Abstract
Liver cirrhosis is characterized by distortion of liver architecture, necrosis of hepatocytes and regenerative nodules formation leading to cirrhosis. Various types of cell sources have been used for the management and treatment of decompensated liver cirrhosis. Knowledge of stem cells has offered a new dimension for regenerative therapy and has been considered as one of the potential adjuvant treatment modality in patients with end stage liver diseases (ESLD). Human fetal hepatic progenitor cells are less immunogenic than adult ones. They are highly propagative and challenging to cryopreservation. In our earlier studies we have demonstrated that fetuses at 10-18 wk of gestation age contain a large number of actively dividing hepatic stem and progenitor cells which possess bi-potent nature having potential to differentiate into bile duct cells and mature hepatocytes. Hepatic stem cell therapy for the treatment of ESLD is in their early stage of the translation. The emerging technology of decellularization and recellularization might offer a significant platform for developing bioengineered personalized livers to come over the scarcity of desired number of donor organs for the treatment of ESLD. Despite these significant advancements long-term tracking of stem cells in human is the most important subject nowadays in order to answer several unsettles issues regarding the route of delivery, the choice of stem cell type(s), the cell number and the time-point of cell delivery for the treatment in a chronic setting. Answering to these questions will further contribute to the development of safer, noninvasive, and repeatable imaging modalities that could discover better cell therapeutic approaches from bench to bed-side. Combinatorial approach of decellularization and nanotechnology could pave a way towards the better understanding in determination of cell fate post-transplantation.
Keywords: Hepatic stem cells, Bioengineering, Liver cirrhosis, Labeling and tracking
Core tip: Liver cirrhosis is characterized by distortion of liver architecture, necrosis of hepatocytes and regenerative nodules formation leading to cirrhosis. The available treatment modalities are not very effective against liver cirrhosis. Stem cells are considered one of the potential adjuvant treatment modality in liver cirrhosis patients. Fetal hepatic stem cells transplantation in liver cirrhosis has emerged as an alternative to organ transplantation. However long-term stem cells labeling and tracking is needed for cell fate determination after transplantation. Decellularization technology provides a novel tool to develop bioengineered personalized livers to accomplish the shortage of donor livers.
INTRODUCTION
The liver is a central metabolic and highly specialized detoxifying organ. Approximate 70%-75% of liver functions are being carried out by the hepatocytes which together with cholangiocytes (5%-10% of hepatic cells) constitute the liver parenchyma. Liver regeneration is a very rapid and well synchronized phenomenon. Liver responds to initial injury compensating the loss of parenchymal mass. If damage perseveres commencement of petite terminal peri-portal oval cells activates which engross mobilization of several vital factors.
EVOLUTION OF CELL THERAPY AND PRECLINICAL INVESTIGATION
Various strategies have been explored to provide functional support to the fading liver. Hepatic cell transplantation has emerged as one of the alternative modality. The experimental data of hepatocyte transplantation in different animal models has proved its efficacy. In early 1980s hepatocyte transplantation in D-galactosamine induced acute liver failure (ALF) model showed survival rate of more than 60%[1-4]. Different routes were used for delivery of cells like intraportal, intrasplenic and intraperitoneal. Intra-peritoneal cell transplantation was found to be more appropriate site for transplantation as large number of cells can be delivered and also provides better engraftment of infused cells[5]. In view of larger number of cells required, Habibullah et al[5], infused 6 × 106 hepatocytes according to body weight (per kg) in ALF animal model and observed > 60% survival post-transplantation.
CLINICAL INVESTIGATION OF HEPATOCYTES TRANSPLANTATION
After preclinical investigations in animal models, first attempt of hepatocytes transplantation in human with liver cirrhosis was carried out by Mito et al[6] which provided proof for functional benefits post-transplantation. This was a landmark study proving a new paradigm for shifting hepatocytes transplantation towards the clinical perception and further followed by studies from our centre. In our preliminary clinical study, human fetal hepatocytes were infused intra-peritoneally into seven ALF patients[7]. The study have demonstrated beneficial effects in ALF patients with grade III and grade IV hepatic encephalopathy because transplanted hepatocytes by virtue of their synthetic, metabolic and detoxifying functions causes clinical and functional improvements. Several other studies have also provided evidence for active bridging of orthotropic liver transplantation in patients though post-transplantation of human hepatocytes[8]. Besides this various other studies have also demonstrated the functional benefits using different other routes of cell transplantation[1,3,5]. We performed a pioneer study by intra-peritoneal hepatocytes transplantation in a 26 year old pregnant patient having acute fatty liver to investigate the functional benefit after transplantation[9]. This study demonstrated significant improvements after 2 d of hepatocytes transplantation and provided an alternative option of liver transplantation and new hope for the patients suffering with end stage liver failure. After this several other studies have been reported from various centers for hepatocytes transplantation in different types of metabolic disorders[10,11]. However, transplantation of large number of human hepatocytes for adequate liver function remains a challenge before its wide clinical applications.
CHRONIC LIVER DISEASE (LIVER CIRRHOSIS)
This category has produced a large burden on the society and has been considered as more appropriate type for cell therapeutic approaches. Liver cirrhosis is being characterized by distortion of liver architecture, necrosis of hepatocytes and regenerative nodules formation leading to cirrhosis. Various types of cell sources have been applied for the management and treatment of decompensated liver cirrhosis.
HEPATIC STEM CELLS AND LIVER CIRRHOSIS
Discovery of stem cells has provided a new dimension for regenerative therapy and are considered one of the potential adjuvant treatment modality in patients with ESLD. With the knowledge of embryonic stem cells, the area of cell biology has revolutionized for its potential in regenerative medicine. It gave a new hope and opened a new area of cellular therapy. However, a variety of stem cell types have been identified from various sources, hepatic stem cells (HSCs) has been potential therapeutic option for the treatment of liver cirrhosis. HSCs are able to give rise mature progenies and have self-renewing capacity. Several studies have demonstrated isolation of HSCs from adult tissues followed by in vitro proliferation and differentiation into functional hepatocytes[12-14]. However, regeneration of diseased liver by human HSCs is still awaited.
Hepatic progenitor cells (HPCs) isolated from human fetal liver is less immunogenic, highly propagative and more challenging for cryopreservation than the adult ones. In our previous study we have demonstrated that fetuses at 10-18 wk of gestation age contain a large amount of dynamic HPCs which possess bi-potent nature giving rise to both hepatocytes and bile duct cells[15]. Fetal liver stem cells has been identified as transition between embryonic cells and adult ones which is mostly non-teratogenic[16]. During third trimester of fetal development, plasticity to form liver parenchyma cells makes fetal HSCs an excellent resource for cellular therapeutic approaches.
SOURCES OF STEM CELLS IN LIVER DISEASES
There are two major sources of stem cells (1) embryonic; and (2) adult which have been considered to have potential in the treatment of various types of diseases. However, fetal liver derived stem cells are the transition between embryonic and adult sources and have several advantages on these two sources. Fetal liver derived cells are also termed as hepatoblasts which appears after the specialization of liver endoderm and growing liver bud. Various types of adult stem cell sources have been investigated for their application in liver regeneration such as mesenchymal stem cells (MSCs) derived from umbilical cord blood/tissue and bone marrow, induced pluripotent cells derived from somatic cells. These extra-hepatic sources have been reported to provide trophic support in injured liver. They also induce the endogenous cell proliferation and survival under diseased environment. But the control of their lineage specificity and plasticity needs more investigation in order to apply them in clinical settings. In this regard, MSCs from bone marrow has already been recognized as major player in clinical regeneration and resolution of liver cirrhosis due to its immunomodulation and regenerative potential.
ISOLATION AND DEPICTION OF HUMAN HEPATIC PROGENITORS FROM FETAL LIVER
After obtaining a human fetus in better clinical form, isolation of cells is performed by two step collagenase digestion method as earlier described by Habibullah et al[7]. Cells within the fetal liver are generally well connected with intercellular links rooted in extra-cellular matrix (ECM) which is required to be destroyed for single cell isolation either by mechanical or enzymatic dissociation. The human fetal liver cells thus isolated can be enriched further for specific progenitor cells using magnetic activated cell sorting (MACS) or fluorescence activated cell sorting (FACS). Enrichment can be done either based on cell size and density or based on cell surface/functional markers. Enriched human HPCs further need to be distinguished from biliary and mature hepatocytes[17]. In our earlier studies human HPCs were first enriched with epithelial cell adhesion molecule (EpCAM) and further identified for the expression of cytokeratin-18; a marker for liver epithelium, cytokeratin-19; a biliary marker and finally albumin and alfa-feto protein for mature hepatocytes using Real-Time quantitative polymerase chain reaction and immunocytochemical (ICC) staining. These explanations were further supported by FACS results indicating positivity for above markers including CD49f, CD29, CD90 and CD34 and negative for CD45 and HLA class II.
IN VIVO DELIVERY OF CELLS WITHIN LIVER
Portal vein
Portal vein is the most often used route for the stem cell transplantation due to multiple vascular accesses. It is admitted either by trans-jugular or trans-hepatic percutaneous approach. However accessing this route by above approaches is difficult in the presence of ascites and has major risk of bleeding, diathesis and coagulopathy.
Accessing through hepatic artery
In our knowledge this is a more suitable route for cellular transplantation as compared to the portal vein[17]. It is accessed through trans-radial, trans-femoral and trans-branchial approaches.
Splenic infusion
Intrasplenic approach has been suggested as better approach compared to the splenic arterial infusion of hepatocytes transplantation[18]. A recent study has proved this concept where the fetal hepatocytes were transplanted via intrasplenic infusion through the splenic artery in a patient with end stage liver cirrhosis leading to the improvement in clinical conditions[19]. The translocation of infused cells through intrasplenic injection has also been demonstrated where cells after infusion first reaches to the splenic veins and then to hepatic sinusoids[20].
CLINICAL STUDIES OF HUMAN HEPATIC STEM/PROGENITOR CELL THERAPY
HSCs-based therapies for the treatment of ESLD are in their early stages of translation. We have done the pioneering studies using HSCs transplantation in the treatment of patients with inborn metabolic errors, liver cirrhosis, hepatitis B/C, and other liver disorders[17,20]. We used EpCAM+ve enriched human HSCs from human fetal livers following the approach described by Schmelzer et al[14]. Remarkably no immune suppressant was required and recipient was not matched for histocompatibility antigens. All the patients with decompensated liver cirrhosis receiving HSCs transplantation through hepatic artery showed significant decrease (P < 0.01) in the mean Mayo-End stage Liver Disease (MELD) score and improvement in multiple diagnostics and biochemical parameters after six-months follow-up[17]. After 6 mo to one year of HSCs post-transplantation angiogram analysis of the patients showed significant improvement in branching of hepatic arteries and veins (Figure 1).
Figure 1.
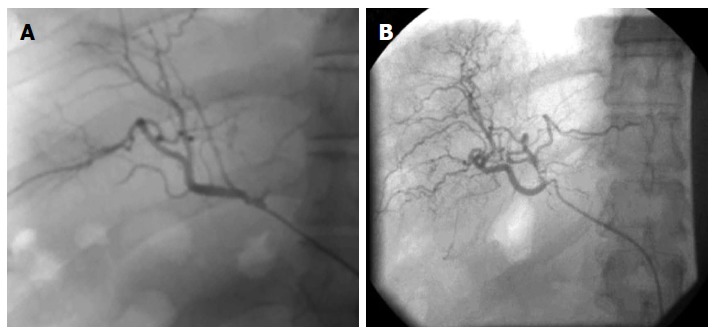
Hepatic artery characterization using angiograph of patients post transplantation after 6 mo (A) and after 12 mo (B).
Recently we accessed cellular immune response in 5 patients with decompensated liver cirrhosis after transplantation of human HPCs. The study showed marked clinical recovery with decline in the MELD score and there was no significant variation found in T-cell subpopulation (CD3, CD4, CD8 and CD4/CD8 ratio), natural killer cells and in serum cytokine levels between pre and post transplantation demonstrating the safety of EpCAM+ve HPCs transplantation for liver cirrhosis (Unpublished).
Future efforts to use human HSCs/HPCs clinically will be facilitated by production of cells at large-scale. The sourcing of donor cells may be fetal tissue in those countries that permits their use. The cells may be utilized as directly isolated, or after in vitro expansion.
IN VIVO TRACKING AND MONITORING OF TRANSPLANTED HEPATIC STEM/PROGENITOR CELLS
Since last few decades’ vast developments have been made for the management of liver disease. However more investigations are desired to direct the better tissue imaging approaches to identify the fate of grafted cells. It is essential for better understanding of cell infusion routes, migration and efficacy of desired cells post-transplantation. Currently various magnetic, fluorescence or radio imaging methods have been used to evaluate efficacy of transplanted cells. However, few of them have proved their prospective in clinical settings but majority of them are still in their preclinical phases of investigations.
Tracking the fate of transplanted cells is vital to monitor the delivery and viability of the grafts over extended time. It could be achieved by merging optimal molecular imaging methods with stem cells. Through advancements in experimental and molecular imaging technologies, it would provide heavy motivation to improvise the molecular imaging approaches for better understanding of the regenerative mechanisms involved in stem cells transplantation.
Molecular imaging
Stem cells labeling and tracking in human tissues/organs is the most important subject nowadays in order to answer several unsettled issues regarding the cell delivery routes, stem cell choice, number of cells to be infused and the time-point of cell delivery for the treatment in a chronic setting. Answering to these questions will further contribute to the development of safer, non-invasive, and repeatable imaging modalities that could discover better cell therapeutic approaches from bench to bed-side. Long-term monitoring of transplanted stem cells continuously with high temporal resolution and good biocompatibility will allow us to better understand the precise regeneration mechanisms in different organs. Nanobiotechnology has emerged as the most immense area for labeling and tracking of cells both in vitro and in vivo.
HM-PAO based tracking of transplanted HSCs: Our initial effort was made to monitor transplanted HSCs in vivo by using HM-PAO (lipid-binding dye). However, existence of short life-span of HM-PAO within the cells, we were able to identify transplanted cells only till 24 h of post-transplantation[18]. However, other methods such as hepatic scintigraphy demonstrated that infused human HPCs homes evenly in both liver lobes with elevated engraftment, thus yet again recalling the efficiency of cell delivery route through hepatic artery. During and after 6 mo of follow-up no other clinical complications were observed[17].
Di-D labeling and tracking of transplanted cells: Due to short life of HM-PAO, it was quite difficult to monitor transplanted cells in long-term. Hence, further we used live cell imaging dye (i.e., Di-D) for labeling and long-term tracking of human HSCs post-transplantation. DiD-labeled EpCAM+ve human HPCs were infused in liver of nude mice to identify its engraftment and survival rate. Post-transplantation labeled cells were tracked and located at different time points by using an animal imaging system (KODAK Fx-PRO). Post-transplantation persistence DiD-labeled cells were observed up to 110 d. X-ray images of animals receiving cells were also captured and overlaid on fluorescence imaging to investigate better consignment (Unpublished). However, additional studies are required with regard to safety and efficacy of such dyes before applying into the medical conditions.
Magnetic resonance imaging of transplanted cells using high contrast nanoparticles: Magnetic resonance imaging (MRI) is widely used technique in preclinical and clinical studies. Particular type of stem cells are enriched with a contrast agent or directly labeled with super paramagnetic iron oxide (SPIO) to track them in damaged tissues post-transplantation. Non-invasive in vivo monitoring can be achieved by MRI using SPIO-labeled cells due to its significant temporal and spatial contrast[21-24]. We have synthesized such high contrast nanoparticles by combining SPIO with Gadolinium which is also detectable in 1.5 T MRI machine. This approach represents one such tool that can provide insight into cell survival and proliferation following transplantation into the tissues.
LIVER BIOENGINEERING: A POTENTIAL APPROACH TO UNDERSTAND THE REGENERATIVE MECHANISMS OF HSCS POST-TRANSPLANTATION
Liver bioengineering is one of the most exciting areas in regenerative medicine. In last few decades various strategies have been attempted to support the liver tissue regeneration either by using artificial devices or two-dimensional culture system. However, three-dimensional architecture, vascular network and dense cellular masses are required to support the functional damage of liver. Hepatocytes are known to be attachment-dependent cells and loss very quickly in absence of suitable medium composition, ECM and cell-cell contacts. Cellular proliferation and differentiation is also influenced by three-dimensional organ architecture[25-27]. Employment of naturally derived matrices have proved their potential in hepatocyte culture[26]. Several studies have demonstrated long-term heterotropic hepatocytes transplantation in a variety of matrices[27,28]. Nonetheless, initial engraftment rates are suboptical. Hence development of a whole tissue engineered liver construct is in urgent need for orthotropic transplantation.
Repopulation of human HSCs in whole decellularized liver scaffold to generate humanized organ
Decellularization is defined as the technology used to remove all parenchyma cells, endothelial cells, myofilaments and other cellular components from the organ while retaining its three dimension architecture and vascular tree. The use of naturally vasculature and rapid separation of debris and waste fluid from organ or tissue significantly improves the quality of organ scaffolds for reconstitution of new organ/tissue. Because of the above facts decellularization technology has been considered an ideal tool for regeneration of whole organ/tissues. Different types of stem cells from a variety of sources either alone or in combination with other cells have been tried to develop the whole functional organ architecture to provide a considerable approach for organ bioengineering.
Recellularization is a process of generating functional cells within the decellularized organ scaffold in order to get fully functional bio-artificial organ. Seeding of specific type of cells with high proliferation and controlled differentiation potential is necessary to repopulate the decellularized organ scaffold. Despite using various types of organs for decellularization and recellularization, none of them have proved their absolute potential to replace the damaged organ/tissue.
Since last two decades’ most of the tissue engineering approaches have involved in reconstruction of thin masses of cells, for instance skin, arteries and bladder[29-31]. Whereas construction of thick masses of cells/tissues such as muscles, liver and kidney, etc. has been proved difficult to mimic due to fractional distribution of oxygen and nutrients inside the engineered scaffold[32]. Several recent studies have proved decellularization and recellularization technologies as a most significant tool for the creation of fully humanized functional organs[33-37].
In our view, these decellularized liver bio-scaffolds have a great potential to ensure an advanced in vitro natural liver cell culture system for toxicology, pharmacology and drug discovery. This may also establish an excellent tool to cram the normal development of organs and diseased tissues. The technology of decellularization and recellularization passes much potential to reach the significant aim of engineering bio-artificial personalized livers for the treatment of acute and chronic liver diseases. Therefore lead has been taken by our centre to utilize the technology of decellularization and repopulation further to improvise the research quality and to reach the goal for generating personalized whole functional liver for patients suffering with end stage liver failure.
CONCLUSION
Human HSCs-based therapies for the treatment of ESLD are in their early stage of the translation. Long-term stem cells labeling and tracking in vivo is the most important subject nowadays. High contrast and paramagnetic nanoparticles with high biocompatibility have emerged a better option in order to answer several unsettled issues regarding the cell delivery routes, better stem cell choice, number of cells to be infused and the time-point of cell delivery for the treatment in a chronic setting. The technology of decellularization and recellularization might offer a significant platform for developing bioengineered personalized livers to come over scarcity of desired number of donor organs for the treatment of ESLD. Combinatorial approach of decellularization and nanotechnology could pave a way towards the better understanding in determination of cell fate post-transplantation.
Footnotes
Conflict-of-interest: The authors have no conflict of interest related to the manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 2, 2014
First decision: January 20, 2015
Article in press: April 20, 2015
P- Reviewer: Asahina K, Guo ZK, Saeki K S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
References
- 1.Sutherland DE, Numata M, Matas AJ, Simmons RL, Najarian JS. Hepatocellular transplantation in acute liver failure. Surgery. 1977;82:124–132. [PubMed] [Google Scholar]
- 2.Sommer BG, Sutherland DE, Matas AJ, Simmons RL, Najarian JS. Hepatocellular transplantation for treatment of D-galactosamine-induced acute liver failure in rats. Transplant Proc. 1979;11:578–584. [PubMed] [Google Scholar]
- 3.Makowka L, Falk RE, Rotstein LE, Falk JA, Nossal N, Langer B, Blendis LM, Phillips MJ. Cellular transplantation in the treatment of experimental hepatic failure. Science. 1980;210:901–903. doi: 10.1126/science.7001630. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner D, LaPlante-O’Neill PM, Sutherland DE, Najarian JS. Effects of intrasplenic injection of hepatocytes, hepatocyte fragments and hepatocyte culture supernatants on D-galactosamine-induced liver failure in rats. Eur Surg Res. 1983;15:129–135. doi: 10.1159/000128344. [DOI] [PubMed] [Google Scholar]
- 5.Habibullah CM, Ayesha Q, Khan AA, Naithani R, Lahiri S. Xenotransplantation of UV-B-irradiated hepatocytes. Survival and immune response. Transplantation. 1995;59:1495–1497. doi: 10.1097/00007890-199505270-00027. [DOI] [PubMed] [Google Scholar]
- 6.Mito M, Kusano M, Kawaura Y. Hepatocyte transplantation in man. Transplant Proc. 1992;24:3052–3053. [PubMed] [Google Scholar]
- 7.Habibullah CM, Syed IH, Qamar A, Taher-Uz Z. Human fetal hepatocyte transplantation in patients with fulminant hepatic failure. Transplantation. 1994;58:951–952. doi: 10.1097/00007890-199410270-00016. [DOI] [PubMed] [Google Scholar]
- 8.Khan AA, Habeeb A, Parveen N, Naseem B, Babu RP, Capoor AK, Habibullah CM. Peritoneal transplantation of human fetal hepatocytes for the treatment of acute fatty liver of pregnancy: a case report. Trop Gastroenterol. 2004;25:141–143. [PubMed] [Google Scholar]
- 9.Khan AA, Habeeb A, Parveen N, Naseem B, Babu RP, Capoor AK, Habibullah CM. Peritoneal transplantation of human fetal hepatocytes for the treatment of acute fatty liver of pregnancy: a case report. Trop Gastroenterol. 2004;25:141–143. [PubMed] [Google Scholar]
- 10.Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- 11.Sokal EM, Smets F, Bourgois A, Van Maldergem L, Buts JP, Reding R, Bernard Otte J, Evrard V, Latinne D, Vincent MF, et al. Hepatocyte transplantation in a 4-year-old girl with peroxisomal biogenesis disease: technique, safety, and metabolic follow-up. Transplantation. 2003;76:735–738. doi: 10.1097/01.TP.0000077420.81365.53. [DOI] [PubMed] [Google Scholar]
- 12.Piscaglia AC, Novi M, Campanale M, Gasbarrini A. Stem cell-based therapy in gastroenterology and hepatology. Minim Invasive Ther Allied Technol. 2008;17:100–118. doi: 10.1080/13645700801969980. [DOI] [PubMed] [Google Scholar]
- 13.Duret C, Gerbal-Chaloin S, Ramos J, Fabre JM, Jacquet E, Navarro F, Blanc P, Sa-Cunha A, Maurel P, Daujat-Chavanieu M. Isolation, characterization, and differentiation to hepatocyte-like cells of nonparenchymal epithelial cells from adult human liver. Stem Cells. 2007;25:1779–1790. doi: 10.1634/stemcells.2006-0664. [DOI] [PubMed] [Google Scholar]
- 14.Schmelzer E, Wauthier E, Reid LM. The phenotypes of pluripotent human hepatic progenitors. Stem Cells. 2006;24:1852–1858. doi: 10.1634/stemcells.2006-0036. [DOI] [PubMed] [Google Scholar]
- 15.Vali SM, Vishwakarma SK, Bardia A, Tiwari SK, Srinivas G, Raj A, Tripura C, Habeeb MA, Khan AA, Pande G. Isolation and characterization of stem cells sub population within the human fetal liver. Cell Biol Res Ther. 2014 [Google Scholar]
- 16.Rao MS, Khan AA, Parveen N, Habeeb MA, Habibullah CM, Pande G. Characterization of hepatic progenitors from human fetal liver during second trimester. World J Gastroenterol. 2008;14:5730–5737. doi: 10.3748/wjg.14.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aleem Khan A, Parveen N, Habeeb MA, Habibullah CM. Journey from hepatocyte transplantation to hepatic stem cells: a novel treatment strategy for liver diseases. Indian J Med Res. 2006;123:601–614. [PubMed] [Google Scholar]
- 18.Khan AA, Shaik MV, Parveen N, Rajendraprasad A, Aleem MA, Habeeb MA, Srinivas G, Raj TA, Tiwari SK, Kumaresan K, et al. Human fetal liver-derived stem cell transplantation as supportive modality in the management of end-stage decompensated liver cirrhosis. Cell Transplant. 2010;19:409–418. doi: 10.3727/096368910X498241. [DOI] [PubMed] [Google Scholar]
- 19.Nagata H, Ito M, Shirota C, Edge A, McCowan TC, Fox IJ. Route of hepatocyte delivery affects hepatocyte engraftment in the spleen. Transplantation. 2003;76:732–734. doi: 10.1097/01.TP.0000081560.16039.67. [DOI] [PubMed] [Google Scholar]
- 20.Gridelli B, Vizzini G, Pietrosi G, Luca A, Spada M, Gruttadauria S, Cintorino D, Amico G, Chinnici C, Miki T, et al. Efficient human fetal liver cell isolation protocol based on vascular perfusion for liver cell-based therapy and case report on cell transplantation. Liver Transpl. 2012;18:226–237. doi: 10.1002/lt.22322. [DOI] [PubMed] [Google Scholar]
- 21.Cheng K, Benten D, Bhargava K, Inada M, Joseph B, Palestro C, Gupta S. Hepatic targeting and biodistribution of human fetal liver stem/progenitor cells and adult hepatocytes in mice. Hepatology. 2009;50:1194–1203. doi: 10.1002/hep.23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilhelm C, Gazeau F, Bacri JC. Magnetophoresis and ferromagnetic resonance of magnetically labeled cells. Eur Biophys J. 2002;31:118–125. doi: 10.1007/s00249-001-0200-4. [DOI] [PubMed] [Google Scholar]
- 24.Mooney D, Hansen L, Vacanti J, Langer R, Farmer S, Ingber D. Switching from differentiation to growth in hepatocytes: control by extracellular matrix. J Cell Physiol. 1992;151:497–505. doi: 10.1002/jcp.1041510308. [DOI] [PubMed] [Google Scholar]
- 25.Fiegel HC, Kaufmann PM, Bruns H, Kluth D, Horch RE, Vacanti JP, Kneser U. Hepatic tissue engineering: from transplantation to customized cell-based liver directed therapies from the laboratory. J Cell Mol Med. 2008;12:56–66. doi: 10.1111/j.1582-4934.2007.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linke K, Schanz J, Hansmann J, Walles T, Brunner H, Mertsching H. Engineered liver-like tissue on a capillarized matrix for applied research. Tissue Eng. 2007;13:2699–2707. doi: 10.1089/ten.2006.0388. [DOI] [PubMed] [Google Scholar]
- 27.Lin P, Chan WC, Badylak SF, Bhatia SN. Assessing porcine liver-derived biomatrix for hepatic tissue engineering. Tissue Eng. 2004;10:1046–1053. doi: 10.1089/ten.2004.10.1046. [DOI] [PubMed] [Google Scholar]
- 28.Kaufmann PM, Kneser U, Fiegel HC, Kluth D, Herbst H, Rogiers X. Long-term hepatocyte transplantation using three-dimensional matrices. Transplant Proc. 1999;31:1928–1929. doi: 10.1016/s0041-1345(99)00208-0. [DOI] [PubMed] [Google Scholar]
- 29.Johnson LB, Aiken J, Mooney D, Schloo BL, Griffith-Cima L, Langer R, Vacanti JP. The mesentery as a laminated vascular bed for hepatocyte transplantation. Cell Transplant. 1994;3:273–281. doi: 10.1177/096368979400300403. [DOI] [PubMed] [Google Scholar]
- 30.Zacchi V, Soranzo C, Cortivo R, Radice M, Brun P, Abatangelo G. In vitro engineering of human skin-like tissue. J Biomed Mater Res. 1998;40:187–194. doi: 10.1002/(sici)1097-4636(199805)40:2<187::aid-jbm3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 31.Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A, et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 33.Griffith LG, Naughton G. Tissue engineering--current challenges and expanding opportunities. Science. 2002;295:1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 34.Khan AA, Vishwakarma SK, Bardia A, Venkateshwarulu J. Repopulation of decellularized whole organ scaffold using stem cells: an emerging technology for the development of neo-organ. J Artif Organs. 2014;17:291–300. doi: 10.1007/s10047-014-0780-2. [DOI] [PubMed] [Google Scholar]
- 35.Vishwakarma SK, Bhavani PG, Bardia A, Abkari A, Murthy GS, Venkateshwarulu J, Khan AA. Preparation of natural three-dimensional goat kidney scaffold for the development of bioartificial organ. Indian J Nephrol. 2014;24:372–375. doi: 10.4103/0971-4065.133008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rout S, Vishwakarma SK, Khan AA. Decellularized heart: A step towards creating personalized bioengineered organs. Cur Sci. 2014:107. [Google Scholar]
- 37.Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53:604–617. doi: 10.1002/hep.24067. [DOI] [PubMed] [Google Scholar]


