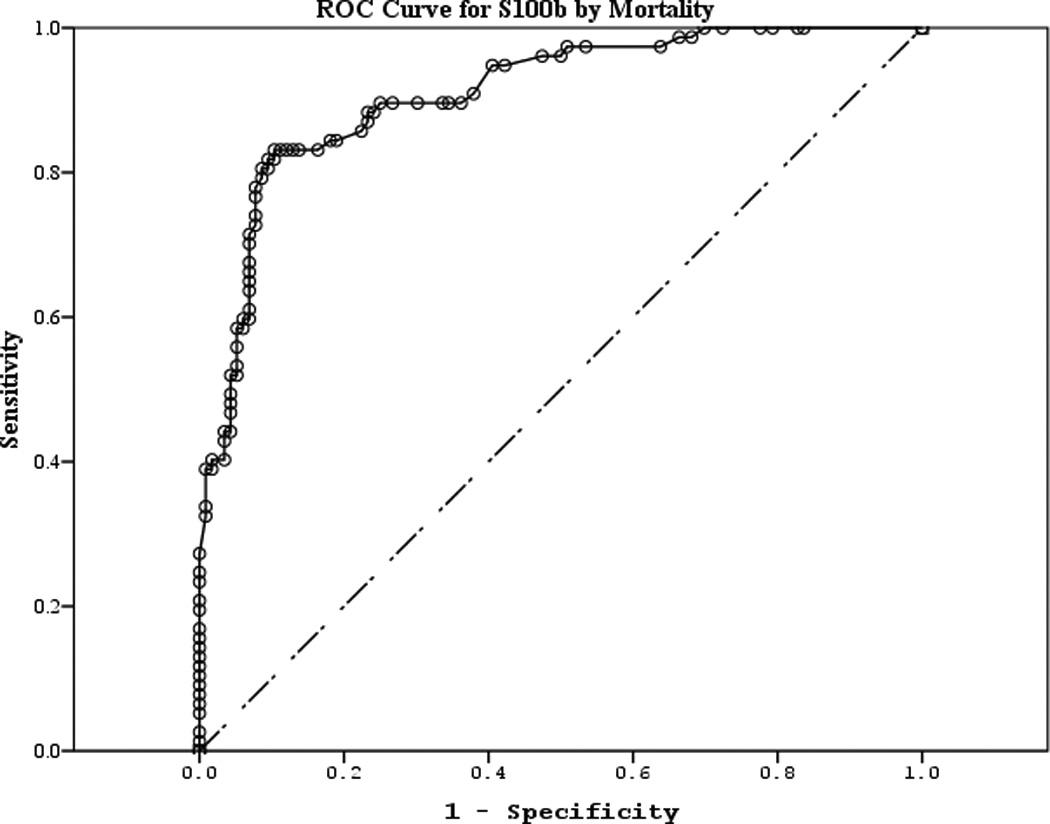
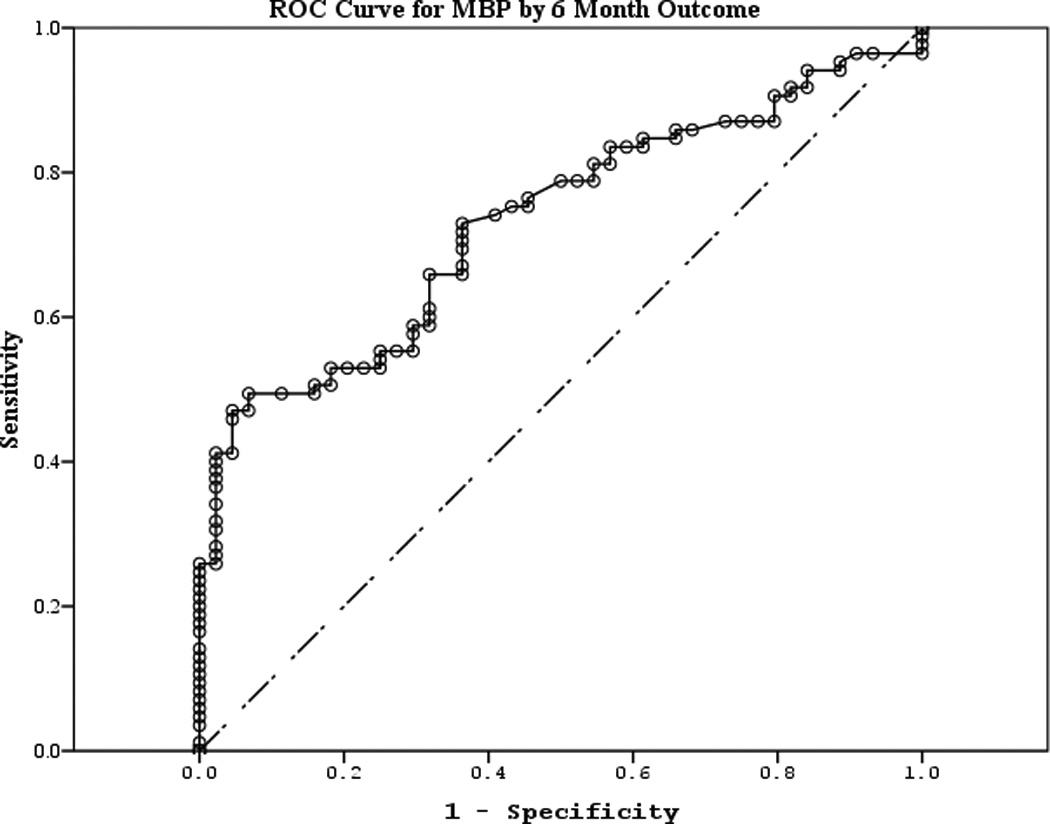
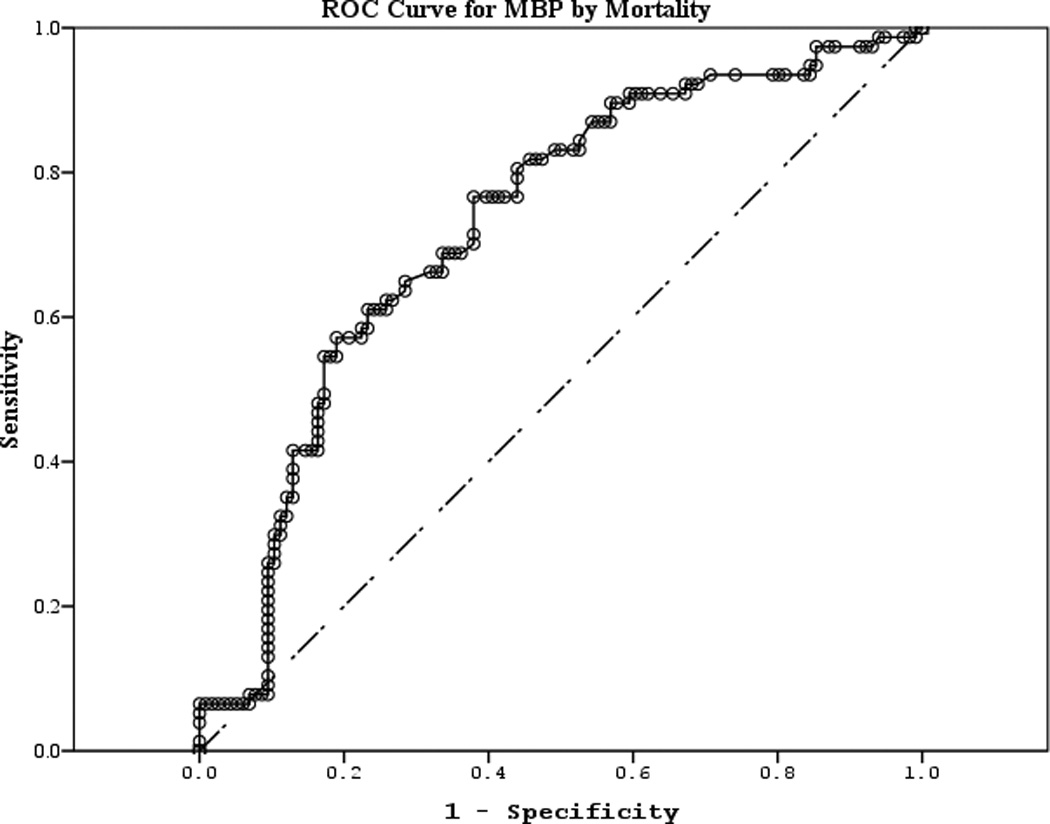
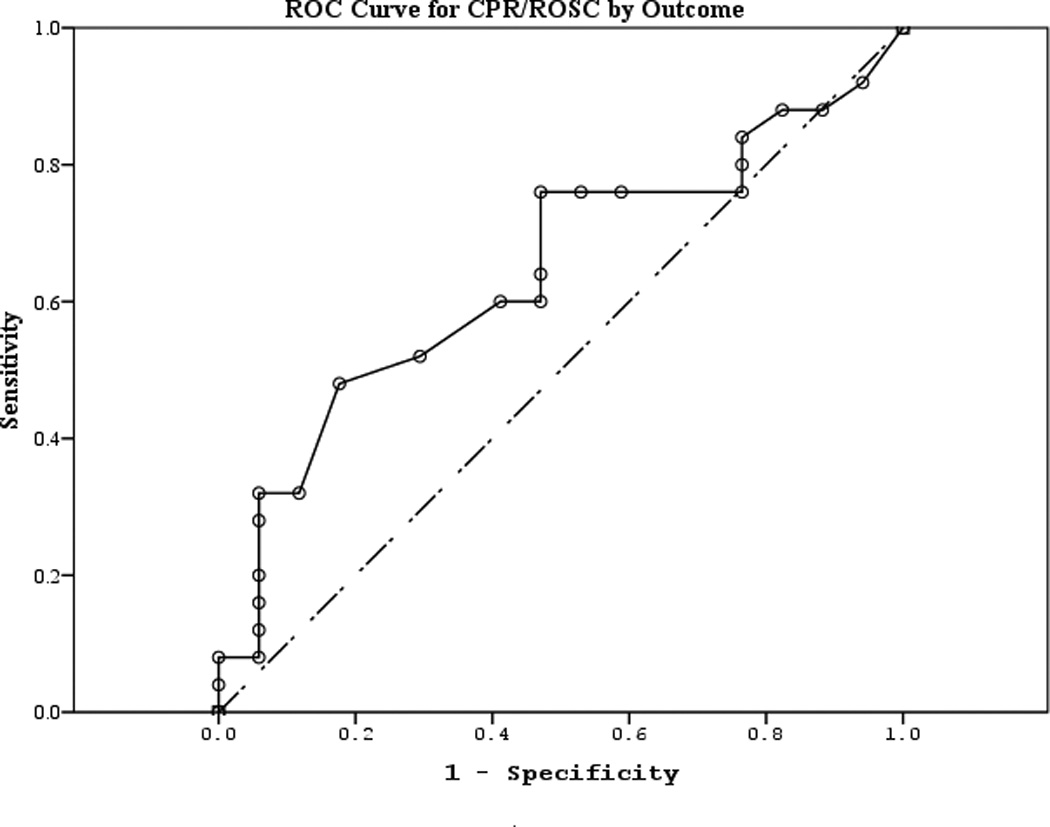
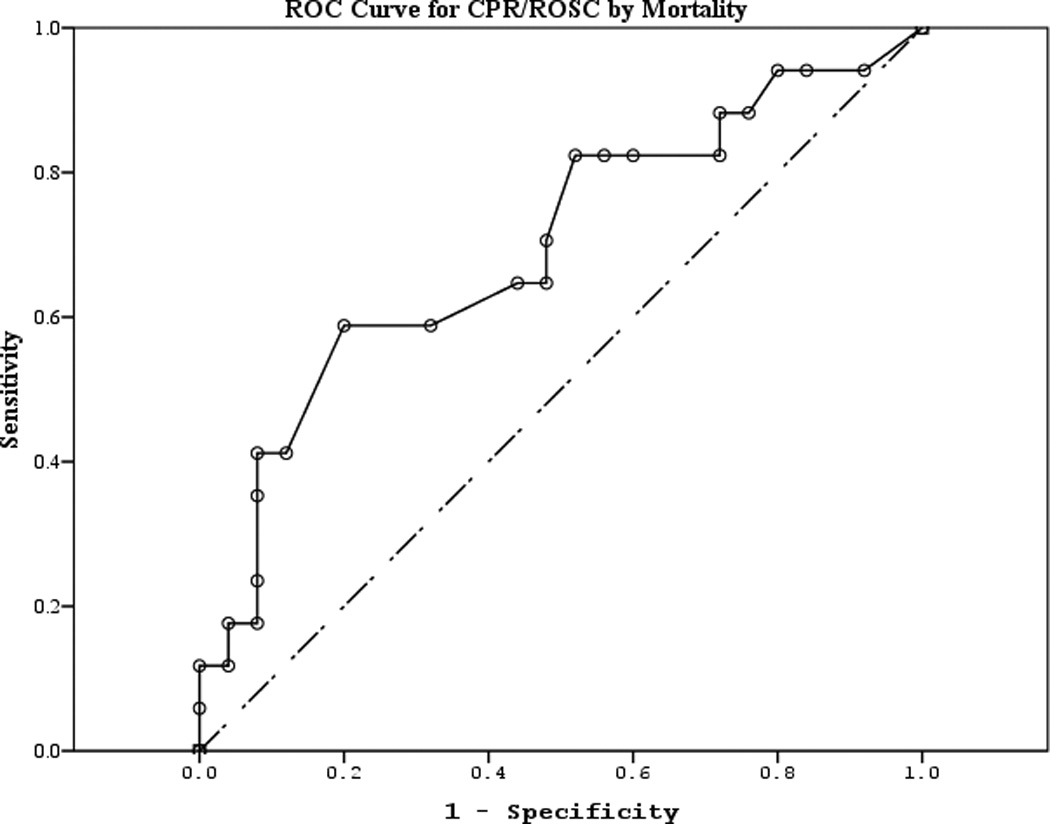
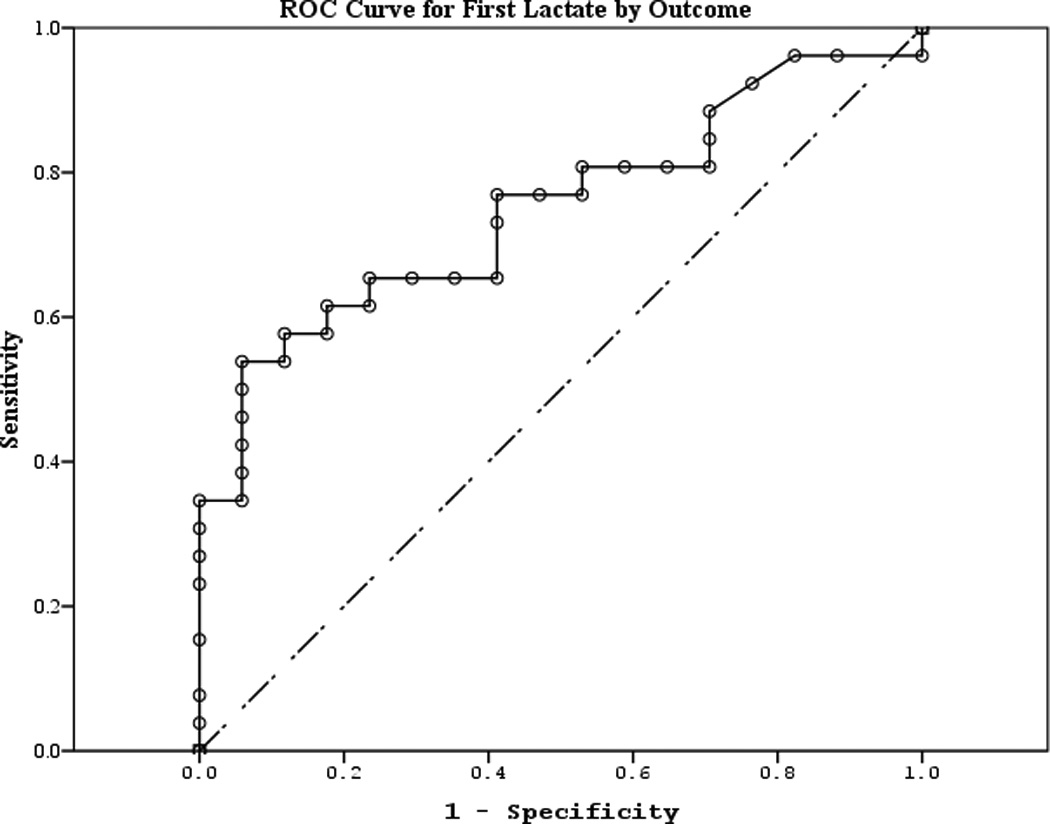
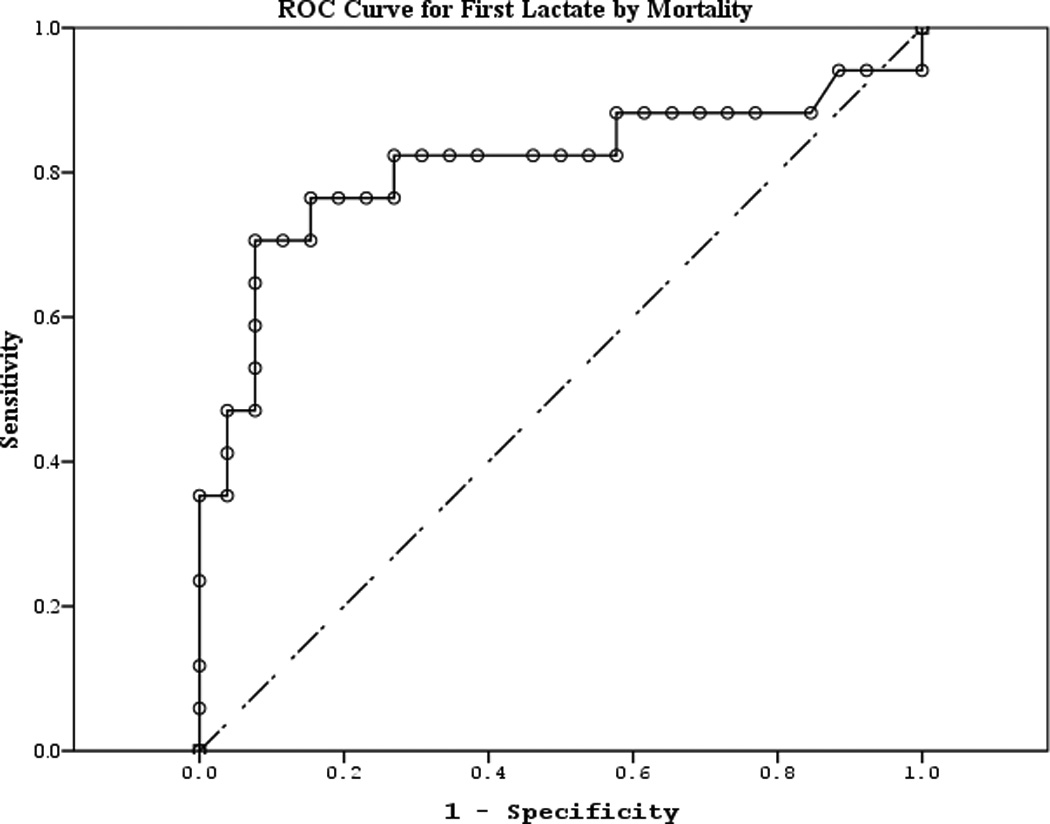
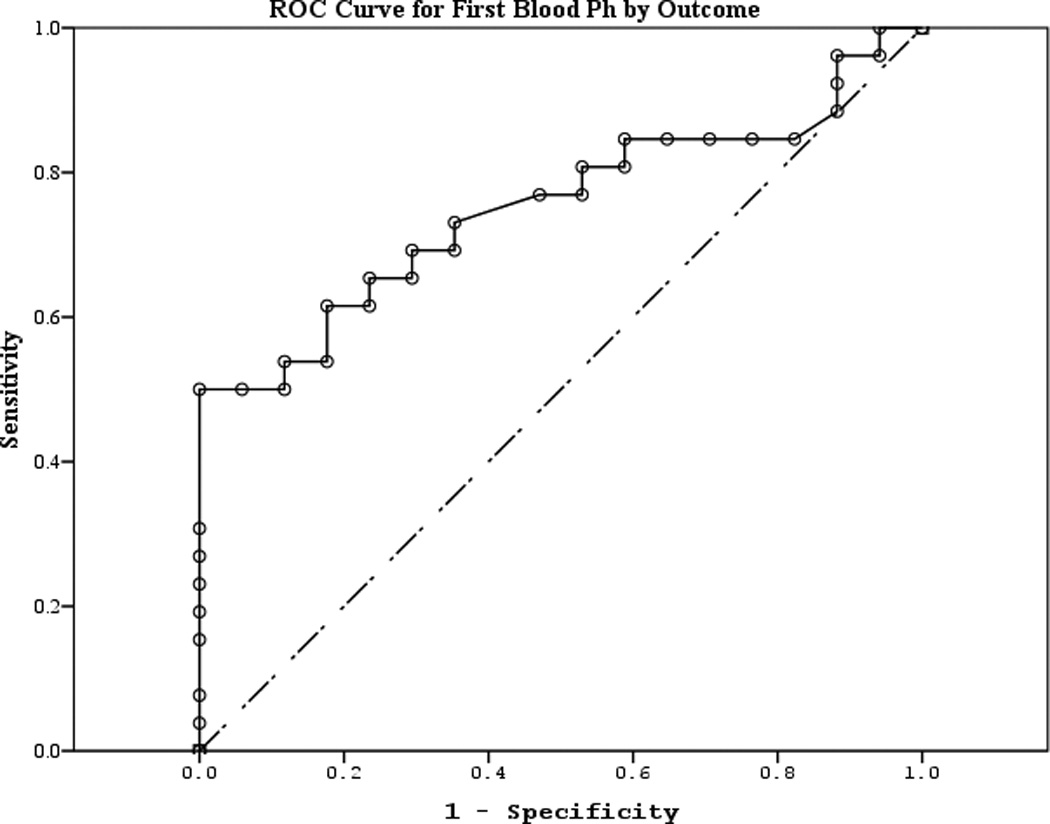
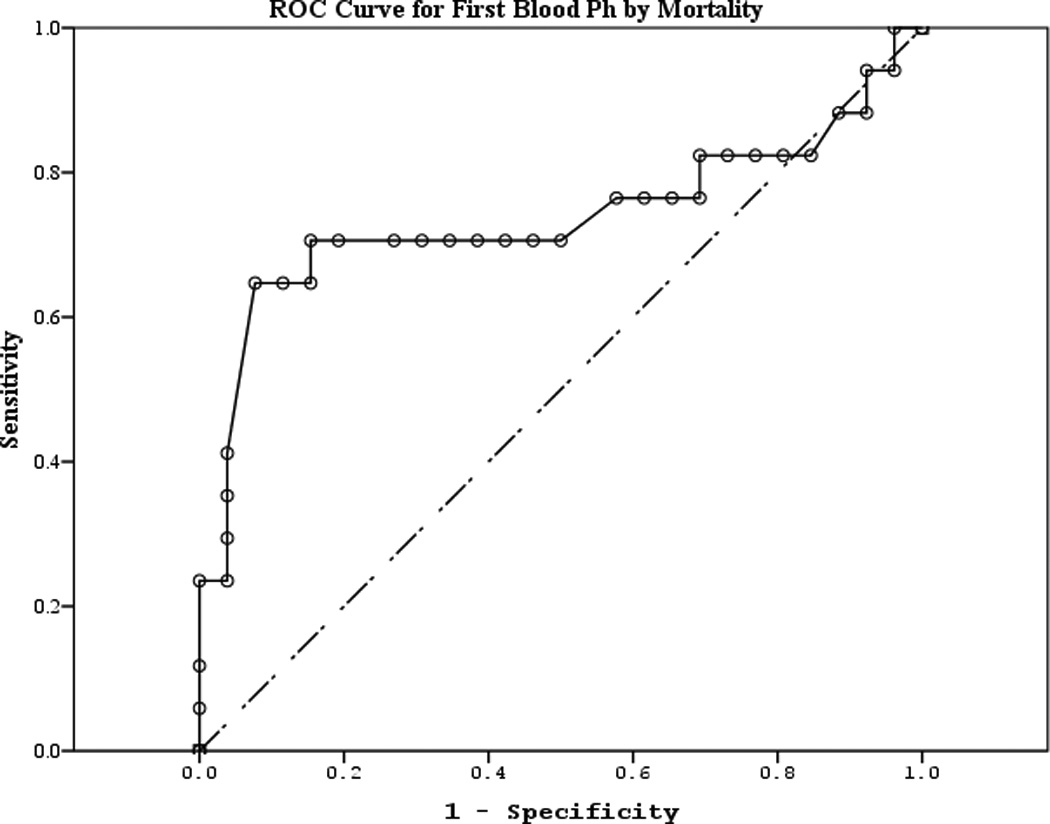
a–l. Receiver operating characteristic curves for serum and clinical variables by good vs. poor outcome and mortality at 6 months.
Table 4.
Area under the curve for serum and clinical biomarkers to predict good vs. poor outcome and mortality at 6 months.
| 95% Confidence Interval | |||||
|---|---|---|---|---|---|
| Biomarker (outcome) | Area | Standard Error | p-value | Lower | Upper |
| NSE (good vs. poor) | 0.859 | 0.032 | <0.001 | 0.796 | 0.922 |
| NSE (mortality) | 0.787 | 0.033 | <0.001 | 0.721 | 0.852 |
| S100b (good vs. poor) | 0.955 | 0.017 | <0.001 | 0.922 | 0.987 |
| S100b (mortality) | 0.908 | 0.021 | <0.001 | 0.866 | 0.950 |
| MBP (good vs. poor) | 0.732 | 0.043 | <0.001 | 0.647 | 0.817 |
| MBP (mortality) | 0.727 | 0.037 | <0.001 | 0.654 | 0.799 |
| CPR-ROSC (good vs. poor) | 0.642 | 0.086 | 0.121 | 0.474 | 0.811 |
| CPR-ROSC (mortality) | 0.696 | 0.086 | 0.032 | 0.528 | 0.865 |
| First lactate (good vs. poor) | 0.749 | 0.074 | 0.006 | 0.604 | 0.893 |
| First lactate (mortality) | 0.809 | 0.079 | 0.001 | 0.654 | 0.964 |
| First blood pH (good vs. poor) | 0.752 | 0.074 | 0.006 | 0.608 | 0.896 |
| First blood pH (mortality) | 0.736 | 0.091 | 0.009 | 0.558 | 0.915 |
NSE, neuron specific enolase; MBP, myelin basic protein; CPR, cardiopulmonary resuscitation; ROSC, return of spontaneous circulation
Tables 5a–b feature biomarker concentration thresholds for best sensitivity and specificity and corresponding positive and negative predictive value at 24 and 48 hour time points. For example, a cut point of 0.008 ng/ml for 24 hour S100b yielded an extremely high probability of correctly classifying good vs. poor outcome using just one biomarker score. Similar results were obtained for mortality. A cut point of 53.10 ng/ml for 24 hour NSE resulted in a very high probability of correctly classifying mortality coupled with the best specificity on that variable.
Table 5.
| a. Best sensitivity and specificity for serum biomarkers to predict good vs. poor outcome at 6 months. | |||||
|---|---|---|---|---|---|
| Concentration (ng/ml) Good/poor outcome |
Sensitivity | Specificity | Positive predictive value |
Negative predictive value |
|
| S100b | |||||
| 24 h (best sensitivity) | 0.001 | 0.96 | 0.29 | 67.6 | 83.3 |
| 24 h (best specificity) | 0.128 | 0.40 | 1.00 | 100.0 | 53.1 |
| 48 h (best sensitivity) | 0.008 | 0.96 | 0.59 | 77.4 | 90.9 |
| 48 h (best specificity) | 0.283 | 0.20 | 1.00 | 100.0 | 45.9 |
| NSE | |||||
| 24 h (best sensitivity) | 2.15 | 0.96 | 0.12 | 62.5 | 66.7 |
| 24 h (best specificity) | 53.10 | 0.20 | 1.00 | 100.0 | 45.9 |
| 48 h (best sensitivity) | 0.48 | 0.96 | 0.18 | 63.2 | 75.0 |
| 48 h (best specificity) | 76.71 | 0.24 | 1.00 | 100.0 | 47.2 |
| MBP | |||||
| 24 h (best sensitivity) | 0.08 | 1.00 | 0.18 | 65.0 | 100.0 |
| 24 h (best specificity) | 5.83 | 0.04 | 1.00 | 100.0 | 40.5 |
| 48 h (best sensitivity) | 0.05 | 1.00 | 0.06 | 61.0 | 100.0 |
| 48 h (best specificity) | 5.43 | 0.12 | 1.00 | 100.0 | 43.6 |
| b. Best sensitivity and specificity for serum biomarkers to predict mortality at 6 months. | |||||
|---|---|---|---|---|---|
| Concentration (ng/ml) Good/poor outcome |
Sensitivity | Specificity | Positive predictive value |
Negative predictive value |
|
| S100b | |||||
| 24 h (best sensitivity) | 0.001 | 0.96 | 0.29 | 45.9 | 100.0 |
| 24 h (best specificity) | 0.128 | 0.40 | 1.00 | 100.0 | 81.3 |
| 48 h (best sensitivity) | 0.008 | 0.96 | 0.59 | 81.8 | 77.4 |
| 48 h (best specificity) | 0.283 | 0.20 | 1.00 | 100.0 | 70.3 |
| NSE | |||||
| 24 h (best sensitivity) | 2.15 | 0.96 | 0.12 | 48.5 | 90.0 |
| 24 h (best specificity) | 53.10 | 0.20 | 1.00 | 100.0 | 73.3 |
| 48 h (best sensitivity) | 0.48 | 0.96 | 0.18 | 53.6 | 92.9 |
| 48 h (best specificity) | 76.71 | 0.24 | 1.00 | 80.0 | 67.6 |
| MBP | |||||
| 24 h (best sensitivity) | 0.08 | 1.00 | 0.18 | 42.5 | 100.0 |
| 24 h (best specificity) | 5.83 | 0.04 | 1.00 | 39.5 | 60.5 |
| 48 h (best sensitivity) | 0.05 | 1.00 | 0.06 | 70.0 | 71.9 |
| 48 h (best specificity) | 5.43 | 0.12 | 1.00 | 100.0 | 63.4 |
NSE, neuron specific enolase; MBP, myelin basic protein
DISCUSSION
In summary, we found that serum biomarkers of brain injury, S100b, NSE, and MBP, have unique patterns of release after pediatric CA. Furthermore, despite a relatively small sample size, biomarker concentrations at multiple time points predicted good or poor outcome and mortality at 6 months with remarkable accuracy. Importantly, the initial and 24 hour biomarker time points have potential value to clinicians in helping to decide whether to initiate a neuroprotective therapy and assisting in counseling families.
Serum S100b can detect early brain injury,11,29 change in response to secondary brain insults30, and predict outcome after various adult and pediatric CNS injuries18,29,31–34. Berger et al found that initial and peak serum S100b concentrations were increased in children with hypoxic-ischemic injury and traumatic brain injury (TBI) but children with TBI peaked earlier (6 vs. 9 hours)20. In our study, S100b was the most robust biomarker to predict outcome at all time points. S100b was also found to be superior to NSE to predict outcome in a study in adults with CA35. In children with CA, Topjian et al found that serum S100b concentrations predicted survival but not good vs. poor outcome when measured at 48 and 72 hours while our study found all time points to be associated with neurological outcome and survival10. S100b consistently peaked prior to the neuronal and myelin biomarkers but the reasons for this are unclear. However, inflammatory mediators released acutely after ischemia-reperfusion by the blood-brain-barrier’s epithelial layer may provoke astrocytes to release cytokines and S100b36,37.
Peak serum NSE concentrations occurred between days 1–2 after CA and tended to remain increased for up to a week or longer in children who fared poorly. The later appearance and often prolonged release of NSE in serum may reflect neuronal cell death from the instigating event as well as ongoing secondary cell death from apoptosis38. This finding of delayed neuronal death is predicted from classic studies of global brain ischemia39. Serum NSE has been evaluated in neonates, children, and adults with hypoxic-ischemic injury and correlates with severity of injury and predicts outcome40,41, 20,42–45. Notably, serum NSE was endorsed by the American Academy of Neurology as an early clinical marker to predict outcome. However, this recommendation was prior to hypothermia becoming standard of care in adults with CA, inviting re-evaluation46. Additionally, in adults surviving CA, NSE concentrations were decreased in subjects randomized to hypothermia compared to subjects in the normothermic group. NSE concentrations correlated with improved gross outcome at 6 months in the hypothermia group, and trended towards improved cognitive and neurophysiological scores in the authors’ follow-up study13,47.
MBP, accounting for 30% of protein in the myelin sheath, peaks late in serum after TBI and can be increased for up to 2 weeks in subjects with poor outcome12,48. MBP has been previously documented in the cerebrospinal fluid in children after severe hypoxic insult49. MBP’s relatively late peak after pediatric CA is consistent with white matter being more resistant to ischemia as compared with gray matter50,51. The pathophysiology of myelin injury after CA is unknown, but presence of white matter injury in the splenium of the corpus callosum suggests a role for Wallerian degeneration after adult CA52. Focal and diffuse white matter injury also occurs after neonatal asphyxia53.
Our analysis suggests that subject temperature did not affect biomarker trajectories. These results could be validated in a larger study or an RCT where subject temperature is controlled.
Initial serum S100b and NSE concentrations were inversely correlated with age in this study. Notably, similar to previous reports, S100b concentrations at later time points also displayed this trend, but NSE did not54. However, population norms have been established for S100b that may allow for its use in all age groups55. Unlike pediatric TBI, younger age was not inversely correlated with MBP concentrations at any time point despite having less brain myelination developmentally56.
Remarkably, serum NSE and S100b performed better than clinical variables in discriminating subject outcome. Clinical predictors of outcome after pediatric CA that have been assessed include duration of pulselessness, first blood gas pH upon ROSC, motor and pupillary examination at 24 hours after ROSC, and EEG57–61.
Evidence suggests that each brain disease requires separate studies to determine their accuracy and cut-off values with and without treatments such as hypothermia to optimize their use. Our findings, in particular the robust ROC results, strongly suggest the need for validation in a larger sample of subjects and consideration for a biomarker panel to maximize accuracy of outcome prediction. The ultimate objective would be development of a point of care test which can be used for rapid results at the bedside for clinical and research purposes.
Study limitations
There were several limitations to the design of this study. Laboratory, imaging, and EEG studies were not mandated in this study. Not all subjects survived to the 7 day study time point, therefore not contributing a full complement of serum biomarkers. However, this is intrinsic to studying a disease with high mortality. Missing data was not imputed. The relatively limited sample size precluded multivariate analysis to see if the highly accurate prediction rate could be improved on by modeling a panel of serum and clinical biomarkers. PCPC was assigned in a non-blinded fashion by the study PI. It was only necessary to contact the families of children who survived to 6 months and the PI was sometimes also part of the clinical team treating study subjects. Finally, the outcome measure we used was the PCPC which is a gross measure of function and may not accurately depict outcome in infants and young children62.
CONCLUSIONS
Our preliminary data show that serum biomarkers S100b, NSE, and MBP have potential to aid in guiding treatment decisions and outcome prediction of children surviving pediatric CA. Modeling and validation of serum and clinical biomarkers may strengthen early prognostication and assist with risk stratification in future clinical studies.
ACKNOWLEGEMENTS
Special thanks to Michelle Dragotta, Christine Kyper, and Alan Abraham for assistance in data acquisition and Keri Feldman for specimen care and measurement. We are grateful to the staff, nurses, and physicians of the ICU for their efforts in subject recruitment and provision of excellent clinical care.
Conflicts of Interest and Source of Funding: Drs. Berger and Kochanek are provisional co-patent holders on a biomarker panel for abusive head trauma in infants. Dr. Callaway has patents and royalties with Medtronic ERS, Inc. related to timing of defibrillation, licensed to a manufacturer of defibrillators.
We appreciate the generous support from the following sources: NICHD K12 HD047349 (E.L.F.), NINDS K23 NS065132 (E.L.F.), and the Laerdal Foundation (E.L.F.). This publication was also made possible by Grant Number 5UL1 RR024153-04 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
REFERENCES
- 1.Kuisma M, Suominen P, Korpela R. Paediatric out-of-hospital cardiac arrests--epidemiology and outcome. Resuscitation. 1995 Oct;30(2):141–150. doi: 10.1016/0300-9572(95)00888-z. [DOI] [PubMed] [Google Scholar]
- 2.Reis AG, Nadkarni V, Perondi MB, Grisi S, Berg RA. A prospective investigation into the epidemiology of in-hospital pediatric cardiopulmonary resuscitation using the international Utstein reporting style. Pediatrics. 2002;109(2):200–209. doi: 10.1542/peds.109.2.200. [DOI] [PubMed] [Google Scholar]
- 3.Young KD, Gausche-Hill M, McClung CD, Lewis RJ. A prospective, population-based study of the epidemiology and outcome of out-of-hospital pediatric cardiopulmonary arrest. Pediatrics. 2004 Jul;114(1):157–164. doi: 10.1542/peds.114.1.157. [DOI] [PubMed] [Google Scholar]
- 4.Nadkarni VM, Larkin GL, Peberdy MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006 Jan 4;295(1):50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 5.de Mos N, van Litsenburg RR, McCrindle B, Bohn DJ, Parshuram CS. Pediatric in-intensive-care-unit cardiac arrest: incidence, survival, and predictive factors. Crit Care Med. 2006 Apr;34(4):1209–1215. doi: 10.1097/01.CCM.0000208440.66756.C2. [DOI] [PubMed] [Google Scholar]
- 6.Hickey RW, Painter MJ. Brain injury from cardiac arrest in children. Neurol Clin. 2006 Feb;24(1):147–158. doi: 10.1016/j.ncl.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Donoghue AJ, Nadkarni V, Berg RA, et al. Out-of-hospital pediatric cardiac arrest: an epidemiologic review and assessment of current knowledge. Ann Emerg Med. 2005 Dec;46(6):512–522. doi: 10.1016/j.annemergmed.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 8.O'Rourke PP. Outcome of children who are apneic and pulseless in the emergency room. Crit Care Med. 1986 May;14(5):466–468. [PubMed] [Google Scholar]
- 9.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005 Feb 19–25;365(9460):663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 10.Topjian AA, Lin R, Morris MC, et al. Neuron-specific enolase and S-100B are associated with neurologic outcome after pediatric cardiac arrest. Pediatr Crit Care Med. 2009 Jul;10(4):479–490. doi: 10.1097/PCC.0b013e318198bdb5. [DOI] [PubMed] [Google Scholar]
- 11.Steffen IG, Hasper D, Ploner CJ, et al. Mild therapeutic hypothermia alters neuron specific enolase as an outcome predictor after resuscitation: 97 prospective hypothermia patients compared to 133 historical non-hypothermia patients. Crit Care. 2010;14(2):R69. doi: 10.1186/cc8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger RP, Adelson PD, Pierce MC, Dulani T, Cassidy LD, Kochanek PM. Serum neuron-specific enolase, S100B, and myelin basic protein concentrations after inflicted and noninflicted traumatic brain injury in children. J Neurosurg. 2005 Jul;103(1) Suppl:61–68. doi: 10.3171/ped.2005.103.1.0061. [DOI] [PubMed] [Google Scholar]
- 13.Tiainen M, Roine RO, Pettila V, Takkunen O. Serum neuron-specific enolase and S-100B protein in cardiac arrest patients treated with hypothermia. Stroke. 2003 Dec;34(12):2881–2886. doi: 10.1161/01.STR.0000103320.90706.35. [DOI] [PubMed] [Google Scholar]
- 14.Berger R, Richichi R. Derivation and validation of an equation for adjustment of neuron-specific enolase concentrations in hemolyzed serum. Pediatr Crit Care Med. 2009 Mar;10(2):260–263. doi: 10.1097/PCC.0b013e31819a376d. [DOI] [PubMed] [Google Scholar]
- 15.Anderson RE, Hansson LO, Nilsson O, Dijlai-Merzoug R, Settergren G. High serum S100B levels for trauma patients without head injuries. Neurosurgery. 2001 Jun;48(6):1255–1258. doi: 10.1097/00006123-200106000-00012. discussion 1258–1260. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Garcia S, Gonzalez-Quevedo A, Fernandez-Concepcion O, et al. Short-term prognostic value of serum neuron specific enolase and S100B in acute stroke patients. Clin Biochem. 2012 Nov;45(16–17):1302–1307. doi: 10.1016/j.clinbiochem.2012.07.094. [DOI] [PubMed] [Google Scholar]
- 17.Fassbender K, Schmidt R, Schreiner A, et al. Leakage of brain-originated proteins in peripheral blood: temporal profile and diagnostic value in early ischemic stroke. J Neurol Sci. 1997 May 1;148(1):101–105. doi: 10.1016/s0022-510x(96)05351-8. [DOI] [PubMed] [Google Scholar]
- 18.Lardner D, Davidson A, McKenzie I, Cochrane A. Delayed rises in serum S100B levels and adverse neurological outcome in infants and children undergoing cardiopulmonary bypass. Paediatr Anaesth. 2004 Jun;14(6):495–500. doi: 10.1111/j.1460-9592.2004.01230.x. [DOI] [PubMed] [Google Scholar]
- 19.Bechtel K, Frasure S, Marshall C, Dziura J, Simpson C. Relationship of serum S100B levels and intracranial injury in children with closed head trauma. Pediatrics. 2009 Oct;124(4):e697–e704. doi: 10.1542/peds.2008-1493. [DOI] [PubMed] [Google Scholar]
- 20.Berger RP, Adelson PD, Richichi R, Kochanek PM. Serum biomarkers after traumatic and hypoxemic brain injuries: insight into the biochemical response of the pediatric brain to inflicted brain injury. Dev Neurosci. 2006;28(4–5):327–335. doi: 10.1159/000094158. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Silverstein FS, Skoff R, Barks JD. Hypoxic-ischemic oligodendroglial injury in neonatal rat brain. Pediatric Research. 2002;51(1):25–33. doi: 10.1203/00006450-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Alix A, Cabanas F, Pellicer A, Hernanz A, Stiris TA, Quero J. Neuron-specific enolase and myelin basic protein: relationship of cerebrospinal fluid concentrations to the neurologic condition of asphyxiated full-term infants. Pediatrics. 1994;93(2):234–240. [PubMed] [Google Scholar]
- 23.Nolan JP, Neumar RW, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication: a scientific statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke (Part II) Int Emerg Nurs. 2010 Jan;18(1):8–28. doi: 10.1016/j.ienj.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Fink EL, Kochanek PM, Clark RS, Bell MJ. Fever control and application of hypothermia using intravenous cold saline. Pediatr Crit Care Med. 2012 Jan;13(1):80–84. doi: 10.1097/PCC.0b013e3181fe27c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langhelle A, Nolan J, Herlitz J, et al. Recommended guidelines for reviewing, reporting, and conducting research on post-resuscitation care: the Utstein style. Resuscitation. 2005 Sep;66(3):271–283. doi: 10.1016/j.resuscitation.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Wernovsky G, Wypij D, Jonas RA, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995 Oct 15;92(8):2226–2235. doi: 10.1161/01.cir.92.8.2226. [DOI] [PubMed] [Google Scholar]
- 27.Nishisaki A, Sullivan J, 3rd, Steger B, et al. Retrospective analysis of the prognostic value of electroencephalography patterns obtained in pediatric in-hospital cardiac arrest survivors during three years. Pediatr Crit Care Med. 2007 Jan;8(1):10–17. doi: 10.1097/01.pcc.0000256621.63135.4b. [DOI] [PubMed] [Google Scholar]
- 28.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992 Jul;121(1):68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 29.Berger RP, Pierce MC, Wisniewski SR, Adelson PD, Kochanek PM. Serum S100B concentrations are increased after closed head injury in children: a preliminary study. J Neurotrauma. 2002 Nov;19(11):1405–1409. doi: 10.1089/089771502320914633. [DOI] [PubMed] [Google Scholar]
- 30.Unden J, Astrand R, Waterloo K, et al. Clinical significance of serum S100B levels in neurointensive care. Neurocrit Care. 2007;6(2):94–99. doi: 10.1007/s12028-007-0005-0. [DOI] [PubMed] [Google Scholar]
- 31.Jauch EC, Lindsell C, Broderick J, Fagan SC, Tilley BC, Levine SR. Association of serial biochemical markers with acute ischemic stroke: the National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator Stroke Study. Stroke. 2006 Oct;37(10):2508–2513. doi: 10.1161/01.STR.0000242290.01174.9e. [DOI] [PubMed] [Google Scholar]
- 32.Petzold A, Green AJ, Keir G, et al. Role of serum S100B as an early predictor of high intracranial pressure and mortality in brain injury: a pilot study. Crit Care Med. 2002 Dec;30(12):2705–2710. doi: 10.1097/00003246-200212000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Foerch C, Otto B, Singer OC, et al. Serum S100B predicts a malignant course of infarction in patients with acute middle cerebral artery occlusion. Stroke. 2004 Sep;35(9):2160–2164. doi: 10.1161/01.STR.0000138730.03264.ac. [DOI] [PubMed] [Google Scholar]
- 34.James ML, Blessing R, Phillips-Bute BG, Bennett E, Laskowitz DT. S100B and brain natriuretic peptide predict functional neurological outcome after intracerebral haemorrhage. Biomarkers. 2009 Sep;14(6):388–394. doi: 10.1080/13547500903015784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shinozaki K, Oda S, Sadahiro T, et al. Serum S-100B is superior to neuron-specific enolase as an early prognostic biomarker for neurological outcome following cardiopulmonary resuscitation. Resuscitation. 2009 Aug;80(8):870–875. doi: 10.1016/j.resuscitation.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006 Jan;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 37.Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000 Apr;20(2):131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beilharz EJ, Williams CE, Dragunow M, Sirimanne ES, Gluckman PD. Mechanisms of delayed cell death following hypoxic-ischemic injury in the immature rat: evidence for apoptosis during selective neuronal loss. Brain Res Mol Brain Res. 1995 Mar;29(1):1–14. doi: 10.1016/0169-328x(94)00217-3. [DOI] [PubMed] [Google Scholar]
- 39.Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982 May 6;239(1):57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- 40.Meynaar IA, Straaten HM, van der Wetering J, et al. Serum neuron-specific enolase predicts outcome in post-anoxic coma: a prospective cohort study. Intensive Care Medicine. 2003;29(2):189–195. doi: 10.1007/s00134-002-1573-2. [DOI] [PubMed] [Google Scholar]
- 41.Tekgul H, Yalaz M, Kutukculer N, et al. Value of biochemical markers for outcome in term infants with asphyxia. Pediatr Neurol. 2004 Nov;31(5):326–332. doi: 10.1016/j.pediatrneurol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Berger RP. The use of serum biomarkers to predict outcome after traumatic brain injury in adults and children. J Head Trauma Rehabil. 2006;21(4):315–333. doi: 10.1097/00001199-200607000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Berger RP, Pierce MC, Wisniewski SR, et al. Neuron-specific enolase and S100B in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatrics. 2002 Feb;109(2):E31. doi: 10.1542/peds.109.2.e31. [DOI] [PubMed] [Google Scholar]
- 44.Satchell MA, Lai Y, Kochanek PM, et al. Cytochrome c, a biomarker of apoptosis, is increased in cerebrospinal fluid from infants with inflicted brain injury from child abuse. J Cereb Blood Flow Metab. 2005 Jul;25(7):919–927. doi: 10.1038/sj.jcbfm.9600088. [DOI] [PubMed] [Google Scholar]
- 45.Schoerkhuber W, Kittler H, Sterz F, et al. Time course of serum neuron-specific enolase. A predictor of neurological outcome in patients resuscitated from cardiac arrest. Stroke. 1999 Aug;30(8):1598–1603. doi: 10.1161/01.str.30.8.1598. [DOI] [PubMed] [Google Scholar]
- 46.Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006 Jul 25;67(2):203–210. doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 47.Tiainen M, Poutiainen E, Kovala T, Takkunen O, Happola O, Roine RO. Cognitive and neurophysiological outcome of cardiac arrest survivors treated with therapeutic hypothermia. Stroke. 2007 Aug;38(8):2303–2308. doi: 10.1161/STROKEAHA.107.483867. [DOI] [PubMed] [Google Scholar]
- 48.Thomas DG, Palfreyman JW, Ratcliffe JG. Serum-myelin-basic-protein assay in diagnosis and prognosis of patients with head injury. Lancet. 1978 Jan 21;1(8056):113–115. doi: 10.1016/s0140-6736(78)90415-4. [DOI] [PubMed] [Google Scholar]
- 49.Kohlschutter A. Myelin basic protein in cerebrospinal fluid from children. Eur J Pediatr. 1978 Mar 13;127(3):155–161. doi: 10.1007/BF00442056. [DOI] [PubMed] [Google Scholar]
- 50.Falcao AL, Reutens DC, Markus R, et al. The resistance to ischemia of white and gray matter after stroke. Ann Neurol. 2004 Nov;56(5):695–701. doi: 10.1002/ana.20265. [DOI] [PubMed] [Google Scholar]
- 51.Bristow MS, Simon JE, Brown RA, et al. MR perfusion and diffusion in acute ischemic stroke: human gray and white matter have different thresholds for infarction. J Cereb Blood Flow Metab. 2005 Oct;25(10):1280–1287. doi: 10.1038/sj.jcbfm.9600135. [DOI] [PubMed] [Google Scholar]
- 52.Bianchi MT, Sims JR. Restricted diffusion in the splenium of the corpus callosum after cardiac arrest. The open neuroimaging journal. 2008;2:1–4. doi: 10.2174/1874440000802010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sugiura H, Kouwaki M, Kato T, et al. Magnetic resonance imaging in neonates with total asphyxia. Brain Dev. 2012 May 12; doi: 10.1016/j.braindev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Shore PM, Berger RP, Varma S, et al. Cerebrospinal fluid biomarkers versus glasgow coma scale and glasgow outcome scale in pediatric traumatic brain injury: the role of young age and inflicted injury. J Neurotrauma. 2007 Jan;24(1):75–86. doi: 10.1089/neu.2006.0062. [DOI] [PubMed] [Google Scholar]
- 55.Bouvier D, Castellani C, Fournier M, et al. Reference ranges for serum S100B protein during the first three years of life. Clin Biochem. 2011 Jul;44(10–11):927–929. doi: 10.1016/j.clinbiochem.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 56.Su E, Bell MJ, Kochanek PM, et al. Increased CSF Concentrations of Myelin Basic Protein After TBI in Infants and Children: Absence of Significant Effect of Therapeutic Hypothermia. Neurocrit Care. 2012 Dec;17(3):401–407. doi: 10.1007/s12028-012-9767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abend NS, Topjian A, Ichord R, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology. 2009 Jun 2;72(22):1931–1940. doi: 10.1212/WNL.0b013e3181a82687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abend NS, Topjian AA, Kessler S, et al. Outcome prediction by motor and pupillary responses in children treated with therapeutic hypothermia after cardiac arrest. Pediatr Crit Care Med. 2012 Apr 14;:32–38. doi: 10.1097/PCC.0b013e3182196a7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moler FW, Donaldson AE, Meert K, et al. Multicenter cohort study of out-of-hospital pediatric cardiac arrest. Crit Care Med. 2011 Jan;39(1):141–149. doi: 10.1097/CCM.0b013e3181fa3c17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meert KL, Donaldson A, Nadkarni V, et al. Multicenter cohort study of in-hospital pediatric cardiac arrest. Pediatr Crit Care Med. 2009 Sep;10(5):544–553. doi: 10.1097/PCC.0b013e3181a7045c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fink EL, Clark RS, Kochanek PM, Bell MJ, Watson RS. A tertiary care center's experience with therapeutic hypothermia after pediatric cardiac arrest. Pediatr Crit Care Med. 2010 Jan;11(1):66–74. doi: 10.1097/PCC.0b013e3181c58237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000 Jul;28(7):2616–2620. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]