Abstract
Stem cells are pluripotent cells, having a property of differentiating into various types of cells of human body. Several studies have developed mesenchymal stem cells (MSCs) from various human tissues, peripheral blood and body fluids. These cells are then characterized by cellular and molecular markers to understand their specific phenotypes. Dental pulp stem cells (DPSCs) are having a MSCs phenotype and they are differentiated into neuron, cardiomyocytes, chondrocytes, osteoblasts, liver cells and β cells of islet of pancreas. Thus, DPSCs have shown great potentiality to use in regenerative medicine for treatment of various human diseases including dental related problems. These cells can also be developed into induced pluripotent stem cells by incorporation of pluripotency markers and use for regenerative therapies of various diseases. The DPSCs are derived from various dental tissues such as human exfoliated deciduous teeth, apical papilla, periodontal ligament and dental follicle tissue. This review will overview the information about isolation, cellular and molecular characterization and differentiation of DPSCs into various types of human cells and thus these cells have important applications in regenerative therapies for various diseases. This review will be most useful for postgraduate dental students as well as scientists working in the field of oral pathology and oral medicine.
Keywords: Human dental pulp stem cells, Mesenchymal stem cells, Dentin, Pluripotency, Stem cell therapy, Molecular markers
Core tip: Human dental pulp stem cells (DPSCs) have shown a potentiality for the treatment of various human diseases including dental related problems. The review will overview the information about DPSCs, their isolation, cellular and molecular characterization, differentiation into various types of cells and their applications in regenerative therapies for various diseases. This review will be most useful for postgraduate dental students as well as the scientists working in the field of oral pathology, oral medicine and regenerative medicine.
INTRODUCTION
Stem cells are unspecialized cells having a property of self renewal and further differentiate into various types of specialized cells[1]. Stem Cells are identified in a number of adult tissues including skin, adipose tissues[2], peripheral blood[3,4], bone marrow, pancreas, intestine, brain, hair follicles, as well as in the dental pulp cells[5-7]. Stem cell research has expanded well due to their usefulness in regenerative therapies for improving the life of patients suffering from various genetical and neurological diseases. Studies have shown that the dental pulp tissue can also be used to derive mesenchymal stem cells (MSCs) when tissue is grown in culture[7]. These MSCs can differentiate into several cell types as shown in Table 1. In year 2012, Shinya Yamanaka and John Gurdon have won Noble prize award for their excellent work on induced pluripotent stem cells (iPSCs) derived from adult somatic cells. This work has resulted into development of innovated technology to make an iPSCs from individual patient who needs treatment for specific disease. It is proposed that dental pulp stem cells (DPSCs) can develop iPSCs which can be used for therapies of various diseases[8]. This review is mainly highlighting the importance of DPSCs, their isolation, characterization by cellular and molecular markers, differentiation and their applications in treatment of various diseases.
Table 1.
Normal differentiation pathways of adult stem cells from various tissues and cells
| Stem cell | Source | Types of cells produced |
| Hematopoietic | All types of blood cells | Red blood cells, B lymphocytes, T lymphocytes, natural killer cells, neutrophils, basophils, eosinophils, monocytes, macrophages and platelets |
| Bone marrow | Connective tissues | Tendons, osteocytes (bone cells), adipocytes (fat cells), chondrocytes (cartilage cells) |
| Stromal cells | ||
| (mesenchymal) | ||
| Neural | Parts of the nervous system | Neurons, astrocytes and oligodendrocytes |
| Epithelial | Lining of the digestive tract | Absorptive cells, goblet cells, paneth cells and endocrine cells |
| Epidermal | Basal layer of epidermis | Keratinocytes and dermal cells |
| Follicular | Base of hair follicles | Hair follicles and epidermis |
| Hepatic | Liver | Hepatocyte cells |
MSCs
MSCs are derived from any part of human body tissues or cells. They have a property of self-renew and differentiation into specific functional cell types as described before[9]. MSCs are useful cells due to their therapeutic potentiality[9,10]. Even though they are not immortal, they grow very well in culture and maintain their pluripotency property. MSCs are identified by their positive expression of CD105 (SH2) and CD13 (SH3/4) and CD73 genes and are negative for the hematopoietic markers such as CD34 and CD45. Animal experimentation has shown that MSCs has migratory property and can reach to the site of injury. Light or phase contrast microscopy studies have shown that MSCs are elongated cells and look like fibroblast cells as shown in Figure 1. After subculture, MSCs exhibit a high expansion potential without loosing their normal karyotype and telomerase activity[10-12].
Figure 1.
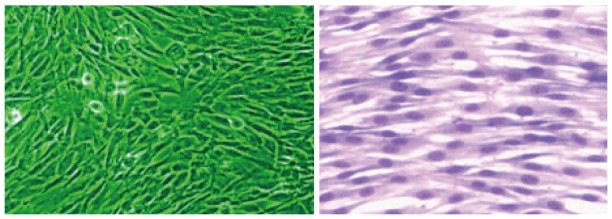
Phase contrast and Giemsa stained picture of Dental pulp stem cells growing in monolayer culture (Dr. Potdar’s, laboratory).
As MSCs are derived from adult tissues, there is no ethical concern required to use these cells for human therapies. Similarly, they have low immunogenicity and therefore they are promising candidates for regenerative therapies. In vitro differentiation of MSCs is mainly dependent on presence of growth factors and cytokines in growth medium. Bone marrow (BM) has been considered as the main source of MSCs, however, collection of BM from patient is a painful and invasive procedure. Therefore, there is a need to find out other sources of tissues which are non invasive and can give similar types of MSCs. Several investigators are presently testing adipose tissue derived stem cells and DPSCs for this purpose. Now it is already established that both these cells have same renewal and differentiating properties of BM cells and thus, these cells can be used for future regenerative therapies of various diseases[12].
DPSCs
Dental Pulp tissue is extracted from the teeth recovered during routine dental procedure throughout the life and these teeth are the most convenient and valuable source of DPSCs which are well characterized as a MSCs as shown in Figure 1.
It is a non invasive process of extraction of MSCs from dental pulp tissue. DPSCs can be cryopreserved and revived whenever; they are needed for future regenerative therapies[13]. Some of the diseases which are being cured by DPSCs include type 1 diabetes, neurological diseases, Immunodeficiency diseases and diseases of bone and cartilages[14-16].
During the development of teeth, there is an interaction between epithelial cells of dental pulp which lead to the differentiation of ameloblasts and odontoblasts, resulting into deposition of specialized mineralized matrices, i.e., enamel and dentin respectively[17]. The inner area of dental pulp chamber contains a highly proliferative stem/progenitor cells possessing a self-renewal and differentiation properties[17]. It has been shown that after teeth eruption, there is an induction of reparatitive dentin formation which protects dental pulp from further degradation[17]. MSCs constitutes a heterogeneous population of cells which are found first in BM and later in multiple tissues like adipose tissue[2,12], skin[18], cartilage, umbilical cord[19], placenta[20,21], and now in dental pulp[13,16]. As DPSCs have comparable therapeutic potential similar to BMMSCs, DPSCs is another alternative noninvasive source to be used for future regenerative therapies[22].
DPSCs or stem cells from human exfoliated deciduous teeth (SHED) cells require a longer time for initial colony formation than other somatic cells[23]. Third molar teeth derived cells differentiate into odontoblasts and secrete 3 D like crystal structure in vitro. Figure 2 shows calcium phosphate crystals secreted from DPSCs cells in culture experiment from my laboratory.
Figure 2.
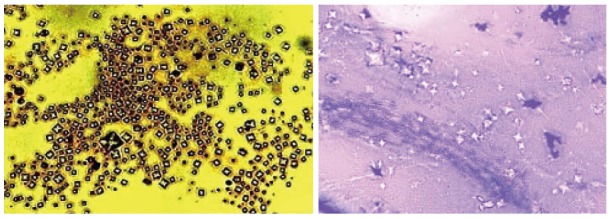
Silver and Giemsa stained Calcium Phosphate crystals secreted by Dental pulp stem cells in culture (Dr. Potdar’s Laboratory).
It has been shown that DPSCs can be differentiated by modulation with growth factors, transcriptional factors, extracellular matrix proteins and receptor molecules into different cell types include odontoblast, osteoblast, chondrocyte, cardiomyocytes, neuron cells, adipocyte, corneal epithelial cell, melanoma cell and insulin secreting Beta cells[24].
DPSCs usually remain quiescent when they are within the dental pulps, but respond quickly after injury. Theses DPSCs have a high proliferative capacity and immediately differentiate into odontoblasts, osteoblasts, and chondrocytes to produce dentin, bone, and cartilage tissues respectively for this repair process. It has been shown that the potentiality of DPSCs differentiation into odontoblastsis reduced after passage 9 and they can only differentiate into osteoblast cells[24,25]. DPSCs are mostly derived from cranial neural crest cells because they expressed GFAP, HNK-1, Nestin, P75 and S-100 which are neural crest- stem cell markers[26]. Our recent studies on DPSCs have shown the differentiation of DPSCs into neuron like cells, cardiomyocytes, adipocytes and insulin secreting beta cells as shown in Figures 3 and 4.
Figure 3.
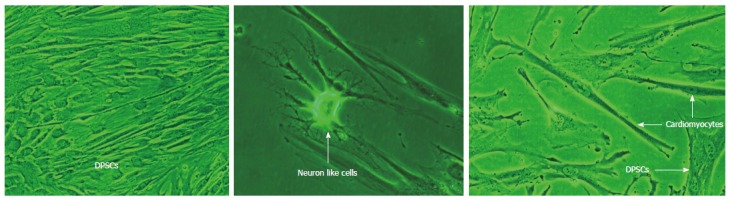
Differentiation of Dental pulp stem cells into neuron like cells and cardiomyocytes (Dr. Potdar’s Laboratory). DPSCs: Dental pulp stem cells.
Figure 4.
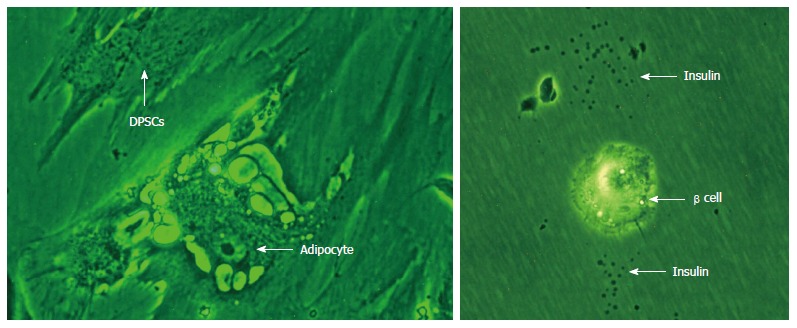
Differentiation of Dental pulp stem cells into adipocytes and insulin secreting beta cells. DPSCs: Dental pulp stem cells.
Almushayt et al[26] have confirmed the expression of odontoblast specific markers and presence of collagenous matrix and calcified deposits in DPSCs to show that these cells differentiate into odontoblast and form dentin material. Mokry et al[27] have shown that due to telomere attrition, human DPSCs have shown extensive cell proliferation in vitro but progressive shortening of telomere length diminishes transplantation capacity of DPSCs and therefore these cells are less suitable for therapeutic applications. They have further proposed that the determination of telomere length and age of these stem cells are as important biomarkers to be assessed before utilization of these cells for regenerative therapies[27]. Smith et al[28] have further shown that molecular signaling is responsible for the differentiation of DPSCs into odontoblast-like cells for reparative dentinogenesis. Therefore, determination of the molecules, responsible for signaling their DPSCs differentiation, may provide a powerful tool for the clinicians to evaluate a regenerative response of these cells appropriately for their application in regenerative therapies[28]. d’Aquino et al[29] have shown that human DPSCs can co-differentiate into osteoblasts and endotheliocytes and form adult bone tissue after transplantation in vivo. This suggests an interesting area for development of three-dimensional tissue scaffold for reconstructing bone tissue engineering using DPSCs in dental disorder[29]. Further it has shown that DPSCs and SHEDs are differentiated into neuron like cells and also shown expression of Nestin as a confirmation of this differentiation. These cells also have constitutive expression of Nestin[30]. Thus, DPSCs have high potential to serve as a good resource for treatment in neurodegenerative diseases[31].
Human DPSCs which are grown by explant culture method have better proliferative capacity and also differentiate into various cell types in vitro[32]. Recent study by Paino et al[33] have shown that DPSCs spontaneously differentiate in vitro toward the melanocytic lineage too. However, SHEDs differ from DPSCs with respect to their growth rate and their property to differentiate in vivo[34]. Similarly, DPSCs have shown the greatest potential to produce a high volume of mineralized matrix suggesting that these cells also show promise for use in regenerative dental therapies[35].
Stem cells from human exfoliated deciduous teeth
Stem cells can be isolated from the pulp of human exfoliated deciduous teeth. These cells induce bone formation and differentiate into other non-dental mesenchymal cells in vitro. SHED have higher proliferation rates, form a sphere-like clusters and differentiate into osteoblasts but they are not able to regenerate complete dentin and pulp-like complexes in vivo. These cells can repair calvarial defects in mice due to their ability to differentiate into osteoblasts. SHED secretes neurotrophic facor for repair of motor neurons following dental injury and therefore it has proposed that SHED can be useful for the treatment of neurodegenerative diseases[23]. Jeon et al[36] have shown differences in in vitro and in vivo characteristics of SHED isolated via enzymatic disaggregation (SHED) and outgrowth (o-SHED) by primary culture method. SHEDs have stemness characteristics, but o-SHEDs are mainly suitable for bone tissue regeneration therapy.
Stem cells from apical papilla
Stem cells from apical papilla (SCAP) are the cells which are found at the tooth root apex. They have higher proliferation rates as well as have a differentiation property in vitro similar to DPSCs. They are capable of differentiating into odontoblast cells and produce dentin in vivo[23]. Due to their higher proliferative potential, SCAPs are also suitable for cell-based therapy for formation of apex roots. White Mineral Trioxide Aggregate (WMTA) is a capping material has a property to differentiate SCAPs into osteoblast cells and thus SCAPs are involved in this regenerative process. WMTA significantly increase the proliferation of SCAPs within 1-5 d whereas, calcium-enriched medium take 7 d to proliferate these cells. SCAPs migrate and proliferate steadily in the presence of 2% and 10% FBS. Thus, this data suggests that WMTA induces early migration and proliferation of SCAPs as compared with late induction by calcium chloride or fetal bovine serum (FBS)[37].
Periodontal ligament stem cells
Human periodontal ligament stem cells (PDLSCs) can differentiate into cementoblast-like cells. They also have a capacity to form connective tissue which is riched in collagen I fiber. Human PDLSCs when seeded on 3D scaffolds such as fibrin sponge, generate bone in vivo and retain stem cell properties and tissue regeneration capacity[23]. It has further been shown that Proteasome inhibitor, Bortezomib has a property of inducing differentiation of PDSLSs into osteoblasts. The main mechanism involved behind this is the mineralization of PDSLSs cells by accumulation of β catenin and significant expression of BMP2 gene. Thus, it is clear that the Bortezomib plays important role in periodontal regenerative therapy[38].
Dental follicle precursor cells
Dental follicle precursor cells (DFPCs) are derived from dental follicle tissue which is a loose connective tissue that surrounds the developing tooth. These cells have an ability to produce bone and cementum. Therefore, these stem cells have great potentiality to use in periodontal and bone regeneration therapies. DFPCs derived from human third molars teeth are rapidly attached to the culture plates and form a calcified nodules. They also exhibits a better plasticity than other dental stem cells[39]. Dental pulp and dental follicle stem cells have similar mesenchymal stem cell characteristics, but DFPCs are easily accessible for cell culture and have a higher proliferation capacity than DPSCs. Therefore it appears that DFPCs might have more advantages as a stem cell resource for regenerative therapies in dental abnormalities[40].
ISOLATION OF DPSCs
Several investigators have described various methods for isolation of stem cells from human dental pulp. Raoof et al[41] have used three different methods for isolation of DPSCs from dental pulp tissue: (1) Dental pulp tissue is digested with collagenase or dispase enzyme and isolated trypsinised cells are plated in culture dishes; (2) They have explanted undigested dental pulp small tissue pieces directly to petridishes; and (3) Dental pulp tissues are initially trypsinised and then small tissue pieces are explanted to petridishes for their outgrowth. They have grown these cultures in Minimum Essential Medium (MEM) supplemented with 20% FBS at 37 °C with 5% CO2 and 90% humidity in CO2 incubator. They have recommended the third method for isolation of DPSCs from dental pulp. This method gives better cell outgrowth with achieving confluency at 60% within 2 d of culture. They have further checked the pluripotency of these cells by studying the expression of Nanog, OCT-4, and Nucleotoxin markers by RT/PCR analysis. Thus, this study proposes the third method for obtaining better DPSCs with high efficacy in a short time[41].
Lindemann et al[42] have evaluated the effect of cryopreservation on DPSCs characteristics. They have isolated dental pulp cells from 7-d old non-cryopreserved and cryopreserved human deciduous teeth and culture them simultaneously. They found that there is no change in differentiating and immunophenotype properties of both these cells. There is a change in the morphology, proliferative capacity of cryopreserved cells than non- cryopreserved cells[42].
Successful and efficient cryopreservation of living cells and organs is a key clinical application of regenerative medicine. Recently, Lin et al[43] 2014 have reported magnetic cryopreservation for intact tooth banking and dental tissue. Human DPSCs isolated from extracted teeth are frozen and then stored at -196 °C for 24 h. During freezing, the cells are suspended in freezing media containing 10% DMSO. The results have shown that when the freezing medium is DMSO-free, the survival rates of revived DPSCs increase by 2 to 2.5-folds[43]. Gioventù et al[44] have developed a new method of cryopreservation of whole dental pulp by using laser beam. They have studied 4 human deciduous whole teeth, cryopreserved by making micro-channels into the tooth with the help of laser beam and then preserve these cells at -80 °C.This method saves a time in isolating DPSCs before cryopreservation and thus reduces the initial costs and workload of tooth banking. The DPSCs cells isolated by this method have shown normal morphology, cell viability and proliferation rate as well as maintain normal mesenchymal phenotype, similar to those of cells isolated from fresh non-cryopreserved teeth. They have further shown that DPSCs isolated without laser piercing have significant loss of cell viability and proliferation rate as compared to teeth cryopreserved by leaser piercing. Thus, this data has suggested the use of Gioventù et al[44] method for whole tooth banking.
MOLECULAR MARKERS FOR DPSCs
Stem cell markers
Since completion of human genome project in year 2003, several molecular markers are established to identify specific cell type. Several investigators have already established many markers but there are few markers which tell us their phenotypes, pluripotency status and differentiating characteristics. Dental pulp cells also should be characterized by such types of markers, some of which are explained in next paragraph of this review.
Mesenchymal stem cell markers
The mesenchymal phenotypes of stem cells can be confirmed by using 3 major genes, CD105 is termed as Endoglin (ENG). This protein is a component of the Transforming Growth Factor β Receptor Complex having high affinity in binding to TGFβ1 and TGFβ3. Figure 5 shows the expression of CD105 in DPSCs suggesting it mesenchymal phenotype.
Figure 5.
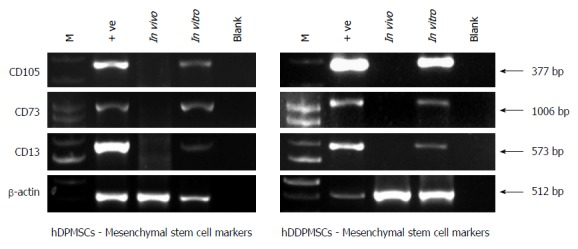
Mesenchymal stem cell markers in dental pulp stem cells.
It has been reported that there is a significant decrease in expression of CD105 gene in differentiated osteoblasts, chondrocytes, adipocytes. Therefore there is a need to check for expression of CD105 gene before use of these stem cells for stem-cell therapy[45,46]. CD13 is another marker which is termed as alanyl (membrane) aminopeptidase (ANPEP).This gene plays very important role in a causation of various types of leukemia or lymphoma[15]. CD73 (Cluster of Differentiation 73) also known as ecto-5’-nucleotidase or 5’-nucleotidase (5’-NT). This is an enzyme, which encodes NT5E gene[27,36]. This enzyme is used as a marker of lymphocyte differentiation. Barry et al[47] (2001) have reported expression of CD73 on MSCs. In our study, we have shown that all three markers such as CD105, CD13 and CD73 are expressed in human dental pulp cells as shown in Figure 5.
Hematopoietic stem cell markers
Blood cells mainly expressed two important hematopoietic markers, i.e., CD45 and CD34[48,49]. CD45 is also called as a Protein Tyrosine Phosphatase, Receptor Type C (PTPRC) gene. CD34 is other marker specifically expressed on human hematopoietic progenitor cells. It is also termed as a RP11-328D5.2 gene. It is a cell surface glycoprotein and functions as a cell-cell adhesive factor. It has function in attaching stem cells to extracellular matrix of bone marrow as well as it can attach directly to stromal cells also[49].
Pluripotency markers
Stem cells are pluripotent cells and express 3 major genes such as OCT4, NANOG and SOX2. The official symbol of OCT4 gene is POU5F1B. OCT4 is a transcriptional factor involved in early embryogenesis and very much essential for maintenance of pluripotency of stem cells. It is well established marker for confirming undifferentiated status of stem cells and always involved in self-renewal capability of undifferentiated stem cells[50]. Study has already shown that gene knockdown of OCT 4 brings about differentiation of undifferentiated stem cells[51]. NANOG is termed as NANOG homoeobox. It is a transcription factor and well involved in self-renewal capacity of undifferentiated stem cells. It maintains pluripotency property of stem cells. The cells with NANOG protein have an ability to form any cell type of three germ layers of human body[52,53]. The third pluripotency gene is Sex determining region Ybox 2 (SOX2). It is also a transcription factor and maintains self-renew capacity of undifferentiated stem cells. This is a intronless gene and is involved in the regulation of embryonic development[54].
Differentiation markers
LIF gene is called as Leukemia Inhibitory Factor and involved in the induction of hematopoietic differentiation in normal and myeloid leukemia cells. It plays a role in immune tolerance at the maternal-fetal interface. LIF derives its name from its ability to induce the terminal differentiation of myeloid leukemic cells[55]. The other differentiating marker is a Keratin18. It is a KAP protein which forms a matrix of keratin intermediate filaments of cell cytoskeleton structure. Keratin 8, Keratin 18, and keratin 19 are used as a marker for epithelial cells and differentiate from hematopoietic cells[56].
Specific markers for DPSCs
Specific markers for Dental pulp cells have been well described by researchers[57,58] 2013.
Dentin sialophosphoprotein (DSPP) and dentin matrix protein 1 (DMP1) are the markers of odontoblast differentiation[58]. DSPP is a major non-collagenous dentin specific protein expressed and secreted by odontoblasts. It is an essential protein for normal tooth development[58]. DMP1 is an another extracellular matrix protein which is involved in differentiation of DPSCs into odontoblasts. It is a member of the small integrin-binding ligand N-linked glycoprotein family and has a critical role for proper mineralization of bone and dentin. It plays a role in regulating expression of osteoblast-specific genes during osteoblast cell differentiation[58]. Telomerase is another dental pulp marker, which is known to play key roles in understanding the differentiating status of DPSCs. It has been reported that undifferentiated DPSCs express high telomerase activity, whereas, it is gradually decreased in differentiated cells[59]. This is an important marker to be taken into consideration while therapy of patients to know undifferentiated status of DPSCs for transplantation[59]. Alkaline Phosphatase (ALP) is also one of the markers of differentiation of DPSCs and plays important role in formation of calcified tissue and extracellular matrix[60]. Similarly, Osteopontin (OPN) is an earlier marker of osteogenic differentiation[61] and Bone Sialoprotein (BSP) is one of the late markers of mineralized tissue differentiation[62]. Recent studies by Yu et al[24] has shown that STRO-1+ DPSCs can differentiate into odontoblasts to form dentin, osteoblasts to form bone and chondrocytes to form a cartilage tissue respectively. This suggests that STRO 1 can be used as a marker for understanding the differentiating potentiality of isolated DPSCs[24].
DIFFERENTIATION OF DPSCs
Biodentine is a bioactive dentine substitute which is used in direct contact with pulp tissue. Luo et al[63] have investigated the response of DPSCs to Biodentine and shown that this material acts via 3 major signaling pathways to induce odontoblast differentiation in DPSCs. They have also shown that Biodentine significantly increases alkaline phosphates activity, OCN, DSPP, DMP1, and BSP gene expression and mineralized nodule formation. Hence they have concluded that Biodentine is a bioactive and biocompatible material capable of inducing odontoblast differentiation of DPSCs[63].
Studies have shown that optimal mechanical compression brings about the induction of cell differentiation[64]. Miyashita et al[64] have shown that the optimal mechanical compression significantly increases expression of the odontoblast-specific markers, i.e., DSPP in dental pulp cells via MAPK signaling pathways. This also results into the expression of the BMP7 and Wnt10a genes[64]. These transcription factors have been implicated in regulating the differentiation of odontoblasts from DPSCs but their regulatory role is not completely understood. New transcriptional factors involved in odontoblast differentiation of DPSCs are analyzed by using a Microarray analysis[65]. Choi et al[65] have also shown that DPSCs strongly express Bobby sox homolog (BBX) gene during odontoblast differentiation. This gene is also expressed by adult molar odontoblasts cells and other tissues of our body. So overall, it is suggested that BBX plays an important role during the odontoblast differentiation of human DPSCs.
Kim et al[66] have cultured dental pulp cells on a conventional surface and nano-patterned surface. It has been shown that cell plated on nano-patterned surface are in linear arrangement as compared to cells plated on a conventional surface. Gene expression analysis also has shown that there is a significantly higher expression of LPL in the nano-patterned group than in the conventional group. Whereas, there is a higher expression of RUNX2 gene in the conventional group than in the nano-patterned group. This study overall suggests that nano-patterned surface enhances adipogenic differentiation of hDPSCs, whereas, normal surface may inhibits osteogenic differentiation of these cells[66,67].
APPLICATIONS OF DPSCs IN REGENERATIVE MEDICINE
Presently therapies for dental pulp degradation are done by conventional methods such as dental pulp capping or by root canal therapy. However, advancement in dental research, dental scientists are focusing on using some of the medical devices in dental tissue engineering and also can use potential dental cells, extracted from dental pulp of patient. They can use biocompatible material as direct capping agents that can supply growth factors or molecules to stimulate reparative dentin formation. As DPSCs cells have property to differentiate into odontoblasts, these cells can be used directly for dental therapy as well as very well used as in vitro model system to evaluate or optimize newly developed bioactive materials for future dental therapy[17]. Regenerative property of the pulp-dentin complex is mainly depending on formation of tertiary dentin, reactionary dentin and reparative dentin. There are two different approaches implemented in regeneration of dentin by the use of tissue engineering techniques[17]. The first approach includes a device which can use as a filling material into a deep cavity of tooth with partial layer of dentin on top of the pulp. In this process, they used some growth factors or molecules that can form reparative dentin. The second approach is to put scaffold on open pulp along with odontoblast-like cells to grow on it. These cells will synthesis reparative dentin. This is somewhat difficult and challenging approach and is being studied extensively for curing dental disorders. It is a mandatory that the scaffold used for clinical application should have capacity to adhere the cells to the surface of this scaffold and should proliferate and differentiate these dental pulp cells into dentin forming odontoblasts. It is also essential to have a good mobility of these cells on this scaffold[17]. Similarly after implantation, the scaffold should be replaced by regenerated tissue without alteration of volume and size of this scaffold material[17].
DPSCs and Deciduous teeth stem cells (DTSCs) are being used as stem cells for regenerative therapies for bone related diseases and orthopedics surgeries[68] because DPSCs and DTSCs can be differentiated into multiple cell types including bone cells such as osteoblast and chondrocytes[68] as well as we can make iPSCs cells for these treatment. Similarly DPSCs can express neural markers and differentiate into functionally active neurons suggesting, the use of DPSCs in cell based therapy for neuronal disorders and in many cases of accidental brain injury[23].
MSCs show different pluripotency in vitro depending on their source of origin, which suggests that they could behave differently in vivo. DPSCs constitute an alternative to BMMSCs in the armamentarium for cardiac repair because DPSCs are able to repair infarcted myocardium, due to their ability to secrete proangiogenic factor. It has also been reported that cardiac repair seen with DPSCs is similar to repair seen by using BMMSCs. Therefore, DPSCs is considered as a good source of stem cells for regenerative therapies of ischemic heart diseases[69].
The clinical studies by d’Aquino et al[70] have shown the use of DPSCs cells in oro-maxillo-facial (OMF) bone repair. They have used DPSCs on collagen sponge’s scaffolds which produces an effective biocomplex which can give an optimal support for regeneration of DSPCs cells for OMF bone repair. Thus autologous transplantation of DPSCs can be used in a low-risk and effective therapeutic strategy for the repair of bone defects[70].
Vasculogenesis is a potential treatment for ischemic heart disease and it is an exciting area of research in regenerative medicine. Iohara et al[71] has shown that side population (SP) of dental pulp cells has a property of Vasculogenesis. They have isolated a highly vasculogenic subfraction of SP cells from dental pulp which are positive for CD31 and CD146 genes. Thus they have further suggested that these SP cells are a newsource of stem cells which stimulate angiogenesis/vasculogenesis in tissue and can be used in cell based treatment of ischemic heart diseases[71]. Under steady condition, EphB/ephrin-B molecule restricts DPSCs cells for their attachment, migration and to maintain within their stem cell niche and thus Eph/ephrin interactions may contribute to the localization and maintenance of DPSCs within adult human teeth. Following injury, the mobilization of DPSCs to the dentine surfaces may be mediated by EphB/ephrin-B interactions within in the adult dental pulp tissue. Therefore this result suggests a role of EphB/ephrin-B molecule in dental pulp development and regeneration[72].
In dentistry, replacement of damaged tooth by functional and living tooth is one of the most promising areas of research in dental therapy. Recent advances in biotechnology have encouraged researchers to explore the possibility of regenerating living teeth with functional properties by use of scaffold and DPSCs cells. It has been seen that dental implants require high-quality bone structures for their support and reconstruction of teeth in these patients without adequate bone support would be a major problem. Thus, Stem cell-mediated root regeneration technology along with clinical crown technology may be a promising approach for functional tooth restoration in these patients[73].
TGPCs cells from the human third molar have high proliferation activity than MSCs isolated from bone marrow. These cells can be frozen and use as per need for auto graft in the process of regenerative medicine. Studies have shown that TGPCs cells can help to stop malignant progression to HCC in hepatitis patients receiving antiviral treatment[74]. Fibroblastic Growth Factor (FGF) helps in enrichment of DPSCs cells in culture and may be useful for regenerative medicine. It has been shown that bFGF signaling is unconditionally required for maintenance of self-renewal and pluripotency of hESCs[75,76]. The DPSCs cultured in the presence of bFGF may obtain more undifferentiated characteristics than those cultured in its absence [75,76].
CLINICAL STATUS OF USES OF DPSCs IN CLINICAL STUDIES
DPSCs have been extensively used in clinical studies by various investigators. Nakashima et al[77] have examined the effect of growth/differentiation factor 11 (GDF11) on differentiation of dental pulp cells in animal model and shown that GDF11 can differentiate DPSCs into odontoblasts. The odontoblasts differentiation is further confirmed by studying the expression of DSPP gene. GDF11 gene is a morphogen and has property of enhancing process of wound healing in dental pulp tissue. They have further shown that in vivo transfer of GDF11 stimulates reparative dentin formation during dental pulp wound healing. Therefore they have suggested that GDF11 can be used for gene therapy for endodontic treatments in dental pulp disorder[77]. Recently Yang et al[78] have studied TGF-β signaling pathways in mesenchymal and epithelial dental stem cells and shown that TGF-β signaling in homeostasis of mesenchymal types dental cells is via Wnt signaling[78]. In regenerative endodontic, it is believed that EDTA induces odontoblast differentiation by releasing growth factors from the dentin matrix. After 3 d of culture, both the cell density and fibronectin expression level are shown to be significantly higher in the EDTA-treated Dental pulp cells. However, after three weeks, EDTA treated DPSCs have shown higher expression of DSPP and DMP1 indicating role of EDTA in inducing cell attachment and differentiation of DPSCs into odontoblasts or osteoblasts. Thus, it suggests an importance role of EDTA in achieving successful outcomes in regenerative endodontics[79].
Estrela et al[8] have shown that DPSCs are differentiated into active neuron like cells in culture condition as well as these cells have expressed neural marker Nestin. This clearly indicates that there is a great use of DPSCs in regenerative therapies in neurological disorder especially, when there is a brain injury[23]. Periodontitis is a chronic inflammatory disease leading to alveolar bone destruction resulting into tooth loss due to infection with periodontopathogenic bacteria as well as some genetic or environmental factors. DPSCs are know to be potent immunomodulators and they can be very well suitable for tissue regeneration[80]. Thus, DPSCs can improve treatment outcome by promoting bone regeneration in these patients when used in combination with conventional treatment modalities for this disorder[80].
Last few decades, the management of facial defects has rapidly changed. This defect is mainly caused due to Loss of vertical alveolar bone height which gives a stability of dental implants in adult patients. At present, there is no cure for the loss of vertical alveolar bone height and scientists are trying to achieve optimal pre-implantological bone regeneration before placement of dental implant in these patients. Recently, it has been shown that stem cells isolated from the dental pulp, dental follicle, and periodontal ligament can be used to treat alveolar bone defects in humans[81].
Amir et al[82] have shown that the Chitosan added in the growth medium significantly increase DPSCs metabolism within 7 to 14 d in culture. Chitosan is responsible to increase in the release of ALP hydrolytic enzyme activity into the medium during the first week of culture resulting into proliferation and early osteogenic differentiation of DPSCs. However, it has been shown that mineralization remains unaffected by Chitosan treatment. Chitosan also has its role as a 3D scaffold for estrogenic cells differentiation in vivo and acts similarly as in in vitro condition[82].
Regenerative endodontic is a replacement of diseased or missing tooth and traumatized dental pulp by new dental tissue or cells. Recently new protocol for management of these cases has been introduced. Shiehzadeh et al[83] 2013 have studied 3 cases of necrotic or immature teeth with periradicular periodontitis with dental MSCs and they are successful in bone healing within 3-4 wk after treatment. They have also discussed the mechanism of bone healing process and development of formation of root end in this paper. The regenerative endodontic techniques involve combination of disinfection or debridement of infected root canal systems along with use of stem cells, scaffolds, and growth factors. The new protocol is possibly involved in combination of disinfection or debridement of infected root canal systems along with the use of stem cells, scaffolds, and growth factors to permit the revascularization of this pulp. Therefore they have suggested that dental stem cells can be used successfully for this type of regenerative endodontic[83].
Human PDLSCs can differentiate into cementoblast-like cells. They also have a capacity to form connective tissue which is richer in collagen I fiber. Human PDLSCs when seeded on 3D scaffolds such as fibrin sponge, generate bone in vivo and retain stem cells differentiating properties[23]. Recent study by Bright et al[84] have shown the use of PDLSCs for periodontal regenerative therapy. This evidence is proved with their latest experimentation of injecting PDLSCs into animal model system. They are successful in periodontal regeneration therapy and shown the formation of bone, cementum and connective tissue fibers in the animals treated with PDLSCs[84]. They have further postulated that this regeneration process is not depending on any type of periodontal ligament defect. Exiting results of this study encourages scientists to go further with transplantation of PDLSCs for periodontal regeneration therapy in human after examining its efficacy, safety, feasibility in clinical trials.
FUTURE DIRECTIONS
The stem-cell-based tissue-engineering approaches are widely applied in establishing functional organs and tissues. Rapid progress in advancement in technology such as development of iPSCs has provided great hopes in regenerative therapies for various diseases. Liu et al[85] have previously reported that iPS could be an appealing stem cells source contributing to tooth regeneration. The application of iPS technology in dental bioengineering for whole tooth regeneration is an interesting area for future work[85]. Advancement in stem cell and scaffold technology, damaged or lost teeth can be replaced by the use of regenerative therapies. Similarly, discovery of iPSCs technology has revolutionized complete treatment protocols in the field of dentistry by using a concept of autologous transplantation[86].
Now day, dentist can very well manage periodontal diseases by using stem cell and scaffold technology[87,88]. However, making whole artificial tooth and periodontal frame work by this technology is a challenge for scientists working in the field dental regenerative therapies. It is now well established that most of the dental related problems can be treated by using DPSCs alone or in combination with scaffold technology[88]. Similar to periodontal disorder, advancement in use of DPSCs have added advantage in the field of Endodontic where, we can develop human dental pulp in the laboratory. These outcomes provide evidence suggesting that it might be feasible to restore viability in a necrotic young permanent tooth by engineering a new dental pulp. The potential impact of such therapies is immense and may allow for the completion and reinforcement of the tooth structure by biological regeneration in near future[89].
ACKNOWLEGDEMENTS
Authors are thankful to the management of Jaslok Hospital and Research Centre for providing the facilities for development of stem cell research laboratory and carrying out stem research in the field of DPSCs and other related disorders. I am also thankful to our staff and students of our laboratory for their technical support.
Footnotes
Supported by Jaslok Hospital and Research Centre, Mumbai, India, Project ni 491, A/C 27814.
Conflict-of-interest: No.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 27, 2014
First decision: January 20, 2015
Article in press: April 20, 2015
P- Reviewer: Chen S, Ferreira MM, Su CQ, Tamama K S- Editor: Ji FF L- Editor: A E- Editor: Wang CH
References
- 1.Potdar PD, Deshpande S. Mesenchymal stem cell transplantation: New, avenues for stem cell therapies. J Transplant Technol Res. 2013;3:1–16. [Google Scholar]
- 2.Potdar P, Sutar J. Establishment and molecular characterization of mesenchymal stem cell lines derived from human visceral & amp; subcutaneous adipose tissues. J Stem Cells Regen Med. 2010;6:26–35. doi: 10.46582/jsrm.0601005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potdar PD, D’souza SB. Isolation of Oct4+, Nanog+ and SOX2- mesenchymal cells from peripheral blood of a diabetes mellitus patient. Hum Cell. 2011;24:51–55. doi: 10.1007/s13577-011-0011-6. [DOI] [PubMed] [Google Scholar]
- 4.Potdar P, Subedi R. Defining Molecular Phenotypes of Mesenchymal and hematopoietic Stem Cells derived from Peripheral blood of Acute Lymphocytic Leukemia patients for regenerative stem cell therapy. J Stem Cells Regen Med. 2011;7:29–40. doi: 10.46582/jsrm.0701004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potdar PD, Chougule S. Establishment and molecular characterization of breast cancer mesenchymal stem cell line derived from human non-metastasis breast cancer tumor. Stem Cell Discovery. 2011;1:21–28. [Google Scholar]
- 6.Potdar PD, Kumar KS. Establishment and molecular characterization of human dermal mesenchymal like stem cells derived from human scalp biopsy of androgenetic alopecia patient. Stem Cell Discovery. 2013;3:77–82. [Google Scholar]
- 7.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 8.Estrela C, Alencar AH, Kitten GT, Vencio EF, Gava E. Mesenchymal stem cells in the dental tissues: perspectives for tissue regeneration. Braz Dent J. 2011;22:91–98. doi: 10.1590/s0103-64402011000200001. [DOI] [PubMed] [Google Scholar]
- 9.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 10.Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood) 2001;226:507–520. doi: 10.1177/153537020122600603. [DOI] [PubMed] [Google Scholar]
- 11.Rebelatto CK, Aguiar AM, Moretão MP, Senegaglia AC, Hansen P, Barchiki F, Oliveira J, Martins J, Kuligovski C, Mansur F, et al. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood) 2008;233:901–913. doi: 10.3181/0712-RM-356. [DOI] [PubMed] [Google Scholar]
- 12.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang AH, Snyder BR, Cheng PH, Chan AW. Putative dental pulp-derived stem/stromal cells promote proliferation and differentiation of endogenous neural cells in the hippocampus of mice. Stem Cells. 2008;26:2654–2663. doi: 10.1634/stemcells.2008-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davila JC, Cezar GG, Thiede M, Strom S, Miki T, Trosko J. Use and application of stem cells in toxicology. Toxicol Sci. 2004;79:214–223. doi: 10.1093/toxsci/kfh100. [DOI] [PubMed] [Google Scholar]
- 15.Drize NJ, Surin VL, Gan OI, Deryugina EI, Chertkov JL. Gene therapy model for stromal precursor cells of hematopoietic microenvironment. Leukemia. 1992;6 Suppl 3:174S–175S. [PubMed] [Google Scholar]
- 16.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauth C, Huwig A, Graf-Hausner U, Roulet JF. Restorative applications for dental pulp therapy topics in tissue engineering. Ashammakhi N, Reis R, Chiellini E, editors. In: Topics in Tissue Engineering; 2007. pp. 1–30. [Google Scholar]
- 18.Young HE, Steele TA, Bray RA, Hudson J, Floyd JA, Hawkins K, Thomas K, Austin T, Edwards C, Cuzzourt J, et al. Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec. 2001;264:51–62. doi: 10.1002/ar.1128. [DOI] [PubMed] [Google Scholar]
- 19.Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 20.Yen BL, Huang HI, Chien CC, Jui HY, Ko BS, Yao M, Shun CT, Yen ML, Lee MC, Chen YC. Isolation of multipotent cells from human term placenta. Stem Cells. 2005;23:3–9. doi: 10.1634/stemcells.2004-0098. [DOI] [PubMed] [Google Scholar]
- 21.Potdar PD, Chaugule S. Development and molecular characterization of human placental mesenchymal stem cells from human aborted fetal tissue as a model to study mechanism of spontaneous abortion. Advances in Stem Cells. 2014;2014:1–17. [Google Scholar]
- 22.Beltrão-Braga PC, Pignatari GC, Maiorka PC, Oliveira NA, Lizier NF, Wenceslau CV, Miglino MA, Muotri AR, Kerkis I. Feeder-free derivation of induced pluripotent stem cells from human immature dental pulp stem cells. Cell Transplant. 2011;20:1707–1719. doi: 10.3727/096368911X566235. [DOI] [PubMed] [Google Scholar]
- 23.Verma K, Bains R, Bains VK, Rawtiya M, Loomba K, Srivastava SC. Therapeutic potential of dental pulp stem cells in regenerative medicine: An overview. Dent Res J (Isfahan) 2014;11:302–308. [PMC free article] [PubMed] [Google Scholar]
- 24.Yu J, He H, Tang C, Zhang G, Li Y, Wang R, Shi J, Jin Y. Differentiation potential of STRO-1+ dental pulp stem cells changes during cell passaging. BMC Cell Biol. 2010;11:32. doi: 10.1186/1471-2121-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu J, Wang Y, Deng Z, Tang L, Li Y, Shi J, Jin Y. Odontogenic capability: bone marrow stromal stem cells versus dental pulp stem cells. Biol Cell. 2007;99:465–474. doi: 10.1042/BC20070013. [DOI] [PubMed] [Google Scholar]
- 26.Almushayt A, Narayanan K, Zaki AE, George A. Dentin matrix protein 1 induces cytodifferentiation of dental pulp stem cells into odontoblasts. Gene Ther. 2006;13:611–620. doi: 10.1038/sj.gt.3302687. [DOI] [PubMed] [Google Scholar]
- 27.Mokry J, Soukup T, Micuda S, Karbanova J, Visek B, Brcakova E, Suchanek J, Bouchal J, Vokurkova D, Ivancakova R. Telomere attrition occurs during ex vivo expansion of human dental pulp stem cells. J Biomed Biotechnol. 2010;2010:673513. doi: 10.1155/2010/673513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith AJ, Scheven BA, Takahashi Y, Ferracane JL, Shelton RM, Cooper PR. Dentine as a bioactive extracellular matrix. Arch Oral Biol. 2012;57:109–121. doi: 10.1016/j.archoralbio.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 29.d’Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A, Papaccio G. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14:1162–1171. doi: 10.1038/sj.cdd.4402121. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg M, Smith AJ. Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Crit Rev Oral Biol Med. 2004;15:13–27. doi: 10.1177/154411130401500103. [DOI] [PubMed] [Google Scholar]
- 31.Kadar K, Kiraly M, Porcsalmy B, Molnar B, Racz GZ, Blazsek J, Kallo K, Szabo EL, Gera I, Gerber G, et al. Differentiation potential of stem cells from human dental origin - promise for tissue engineering. J Physiol Pharmacol. 2009;60 Suppl 7:167–175. [PubMed] [Google Scholar]
- 32.Spath L, Rotilio V, Alessandrini M, Gambara G, De Angelis L, Mancini M, Mitsiadis TA, Vivarelli E, Naro F, Filippini A, et al. Explant-derived human dental pulp stem cells enhance differentiation and proliferation potentials. J Cell Mol Med. 2010;14:1635–1644. doi: 10.1111/j.1582-4934.2009.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paino F, Ricci G, De Rosa A, D’Aquino R, Laino L, Pirozzi G, Tirino V, Papaccio G. Ecto-mesenchymal stem cells from dental pulp are committed to differentiate into active melanocytes. Eur Cell Mater. 2010;20:295–305. doi: 10.22203/ecm.v020a24. [DOI] [PubMed] [Google Scholar]
- 34.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies OG, Cooper PR, Shelton RM, Smith AJ, Scheven BA. A comparison of the in vitro mineralisation and dentinogenic potential of mesenchymal stem cells derived from adipose tissue, bone marrow and dental pulp. J Bone Miner Metab. 2014:Epub ahead of print. doi: 10.1007/s00774-014-0601-y. [DOI] [PubMed] [Google Scholar]
- 36.Jeon M, Song JS, Choi BJ, Choi HJ, Shin DM, Jung HS, Kim SO. In vitro and in vivo characteristics of stem cells from human exfoliated deciduous teeth obtained by enzymatic disaggregation and outgrowth. Arch Oral Biol. 2014;59:1013–1023. doi: 10.1016/j.archoralbio.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Schneider R, Holland GR, Chiego D, Hu JC, Nör JE, Botero TM. White mineral trioxide aggregate induces migration and proliferation of stem cells from the apical papilla. J Endod. 2014;40:931–936. doi: 10.1016/j.joen.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitagaki J, Miyauchi S, Xie CJ, Yamashita M, Yamada S, Kitamura M, Murakami S. Effects of the proteasome inhibitor, bortezomib, on cytodifferentiation and mineralization of periodontal ligament cells. J Periodontal Res. 2015;50:248–255. doi: 10.1111/jre.12202. [DOI] [PubMed] [Google Scholar]
- 39.Lizier NF, Kerkis I, Wenceslau CV. Generation of induced pluripotent stem cells from dental pulp somatic cells. Bhartiya D, Lenka N, editors. ISBN 978-953-51-1192-4. August 28, 2013. Pluripotent Stem Cells. America: InTech; 2013. [Google Scholar]
- 40.Shoi K, Aoki K, Ohya K, Takagi Y, Shimokawa H. Characterization of pulp and follicle stem cells from impacted supernumerary maxillary incisors. Pediatr Dent. 2014;36:79–84. [PubMed] [Google Scholar]
- 41.Raoof M, Yaghoobi MM, Derakhshani A, Kamal-Abadi AM, Ebrahimi B, Abbasnejad M, Shokouhinejad N. A modified efficient method for dental pulp stem cell isolation. Dent Res J (Isfahan) 2014;11:244–250. [PMC free article] [PubMed] [Google Scholar]
- 42.Lindemann D, Werle SB, Steffens D, Garcia-Godoy F, Pranke P, Casagrande L. Effects of cryopreservation on the characteristics of dental pulp stem cells of intact deciduous teeth. Arch Oral Biol. 2014;59:970–976. doi: 10.1016/j.archoralbio.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 43.Lin SL, Chang WJ, Lin CY, Hsieh SC, Lee SY, Fan KH, Lin CT, Huang HM. Static magnetic field increases survival rate of dental pulp stem cells during DMSO-free cryopreservation. Electromagn Biol Med. 2014:1–7. doi: 10.3109/15368378.2014.919588. [DOI] [PubMed] [Google Scholar]
- 44.Gioventù S, Andriolo G, Bonino F, Frasca S, Lazzari L, Montelatici E, Santoro F, Rebulla P. A novel method for banking dental pulp stem cells. Transfus Apher Sci. 2012;47:199–206. doi: 10.1016/j.transci.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Koç ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 46.Kaplan R, Morse B, Huebner K, Croce C, Howk R, Ravera M, Ricca G, Jaye M, Schlessinger J. Cloning of three human tyrosine phosphatases reveals a multigene family of receptor-linked protein-tyrosine-phosphatases expressed in brain. Proc Natl Acad Sci USA. 1990;87:7000–7004. doi: 10.1073/pnas.87.18.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Misumi Y, Ogata S, Ohkubo K, Hirose S, Ikehara Y. Primary structure of human placental 5’-nucleotidase and identification of the glycolipid anchor in the mature form. Eur J Biochem. 1990;191:563–569. doi: 10.1111/j.1432-1033.1990.tb19158.x. [DOI] [PubMed] [Google Scholar]
- 48.Simmons DL, Satterthwaite AB, Tenen DG, Seed B. Molecular cloning of a cDNA encoding CD34, a sialomucin of human hematopoietic stem cells. J Immunol. 1992;148:267–271. [PubMed] [Google Scholar]
- 49.Satterthwaite AB, Burn TC, Le Beau MM, Tenen DG. Structure of the gene encoding CD34, a human hematopoietic stem cell antigen. Genomics. 1992;12:788–794. doi: 10.1016/0888-7543(92)90310-o. [DOI] [PubMed] [Google Scholar]
- 50.Hu W, Feng Z, Teresky AK, Levine AJ. p53 regulates maternal reproduction through LIF. Nature. 2007;450:721–724. doi: 10.1038/nature05993. [DOI] [PubMed] [Google Scholar]
- 51.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 52.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 53.Looijenga LH, Stoop H, de Leeuw HP, de Gouveia Brazao CA, Gillis AJ, van Roozendaal KE, van Zoelen EJ, Weber RF, Wolffenbuttel KP, van Dekken H, et al. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 2003;63:2244–2250. [PubMed] [Google Scholar]
- 54.Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 55.Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 56.Doherty GJ, McMahon HT. Mediation, modulation, and consequences of membrane-cytoskeleton interactions. Annu Rev Biophys. 2008;37:65–95. doi: 10.1146/annurev.biophys.37.032807.125912. [DOI] [PubMed] [Google Scholar]
- 57.Guo L, Li J, Qiao X, Yu M, Tang W, Wang H, Guo W, Tian W. Comparison of odontogenic differentiation of human dental follicle cells and human dental papilla cells. PLoS One. 2013;8:e62332. doi: 10.1371/journal.pone.0062332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tziafas D, Kodonas K. Differentiation potential of dental papilla, dental pulp, and apical papilla progenitor cells. J Endod. 2010;36:781–789. doi: 10.1016/j.joen.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Odorico JS, Kaufman DS, Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19:193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- 60.Liu Q, Cen L, Yin S, Chen L, Liu G, Chang J, Cui L. A comparative study of proliferation and osteogenic differentiation of adipose-derived stem cells on akermanite and beta-TCP ceramics. Biomaterials. 2008;29:4792–4799. doi: 10.1016/j.biomaterials.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 61.Jiang X, Zhao J, Wang S, Sun X, Zhang X, Chen J, Kaplan DL, Zhang Z. Mandibular repair in rats with premineralized silk scaffolds and BMP-2-modified bMSCs. Biomaterials. 2009;30:4522–4532. doi: 10.1016/j.biomaterials.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee JS, Lee JM, Im GI. Electroporation-mediated transfer of Runx2 and Osterix genes to enhance osteogenesis of adipose stem cells. Biomaterials. 2011;32:760–768. doi: 10.1016/j.biomaterials.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 63.Luo Z, Kohli MR, Yu Q, Kim S, Qu T, He WX. Biodentine induces human dental pulp stem cell differentiation through mitogen-activated protein kinase and calcium-/calmodulin-dependent protein kinase II pathways. J Endod. 2014;40:937–942. doi: 10.1016/j.joen.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 64.Miyashita S, Ahmed NE, Murakami M, Iohara K, Yamamoto T, Horibe H, Kurita K, Takano-Yamamoto T, Nakashima M. Mechanical forces induce odontoblastic differentiation of mesenchymal stem cells on three-dimensional biomimetic scaffolds. J Tissue Eng Regen Med. 2014:Epub ahead of print. doi: 10.1002/term.1928. [DOI] [PubMed] [Google Scholar]
- 65.Choi YA, Seol MY, Shin HI, Park EK. Bobby Sox homology regulates odontoblast differentiation of human dental pulp stem cells/progenitors. Cell Commun Signal. 2014;12:35. doi: 10.1186/1478-811X-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim D, Kim J, Hyun H, Kim K, Roh S. A nanoscale ridge/groove pattern arrayed surface enhances adipogenic differentiation of human supernumerary tooth-derived dental pulp stem cells in vitro. Arch Oral Biol. 2014;59:765–774. doi: 10.1016/j.archoralbio.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 67.Lei M, Li K, Li B, Gao LN, Chen FM, Jin Y. Mesenchymal stem cell characteristics of dental pulp and periodontal ligament stem cells after in vivo transplantation. Biomaterials. 2014;35:6332–6343. doi: 10.1016/j.biomaterials.2014.04.071. [DOI] [PubMed] [Google Scholar]
- 68.Yamada Y, Ito K, Nakamura S, Ueda M, Nagasaka T. Promising cell-based therapy for bone regeneration using stem cells from deciduous teeth, dental pulp, and bone marrow. Cell Transplant. 2011;20:1003–1013. doi: 10.3727/096368910X539128. [DOI] [PubMed] [Google Scholar]
- 69.Gandia C, Armiñan A, García-Verdugo JM, Lledó E, Ruiz A, Miñana MD, Sanchez-Torrijos J, Payá R, Mirabet V, Carbonell-Uberos F, et al. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells. 2008;26:638–645. doi: 10.1634/stemcells.2007-0484. [DOI] [PubMed] [Google Scholar]
- 70.d’Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, Graziano A, Desiderio V, Laino G, Papaccio G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;18:75–83. doi: 10.22203/ecm.v018a07. [DOI] [PubMed] [Google Scholar]
- 71.Iohara K, Zheng L, Wake H, Ito M, Nabekura J, Wakita H, Nakamura H, Into T, Matsushita K, Nakashima M. A novel stem cell source for vasculogenesis in ischemia: subfraction of side population cells from dental pulp. Stem Cells. 2008;26:2408–2418. doi: 10.1634/stemcells.2008-0393. [DOI] [PubMed] [Google Scholar]
- 72.Stokowski A, Shi S, Sun T, Bartold PM, Koblar SA, Gronthos S. EphB/ephrin-B interaction mediates adult stem cell attachment, spreading, and migration: implications for dental tissue repair. Stem Cells. 2007;25:156–164. doi: 10.1634/stemcells.2006-0373. [DOI] [PubMed] [Google Scholar]
- 73.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ikeda E, Yagi K, Kojima M, Yagyuu T, Ohshima A, Sobajima S, Tadokoro M, Katsube Y, Isoda K, Kondoh M, et al. Multipotent cells from the human third molar: feasibility of cell-based therapy for liver disease. Differentiation. 2008;76:495–505. doi: 10.1111/j.1432-0436.2007.00245.x. [DOI] [PubMed] [Google Scholar]
- 75.Morito A, Kida Y, Suzuki K, Inoue K, Kuroda N, Gomi K, Arai T, Sato T. Effects of basic fibroblast growth factor on the development of the stem cell properties of human dental pulp cells. Arch Histol Cytol. 2009;72:51–64. doi: 10.1679/aohc.72.51. [DOI] [PubMed] [Google Scholar]
- 76.Boskey AL. Mineralization of Bones and Teeth. Elements. 2007;3:387–393. [Google Scholar]
- 77.Nakashima M, Mizunuma K, Murakami T, Akamine A. Induction of dental pulp stem cell differentiation into odontoblasts by electroporation-mediated gene delivery of growth/differentiation factor 11 (Gdf11) Gene Ther. 2002;9:814–818. doi: 10.1038/sj.gt.3301692. [DOI] [PubMed] [Google Scholar]
- 78.Yang G, Zhou J, Teng Y, Xie J, Lin J, Guo X, Gao Y, He M, Yang X, Wang S. Mesenchymal TGF-β signaling orchestrates dental epithelial stem cell homeostasis through Wnt signaling. Stem Cells. 2014;32:2939–2948. doi: 10.1002/stem.1772. [DOI] [PubMed] [Google Scholar]
- 79.Pang NS, Lee SJ, Kim E, Shin DM, Cho SW, Park W, Zhang X, Jung IY. Effect of EDTA on attachment and differentiation of dental pulp stem cells. J Endod. 2014;40:811–817. doi: 10.1016/j.joen.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 80.Racz GZ, Kadar K, Foldes A, Kallo K, Perczel-Kovach K, Keremi B, Nagy A, Varga G. Immunomodulatory and potential therapeutic role of mesenchymal stem cells in periodontitis. J Physiol Pharmacol. 2014;65:327–339. [PubMed] [Google Scholar]
- 81.Grimm WD, Dannan A, Giesenhagen B, Schau I, Varga G, Vukovic MA, Sirak SV. Translational Research: Palatal-derived Ecto-mesenchymal Stem Cells from Human Palate: A New Hope for Alveolar Bone and Cranio-Facial Bone Reconstruction. Int J Stem Cells. 2014;7:23–29. doi: 10.15283/ijsc.2014.7.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amir LR, Suniarti DF, Utami S, Abbas B. Chitosan as a potential osteogenic factor compared with dexamethasone in cultured macaque dental pulp stromal cells. Cell Tissue Res. 2014;358:407–415. doi: 10.1007/s00441-014-1938-1. [DOI] [PubMed] [Google Scholar]
- 83.Shiehzadeh V, Aghmasheh F, Shiehzadeh F, Joulae M, Kosarieh E, Shiehzadeh F. Healing of large periapical lesions following delivery of dental stem cells with an injectable scaffold: new method and three case reports. Indian J Dent Res. 2014;25:248–253. doi: 10.4103/0970-9290.135937. [DOI] [PubMed] [Google Scholar]
- 84.Bright R, Hynes K, Gronthos S, Bartold PM. Periodontal ligament-derived cells for periodontal regeneration in animal models: a systematic review. J Periodontal Res. 2015;50:160–172. doi: 10.1111/jre.12205. [DOI] [PubMed] [Google Scholar]
- 85.Liu P, Zhang Y, Chen S, Cai J, Pei D. Application of iPS cells in dental bioengineering and beyond. Stem Cell Rev. 2014;10:663–670. doi: 10.1007/s12015-014-9531-2. [DOI] [PubMed] [Google Scholar]
- 86.Otsu K, Kumakami-Sakano M, Fujiwara N, Kikuchi K, Keller L, Lesot H, Harada H. Stem cell sources for tooth regeneration: current status and future prospects. Front Physiol. 2014;5:36. doi: 10.3389/fphys.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abou Neel EA, Chrzanowski W, Salih VM, Kim HW, Knowles JC. Tissue engineering in dentistry. J Dent. 2014;42:915–928. doi: 10.1016/j.jdent.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 88.Ji J, Sun W, Wang W, Munyombwe T, Yang XB. The effect of mechanical loading on osteogenesis of human dental pulp stromal cells in a novel in vitro model. Cell Tissue Res. 2014;358:123–133. doi: 10.1007/s00441-014-1907-8. [DOI] [PubMed] [Google Scholar]
- 89.Demarco FF, Conde MC, Cavalcanti BN, Casagrande L, Sakai VT, Nör JE. Dental pulp tissue engineering. Braz Dent J. 2011;22:3–13. doi: 10.1590/s0103-64402011000100001. [DOI] [PMC free article] [PubMed] [Google Scholar]


