Abstract
Use of antifungal agents has increased over past few decades. A number of risk factors such as immunosuppression, broad spectrum antibiotics, dialysis, pancreatitis, surgery, etc., have been linked with the increased risk of invasive candidiasis. Though there are various guidelines available for the use of antifungal therapy, local/regional epidemiology plays an important role in determining the appropriate choice of agent in situations where the offending organism is not known (i.e. empirical, prophylactic or preemptive therapy). Developing countries like India need to generate their own epidemiological data to facilitate appropriate use of antifungal therapy. In this article, the authors have highlighted the need for region-specific policies/guidelines for treatment of invasive candidiasis. Currently available Indian literature on candidemia epidemiology has also been summarized here.
Keywords: Antifungal prescription, candidemia, critically ill, epidemiology
Introduction
With the ongoing evolution of medical science, evidence-based medicine has become the cornerstone of art of patient management. Ideally, every drug prescription should be backed by hard core scientific evidence generated from randomized controlled trials. Ironically such trials are difficult to conduct, and most of the current literature on antifungal therapy is generated on western population. However, differences in disease burden, health care practices, economic and geographical conditions may require local bodies to format and design region-specific policies and guidelines.
Invasive candidiasis is associated with increased mortality, length of hospital stay and cost of care.[1] Delay in initiation of appropriate antifungal therapy is associated with increased mortality.[2] Blood culture sensitivity for detection of invasive candidiasis ranges from 21% to 71% in studies of autopsy proven cases. Sensitivity is better in patients with candidemia as compared to patients with deep-seated candidiasis.[3] Waiting for positive culture can cause a significant delay in initiation of antifungal therapy and hence increased mortality and morbidity. Identifying the high-risk patients and early initiation of antifungal therapy has become an important strategy for such infections. Factors associated with increased risk of invasive candidiasis include immunosuppression, organ transplant, broad-spectrum antibiotic use, severe sepsis, total parenteral nutrition, surgery, pancreatitis, diabetes mellitus, dialysis, mechanical ventilation, multiple site colonization with Candida etc., Various risk factors have been studied and grouped together to design risk prediction models/scores (Candida score, Ostrosky's clinical prediction rule, colonization index etc.) for invasive candidiasis.[4] These scores are being used to guide prophylactic, preemptive and empirical antifungal therapies. Broad-spectrum antibiotic use is an important risk factor for invasive candidiasis, and this risk factor is present in a large number of Intensive Care Unit (ICU) patients. Antifungal agents are being frequently used for critically ill patients, but region-specific and cohort-specific epidemiological studies are lacking to support our current prescription practices, especially in developing countries like India. This article is an attempt to study the issues regarding antifungal prescription with respect to geographical variation.
Recently, a 17 member expert panel from Iran reviewed the currently available international guidelines and gave their consensus statement on management of invasive candidiasis in ICU. They emphasized the need for prompt identification of high-risk patients and institution of prophylactic and empirical therapy.[5]
This is in contrast to the practice recommended by FIRE study group in UK. The FIRE study group from UK studied the epidemiology of the invasive fungal disease (IFD) in nonneutropenic adults admitted to UK critical care units. Of 60,778 admissions, 383 patients (0.6%) were admitted with or developed IFD. About 94% of these infections were due to Candida species. The group developed a risk prediction model for identification of high-risk patients for invasive candidiasis. However, the economic model did not find it to be cost effective strategy and, therefore, a strategy of no risk assessment and no antifungal prophylaxis was recommended by the study group.[6]
Differences in Recommendations of International Guidelines
A number of societies have given their guidelines for the management of candidiasis in critically ill, summary of which can be found in Table 1.[7,8,9,10] Due to delay in diagnosis and associated high mortality with candidemia, prophylactic (risk factor driven therapy), preemptive (laboratory parameters driven like colonization or beta D glucan [BDG]) and empirical therapy (fever driven therapy) are important strategies in the management of patients at high risk for Candida infections. Prophylactic therapy is given to patients who qualify one or more of risk prediction models. Empirical therapy is given to patients who qualify these models and also show features of sepsis and/or septic shock.
Table 1.
Guidelines at a glance for treatment of invasive candidiasis in nonneutropenic adult
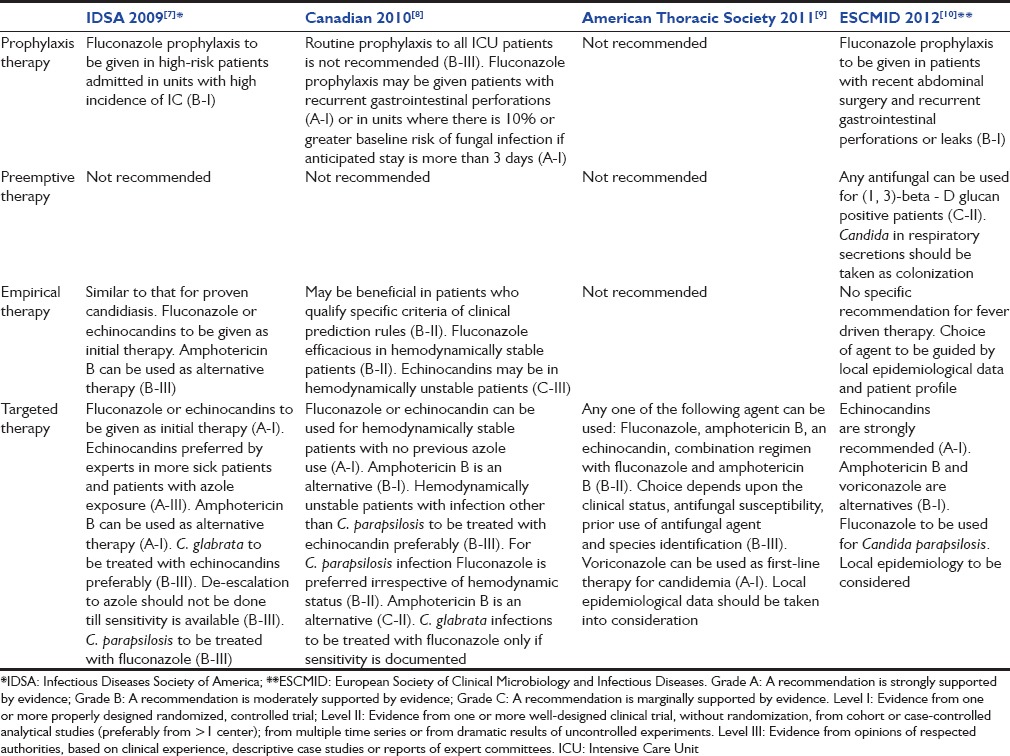
Infectious disease society of America, Canadian and European guidelines recommend prophylaxis as well as empirical therapy in selected group of patients, while American thoracic society guideline has no such recommendations.[7,8,9,10] Preemptive therapy in BDG positive patients is recommended only by European guidelines though the level of recommendation is C-II. Fluconazole is the preferred agent for targeted and empirical therapy in hemodynamically stable patients while echinocandins are preferred in hemodynamically unstable patients. Guidelines have emphasized the role of local epidemiology data in appropriate selection of therapy.
Differences in Regional Epidemiology of Candidemia
Species distribution of Candida shows geographical variation [Table 2]. SENTRY Antimicrobial Surveillance Program (2008–2009) evaluated a total of 2085 clinical Candida isolates collected from 79 different medical centers in Asia, Europe, Latin America and North America. These isolates were either from blood or any other sterile body site, thus representing an infectious event. The most common species isolated from Asia-pacific region was Candida albicans (56.9%) followed by Candida glabrata (13.7%), Candida parapsilosis (13.7%) and Candida tropicalis (11.7%). North America had 43.4% of C. albicans, 23.5% of C. glabrata while Europe had 55% of C. albicans and 15.7% of C. glabrata.[11]
Table 2.
Commonest species causing candidemia as per SENTRY survey (2008-2009)[11]
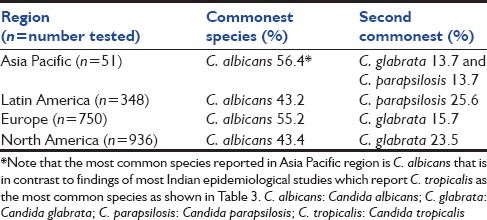
In a recently published laboratory based multicentric survey from Italy, 462 episodes of candidemia were evaluated. They reported C. albicans (49.2%) as the most common isolate, followed by C. parapsilosis (26.2%) and C. glabrata (10.4%). They also reviewed European literature to study distribution and frequency of Candida spp. from 2000 to 2013. C glabrata was found to be common in France, UK and North Europe and C. parapsilosis in Turkey, Spain and Greece.[12]
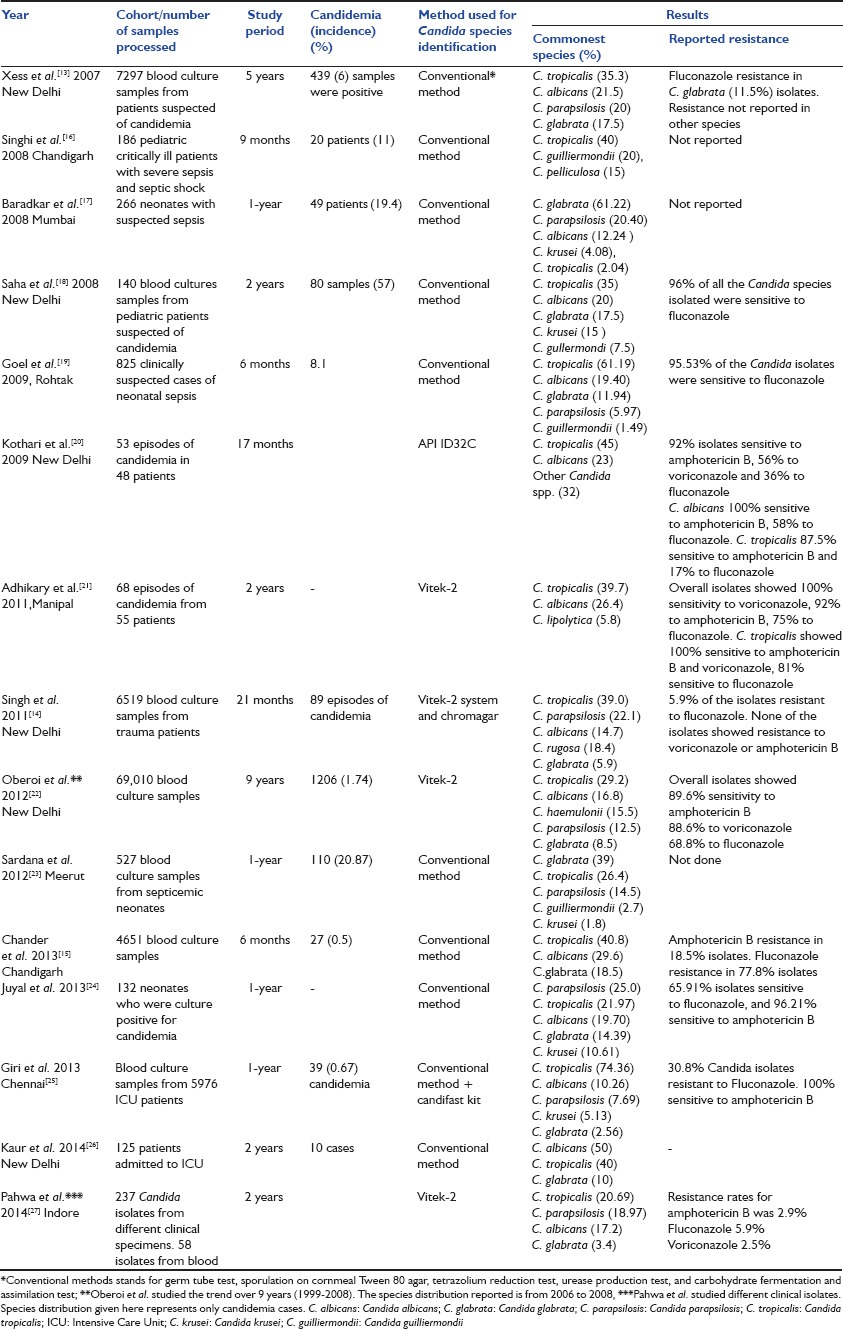
Epidemiological data regarding Indian population is scarce. Candidemia has been more extensively studied in Indian pediatric population as compared to adult population. Most of the Indian data have been generated by retrospective analysis of microbiological records and largely comprise of short communication, correspondence and letter to editor. The most common species reported in Asia-Pacific region in SENTRY survey is C. albicans that is different from the findings of Indian literature which report C. tropicalis as the most common offending species.
Xess et al. evaluated 7297 samples blood culture samples, of which 465 were positive for Candida.[13] C. tropicalis (35.3%) was the most common reported species followed by C. albicans (21.5%). Singh et al. studied 6519 samples in trauma patients and found 89 to be positive for Candida.[14] They reported C. tropicalis (39.0%) as the most common species followed by C. parapsilosis (22.1%) and Candida rugosa (18.4%). Fluconazole resistance was present in 5.9% isolates. This is in contrast to findings of Chander et al. who reported 77.8% fluconazole resistance in 27 Candida isolates from 4651 blood culture samples.[15]
Although, most of the studies have reported C. tropicalis as the most common species in India [Table 3],[13,14,15,16,17,18,19,20,21,22,23,24,25,26,27] at least two studies on neonatal sepsis have reported C. glabrata as the commonest species.[17,23] These findings are important because C. glabrata may show resistance to amphotericin B and fluconazole, and this may represent beginning of epidemiological shift toward more resistant species.
Table 3.
Epidemiology of candidemia in India from 2007 onward
Differences in Diagnostic Techniques
Most of the epidemiological studies in Indian set up have used conventional methods for species identification. Conventional methods include germ tube test, sporulation on cornmeal Tween 80 agar, tetrazolium reduction test, urease production test, and carbohydrate fermentation and assimilation test. These conventional tests have been replaced by automated systems (Vitek-2, matrix-assisted laser desorption/ionization time of flight mass spectrometry [MALDI-TOF MS]) in developed countries.
Conventional methods are manual, labor intensive, time-consuming and dependent upon the efficiency of the laboratory. Automated systems have better quality control but more costly. In spite of the development of commercial automated systems, correct and rapid identification of fungal species remains an evolving domain. Iriart et al. compared Vitek MS with conventional laboratory identification and Vitek 2.[28] Correct identification was reported as 93.2% by Vitek MS, compared to 94.1% by the conventional method and 88.0% by Vitek-2. Castanheira et al. reported that 53 strains were wrongly identified as Candida famata by various commercial systems (Vitek, Microspan, etc.) during the SENTRY and ARTEMIS surveillance programs.[29] The authors concluded that commercial systems lack accuracy in identification of fungal species except for MALDI-TOF instruments. MALDI-TOF instruments are cost effective, but they require 1 time huge investment though the recurring cost is minimal.
Differences in Antifungal Sensitivity of Various Candida Species
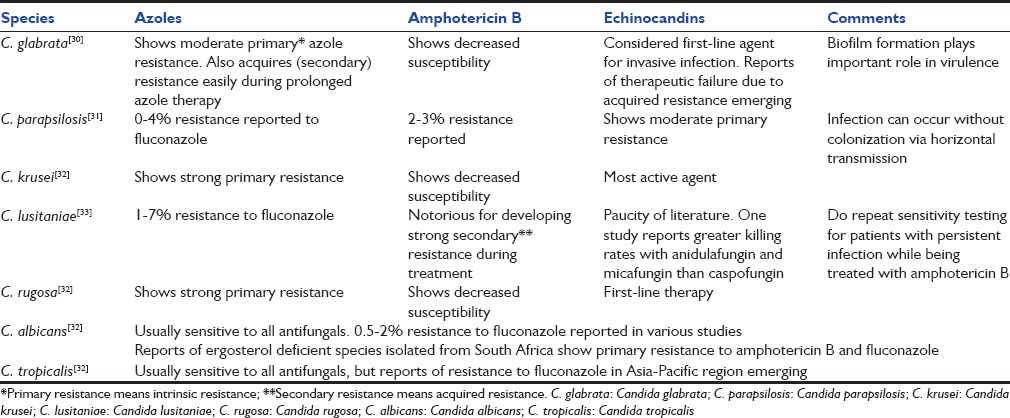
Various Candida species vary in their susceptibility for different antifungal agents [Table 4].[30,31,32,33] As the number of antifungal agents is limited, their irrational use can lead to epidemiological shift toward resistant organisms. During past two decades, there is a rising trend in non albicans Candida (NAC) species all over the world. Many of the NAC species show primary resistance for various antifungal agents. For example, C. glabrata shows primary resistance for azoles and polyenes. C. parapsilosis and Candida guilliermondii exhibit inherently reduced sensitivity to echinocandins while C. rugosa and Candida krusei show resistance for azoles and polyenes. Candida lusitaniae is resistant to amphotericin B and should be treated with fluconazole.
Table 4.
Antifungal resistance pattern among various Candida isolates
The above insights emphasize that prophylactic or empirical therapies require smart guess based on knowledge of risk factors, epidemiological data and resistance patterns of common species.
Differences in Economic Conditions
Economic factor is a crucial determinant of diagnostic and therapeutic approach for IFD.
Nonculture based techniques like BDG assay and polymerase chain reaction are used in many developed countries as adjuncts to blood culture. However, high cost limits their role in areas of poor resources.
Similarly, economic factor also plays role in deciding the choice of antifungal therapy. By and large echinocandins are preferred drugs for hemodynamically unstable patients but the cost of the therapy is high. Amphotericin B deoxycholate remains a reasonable option in such patients. Therefore, local bodies should look into all such considerations before formulating policies in their areas.
Differences in Prescription Practices
Despite the complexity in diagnosis and treatment of fungal infections, there is limited awareness among the clinicians. For example, central line removal within 48 h of diagnosis of candidemia and eye examination by a skilled physician are standard recommendations for all candidemia patients but not always practiced. Disseminated candidiasis may manifest only with ocular findings and may result in blindness.
An electronic survey conducted on antifungal prescription practice in United Kingdom published in 2011 showed that 57.7% of ICU units had no documented policy on the use of antifungal agents. About 85% units used empirical antifungal therapy. Presence of multiple risk factors in combination was the most common trigger for starting antifungal therapy. Multifocal colonization triggered antifungal therapy even when present alone. Fluconazole was the most commonly used antifungal agent for empirical therapy, as well as proven candidemia. Central line removal was practiced by 73.5% and ophthalmology consultation was taken by 15.1% practitioners.[34]
Swoboda et al. showed that implementation of standard practice antifungal guidelines was associated with 50% reduction in cost and a significant decrease in antifungal prescription.[35]
To the best of our knowledge, there is no published literature on antifungal prescription practice in Indian set up. In India, with limited resources and limited patient finances it is extremely important to justify the use of antifungal prescription.
Conclusion
Antifungal prescription practice needs to be streamlined, more so in developing countries like India. Epidemiological studies on colonization and candidemia with automated method of species detection are urgently needed. We need to formulate and execute region-specific and cohort-specific guidelines after collection of epidemiological data. Bed side risk prediction models/scores should be used for choosing the right patient for prophylaxis or empirical therapy. This practice will help to bring down indiscriminate use of antifungal agents.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Rentz AM, Halpern MT, Bowden R. The impact of candidemia on length of hospital stay, outcome, and overall cost of illness. Clin Infect Dis. 1998;27:781–88. doi: 10.1086/514955. [DOI] [PubMed] [Google Scholar]
- 2.Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida blood stream infection until positive blood culture results are obtained: A potential risk factor of hospital mortality. Antimicrob Agents Chemother. 2005;49:3640–5. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clancy CJ, Nguyen M H. Finding the “Missing 50%” of Invasive Candidiasis: How Nonculture Diagnostics Will Improve Understanding of Disease Spectrum and Transform Patient Care. Clin Infect Dis. 2013;56:1284–92. doi: 10.1093/cid/cit006. [DOI] [PubMed] [Google Scholar]
- 4.Muskett H, Shahin J, Eyres G, Harvey S, Rowan K, Harrison D. Risk factors for invasive fungal disease in critically ill adult patients: A systematic review. Crit Care. 2011:15. doi: 10.1186/cc10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmadi A, Ardehali SH, Beigmohammadi MT, Hajiabdolbaghi M, Hashemian SM, Kouchek M, et al. Invasive candidiasis in intensive care unit; consensus statement from an Iranian panel of experts, July 2013. JRSM Open. 2014;26:5. doi: 10.1177/2042533313517689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison D, Muskett H, Harvey S, Grieve R, Shahin J, Patel K, et al. Development and validation of a risk model for identification of non-neutropenic, critically ill adult patients at high risk of invasive Candida infection: The Fungal Infection Risk Evaluation (FIRE) Study. Health Technol Assess. 2013;17:1–156. doi: 10.3310/hta17030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, et al. Infectious Diseases Society of America. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bow EJ, Evans G, Fuller J, Laverdière M, Rotstein C, Rennie R, et al. Canadian clinical practice guidelines for invasive candidiasis in adults. The Canadian Journal of Infectious Diseases and Medical Microbiology. 2010;21:122–150. doi: 10.1155/2010/357076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limper AH, Knox KS, Sarosi GA, Ampel NM, Bennett JE, Catanzaro A, et al. An Official American Thoracic Society Statement: Treatment of Fungal Infections in Adult Pulmonary and Critical Care Patients. Am J Respir Crit Care Med. 2011;183:96–128. doi: 10.1164/rccm.2008-740ST. [DOI] [PubMed] [Google Scholar]
- 10.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin Microbiol Infect. 2012;18(Suppl 7):19–37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 11.Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: Report from the SENTRY Antimicrobial Surveillance Program (2008 to 2009) J Clin Microbiol. 2011;49:396–9. doi: 10.1128/JCM.01398-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montagna MT, Lovero G, Borghi E, Amato G, Andreoni S, Campion L, et al. Candidemia in intensive care unit: A nationwide prospective observational survey (GISIA-3 study) and review of the European literature from 2000 through 2013. Eur Rev Med Pharmacol Science. 2014;18:661–74. [PubMed] [Google Scholar]
- 13.Xess I, Jain N, Hasan F, Mandal P, Banerjee U. Epidemiology of candidemia in a tertiary care centre of north India: 5-year study. Infection. 2007;35:256–9. doi: 10.1007/s15010-007-6144-6. [DOI] [PubMed] [Google Scholar]
- 14.Singh RI, Xess I, Mathur P, Behera B, Gupta B, Misra MC. Epidemiology of candidaemia in critically ill trauma patients: Experiences of a level I trauma centre in North India. J Med Microbiol. 2011;60:342–8. doi: 10.1099/jmm.0.023739-0. [DOI] [PubMed] [Google Scholar]
- 15.Chander J, Singla N, Sidhu SK, Gombar S. Epidemiology of Candida blood stream infections: Experience of a tertiary care centre in North India. J Infect Dev Ctries. 2013;16;7:670–5. doi: 10.3855/jidc.2623. [DOI] [PubMed] [Google Scholar]
- 16.Singhi S, Rao DS, Chakrabarti A. Candida colonization and candidemia in a pediatric intensive care unit. Pediatr Crit Care Med. 2008;9:91–5. doi: 10.1097/01.PCC.0000298643.48547.83. [DOI] [PubMed] [Google Scholar]
- 17.Baradkar VP, Mathur M, Kumar S, Rathi M. Candida glabrata emerging pathogen in neonatal sepsis. Ann Trop Med Pub Health. 2008;1:5–8. [Google Scholar]
- 18.Saha R, Das Das S, Kumar A, Kaur IR. Pattern of Candida isolates in hospitalized children. Indian J Pediatr. 2008;75:858–60. doi: 10.1007/s12098-008-0159-6. [DOI] [PubMed] [Google Scholar]
- 19.Goel N, Ranjan PK, Aggarwal R, Chaudhary U, Sanjeev N. Emergence of non albicans Candida in neonatal septicemia and antifungal susceptibility: Experience from a tertiary care center. J Lab Physicians. 2009;1:53–5. doi: 10.4103/0974-2727.59699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kothari A, Sagar V. Epidemiology of Candida blood stream infections in a tertiary care institute in India. Indian J Med Microbiol. 2009;27:171–172. doi: 10.4103/0255-0857.49440. [DOI] [PubMed] [Google Scholar]
- 21.Adhikary R, Joshi S. Species distribution and anti-fungal susceptibility of Candidaemia at a multi super-specialty center in Southern India. Indian J Med Microbiol. 2001;29:309–11. doi: 10.4103/0255-0857.83920. [DOI] [PubMed] [Google Scholar]
- 22.Oberoi JK, Wattal C, Goel N, Raveendran R, Datta S, Prasad K. Non-albicans Candida species in blood stream infections in a tertiary care hospital at New Delhi, India. Indian J Med Res. 2012;136:997–1003. [PMC free article] [PubMed] [Google Scholar]
- 23.Sardana V, Pandey A, Madan M, Goel SP, Asthana AK. Neonatal candidemia: A changing trend. Indian J Pathol Microbiol. 2012;55:132–3. doi: 10.4103/0377-4929.94900. [DOI] [PubMed] [Google Scholar]
- 24.Juyal D, Sharma M, Pal S, Rathaur VK, Sharma N. Emergence of non-albicans Candida species in neonatal candidemia. N Am J Med Sci. 2013;5:541–5. doi: 10.4103/1947-2714.118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giri S, Kindo AJ, Kalyani J. Candidemia in intensive care unit patients: A one year study from a tertiary care center in South India. J Postgrad Med. 2013;59:190–5. doi: 10.4103/0022-3859.118036. [DOI] [PubMed] [Google Scholar]
- 26.Kaur R, Goyal R, Dhakad MS, Bhalla P, Kumar R. Epidemiology and Virulence Determinants including Biofilm Profile of Candida Infections in an ICU in a Tertiary Hospital in India. Journal of Mycology 2014. 2014:1. [Google Scholar]
- 27.Pahwa N, Kumar R, Nirkhiwale S, Bandi A. Species distribution and drug susceptibility of candida in clinical isolates from a tertiary care centre at Indore. Indian J Med Microbiol. 2014;32:44–8. doi: 10.4103/0255-0857.124300. [DOI] [PubMed] [Google Scholar]
- 28.Iriart X, Lavergne RA, Fillaux J, Valentin A, Magnaval JF, Berry A, et al. Routine identification of medical fungi by the new Vitek MS matrix-assisted laser desorption ionization-time of flight system with a new time-effective strategy. J Clin Microbiol. 2012;50:2107–10. doi: 10.1128/JCM.06713-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castanheira M, Woosley LN, Diekema DJ, Jones RN, Pfaller MA. Candida guilliermondii and other species of candida misidentified as Candida famata: Assessment by vitek 2, DNA sequencing analysis, and matrix-assisted laser desorption ionization-time of flight mass spectrometry in two global antifungal surveillance programs. J Clin Microbiol. 2013;51:117–24. doi: 10.1128/JCM.01686-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodrigues CF, Silva S, Henriques M. Candida glabrata: A review of its features and resistance. Eur J Clin Microbiol Infect Dis. 2014;33:673–88. doi: 10.1007/s10096-013-2009-3. [DOI] [PubMed] [Google Scholar]
- 31.Trofa D, Gácser A, Nosanchuk JD. Candida parapsilosis, An Emerging Fungal Pathogen. Clin Microbiol Rev. 2008;21:606–25. doi: 10.1128/CMR.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: A persistent public health problem. Clin Microbiol Rev. 2007;20:133–63. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cantón E, Pemán J, Hervás D, Espinel-Ingroff A. Examination of the in vitro fungicidal activity of echinocandins against Candida lusitaniae by time-killing methods. J Antimicrob Chemother. 2013;68:864–8. doi: 10.1093/jac/dks489. [DOI] [PubMed] [Google Scholar]
- 34.Chalmers CM, Bal AM. Management of fungal infections in the intensive care unit: A survey of UK practice. Br. J. Anaesth. 2011;106:827–31. doi: 10.1093/bja/aer089. [DOI] [PubMed] [Google Scholar]
- 35.Swoboda S, Lichtenstern C, Ober MC, Taylor LA, Störzinger D, Michel A, et al. Implementation of practice guidelines for antifungal therapy in a surgical intensive care unit and its impact on use and costs. Chemotherapy. 2009;55:418–24. doi: 10.1159/000264672. [DOI] [PubMed] [Google Scholar]


