Abstract
Though snake antivenom (SAV) is the mainstay of therapy for poisonous snake bites, there is no universally accepted standard regimen regarding the optimum dose (low vs. high). We therefore, undertook this systematic review to address this important research question. We searched all the published literature through the major electronic databases till August 2014. Randomized clinical trials (RCTs) were included. Eligible trials compared low versus high dose SAV in poisonous snake bite. The review has been registered at PROSPERO (Registration number: CRD42014009700). Of 36 citations retrieved, a total of 5 RCTs (n = 473) were included in the final analyses. Three trials were open-label, 4 conducted in Indian sub-continent and 1 in Brazil. The doses of SAV varied in the high dose group from 40 ml to 550 ml, and in the low dose group from 20 ml to 220 ml. There was no significant difference between the two groups for any of the outcomes except duration of hospital stay, which was lower in the low dose group. The GRADE evidence generated was of “very low quality.” Low-dose SAV is equivalent or may be superior to high-dose SAV in management of poisonous snake bite. Low dose is also highly cost-effective as compared to the high dose. But the GRADE evidence generated was of “very low quality” as most were open label trials. Further trials are needed to make definitive recommendations regarding the dose and these should also include children <9 years of age.
Keywords: Clinical trials, evidence, snake anti-venom, snake bite
Introduction
Globally, between 1.2 million and 5.5 million snakebites occur annually leading to as high as 1,84,1000 envenomings and 94,000 deaths.[1] The highest burden exists in South Asia, Southeast Asia, and sub-Saharan Africa.[1] The management of poisonous snake bite includes local wound management, ventilatory support, and administration of snake antivenom (SAV). The polyvalent SAV (effective against neurotoxic and hemotoxic poisons) is the mainstay of therapy, is expensive, and scarce especially in high-risk areas.[2]
Though SAV is the mainstay of therapy, there is no universally accepted standard regimen regarding the optimum dose, frequency of administration, and duration of therapy. Ideally, the dose of SAV should be based on the measurement of serial venom concentrations in patients and determining when free venom concentrations are undetectable, but this is hardly clinically feasible.[3] That's why most recommendations are based on mouse assays, where the lethal dose is estimated to be around 120 mg of cobra venom and 60 mg of krait venom.[4] The amount of venom neutralized by 1 ml of SAV is around 0.6 mg for cobra and 0.45 mg for krait bite. Hence, the total empirical dose of SAV for a fatal cobra and krait bite is 200 ml and 134 ml, respectively.[5] But, this may not be true for human bites, as the exact amount of venom injected by the snake at the time of bite varies as per the species of the snake, efficiency (depends upon the size of snake) and mode (whether one fang or two fangs penetrated the skin, single or repeated bite) of bite.
Different clinical trials have found different mean effective dose of SAV (varying from 47 ml to about 180 ml) required in cases with envenomation.[6,7] However, there is no consensus regarding the mean effective dose (low vs. high) required in such cases. A recent retrospective descriptive analysis from India demonstrated that the low dose SAV regimen may be as efficacious as high dose SAV regimen.[8] We therefore, undertook this systematic review of randomized clinical trials (RCTs) to address this important research question.
Methods
This systematic review has been registered at PROSPERO (Registration number: CRD42014009700).
Types of studies
Randomized clinical trials were included.
Types of participants
Participants were having evidence of envenomation, irrespective of whether the bite was from a viper, cobra, or krait. Commonly, viper bite causes hematotoxicity, cobra bite produces neurotoxicity, and krait bite causes a combination of both. Exclusion criteria were, presentation 24 h after the bite, history of any bleeding diathesis or any other previous neurological abnormality, and manifested allergy to the SAV.
Types of interventions
The intervention commenced after the patient is hospitalized, and consisted of administration of high or low dose of SAV as an adjuvant to standard hospital treatment of snake bite. The trial had to compare high versus low dose of SAV only. All methods of administration of SAV in all grades of envenomation (mild, moderate or severe) were considered.
Types of outcome measures
Outcome measures frequently used to determine the clinical efficacy of SAV are the mortality rate, time to normalization of clotting time (CT) and neurological parameters, proportions of patients developing neurological complications and experiencing any adverse events. Accordingly, trials measuring following outcomes were included in the review.
Primary outcome measure
Mortality rate.
Secondary outcome measures
Time to normalization of CT
Neurological complication rate
Rate of other complications (acute renal failure [ARF], bleeding or disseminated intravascular coagulation [DIC], and shock)
Duration of hospital stay (days)
Adverse-events
Cost-effectiveness.
Definitions
Abnormal hematological parameters or hematotoxicity are said to be present if the patient's bleeding time is >8 min, prothrombin time >16 s or the CT >30 min or if there was abnormal lysis of the clot.[7,8,9,10] Severity of coagulation dysfunction due to systemic envenomation (mild, moderate and severe) was graded according to the degree of CT prolongation (mild: 11–15 min, moderate: 15–30 min and severe: Incoagulable blood, respectively).[11] Neurotoxic features are said to be present when respiratory distress due to weakness of the respiratory muscles, dysarthria, dysphonia, blurring of vision or diplopia occurs. ARF was defined as oliguria for 24 h (urine output below 400 ml) or more with raised blood urea or serum creatinine concentrations.
Search methodology
We performed a systematic search of the published literature. We searched the Medline/PubMed (1970 to August 2014), the CENTRAL Registry of Clinical Trials (Issue 8, August 2014), the Cochrane Injuries Group database (Issue 8, August 2014), http://www.clinicaltrials.gov Google Scholar (till September 2014), and the reference list of identified articles. Following keywords: ([“snake bite” OR “cobra” OR “krait” OR “viper” OR “SAV” OR “antisnake venom”] AND [“envenomation” OR “envenoming”] AND [“child” OR “children” OR “pediatric” OR “paediatric” OR “adult”) AND (“clinical trial” OR “randomized controlled trial”]) were used for retrieval of relevant articles. No language restrictions were applied. Two reviewers reviewed the search results to identify relevant original human clinical trials. After the search, each author was advised to screen the titles and abstracts for eligibility, and to retrieve full text articles. In case of any disagreement, a consensus was reached after discussion with the third author. If the required data were not available, we contacted the authors and tried to resolve discrepancies.
Data extraction
Data extraction was done using a data extraction form that was designed and pilot tested a priori. The two authors independently extracted data including author, year, setting (country, type of population), type of snake bite including the toxic features observed, exposure/intervention (dose of SAV required, schedule followed), results (outcome measures, effect, significance), and sources of funding/support. Any disagreement in the extracted data was resolved through discussion.
Assessment of risk of bias in the included studies
Two review authors independently assessed the methodological quality of the selected trials using methodological quality assessment forms. We undertook quality assessment of the trials using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions.[12] Any disagreements between the two review authors were resolved through discussion. The potential for selection (systematic differences in the comparison groups), performance (systematic difference in the care provided apart from the intervention being evaluated), exclusion (systematic differences in withdrawals from the trial), and detection (systematic differences in outcome assessment) bias was assessed.
Data synthesis
The data from various studies were pooled and expressed as mean difference (MD) with 95% confidence interval (CI) in case of continuous data and risk ratio (RR) with 95% CI in case of categorical data. P < 0.05 was considered significant. Assessment of heterogeneity was done by I-squared statistics. If there was a high level of heterogeneity (>50%), we tried to explore the cause. A fixed effects model was initially conducted. If significant heterogeneity existed between trials, potential sources of heterogeneity were considered and where appropriate a random effects model was used. RevMan (Review Manager) version 5 was used for all the analyses.[12]
Results
Description of studies
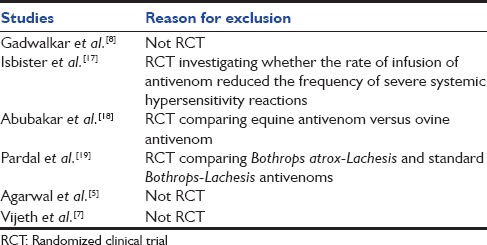
Of 36 citations retrieved, full text of 9 articles was assessed for eligibility [Figure 1]. Of these, a total of 5 RCTs were included [Table 1].[6,13,14,15,16] Five studies were excluded because of the following reasons: Compared two different antivenom preparations (n = 2), not RCTs (n = 2), and compared two different infusion rates (n = 1) [Table 2].[5,7,8,17,18,19] Three of the included studies were open label trials,[13,14,15] four studies were conducted in India,[6,13,14,15] and one in Brazil.[16] Two were double-blinded studies.[6,16] A total of 473 participants including both children and adults were included in the analysis. The age of included children was ≥ 9 years. The doses of SAV varied in the high dose group from 40 ml to 550 ml, and in the low dose group from 20 ml to 220 ml. Only one trial tested the use of both dose schedules in all severity of snake envenomation (mild, moderate, and severe).[13]
Figure 1.
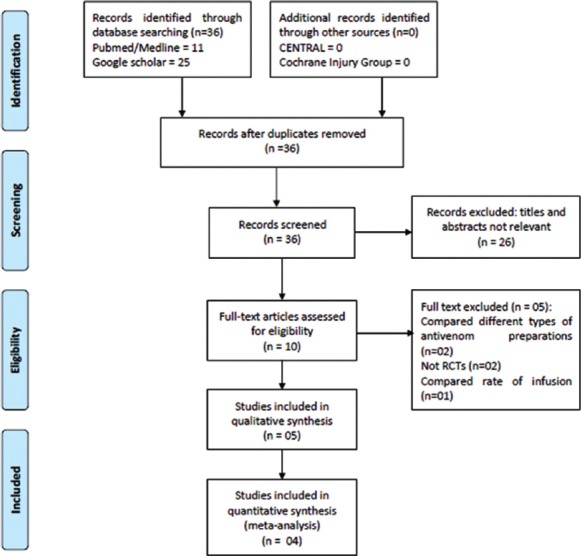
Study flow (PRISMA Flow-chart)
Table 1.
Characteristics of included studies
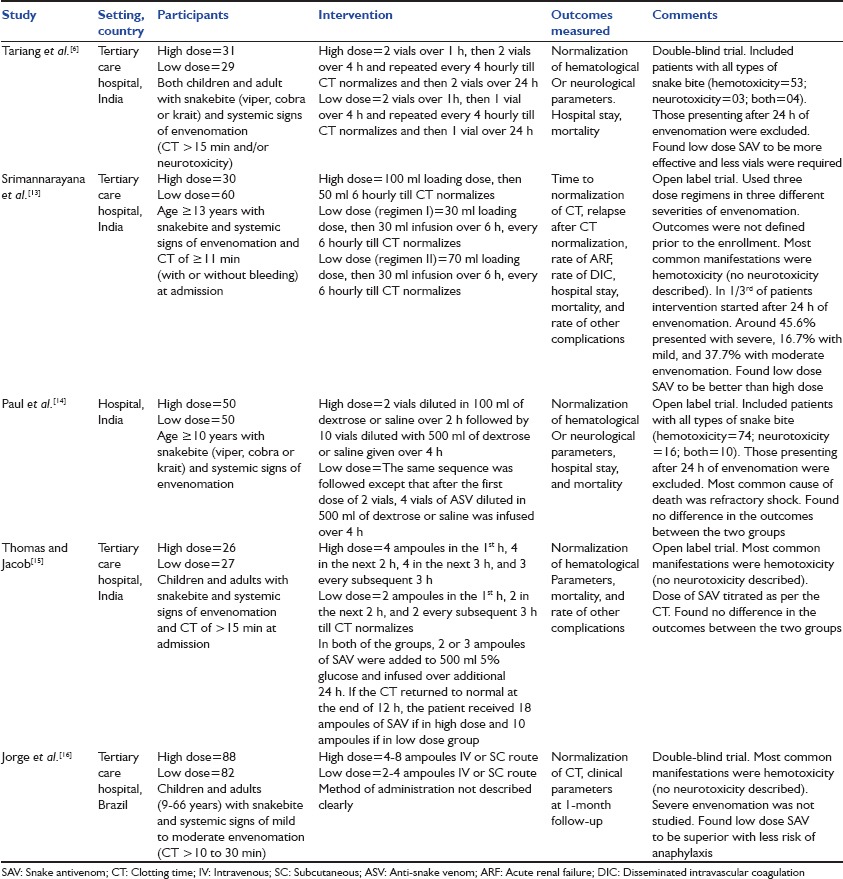
Table 2.
Characteristics of excluded studies
Risk of bias in included studies
The risk of bias in the included trials was of moderate degree as most were open label trials.
Effect of interventions
Primary outcome measure
Mortality rate
This was reported in all the 4 trials. But the result could be pooled from 3 trials, as the fourth one did not find any mortality in either of the groups.[6] The pooled result showed no significant difference in the risk of mortality, with the point estimate favoring the low dose group (RR, 0.69; 95% CI, 0.38–1.26) [Figure 2].
Figure 2.
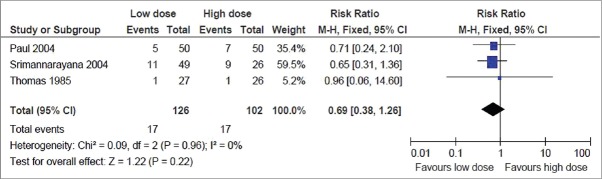
Primary outcome: Mortality rate
Secondary outcome measures
Time to normalization of clotting time
This was reported in two trials.[13,15] In one trial, the mean ± standard deviation (SD) time to normalization of CT was 20.67 ± 9.61 h in high dose group (regimen I), 16.55 ± 9.84 h in low dose (regimen II), and 13.4 ± 7.16 h in low dose (regimen III).[13] There was significant difference between regimen I and III, with the CT getting normalized earlier in regimen I. In another trial, the time for normalization of CT was 10 h 23 min for high dose group and 9 h for low dose group, the difference being nonsignificant.[15]
Neurological complication rate
This was reported in two trials.[6,14] The pooled result showed no significant difference in the neurological complications rate, with the point estimate favoring the low dose group (RR, 0.82; 95% CI, 0.23–2.94) [Figure 3].
Figure 3.
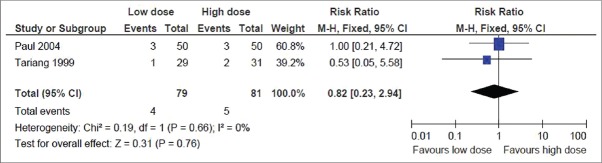
Secondary outcome: Neurological complication rate
Other complications rate
Rate of ARF: This was reported in all the 4 trials. But the result could be pooled from 3 trials, as the fourth-one did not find any ARF in either of the groups.[6] The pooled result showed no significant difference in the rate of ARF, with the point estimate favoring the low dose group (RR, 0.87; 95% CI, 0.62–1.21) [Figure 4]
Bleeding or DIC: This was reported in 4 trials.[6,13,14,16] But the result could be pooled from 2 trials that showed no significant difference in the bleeding or DIC rate,[6,13] with the point estimate favoring the low dose group (RR, 0.77; 95% CI, 0.46–1.29) [Figure 5]. Of the other 2 trials, one did not find any bleeding or DIC in the low dose group,[14] where as in the other largest trial (n = 170), majority showed rapid clinical improvement after treatment with either dose regimen (rapid restoration of blood coagulability and cessation of bleeding)[16]
Shock: This was reported in 2 trials.[14,15] In one trial, 4 out of 50 patients developed refractory shock in the low dose group versus 6 out of 50 in high dose group.[14] In other trial, 1 case of peripheral circulatory failure occurred in the low dose group.[15]
Figure 4.
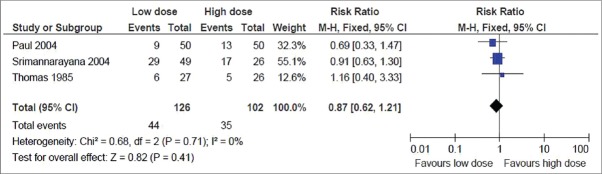
Secondary outcome: Rate of acute renal failure
Figure 5.
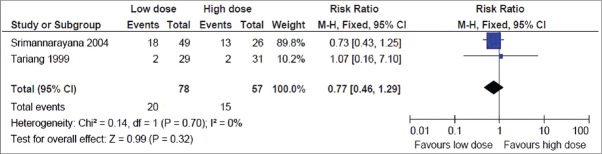
Secondary outcome: Bleeding or disseminated intravascular coagulation rate
Duration of hospital stay (days)
This was reported in 3 trials.[6,13,14] But the result could be pooled from 2 trials, as one trial did not report the SD value.[14] In the later trial, there was no difference in the average hospital stay (days) between the low dose (8.42 days) and high dose group (9.02 days) (P > 0.05). The pooled result from 2 trials showed 1.27 less days of hospital stay in the low dose group compared to the high dose group and the result was statistically significant (MD, −1.27; 95% CI, −2.05 to − 0.5) (P = 0.001) [Figure 6].
Figure 6.
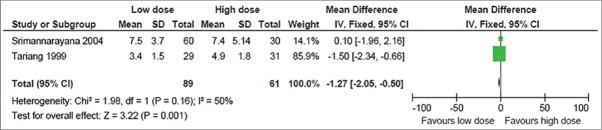
Secondary outcome: Duration of hospital stay
Adverse-events
No major adverse-events were reported in 3 trials that reported the same. In one trial, allergic reactions (skin rash and febrile reactions) occurred overall in both the groups.[6] In the other trial, adverse SAV reactions (itching, urticaria, and erythema) occurred in 8 out of 30 patients in the high dose group and 8 out of 60 patients in the low dose group.[13] Another trial reported that none of the patients were sensitive to antivenom on skin testing, and serum reactions to antivenom were transient and did not necessitate treatment being stopped.[15]
Cost-effectiveness
The MD in the number of SAV vials required between high dose and low dose group among different trials varied from 4.2 to 18 vials. The cost of SAV supplied by Indian antivenom producers to government hospitals as per the recent data are at 115 rupees per vial (US$ 2.50).[20] Hence, by using the low dose schedule, the savings can vary from 500 to 2000 rupees (10–40 US$) per patient excluding the other expenditures (including that for hospitalization, and other therapies).
Publication bias
To assess whether there was a bias in the published literature, funnel plot was constructed using the RR and 1/SE values obtained from studies measuring the primary outcome (mortality rate). In the absence of a publication bias, such a plot is expected to have a shape resembling an inverted funnel.[21] From the funnel plot generated, the possibility of publication bias cannot be ruled out [Figure 7].
Figure 7.
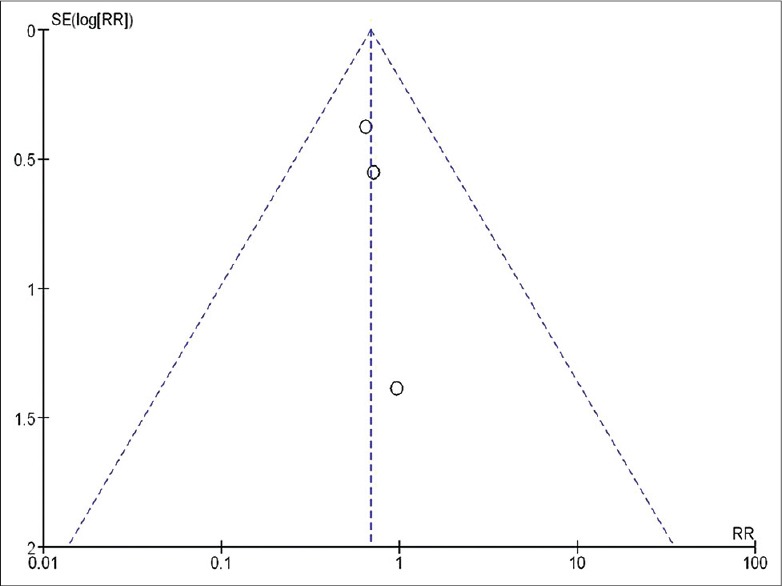
Funnel plot assessing the publication bias
Grade of evidence
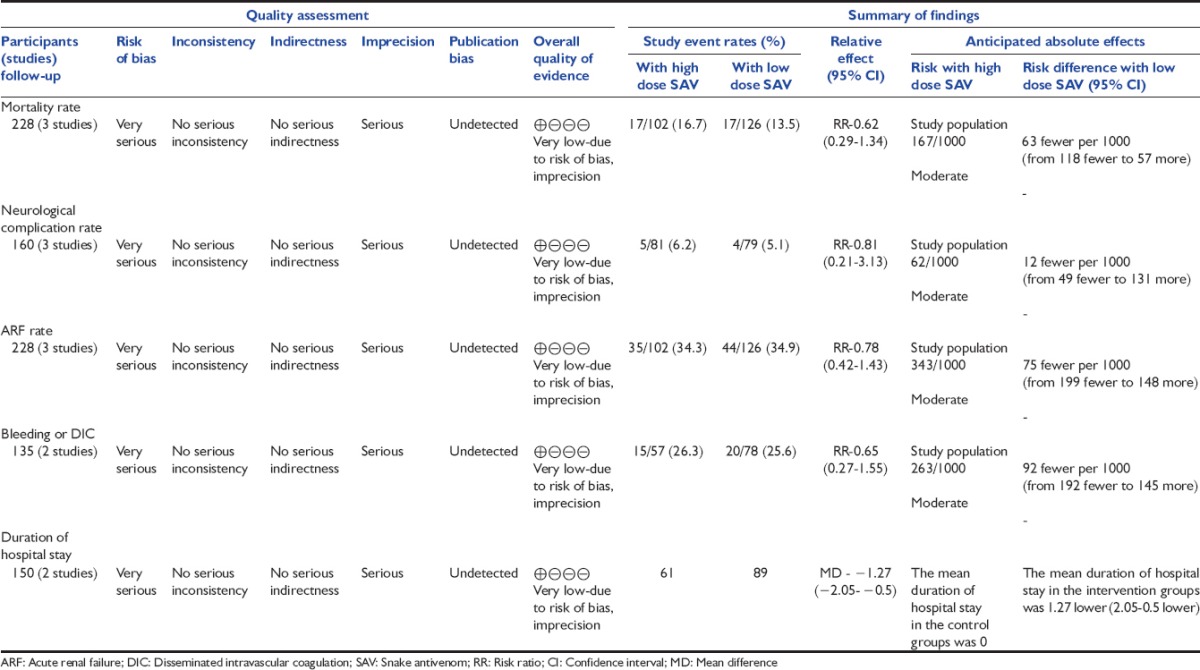
For assessment of the quality of evidence we used GRADE Profiler software (version 3.2).[22] The software uses five parameters for rating the quality of evidence. The parameters used are-limitations to design of randomized controlled trials, inconsistency of results or unexplained heterogeneity, indirectness of evidence, imprecision of results, and publication bias. The rating is done as – no, serious, and very serious limitation. The evidence generated was of “very low quality” for following outcomes: Mortality rate, neurological complication rate, ARF rate, bleeding or DIC, and duration of hospital stay [Table 3]. This was mainly because of most of the trials being open label.
Table 3.
Low dose SAV versus high dose SAV for poisonous snake bite (GRADE evidence)
Discussion
Summary of evidence
After an extensive search of the literature, we could find only 5 trials to be eligible for inclusion in the present systematic review. Our results indicate that there is no difference between the high dose versus low dose SAV for treatment of poisonous snake bite. In fact, use of low dose SAV was associated with a shorter duration of hospital stay. When we constructed the GRADE of evidence from the available evidence, it was found to be of low quality.
The optimal management strategy for poisonous snake bite is still a matter of controversy. SAV has always been the cornerstone of management, but the dose and schedule of administration in a given case varies from place to place. As there was no clear cut guideline regarding the same, the current systematic review was planned to address the same. After undertaking the analyses, we found that there is no difference between the high versus the low dose SAV. Though this might be fascinating, the optimal dose schedule still remains a jigsaw puzzle considering the different “low dose schedule” defined in different trials. Moreover, only one trial compared the effect of both high and low dose schedules in different grades of severity of envenomation.
The type of snake envenomation was also different in all the trials, with hemotoxic manifestations dominating the clinical and laboratory pictures in most of the trials. One study found that hemotoxic manifestations can be reversed by a low dose schedule, but neurotoxic manifestations might require a higher dose to reverse the effect.[6] This is based on the following two facts. First, the neuropathic effects of bound toxin may last for a longer time. Second, the effectiveness of antivenom is based on the contact of antibodies with venom antigens (allowing the formation of antigen-antibody complexes, their precipitation and clearance). The antigens should be present in the same compartment as the antibodies. Most antigens from viper venoms are probably in the vascular compartment and get rapidly complexed by the antivenom. In contrast, the elapid neurotoxins are widely distributed, particularly in the lymphatic system (where the antivenom is not present, if administered intravenously). This could explain the difference observed in the dosages effective to achieve neutralization of the respective venoms. But, another study found that hemotoxic viper envenomation requires a higher dose of SAV than the neurotoxic envenomation.[14] Since the dosing strategy in each study involved repeated administration of SAV until the desired clinical endpoint is reached, one reason for the null results may be that subjects in each group received similar doses of SAV. However, we were not able to calculate the total doses in each group (low and high dose), as the time to achieve the clinical end points were not clear in all the trials. We need more trials in future to address this issue.
Besides measuring the clinical and laboratory outcomes, we also estimated the cost-effectiveness of low dose versus high dose schedule, though the included trials did not provide any data on this. Actually, by using the low dose schedule, the savings can vary from 500 to 2000 rupees (10–40 US$) per patient (excluding that for hospital stay and other therapies). This is important for Asian and African countries, where cost is an issue. Regarding the difference in the rate of adverse events, actually, no trial reported the adverse events separately for both the groups. But all the trials reported minor overall adverse events.
Limitations
Most were open label trials, so the evidence generated was of “very low quality.” We could not take into account the type of envenomation (hemotoxic or neurotoxic) separately as none of the trial reported the outcome separately for both of them. As the venom level was not measured in any of the trials, it was difficult for us to make any suggestion about the dose-response effect. Moreover, the results of a clinical trial are only valid for the antivenom- and venom-considered. Its validity could not be extended to other antivenoms, or for other snake species. Our results may not be applicable to other parts of the world as all the trials were conducted in India. Similarly, as no trial included children <9 years age, it is also difficult to make any recommendation in this age group.
Further area of research
Future trials should report about the type of envenomation and the dose of SAV required reversing the toxic effect of individual type of envenomation. The low dose of SAV varied among all the trials. Hence, the future trials should focus on a single low dose formulation. Simultaneously, they should measure the venom level in blood to corroborate the clinical findings. Finally, future trials should also report the findings from other parts of the world and should include children <9 years of age.
Conclusion
Low-dose SAV is equivalent or may be superior to high dose in management of poisonous snake bite. Low dose is also highly cost-effective as compared to the high dose. But the GRADE evidence generated was of “very low quality” as most were open label trials. Future trials should try to report the findings from other parts of the world and should include children <9 years of age.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, et al. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:e218. doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warrell DA. WHO/SEARO. Guidelines for the Clinical Management of Snake Bites in the Southeast Asian Region. [Last accessed on 2014 Aug 21]. Available from: http://www.searo.who.int/linkfiles/bct_snake_bite_guidelines.pdf .
- 3.Rivière G, Choumet V, Audebert F, Sabouraud A, Debray M, Scherrmann JM, et al. Effect of antivenom on venom pharmacokinetics in experimentally envenomed rabbits: Toward an optimization of antivenom therapy. J Pharmacol Exp Ther. 1997;281:1–8. [PubMed] [Google Scholar]
- 4.Bawaskar HS. Snake venoms and antivenoms: Critical supply issues. J Assoc Physicians India. 2004;52:11–3. [PubMed] [Google Scholar]
- 5.Agarwal R, Aggarwal AN, Gupta D, Behera D, Jindal SK. Low dose of snake antivenom is as effective as high dose in patients with severe neurotoxic snake envenoming. Emerg Med J. 2005;22:397–9. doi: 10.1136/emj.2004.020727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tariang DD, Philip PJ, Alexander G, Macaden S, Jeyaseelan L, Peter JV, et al. Randomized controlled trial on the effective dose of anti-snake venom in cases of snake bite with systemic envenomation. J Assoc Physicians India. 1999;47:369–71. [PubMed] [Google Scholar]
- 7.Vijeth SR, Dutta TK, Shahapurkar J, Sahai A. Dose and frequency of anti-snake venom injection in treatment of Echis carinatus (saw-scaled viper) bite. J Assoc Physicians India. 2000;48:187–91. [PubMed] [Google Scholar]
- 8.Gadwalkar SR, Kumar NS, Kushal DP, Shyamala G, Mohammad MZ, Vishwanatha H. Judicious use of antisnake venom in the present period of scarcity. Indian J Crit Care Med. 2014;18:722–7. doi: 10.4103/0972-5229.144014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yap CH, Ihle BU. Coagulopathy after snake envenomation. Neurology. 2003;61:1788. doi: 10.1212/01.wnl.0000103858.41940.5f. [DOI] [PubMed] [Google Scholar]
- 10.Vijeth SR, Dutta TK, Shahapurkar J. Correlation of renal status with hematologic profile in viperine bite. Am J Trop Med Hyg. 1997;56:168–70. doi: 10.4269/ajtmh.1997.56.168. [DOI] [PubMed] [Google Scholar]
- 11.Reid HA, Chan KE, Thean PC. Prolonged coagulation defect (defibrination syndrome) in Malayan viper bite. Lancet. 1963;1:621–6. doi: 10.1016/s0140-6736(63)91269-8. [DOI] [PubMed] [Google Scholar]
- 12.Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2012. The Nordic Cochrane Centre The Cochrane Collaboration. Review Manager (RevMan). 5.2. [Google Scholar]
- 13.Srimannarayana J, Dutta TK, Sahai A, Badrinath S. Rational use of anti-snake venom (ASV): Trial of various regimens in hemotoxic snake envenomation. J Assoc Physicians India. 2004;52:788–93. [PubMed] [Google Scholar]
- 14.Paul V, Pratibha S, Prahlad KA, Earali J, Francis S, Lewis F. High-dose anti-snake venom versus low-dose anti-snake venom in the treatment of poisonous snake bites – A critical study. J Assoc Physicians India. 2004;52:14–7. [PubMed] [Google Scholar]
- 15.Thomas PP, Jacob J. Randomised trial of antivenom in snake envenomation with prolonged clotting time. Br Med J (Clin Res Ed) 1985;291:177–8. doi: 10.1136/bmj.291.6489.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jorge MT, Cardoso JL, Castro SC, Ribeiro L, França FO, de Almeida ME, et al. A randomized ‘blinded’ comparison of two doses of antivenom in the treatment of Bothrops envenoming in São Paulo, Brazil. Trans R Soc Trop Med Hyg. 1995;89:111–4. doi: 10.1016/0035-9203(95)90678-9. [DOI] [PubMed] [Google Scholar]
- 17.Isbister GK, Shahmy S, Mohamed F, Abeysinghe C, Karunathilake H, Ariaratnam A. A randomised controlled trial of two infusion rates to decrease reactions to antivenom. PLoS One. 2012;7:e38739. doi: 10.1371/journal.pone.0038739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abubakar IS, Abubakar SB, Habib AG, Nasidi A, Durfa N, Yusuf PO, et al. Randomised controlled double-blind non-inferiority trial of two antivenoms for saw-scaled or carpet viper (Echis ocellatus) envenoming in Nigeria. PLoS Negl Trop Dis. 2010;4:e767. doi: 10.1371/journal.pntd.0000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pardal PP, Souza SM, Monteiro MR, Fan HW, Cardoso JL, França FO, et al. Clinical trial of two antivenoms for the treatment of Bothrops and Lachesis bites in the north eastern Amazon region of Brazil. Trans R Soc Trop Med Hyg. 2004;98:28–42. doi: 10.1016/s0035-9203(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 20.Whitaker R, Whitaker S. Venom, antivenom production and the medically important snakes of India. Curr Sci. 2012;103:635–43. [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schünemann H, Brozek J, Oxman A, editors. Jan Brozek, Andrew Oxman, Holger Schünemann; 2008. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendation. Version 3.2. [Google Scholar]


