Abstract
Background:
Therapeutic hypothermia (TH) may improve neurological outcome in comatose patients following out of hospital cardiac arrest (OHCA). The reliability of clinical prediction of neurological outcome following TH remains unclear. In particular, there is very limited data on survival and predictors of neurological outcome following TH for OHCA from resource-constrained settings in general and South Asia in specific.
Objective:
The objective was to identify factors predicting unfavorable neurological outcome at hospital discharge in comatose survivors of OHCA treated with hypothermia.
Design:
Retrospective chart review.
Setting:
Urban 200-bed hospital in Chennai, India.
Methods:
Predictors of unfavorable neurological outcome (cerebral performance category score [3–5]) at hospital discharge were evaluated among patients admitted between January 2006 and December 2012 following OHCA treated with TH. Hypothermia was induced with cold intravenous saline bolus, ice packs and cold-water spray with bedside fan. Predictors of unfavorable neurological outcome were examined through multivariate exact logistic regression analysis.
Results:
A total of 121 patients were included with 106/121 (87%) experiencing the unfavorable neurological outcome. Independent predictors of unfavorable neurological outcome included: Status myoclonus <24 h (odds ratio [OR] 21.79, 95% confidence interval [CI] 2.89-Infinite), absent brainstem reflexes (OR 50.09, 6.55-Infinite), and motor response worse than flexion on day 3 (OR 99.41, 12.21-Infinite). All 3 variables had 100% specificity and positive predictive value.
Conclusion:
Status myoclonus within 24 h, absence of brainstem reflexes and motor response worse than flexion on day 3 reliably predict unfavorable neurological outcome in comatose patients with OHCA treated with TH.
Keywords: Neurological outcome, out of hospital cardiac arrest, therapeutic hypothermia
Introduction
Out of hospital cardiac arrest (OHCA) is an important cause for the admission of patients to intensive care units (ICUs) in the developing world and is a major cause of mortality and serious morbidity worldwide.[1] Despite major advances in resuscitation practice, the outcome of patients with OHCA has generally remained poor for more than 30 years, with <10% typically surviving to hospital discharge.[1,2] The outcome following cardiac arrest (CA) is largely dependent on the arrest rhythm (shockable vs. nonshockable) and the provision of early cardiopulmonary resuscitation (CPR) and defibrillation indicating the importance of time to resuscitation and prehospital management.[3,4] Therapeutic hypothermia (TH) has been shown to improve neurological outcome in comatose survivors of CA associated with ventricular fibrillation (VF) and pulseless ventricular tachycardia.[5,6] In order to accurately counsel family of survivors of CA and in the interest of ICU resource utilization, it is important to reliably predict neurological outcome as early as possible following OHCA. This is particularly true in resource-constrained settings, common in the developing world, where there is frequently a shortage of ICU beds, supplies and clinical staffing as well as financial resources to support extended ICU and inpatients stays. The American Academy of Neurology guidelines for prediction of neurological outcome following CA were primarily developed based on evidence from the pre-TH era.[7] There has therefore been interest in the validation of these clinical signs as predictors of outcome in cohorts of CA patients treated with TH.[8,9,10,11,12,13] Some authors advocate the use of sophisticated tools like continuous electroencephalography (EEG) monitoring and evoked response potential for accurate prediction of neurological prognosis following CA.[8,9,10,11,12,13] However, these tools are not routinely available and are expensive, limiting their value in the developing world.
In addition to the uncertainty regarding accuracy of clinical prediction following TH, there is very limited published data on the incidence and predictors of unfavorable neurological outcome following TH for OHCA from the developing world, and none at all from South Asia.[14] Several factors, such as the incidence of nonshockable rhythms and limited availability and effectiveness of prehospital care (emergency medical services and bystander CPR), may have a significant impact on OHCA outcomes and on predictors.[15,14]
Objective
To determine early predictors of unfavorable neurological outcome (cerebral performance category [CPC] 3–5) at hospital discharge in patients with OHCA treated with TH.
Methods
Setting
Urban, 200-bed community hospital in Chennai, India, with a full-facility 12-bed critical care unit. A “code blue” alarm is triggered for all CAs in the emergency department (ED). A resuscitation team led by an advance cardiac life support (ACLS) certified critical care consultant or senior registrar responds to the “code blue” at all times.
Design
This was a retrospective study of a cohort of comatose patients with OHCA, who presented to the ED between January 1, 2006, and December 31, 2012. Approval from the hospital ethics committee was obtained.
Patients
Inclusion criteria: Adult patients (age ≥16 years) with OHCA and return of spontaneous circulation (ROSC) who remained comatose with Glasgow Coma Scale (GCS) <5 (E1 V1 M ≤2) and received TH.
Exclusion criteria: In-hospital, ED and trauma related CAs, patients who did not receive TH due to signs of awakening post-ROSC, death within 24 h, prolonged arrest time evidenced by the presence of rigor mortis, any disease with terminal illness and no hope for survival due to futility of treatment.
Data collection and variables definition
The hospital maintains a registry with ICD coding for “cardiac and cardiopulmonary arrest” (I 46.9) from which details of all patients who presented with CA to the ED were collected. Medical records of the patients identified in the registry to have suffered “out of hospital cardiac or cardiopulmonary arrest” were examined. These details were further verified in a database maintained by the ICU that contains information on all procedures undertaken by the ICU team including the performance of CPR in the ED. Data regarding prehospital care (bystander CPR), CA rhythm on presentation, and duration of circulatory arrest as documented by the resuscitation team were noted. Clinical and demographic data were collected using Utstein's style recommendations.[16] Charlson's comorbidity index was used to assess the premorbid status.[17] Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) II score at admission was calculated to measure the severity of acute illness. The etiology of CA was categorized into cardiac and noncardiac based on the circumstances leading to the event and the patient's past medical history. The CA rhythm was dichotomized as shockable (VF and pulseless ventricular tachycardia) and nonshockable (asystole and pulseless electrical activity). The duration of CA (time taken to achieve ROSC) was calculated as a sum of arrest-CPR interval (time interval between the onset of collapse and onset of CPR, also referred to as the “no-flow state”) and the duration of CPR (“low-flow state”). It was dichotomized to ≤25 min and >25 min based on a previous study.[8] Following ROSC, defined as a return of pulse and its maintenance for ≥30 min, the patients were admitted to ICU.
Induced hypothermia, sedation, analgesia and neuromuscular blockade
Therapeutic hypothermia (core temperature 32°C–34°C) was immediately commenced on admission to ED and applied for 24 h with surface cooling (ice packs over axilla, neck and groin, cold water spray, and fan) and intravenous bolus of cold (0°C–4°C) normal saline (500–1000 ml). Midazolam (sedation) and morphine (analgesia) as a continuous infusion with or without paralytics (vecuronium) were used to control severe shivering, status myoclonus and ventilator dyssynchrony. Intermittent boluses of sedation (if required/PRN) were given to patients without the above features. Sedation and neuromuscular blockade were stopped following cessation of TH. The unit practice was to use minimal or no sedation in patients who remained comatose post-rewarming to allow meaningful neurological assessment. Rewarming was passive through the cessation of cooling measures.
General patient management
Management of the CA was in accordance to the American Heart Association's ACLS guidelines.[3,4] Organ failure support was provided as required. Blood sugar control was achieved with insulin infusion (Human Actrapid®). It was considered as “favorable” if the mean sugar levels were 6–10 mmol/l/day and “unfavorable” if mean blood sugar levels were >10 mmol/l/day.[18] Status myoclonus was treated with sodium valproate and levetiracetam as first-line therapy with the addition of phenytoin or clonazepam as required. The use of midazolam and paralytics were reserved for severe status myoclonus unresponsive to the above measures.
Neurological and outcome assessment
Neurological outcome was collected from the review of the entire hospital record for the admission including the progress notes entered by the intensivist's, nursing and ancillary services, consultation and diagnostic reports, and discharge summaries. The bedside nurse recorded GCS and pupillary response every hour. The presence of status myoclonus, defined as spontaneous or sound sensitive, repetitive, irregular brief jerks in both face and limb present most of the day within 24 h post-CA was noted.[19] Data on brainstem reflexes (pupillary, corneal, and cough) and motor response to painful stimulus on day 3 were derived from the intensivist's daily documentation of the neurological examination. Families received daily counseling on the clinical condition of the patients. Life-sustaining treatment was withdrawn when requested by the surrogate decision makers following counseling. Brain imaging was not performed for the purpose of prognostication. Continuous EEG monitoring and somatosensory evoked response potential testing was not obtained. CPC was used to define neurological outcome at hospital discharge, which was shown to be a useful surrogate measure of long-term survival[20] [Table 1]. CPC 1, 2 was considered as a favorable and CPC 3, 4, and 5 were considered as an unfavorable neurological outcome.[4,16,20]
Table 1.
CPC scale

Statistical analysis
Descriptive statistics are summarized for categorical variables as frequencies (%) and were compared between groups using Fisher's exact test. Continuous variables, expressed as median (inter-quartile range [IQR]), were compared between groups using Wilcoxon rank sum test. All comparisons were two-sided, and P < 0.05 was considered significant. Univariate exact logistic regression models were used to determine the association of unfavorable neurological outcome (CPC 3–5) with several clinical variables.[21] Exact logistic regression modeling, which is based on permutation resampling, is the preferred analytical technique that has replaced the standard asymptotic logistic regression for analyzing small, skewed, or sparse data sets.[21] A multivariate exact logistic regression was used to identify independent predictors of unfavorable neurological outcome among select variables found to have a P < 0.05 on univariate analysis. Due to quasi-complete separation (i.e., 100% specificity) of each of the neurological assessment variables (presence of status myoclonus <24 h, absence of brainstem reflexes and motor response worse than flexion on day 3) it was not possible to include all of them in the multivariate exact logistic regression model as they led to a degenerate conditional distribution and inability to generate estimates. As such, separate multivariate analyses were undertaken for each of the neurological assessments, including an adjustment for the nonshockable rhythm, duration of CA >25 min and APACHE II score. Results are reported as exact odds ratios (OR) with 95% confidence intervals (CIs). The accuracy of neurological assessment was further evaluated by calculating sensitivity, specificity, positive predictive value (PPV), negative predictive value, and likelihood ratios with associated exact binomial 95% CIs. The data were analyzed using Stata IC 12 (StataCorp, College Station, TX, USA).
Results
During the study period, 1112 patients presented to the ED with CA [Figure 1]. CPR was performed on 1000 patients and ROSC was achieved in 151 patients (15.1%). 27 patients died within 24 h and 3 patients did not receive TH as they showed signs of awakening even before TH was commenced. Data were analyzed on the remaining 121 patients who survived >24 h, remained comatose and received TH for 24 h. CA was un-witnessed in 5 patients (4%).
Figure 1.

The outcome of all patients presenting with out of hospital cardiac arrest to the emergency department
General characteristics
Patients were predominantly females (64%), with median age 62 (IQR 51–69) years. Baseline characteristics are depicted in Table 2. Noncardiac cause accounted for majority (73/121, 60%) of CA and the leading causes were: Septic shock (17/73), drug/toxin overdose and hanging (12/73), pneumonia (10/73), renal failure (10/73), and acute severe asthma (10/73). The predominant arrest rhythm was nonshockable (90%). Only 3.3% (4/121) received bystander CPR and 6.6% (8/121) were transferred to the hospital by ambulance. Continuous infusion of midazolam and morphine (maximum 3 mg/h, respectively) was required in 70/121 (58%) patients. Status myoclonus was seen in 52/121 (45%) patients, with onset in the first 24 h in all cases.
Table 2.
General and neurological characteristics of patients according to neurological outcome

Clinical characteristics and unfavorable neurological outcome
A number of clinical characteristics were strongly associated with unfavorable neurological outcome. They were grouped into three phases: Prearrest, peri-arrest, and postarrest phase [Table 3]. Univariate associations with unfavorable neurological outcome were observed with APACHE II score, duration of CA >25 min, nonshockable rhythm, unfavorable glycemic control, presence of status myoclonus within 24 h, absence of at least 1 of the brainstem reflexes, and motor response worse than flexion on day 3. On multivariate analysis, status myoclonus <24 h (OR 21.79, 95% CI 2.89-Infinite), absent brainstem reflexes (OR 50.09, 95% CI 6.55-Infinite)and motor response worse than flexion on day 3 (OR 99.41, 95% CI 12.21-Infinite) independently predicted unfavorable neurological outcome [Table 4]. The presence of status myoclonus within 24 h, absence of more than 1 brain stem reflex and a motor response that was worse than flexion on day 3 were extremely specific (1.00, 95% CI 0.78–1.00) in predicting unfavorable neurological outcome with 100% PPV (1.00, 95% CI 0.96–1.00) [Table 5].
Table 3.
Univariate analysis of clinical characteristics and risk of unfavorable neurological outcome (CPC 3-5)
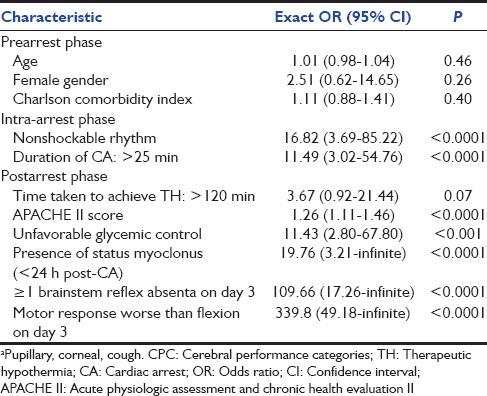
Table 4.
Multivariate analysis of selected clinical characteristics and risk of unfavorable neurological outcome (CPC 3-5)
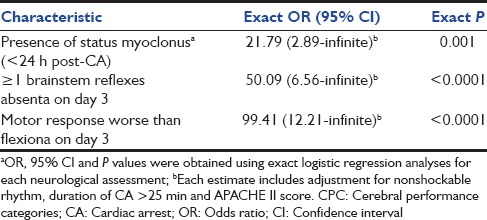
Table 5.
Accuracy of neurological assessments and prediction of poor neurological outcomes (CPC 3-5) among patients who recovered spontaneous circulation after out-of-hospital cardiac arrest

Survival, mortality, and morbidity
Out of the 121 patients, 20 patients (16.5%) awakened from coma by day 3 (with preserved brainstem reflexes and motor response better than extension). Fifteen of these were discharged from the hospital with the favorable neurological outcome (CPC 1, 2). The remaining 5 patient's died (median 17 ICU days) due to severe nosocomial septic shock, multi-organ dysfunction, and ventilator-associated pneumonia with severe acute respiratory distress syndrome.
A total of 101/121 (83.5%) patients remained in coma on day 3 of whom 46 (45%) died (CPC 5) despite aggressive treatment due to recurrent CA, post-CA syndrome, severe cardiogenic and septic shock with multi-organ failure, and brain death due to severe anoxic encephalopathy. Fifty-three patients (52%) died (CPC 5) following the withdrawal of life-sustaining treatment. Two (2%) patients were discharged in persistent vegetative state (CPC 4).
Discussion
Our study has shown that early prediction of unfavorable neurological outcome in post-CA patients treated with hypothermia is feasible at the bedside using the neurological examination. This is the first study to report any data on neurological outcome following OHCA from South Asia.[14] An ideal predictor of unfavorable neurological outcome should be extremely specific with zero false positive rates. Our study has shown that the presence of status myoclonus on the day of CA, absence of brainstem reflexes, and a motor response worse than flexion on day 3 are extremely specific with zero false positive rates.
These findings are consistent with results of Fugate et al. who in their prospective study of 103 post hypothermia patients following OHCA concluded that clinical examination (brainstem reflexes, motor response, and presence of myoclonus) on day 3 after CA remained an accurate predictor of outcome after TH.[10] Three other studies, however, have concluded that neurological examination might not be accurate in predicting unfavorable neurological outcome in OHCA patients treated with TH.[8,9,13] The reasons for this inaccuracy of prediction by neurological examination may have been associated administration of sedative and paralytic agents that may delay neurological recovery and alter the optimal timing of clinical prognostication. The findings of these studies must be viewed with caution as the practice of sedation and analgesia is highly variable across different ICUs. In our study, continuous sedation and analgesia were given only to a selected group of patients and the dose of sedation used was lower than reported in some of these studies.[8,13] Sedation was withheld in patients who remained comatose post-rewarming. We believe, therefore, that sedation and analgesia are less likely to have significantly influenced the results in our study.
The predominant cause for CA was noncardiac (60%), which is different from other global studies where the majority of the arrests were from a cardiac cause.[14,22,23,24] Patients in our study were relatively young. This was consistent with the data from the UK registry where the mean age of patients with noncardiac cause for CA was 59.5 years.[25] The rates of nonshockable rhythm were higher and ROSC rates were lower, most likely due to the virtual absence of prehospital care and limited utilization of EMS.[8,9,10] Documented bystander CPR rates were very low compared to other studies.[23,24] The possible reasons for poor prehospital care and low rates of bystander CPR may include lack of public awareness of basic life support and the logistics involved in the transportation to appropriate medical facility. In our study, mortality was higher, probably due to increased severity of illness at admission, noncardiac etiology in majority of patients, deeper coma, and preponderance of nonshockable rhythm.[8,13,26] Importantly, there has been no uniform definition of coma post-CPR across many studies and guidelines.[5,6,8,13,27] This may have resulted in heterogeneity in the application of TH, which in turn could potentially lead to varied results of survival and neurological outcome. The cumulative (VF and non-VF arrests) rate of survival to discharge with favorable neurological outcome in our study was similar to that reported earlier (12.3% vs. 0.6–31%).[21]
Of note, the goal temperature was achieved within 120 min in more than two-thirds of patients, demonstrating the feasibility of rapid induction of hypothermia using low-technology solutions in resource-limited settings. We, therefore, believe that achieving good neurological outcome after CPR is highly feasible in resource-constrained settings in the developing world such as ours, for those patients who reach appropriate medical centers early and are managed aggressively. The implications of our study are particularly relevant to the developing world wherein the ability to predict unfavorable neurological outcome within 72 h is of specific importance especially in the context of limited resources (trained manpower, equipment, and financial).
This study has limitations. It is a single-center retrospective study. Similar to other studies on prognostication after CA, mortality in our study was most often preceded by withdrawal of life support raising the possible impact of the self-fulfilling prophecy.[10,28] This might have magnified the strengths of associations between predictors and unfavorable outcome. We believe that this may be the biggest limitation in studies evaluating predictors of death/unfavorable outcome following CA. The only definitive way to avoid self-fulfilling prophecy may be to forbid all withdrawal of life support for a defined period time period (3–6 months), which is implausible from both ethical and practical standpoints. The outcomes reported here might not be representative of outcomes following OHCA at other resource-constrained settings, including in India because of the variations that exist in the availability of auxiliary equipment and trained personnel.
Conclusion
Early clinical prediction of unfavorable neurological outcome following OHCA treated with TH is feasible by bedside clinical examination. Neurological examination on day 3 appears to strongly predict the unfavorable neurological outcome. These findings need to be confirmed by larger, multi-center, prospective studies taking similar conditions, and situations into consideration.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflict of interest.
References
- 1.Nolan JP, Lyon RM, Sasson C, Rossetti AO, Lansky AJ, Fox KA, et al. Advances in the hospital management of patients following an out of hospital cardiac arrest. Heart. 2012;98:1201–6. doi: 10.1136/heartjnl-2011-301293. [DOI] [PubMed] [Google Scholar]
- 2.Sasson C, Rogers MA, Dahl J, Kellermann AL. Predictors of survival from out-of-hospital cardiac arrest: A systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2010;3:63–81. doi: 10.1161/CIRCOUTCOMES.109.889576. [DOI] [PubMed] [Google Scholar]
- 3.American Heart Association Guidelines on Cardiopulmonary Resuscitation: Overview of CPR. Circulation. 2005;112:12–8. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 4.Travers AH, Rea TD, Bobrow BJ, Edelson DP, Berg RA, Sayre MR, et al. Part 4: CPR overview: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(18 Suppl 3):S676–84. doi: 10.1161/CIRCULATIONAHA.110.970913. [DOI] [PubMed] [Google Scholar]
- 5.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 6.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 7.Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: Prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67:203–10. doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 8.Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: A prospective study. Ann Neurol. 2010;67:301–7. doi: 10.1002/ana.21984. [DOI] [PubMed] [Google Scholar]
- 9.Bouwes A, Binnekade JM, Kuiper MA, Bosch FH, Zandstra DF, Toornvliet AC, et al. Prognosis of coma after therapeutic hypothermia: A prospective cohort study. Ann Neurol. 2012;71:206–12. doi: 10.1002/ana.22632. [DOI] [PubMed] [Google Scholar]
- 10.Fugate JE, Wijdicks EF, Mandrekar J, Claassen DO, Manno EM, White RD, et al. Predictors of neurologic outcome in hypothermia after cardiac arrest. Ann Neurol. 2010;68:907–14. doi: 10.1002/ana.22133. [DOI] [PubMed] [Google Scholar]
- 11.Samaniego EA, Mlynash M, Caulfield AF, Eyngorn I, Wijman CA. Sedation confounds outcome prediction in cardiac arrest survivors treated with hypothermia. Neurocrit Care. 2011;15:113–9. doi: 10.1007/s12028-010-9412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rittenberger JC, Sangl J, Wheeler M, Guyette FX, Callaway CW. Association between clinical examination and outcome after cardiac arrest. Resuscitation. 2010;81:1128–32. doi: 10.1016/j.resuscitation.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bisschops LL, van Alfen N, Bons S, van der Hoeven JG, Hoedemaekers CW. Predictors of poor neurologic outcome in patients after cardiac arrest treated with hypothermia: A retrospective study. Resuscitation. 2011;82:696–701. doi: 10.1016/j.resuscitation.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 14.Berdowski J, Berg RA, Tijssen JG, Koster RW. Global incidences of out-of-hospital cardiac arrest and survival rates: Systematic review of 67 prospective studies. Resuscitation. 2010;81:1479–87. doi: 10.1016/j.resuscitation.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Rajaram R, Rajagopalan RE, Pai M, Mahendran S. Survival after cardiopulmonary resuscitation in an urban Indian hospital. Natl Med J India. 1999;12:51–5. [PubMed] [Google Scholar]
- 16.Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: Update and simplification of the Utstein templates for resuscitation registries: A statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, Inter-American Heart Foundation, Resuscitation Councils of Southern Africa) Circulation. 2004;110:3385–97. doi: 10.1161/01.CIR.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 17.Christensen S, Johansen MB, Christiansen CF, Jensen R, Lemeshow S. Comparison of Charlson comorbidity index with SAPS and APACHE scores for prediction of mortality following intensive care. Clin Epidemiol. 2011;3:203–11. doi: 10.2147/CLEP.S20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–97. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 19.Wijdicks EF, Parisi JE, Sharbrough FW. Prognostic value of myoclonus status in comatose survivors of cardiac arrest. Ann Neurol. 1994;35:239–43. doi: 10.1002/ana.410350219. [DOI] [PubMed] [Google Scholar]
- 20.Phelps R, Dumas F, Maynard C, Silver J, Rea T. Cerebral Performance Category and long-term prognosis following out-of-hospital cardiac arrest. Crit Care Med. 2013;41:1252–7. doi: 10.1097/CCM.0b013e31827ca975. [DOI] [PubMed] [Google Scholar]
- 21.Mehta CR, Patel NR. Exact logistic regression: Theory and examples. Stat Med. 1995;14:2143–60. doi: 10.1002/sim.4780141908. [DOI] [PubMed] [Google Scholar]
- 22.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–31. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung W, Flynn M, Thanakrishnan G, Milliss DM, Fugaccia E. Survival after out-of-hospital cardiac arrest in Sydney, Australia. Crit Care Resusc. 2006;8:321–7. [PubMed] [Google Scholar]
- 24.Jennings PA, Cameron P, Walker T, Bernard S, Smith K. Out-of-hospital cardiac arrest in Victoria: Rural and urban outcomes. Med J Aust. 2006;185:135–9. doi: 10.5694/j.1326-5377.2006.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 25.Kendal S, PHillipson A, Wright J. British Heart Foundation; 2011. Out of Hospital Cardiac Arrest Registry: First Year of Data Report 2011. [Google Scholar]
- 26.Grmec S, Gasparovic V. Comparison of APACHE II, MEES and Glasgow Coma Scale in patients with nontraumatic coma for prediction of mortality. Acute Physiology and Chronic Health Evaluation. Mainz Emergency Evaluation System. Crit Care. 2001;5:19–23. doi: 10.1186/cc973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deakin CD, Morrison LJ, Morley PT, Callaway CW, Kerber RE, Kronick SL, et al. 2010 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendation (ILCOR guidelines) Resuscitation. 2010;19:e93–174. doi: 10.1016/j.resuscitation.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 28.Perman SM, Kirkpatrick JN, Reitsma AM, Gaieski DF, Lau B, Smith TM, et al. Timing of neuroprognostication in postcardiac arrest therapeutic hypothermia*. Crit Care Med. 2012;40:719–24. doi: 10.1097/CCM.0b013e3182372f93. [DOI] [PMC free article] [PubMed] [Google Scholar]


