Abstract
The tsetse fly vector transmits the protozoan Trypanosoma brucei, responsible for Human African Trypanosomiasis, one of the most neglected tropical diseases. Despite a recent decline in new cases, it is still crucial to develop alternative strategies to combat this disease. Here, we review the literature on the factors that influence trypanosome transmission from the fly vector to its vertebrate host (particularly humans). These factors include climate change effects to pathogen and vector development (in particular climate warming), as well as the distribution of host reservoirs. Finally, we present reports on the relationships between insect vector nutrition, immune function, microbiota and infection, to demonstrate how continuing research on the evolving ecology of these complex systems will help improve control strategies. In the future, such studies will be of increasing importance to understand how vector-borne diseases are spread in a changing world.
Introduction
African unicellular protozoa belonging to the genus Trypanosoma are the causative agents of sleeping sickness in humans, where it is known as Human African Trypanosomiasis (HAT), as well as in animals, where it is known as Animal African Trypanosomiasis (also known as Nagana). They infect successively two hosts during their life cycle (thus they are called ‘digenetic' parasites): an insect, the tsetse fly (Glossina spp.) which is required for their transmission to the second host, most often a mammal (and for the transmission from one mammal host to another). After the strictly hematophagous tsetse fly bites a trypanosome-infected mammal and takes an infected blood meal, the ingested trypanosomes reach the fly midgut, where they differentiate from the bloodstream form (short stumpy trypomastigotes—present in the mammal blood) into the early procyclic form (Sbicego et al., 1999). The parasites then differentiate into several forms during their migration from the gut to the salivary glands. This sequential process includes differentiation from the early procyclic form into the late procyclic form (which is established in the gut), followed by the mesocyclic form (a maturation step in the anterior midgut), followed by the proliferating epimastigote form (in the salivary glands or proboscis, depending on the trypanosome species), and finally differentiation into the non-proliferating metacyclic form (in the salivary glands or proboscis, depending on the trypanosome species) (Van den Abbeele et al., 1999). This last form is the only one that is infective for mammals, and is transmitted from the fly's saliva into a subsequent mammal host's bloodstream during ingestion of a new blood meal. If susceptible, this host will possibly become infected and develop sleeping sickness. Thus, in addition to its role as a trypanosome ‘transporter', the tsetse fly is crucial in providing a milieu where the parasite can differentiate, multiply and become infective to mammals. The ability of the fly to acquire the parasite, favor its maturation, and transmit it to a mammalian host is called ‘vector competence', and depends on both the Glossina and trypanosome species, among other factors.
Interestingly, when flies are fed on trypanosome-infected blood under optimal laboratory conditions, less than 50% become infected. This demonstrates that resistance (usually designated as ‘refractoriness') to trypanosome infection is the normal status of the fly. Midgut infection rates rarely exceed 10% in natural fly populations. In addition, many midgut-infected flies do not produce mature parasites, indicating that they will never become infective (Moloo et al., 1986; Dukes et al., 1989; Maudlin and Welburn, 1994).
Four species groups, morsitans, palpalis, austeni and fusca, within tsetse flies are known to transmit different species or subspecies of trypanosomes. The ‘morsitans group' is the major vector for trypanosomes of the subgenera Trypanozoon and Nannomonas, which respectively include Trypanosoma brucei brucei (Tbb) and Trypanosoma congolense (Tc; the savanah type being the most prevalent in cattle); these are the main nagana-causing parasites in sub-Saharan Africa (Nyeko et al., 1990; Reifenberg et al., 1997; Solano et al., 1999). This disease is responsible for dramatic losses in livestock production, estimated at US$ 4.5 billion/year (Reinhardt, 2002). Furthermore, the morsitans group is the vector of Trypanosoma brucei rhodesiense (Tbr), the causative agent of the acute form of HAT that is endemic in 13 east African countries (Welburn et al., 2009). On the other hand, the palpalis group, which poorly transmits Tbb and Tc (Kazadi, 2000), is the vector of Trypanosoma brucei gambiense, which is responsible for the chronic form of HAT in 24 countries of western and central Africa (Hoare, 1972; Kennedy, 2008; Welburn et al., 2009).
HAT develops in two phases. During the first stage (which is hematolymphatic), the parasite proliferates in the blood and the lymph. This may progress into the second stage (which is meningoencephalitic) if trypanosomes cross the blood–brain barrier and subsequently invade the central nervous system. The second stage is usually characterized by severe neurological disorders and is frequently fatal if not treated. The signs and symptoms are generally similar for the acute and chronic form of HAT. They differ however in frequency, severity and kinetic appearance. Acute form usually progresses to death within 6 months. Chronic form has a more progressive course with an average duration of almost 3 years (reviewed in Dumas and Bouteille, 1996 and reviewed in Franco et al., 2014).
HAT is one of the most neglected tropical diseases in the world (Brun et al., 2010), even though in terms of mortality it ranks ninth out of 25 human infectious and parasitic diseases in Africa (Welburn et al., 2009). To this day, sleeping sickness is responsible for major disruptions to social, agricultural and economic development in Africa (Simarro et al., 2011). For instance, the disease was recently estimated to cause the loss of 1.5 million disability-adjusted life years per year (Hotez et al., 2009). As sleeping sickness mostly affects marginalized populations living in isolated rural areas, the disease is a severe burden to rural poor populations that often do not have access to health facilities (Odiit et al., 2004).
The serious nature of this disease has led to its targeting for elimination by the WHO and PATTEC (Pan-African Tsetse and Trypanosomiasis Eradication Campaign), and subsequently by the London Declaration on Neglected Tropical Diseases. The number of new cases has begun to decrease in recent years, mirroring a situation that was observed in the 1960s before the last heavy outbreak in the 1990s. So, in spite of this decrease, the severity of the situation demands a deeper understanding of HAT to improve current treatment approaches, as well as to help design novel strategies to control the disease. These goals are in line with PATTEC and the WHO-fixed objective to eliminate HAT. As sleeping sickness is a vector-borne disease, control strategies can be focused on the patient (by developing preventive and/or curative approaches) and/or on the tsetse fly vector (to eradicate or impede its vector competence). Unfortunately, these approaches are hindered by a lack of vaccines and a limited drug toolbox that produces harmful side effects (Simarro et al., 2008). To complicate matters, current drug treatments have led to the emergence of resistant trypanosome strains (Baker et al., 2013).
Domestic (that is, pigs) and wild animals (that is, diverse rodents, carnivores and primates) found within HAT zones are a valuable nutritional asset for people living in these areas. At the same time, these animals present a risk to humans, since they may harbor different trypanosome species, including those specific to HAT. This unfortunately creates a situation where these animals act as trypanosome reservoirs (Njiokou et al., 2006; Simo et al., 2006; Farikou et al., 2010a), from which their parasites are spread by tsetse flies that feed indifferently on humans or other domestic or wild mammals.
The cyclical transmission of trypanosomes is highly dependent on the biochemical and physiological interactions that occur between the parasite and its insect host. These in turn depend on a variety of biotic and abiotic factors including: climate change; geographical distribution and environmental conditions of HAT foci; tsetse fly population flow between foci; disease epidemiology; type and distribution of trypanosome reservoirs; changes in tsetse fly nutritional behavior; and finally the nature and diversity of fly intestinal microbiota, including symbiotic (Wigglesworthia glossinidia, Sodalis glossinidius and Wolbacchia spp.) and diverse non-symbiotic bacteria (Dale and Maudlin, 1999; Wang et al., 2013a). Investigations of these factors have already begun in the past several years.
By revisiting the existing literature, the present review on microbial ecology aspects of HAT aims to advance research on how changes in environmental conditions can affect trypanosome–tsetse fly–gut microbiota interactions, and consequently, the dynamics of the disease. Successful pursuits in these areas will enable the design of novel strategies for disease control.
Global changes and sleeping sickness transmission
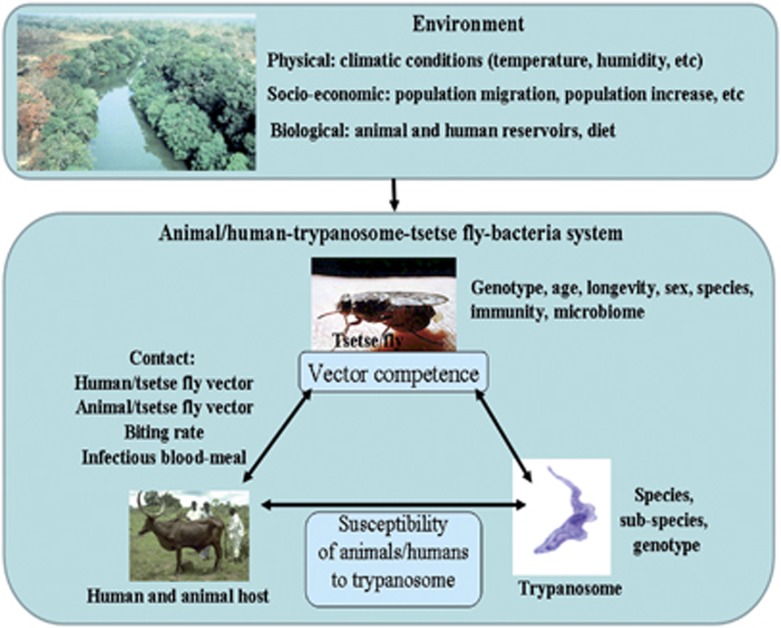
Global climate changes are of particular importance to arthropod-borne diseases (Rogers and Randolph, 2006; Moore et al., 2012). The spread of sleeping sickness is tripartite, involving the trypanosome, the tsetse fly vector (and its symbionts) and the vertebrate hosts. The perpetuation of the parasite itself relies on two connected populations, the adult tsetse flies as well as the mammals from which they take their blood meal. Both the fly vectors and vertebrate hosts require specific climatic conditions (for example, temperature and humidity) for their survival, reproduction and propagation (Dean et al., 1969). The ability of trypanosomes to establish in the midgut, and then to migrate to and mature within the salivary glands, depends on several biotic and abiotic factors. Modification of these factors may affect vector competence, which may then impact trypanosome transmission to host vertebrates and thus the spread of the disease (Figure 1). There is clearly a need for interdisciplinary investigations to determine how global changes (that is, changing temperature, rainfall patterns, increasing urbanization, deforestation, grassland degradation and overgrazing) could affect a variety of factors that include: the geographical distribution of trypanosome vertebrate-host reservoirs; the nutritional behavior of tsetse flies, the development of trypanosome and tsetse flies; and interactions between the tsetse fly vector, the vertebrate hosts and the trypanosome. Detailed studies of these factors would improve our understanding of how the disease is spread in environments affected by socioeconomic, environmental and climatic changes. So, the increasing world population that will, soon, reach seven billion people (Tollefson, 2011) requires an ever-increasing number of livestock and new farmlands to satisfy our nutritional needs. Increasing the number of livestock in farmlands drastically alters the sub-Saharan African environment by modifying not only the livestock distribution, but also the distribution of tsetse flies that feed on it (and possibly their nutritional behavior). Indeed, the importance of environmental factors to transmission intensity and trypanosome distribution is increasingly being recognized (Van den Bossche et al., 2010; Bouyer et al., 2013). Accordingly, a recent study of climate change effects on the evolution of African trypanosomiasis predicts that 46–77 million additional people will be at risk of sleeping sickness by 2090 (Moore et al., 2012).
Figure 1.
Factors that may influence tsetse fly susceptibility for trypanosome infection. For trypanosome transmission to occur, the parasite must first be established in the tsetse fly midgut following an infective blood meal taken from a mammal source acting as a host reservoir of trypanosomes; the trypanosome must then mature in the salivary glands or the mouthparts, depending on the trypanosome species. Many abiotic and biotic factors may affect the success or failure of trypanosome development. The role of these factors in vector competence depends on how they affect trypanosome development in the tsetse fly, and the fly's susceptibility or resistance to trypanosome infection.
Impact of global changes on the developmental rates of trypanosomes and tsetse flies
In vector-borne diseases, temperatures above 34 °C frequently have a negative impact on the survival of both the insect vectors and the parasites (Rueda et al., 1990). Thus, such high temperature may also impact unfavorably tsetse fly and trypanosome populations. Tsetse fly pupation and survival requires favorable environmental conditions, including moderate temperature (23–25 °C), high relative humidity (75–90%) with weak saturation deficit (to avoid high evaporation power) and shade (Ndegwa et al., 1992; Courtin et al., 2010; Pagabeleguem et al., 2012). Nevertheless, higher temperatures induce a more rapid blood meal digestion by the tsetse fly; consequently, the fly may feed more frequently, which can increase both the rate of trypanosome ingestion (when the fly feeds on an infected host) and transmission (when the fly have become trypanosome infected and feeds on a non trypanosome-infected host) (Terblanche et al., 2008). Thus, higher temperatures could have both positive and negative effects on HAT transmission. Finally, there may exist an optimal temperature that would favor an optimal balance between vector and parasite populations development rates and the parasite transmission rate.
Moreover, seasonal alternation, local environmental changes and differences between geographic areas may modify the balance between the different species; this is particularly relevant among wild vertebrates that are fed upon by tsetse flies (Staak et al., 1986; Mukabana et al., 2002; Farikou et al., 2010a). Any modification of tsetse fly nutritional behavior may impact trypanosome transmission, as well as the spread of HAT and Animal African Trypanosomiasis. A fly's nutritional behavior can be determined by identifying the origin of its ingested blood meal and which residues are still present in the gut. Such an investigation was performed in the Bipindi and Campo HAT foci of south Cameroon in 2008, which established that the collected flies had taken their blood meals from humans (46%), pigs (37%) and wild mammals (17%). Notable differences between the two foci were nevertheless recorded: in Bipindi, 23% and 67% of flies took their blood meal from humans and pigs, respectively, whereas these figures were respectively 63% and 23% for flies in Campo. There were also substantial differences observed between years: in 2004, 45% and 7% of flies respectively from Bipindi and Campo took their blood meal from pigs, versus 67% and 23%, respectively, in 2008 (Simo et al., 2008, Farikou et al., 2010a). These results illustrate how the nutritional behavior of tsetse flies depends on the geographical area and how quickly it can change over a relatively short period.
Developmental and immune responses in the trypanosome–tsetse fly association
Trypanosomes undergo several rounds of differentiation and proliferation during their life cycle. Although the development cycle differs somewhat between trypanosome species, the two main stages consist of establishment and maturation steps. Both T. congolense (subgenus Nannomonas) and T. brucei (subgenus Trypanozoon) establish within the fly midgut (midgut colonization step) but mature in the tsetse proboscis and salivary glands, respectively; the development cycle then culminates with the metacyclic form that is infective for mammals (humans, wild or domestic animals) (Van den Abbeele et al., 1999).
As stated in the introduction, the rate of trypanosome midgut colonization in the tsetse fly is generally low (Moloo et al., 1986; Maudlin and Welburn, 1994). During this midgut colonization step, tsetse flies employ mechanisms for eliminating the trypanosomes, whereas the parasites attempt to evade the tsetse fly immune system for their own survival (Aksoy et al., 2003). Several molecules can be released during the time course of these interactions (Table 1), either by tsetse flies or by trypanosomes. This release can be modified according to fly-intrinsic (for example, sex of flies) and fly-extrinsic factors (for example, trypanosome species; starvation). Therefore, successful establishment (that is, midgut colonization) of trypanosomes in tsetse depends on their ability to adapt, transform, survive and grow rapidly after their quick transition from the vertebrate host blood to the different environment of the tsetse gut (Simo et al., 2010).
Table 1. Effects of various intrinsic and extrinsic factors on trypanosome development within the tsetse fly vector.
| Factors | Produced by | Effect | References |
|---|---|---|---|
| Attacin | Tsetse fly | Trypanocidal activity | Hao et al., 2001; Hu and Aksoy, 2006 |
| Diptericin | Tsetse fly | Trypanocidal activity | Hao et al., 2001; Hu and Aksoy, 2006 |
| Glutamin/proline-rich (EP) protein | Tsetse fly | Inhibits trypanosome establishment | Haines et al., 2010 |
| Reactive oxygen species | Tsetse fly | Inhibits trypanosome establishment | Hao et al., 2001; Lehane et al., 2003; Hu and Aksoy, 2006; Nayduch and Aksoy, 2007; MacLeod et al., 2007a |
| Nitrogen monoxide (NO) | Tsetse fly | Promotes trypanosome migration to salivary glands and maturation | MacLeod et al., 2007b |
| L-Cysteine | Tsetse fly | Promotes trypanosome migration to salivary glands and maturation | MacLeod et al., 2007b |
| Purines | Tsetse fly | Promotes trypanosome survival | Henriques et al., 2003 |
| Heat-shock protein 70 Heat-shock protein 83 | Trypanosome | Reaction against stress in fly midgut | Simo et al., 2010 |
| Starvation | Tsetse fly | Increases establishment and/or maturation of trypanosome in tsetse fly and offspring | Kubi et al., 2006 |
Trypanosome invasion activates innate immune responses within tsetse flies (Hao et al., 2001; Hu and Aksoy, 2006) by inducing the tsetse production of several molecules including: antimicrobial peptide; glutamine/proline-rich (EP) protein; reactive oxygen species; and several other molecules involved in the immunodeficiency (Imd) pathway (Hao et al., 2001; Lehane et al., 2003; Hu and Aksoy, 2006; Nayduch and Aksoy, 2007; MacLeod et al., 2007a; Haines et al., 2010). In fact, the balance between the released molecules has an important role in the success or failure of trypanosome establishment within the tsetse fly midgut, as it is crucial for creating a suitable environment for their survival and development. For example, the prevalence of trypanosomes increased in flies when the expression of either the Imd pathway or the downstream-expressed antimicrobial peptide effector was downregulated by RNAi (Hao et al., 2001; Hu and Aksoy, 2006). MacLeod et al. (2007a) have also shown that antioxidants promote the establishment of trypanosome infections in tsetse flies. Subsequently, trypanosomes require signals such as L-cysteine and/or nitric oxide, as well as environmental stimuli, for their migration to the salivary glands, where they mature (MacLeod et al., 2007b).
In the presence of trypanosomes, tsetse flies will modify the expression of several of their genes. In response to this differential gene regulation, trypanosomes regulate the expression of their own genes for their survival (Savage et al., 2012). For example, T. b. gambiense and T. b. rhodesiense express genes associated with reactions against stress (Simo et al., 2010), indicating that trypanosomes are exposed to environmental stress within the tsetse fly midgut. Owing to the delicate equilibrium governing these molecular interactions, any internal or external perturbation may impact the fly–trypanosome relationship. For instance, injecting tsetse flies with E. coli was shown to stimulate their immune system, resulting in a severe blocking of trypanosome establishment subsequent to any infected blood meal (Hao et al., 2001). Therefore, the upregulation of several immune responsive genes early in infection can act to block parasite transmission. These results have previously been discussed in the context of potentially using transgenic approaches to modulate tsetse fly vector competence (Hao et al., 2001). A deficit in mammal blood meal availability, and other environmental factors, can also cause nutritional stress in a tsetse population; in addition to making tsetse flies significantly more susceptible to midgut infection, these factors boost the maturation of midgut infections (Akoda et al., 2009a). These examples illustrate how external factors, which do not depend on the trypanosome or tsetse fly, can modify their association. In this context, environmental changes can impact the biochemistry, physiology and even survival of tsetse flies and trypanosomes. Their interactions will also be affected as a consequence, since both organisms must first adapt their physiology to the modified environmental conditions. Very recent developments in the genomics of the tsetse fly (International Glossina Genome Initiative, 2014) now provide novel ways to further investigate these tsetse–trypanosome interactions through comparative and functional genomics.
Impacts of the tsetse fly microbiome and nutrition on fly physiology and Trypanosoma transmission
Tsetse flies harbor three bacterial symbionts, including the obligate primary (essential) symbiont Wigglesworthia glossinidia (Wang et al., 2013a) and the secondary (non-essential) symbiont Sodalis glossinidius (Dale and Maudlin, 1999). Both symbionts, which belong to the Enterobacteriaceae family, colonize the tsetse fly gut (Aksoy et al., 2013) and are vertically transmitted to the intrauterine-developing larvae via milk gland secretions (Wang et al., 2013a). Wigglesworthia encodes vitamins that may promote host reproduction as well as fly nutrition throughout its development (Nogge, 1982; Rio et al., 2012). In addition to the midgut, Sodalis develops in several other tsetse fly (Glossina spp.) organs (Wang et al., 2013a). The specific elimination of Sodalis has been reported to result in reduced tsetse fly longevity (Dale and Welburn, 2001; Wang et al., 2013b).
Tsetse flies can also harbor a third symbiont, the α-proteobacterium Wolbachia (O'Neill et al., 1993), which is a non-essential bacterium that infects many different invertebrates (Werren et al., 2008). The presence of this bacterium is restricted to the reproductive organs of the tsetse fly and is transmitted transovarially (Wang et al., 2013a). Although it is highly prevalent within laboratory-reared tsetse fly colonies (Cheng et al., 2000), its prevalence in natural tsetse fly populations is variable (Doudoumis et al., 2012). Wolbachia has also been shown to induce strong cytoplasmic incompatibility in tsetse, as by the second gonotrophic cycle, none of the females in an incompatible cross yield any progeny (Alam et al., 2011). This phenomenon occurs when a Wolbachia-infected male mates with an uninfected female resulting in degeneration of the future embryo. In contrast, when a Wolbachia-infected female mates with either an uninfected male or a male infected with the same strain as the female, the female will produce viable Wolbachia-infected offspring. Furthermore, these offspring will be more numerous than those produced by a non Wolbachia-infected female after mating with a non Wolbachia-infected male (Alam et al., 2011). This reproductive advantage for infected females has two implications. First, it results in the spread of Wolbachia infections along with the other traits (Sodalis) that the infected insects might display (Hoffman et al., 1998; Dobson et al., 2002). Second, it produces a progressive replacement of the initial fly population by a population of Wolbachia-infected flies (Alam et al., 2011; Medlock et al., 2013).
Recent investigations of the midgut microbiota composition in natural tsetse fly populations collected from HAT foci in three African countries (Angola, Cameroon and Kenya) have revealed the presence of an unexpectedly diverse bacterial community (more than 10 bacteria species in Glossina fuscipes fuscipes from Kenya (identified using culture-depending and non culture-depending methods), more than 5 bacteria species in G. p. palpalis from Cameroon (using culture-depending methods)) (Geiger et al., 2009, 2011; Lindh and Lehane, 2011). Their diversity was shown to depend on the tsetse species or subspecies, as well as on the geographic origin, although differences in environmental conditions and food supply may also influence the diversity of the harbored bacterial communities. Recently, Aksoy et al. (2014) investigated the different levels and patterns of gut microbial diversity among individuals from tsetse fly populations in Uganda (Glossina morsitans morsitans, G. f. fuscipes and Glossina pallidipes), using multiple approaches such as deep sequencing of the V4 hypervariable region of the 16S rRNA gene, 16S rRNA gene clone libraries, and bacterium-specific quantitative PCR. In contrast to the former, this study revealed an extremely limited microbiota diversity in the investigated flies. The obligate endosymbiont Wigglesworthia was dominant in all samples (>99%), and a wide prevalence of low-density Sodalis infections (<0.05%) was also observed. However, 22% of the samples displayed high Sodalis density colonization; they also carried co-infections with Serratia. The wild fly microbiomes display more bacterial species than insectary-reared flies, where, by now, only one species, a novel one named Serratia glossinae, has been previously identified using a culture-dependent method (Geiger et al., 2010). Finally, bacteria diversity characterized in wild flies was very variable depending on fly species, geographical origin as well as on the different microbiome analysis techniques used. Thus, the need to pursue and extend such investigations.
Colonization of the gut by microbial communities may or may not increase tsetse fly resistance against trypanosome invasion. Underlying mechanisms include competition for nutrients, niche occupation and stimulation of immune responses (Stecher and Hardt, 2011; Brestoff and Artis, 2013; Engel and Moran, 2013; Furusawa et al., 2014). Recent data suggest that Sodalis and Wigglesworthia can modulate trypanosome development (Table 2). Wigglesworthia must be present during the immature larval stages for the adult tsetse fly immune system to develop and function properly (Weiss et al., 2011). The artificial elimination of Wigglesworthia from flies does not only render them sterile, but will also compromise their immune system development. This in turn increases fly susceptibility to gut trypanosome infection; in contrast, flies carrying Wigglesworthia are highly resistant (Wang et al., 2009). Furthermore, comparisons between Wigglesworthia spp. from G. morsitans morsitans and from G. brevipalpis have revealed metabolic variations. These differences involve the chorismate, phenylalanine and folate biosynthetic pathways, which are only present in Wigglesworthia from G. morsitans morsitans. African trypanosomes are auxotrophic for these molecules and salvage them exogenously. This could explain the differences observed in trypanosome susceptibility between these two tsetse species (Rio et al., 2012).
Table 2. Effects of various tsetse fly symbionts on tsetse fly susceptibility to trypanosome infection.
| Factors | Symbiont | Effect | References |
|---|---|---|---|
| Unknown | Wigglesworthia glossinidia | Development of tsetse immune system and resistance to trypanosome infection | Wang et al., 2009; Weiss et al., 2011 |
| Chorismate, phenylalanine, folate | Wigglesworthia glossinidia strain | Increases susceptibility of Glossina morsitans morsitans species to trypanosomes | Rio et al., 2012 |
| Chitinase | Sodalis glossinidius | Increases susceptibility of tsetse to trypanosomes | Welburn et al., 1993 |
| Density | Sodalis glossinidius | Increases susceptibility of tsetse to trypanosomes | Cheng and Aksoy, 1999 |
| Genotypes | Sodalis glossinidius | Associated with tsetse fly infection by different trypanosome species | Geiger et al., 2007 |
| Unknown | Sodalis glossinidius | Effect on fly infection | Farikou et al., 2010b |
| Haplotypes | Sodalis glossinidius | Associated with prevalence of tsetse fly infection | Farikou et al., 2011 |
| Phage | Sodalis glossinidius | Associated with tsetse fly resistance to trypanosome infection | Hamidou Soumana et al., 2014 |
| Unknown | Wolbachia sp. | Inhibits development of trypanosome in tsetse fly | Aksoy et al., 2013 |
Colonization with S. glossinidius has been shown to increase susceptibility for trypanosomes in tsetse flies (Welburn et al., 1993) through a mechanism involving the production of N-acetyl glucosamine (Maudlin and Ellis, 1985; Welburn and Maudlin, 1999). This sugar results from the hydrolysis of pupae chitin, by an endochitinase from S. glossinidius. Furthermore, this sugar is reported to inhibit a midgut lectin from the tsetse fly, which is lethal to trypanosome procyclic forms (Welburn and Maudlin, 1999; Dale and Welburn, 2001). More recently, it was reported that the ability of two different trypanosome subspecies to establish in the tsetse fly midgut is significantly linked to the presence of S. glossinidius-specific genotypes (Geiger et al., 2007). This suggests that different Sodalis genotypes might be associated with differing capacities for trypanosome-establishment facilitation. In addition, susceptibility may increase in response to a greater density of the symbiont in the fly gut (Cheng and Aksoy, 1999). The favorable effect of Sodalis on fly infection by trypanosomes has recently been assessed in large tsetse fly sampling campaigns conducted in two sleeping sickness foci in southern Cameroon (Farikou et al., 2010b). Finally, additional diversity analyses have shown that the geographical isolation of the two foci may have induced the independent evolution of Sodalis and tsetse fly populations, suggesting a probable coevolution between Sodalis and tsetse flies (Farikou et al., 2011). Taken together, these data could help explain reported epidemiological differences in HAT cases between HAT foci. Recently, the transcriptional signature of Sodalis hosted by trypanosome-infected flies was compared with that of Sodalis hosted by refractory flies (that is, flies that were not infected despite having taken a trypanosome-infected blood meal). Many of the modulated transcripts in the symbiont population within flies refractory to trypanosome infection cluster within networks involving lysozyme activity, bacteriolytic enzymes, bacterial cytolysis and cell wall macromolecule catabolic processes. These observations suggest the possible involvement of a Sodalis-hosted prophage in tsetse Trypanosoma resistance (Hamidou Soumana et al., 2014).
Several other mechanisms may be involved in the modulation of trypanosome infection by midgut microbiota including the production of anti-parasitic molecules by the bacteria inhabiting the tsetse fly vector gut (reviewed in Azambuja et al., 2005). For example, pigment-producing bacteria have already been identified in the tsetse fly midgut (Geiger et al., 2009, 2010, 2011; Lindh and Lehane, 2011) and the prodigiosin pigment is reported to be toxic to Plasmodium falciparum (Lazaro et al., 2002) and Trypanosoma cruzi (Azambuja et al., 2004).
Nutrition also affects the susceptibility of tsetse flies to trypanosomal infections, since extreme starvation periods in teneral (young flies that have never taken a blood meal) and non-teneral tsetse flies can increase the proportion of adult flies, and their offspring, that will develop mature trypanosome infections that can be transmitted to humans (Kubi et al. 2006; Akoda et al., 2009b). Previous studies have suggested that immune function is affected by the nutritional state of the fly, as well (Attardo et al., 2006). Bacterial populations may also vary in persistence, abundance and species composition within the tsetse fly host; the host environment and nutrition are major determinants in this case (Chandler et al., 2011). This underscores the importance of defining the relationships between diet and the composition and function of gut microbiota (Ponton et al., 2011, 2013). For example, blood meals have been associated with massive proliferation of bacteria residing in the digestive tract (Kumar et al., 2010; Oliveira et al., 2011; Wang et al., 2011) through the effects of reactive oxygen species levels (Oliveira et al., 2011). This process has been demonstrated in the mosquito midgut, where a blood meal immediately decreases the level of reactive oxygen species through a mechanism involving heme-mediated protein kinase C activation, creating a favorable environment for bacterial proliferation (Oliveira et al., 2011).
As a food source, blood contains a number of components that can interfere with insect physiology (Luckhart and Riehle, 2007; Kang et al., 2008; Pakpour et al., 2013), such that the quality of ingested blood can be just as important as the quantity (Broderick et al., 2004; Chandler et al., 2011). Preference for tsetse fly mammalian hosts (human, wild or domestic animals) can differ greatly according to Glossina species, wild life and geographical locations (Omolo et al., 2009; Farikou et al., 2010a; Muturi et al., 2011). Due to the difference in blood composition between different mammalian hosts (human, wild or domestic animals), it can be expected that blood meals taken from different host types will differentially influence gut microbiota composition in tsetse flies, which might explain some of the geographical variation previously observed (Geiger et al., 2009, 2011). In addition, flies may ingest bacteria within the environment, particularly from the skin surface of hosts during blood meals (Poinar et al., 1979; Simo et al., 2008, Farikou et al., 2010a). Blood composition and sources may therefore be important factors that modulate vector competence, through complex interactions between nutrients, immunity and bacterial communities.
Implications for large-scale tsetse fly control
Current strategies for insect pest control management include the application of chemical insecticides, the dissemination of sterile male insects and the introduction of natural predators (including lady beetles) or parasites (including parasitic wasps) (Engel and Moran, 2013). Environmental factors influence microbial interactions and the resilience of a community, which is in turn influenced by microbial diversity (Masurekar, 2008). These factors will then influence vector competence. Previous insight on the interactions between tsetse flies, trypanosomes, microbiota and the environment can also be used to improve the control of tsetse flies, by providing clues on how to manipulate tsetse fly gut microorganisms. This approach may also be of practical value for generating novel modes of pest biocontrol. A number of bacteria-based approaches have also been suggested, some of which have been successfully implemented (Aksoy et al., 2008; Engel and Moran, 2013).
One recent review (Engel and Moran, 2013) has reported that the composition of the gut microbiota in invertebrate hosts could influence vector competence via different approaches including the modulation of immune responses, niche competition or production of inhibitory molecules (Azambuja et al., 2005; Dong et al., 2009; Hoffmann et al., 2011; Cirimotich et al., 2011a, 2011b). Therefore, it is likely that induced modifications of the gut microbiota composition could impact the vector competence of flies. As mentioned above, natural tsetse flies are colonized by a taxonomically diverse array of microbiota (Geiger et al., 2009, 2011; Aksoy et al., 2014).
One pest control method in particular, paratransgenesis (Aksoy et al., 2008), uses modified symbionts to express molecules that could increase tsetse fly resistance to trypanosomes, by stopping the development of parasites. This method is suggested instead of fly transgenesis, since tsetse flies are viviparous (Attardo et al., 2006): their embryos and larvae develop in utero, rendering microinjection of transgenes into the embryo very difficult. The symbiont Sodalis may be used for paratransgenesis (Medlock et al., 2013), as it is cultivable and thus suitable for genetic manipulation in vitro (Aksoy et al., 2008). Wigglesworthia, by contrast, cannot be used as it is uncultivable. Importantly, the Sodalis symbiont inhabits the tsetse gut in immediate proximity with trypanosomes, thereby directly exposing them to Sodalis effector proteins (De Vooght et al., 2012). Sodalis is vertically transmitted to tsetse offspring and can thus transmit the manipulated character from one generation to the next. Finally, the Sodalis genome is rich in pseudogenes, making it susceptible to large-scale gene erosion (Toh et al., 2006). Due to its reduced functional genome, Sodalis is metabolically dependent on tsetse flies for survival; it has never been found associated with other insects. This makes Sodalis a potentially safe candidate for a paratransgenesis approach. In practice, tsetse flies harboring a recombinant Sodalis strain encoding trypanosome resistance genes must be disseminated into natural fly populations, so as to replace the current susceptible population (Alam et al., 2011). Wolbachia-induced cytoplasmic incompatibility can be exploited to drive this rapid dissemination within natural populations of tsetse fly vectors and progressively replace it, thereby allowing disease control (Alam et al., 2011).
In addition to its role in the Sodalis/Wolbachia couple (dissemination of flies harboring the recombinant Sodalis), Wolbachia may be used alone. The embryonic death caused by Wolbachia-induced cytoplasmic incompatibility can be applied to suppress tsetse fly populations (Alam et al., 2011; Aksoy et al., 2013). Another advantage of the Wolbachia symbiont is its capability to inhibit the development of trypanosomes in tsetse (Table 2) (Aksoy et al., 2013).
One alternative to paratransgenesis could be to increase the prevalence of gut bacteria that are naturally present in tsetse flies and that are capable of reducing the trypanosome load in tsetse fly natural populations.
Finally, several examples of the effect of insect gut bacteria on the fitness or sexual competitiveness of insect vectors have been reported. One promising approach has been observed by feeding the tephritid fly Ceratitis capitata with a specific bacterial diet that can improve the fitness and sexual competitiveness of γ-irradiated sterile male insects (Gavriel et al., 2011; Engel and Moran, 2013). Similar experiments could be conducted on γ-irradiated tsetse flies, an approach used to eradicate isolated tsetse populations (Vreysen et al., 2000), since irradiation may modify their intestinal bacterial community content and so decrease their fitness and sexual competitiveness.
Concluding remarks and future perspectives
In spite of the recent decline in cases, the possibility of an expansion of HAT and other diseases must not be underestimated, as reflected by the recent outbreak of Ebola virus disease in western Africa. Today, a broad range of data are available in disease fields concerned with sleeping sickness. However, these data are dispersed and one major difficulty is to assemble them into an integrated and dynamic overview that depicts the events during disease development, as well as guidelines to detect the factors that control them. Globally, the disease develops in two distinct phases: one within the insect host, the tsetse fly, another in the mammal host, including humans. These two phases are closely interconnected by the exchange of the trypanosome between tsetse flies and the mammal hosts. The interruption of this active and necessary ‘exchange' step could lead to parasite elimination, and consequently the disappearance of the disease from the mammal hosts. Tsetse flies clearly have a central role in parasite exchange, due to the intimate link between their feeding behavior and disease transmission. Thus, the primary focus of interrupting parasite exchange should be placed on anti-vector strategies. These could include eradicating flies or blocking their vector capabilities, as well as: research on the ecological/climatic/nutritional conditions favoring fly population development and spread; trypanosome survival; and fly infection processes. Some of these approaches could be used in the future to determine possible geographic areas, and the corresponding human populations at risk of sleeping sickness. Exceptional recent progress in genomics, transcriptomics, proteomics and biotechnology provides hope for characterizing the factors governing the tripartite interaction between the fly, its microbiota and the parasite, which must then be confirmed by functional analyses. At the same time, progress in diverse biotechnologies may open novel uses for practical application based on former findings. The collection of environmental data and analyses of its effects on disease development and propagation will similarly benefit from further studies. We have provided several points for consideration in Box 1, which may yield success with these approaches.
Box 1. Further issues to develop.
Some proposals we provide have immediate practical applications, whereas others will first require fundamental investigations.
Realize an exhaustive cartography of the HAT foci, especially in isolated bush areas.
Evaluate the asymptomatic HAT prevalence, and the expansion of this disease.
Develop dispensaries in isolated areas.
Generate a ‘sentinel' network.
Develop multidisciplinary investigations to improve our understanding of the environmental and human factors that favor the maintaining of the endemic stage of the disease, or epidemic outbreaks.
On the basis of (5), develop predictive approaches to identify areas potentially at risk of sleeping disease following environmental modifications.
More extensive characterization of the composition of tsetse fly microbiota.
Develop integrated investigations of the interactions between the fly, its microbiome and the trypanosome, to identify genes involved in susceptibility/refractoriness of the fly to trypanosome infection.
Investigate the vertical and horizontal transmission of the bacteria species hosted by the female fly.
Characterize the genetic diversity within populations of the different fly species, as well as their indigenous symbiont Sodalis glossinidius populations, to detect a possible relationship between tsetse fly and Sodalis genotypes.
Evaluate the feasibility of using genetically engineered bacteria that would express trypanocidal molecules in the fly's gut or molecules capable of blocking the trypanosome development cycle, to block the fly's vector competence.
Acknowledgments
We thank the Institut de Recherche pour le Développement, the University of Dschang, as well as the School of Biological Sciences, the Charles Perkins Centre and the University of Sydney for their support.
The authors declare no conflict of interest.
References
- Akoda K, Van den Bossche P, Lyaruu EA, De Deken R, Marcotty T, Coosemans M, et al. Maturation of a Trypanosoma brucei infection to the infectious metacyclic stage is enhanced in nutritionally stressed tsetse flies. J Med Entomol. 2009;46:1446–1449. doi: 10.1603/033.046.0629. [DOI] [PubMed] [Google Scholar]
- Akoda K, Van den Bossche P, Marcotty T, Kubi C, Coosemans M, De Deken R, et al. Nutritional stress affects the tsetse fly's immune gene expression. Med Vet Entomol. 2009;23:195–201. doi: 10.1111/j.1365-2915.2009.00799.x. [DOI] [PubMed] [Google Scholar]
- Aksoy E, Telleria EL, Echodu R, Wu Y, Okedi LM, Weiss BL, et al. Analysis of multiple tsetse fly populations in Uganda reveals limited diversity and species-specific gut microbiota. Appl Environ Microbiol. 2014;80:4301–4312. doi: 10.1128/AEM.00079-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy S, Caccone A, Galvani AP, Okedi LM. Glossina fuscipes populations provide insights for human African trypanosomiasis transmission in Uganda. Trends Parasitol. 2013;29:394–406. doi: 10.1016/j.pt.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy S, Gibson WC, Lehane MJ. Interactions between tsetse and trypanosomes with implications for the control of trypanosomiasis. Adv Parasitol. 2003;53:1–83. doi: 10.1016/s0065-308x(03)53002-0. [DOI] [PubMed] [Google Scholar]
- Aksoy S, Weiss B, Attardo G. Paratransgenesis applied for control of tsetse transmitted sleeping sickness. Adv Exp Med Biol. 2008;627:35–48. doi: 10.1007/978-0-387-78225-6_3. [DOI] [PubMed] [Google Scholar]
- Alam U, Medlock J, Brelsfoard C, Pais R, Lohs C, Balmand S, et al. Wolbachia symbiont infections induce strong cytoplasmic incompatibility in the tsetse fly Glossina morsitans. PLoS Pathog. 2011;7:e1002415. doi: 10.1371/journal.ppat.1002415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardo GM, Strickler-Dinglasan P, Perkin SA, Caler E, Bonaldo MF, Soares MB, et al. Analysis of fat body transcriptome from the adult tsetse fly, Glossina morsitans morsitans. Insect Mol Biol. 2006;15:411–424. doi: 10.1111/j.1365-2583.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- Azambuja P, Feder D, Garcia ES. Isolation of Serratia Marcescens in the midgut of Rhodnius prolixus: impact on the establishment of the parasite Trypanosoma cruzi in the vector. Exp Parasitol. 2004;107:89–96. doi: 10.1016/j.exppara.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Azambuja P, Garcia ES, Ratcliffe NA. Gut microbiota and parasite transmission by insect vectors. Trends Parasitol. 2005;21:568–572. doi: 10.1016/j.pt.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Baker N, de Koning HP, Mäser P, Horn D. Drug resistance in African trypanosomiasis: the melarsoprol and pentamidine story. Trends Parasitol. 2013;29:110–118. doi: 10.1016/j.pt.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer J, Koné N, Bengaly Z. Dynamics of tsetse natural infection rates in the Mouhoun river, Burkina Faso, in relation with environmental factors. Front Cell Infect Microbiol. 2013;3:47. doi: 10.3389/fcimb.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol. 2013;14:676–684. doi: 10.1038/ni.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick NA, Raffa KF, Goodman RM, Handelsman J. Census of the bacterial community of the gypsy moth larval midgut by using culturing and culture-independent methods. App Environ Microbiol. 2004;70:293–300. doi: 10.1128/AEM.70.1.293-300.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun R, Blum J, Chappuis F, Burri C. Human African trypanosomiasis. Lancet. 2010;375:148–159. doi: 10.1016/S0140-6736(09)60829-1. [DOI] [PubMed] [Google Scholar]
- Chandler JA, Lang JM, Bhatnagar S, Eisen JA, Kopp A. Bacterial communities of diverse Drosophila species: ecological context of a host-microbe model system. PLoS Genet. 2011;7:e1002272. doi: 10.1371/journal.pgen.1002272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Aksoy S. Tissue tropism, transmission and expression of foreign genes in vivo in midgut symbionts of tsetse flies. Insect Mol Biol. 1999;8:125–132. doi: 10.1046/j.1365-2583.1999.810125.x. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Ruel TD, Zhou W, Moloo SK, Majiwa P, O'Neill SL, et al. Tissue distribution and prevalence of Wolbachia infections in tsetse flies, Glossina spp. Med Vet Entomol. 2000;14:44–50. doi: 10.1046/j.1365-2915.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- Cirimotich CM, Dong Y, Clayton AM, Sandiford SL, Souza-Neto JA, Mulenga M, et al. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science. 2011;332:855–858. doi: 10.1126/science.1201618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirimotich CM, Ramirez JL, Dimopoulos G. Native microbiota shape insect vector competence for human pathogens. Cell Host Microbe. 2011;10:307–310. doi: 10.1016/j.chom.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtin F, Rayaissé JB, Tamboura I, Serdébéogo O, Koudougou Z, Solano P, et al. Updating the northern tsetse limit in Burkina Faso (1949-2009): impact of global change. Int J Environ Res Public Health. 2010;7:1708–1719. doi: 10.3390/ijerph7041708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale C, Maudlin I. Sodalis gen. nov. and Sodalis glossinidius sp. nov., a microaerophilic secondary endosymbiont of the tsetse fly Glossina morsitans morsitans. Int J Syst Bacteriol. 1999;49:267–275. doi: 10.1099/00207713-49-1-267. [DOI] [PubMed] [Google Scholar]
- Dale C, Welburn SC. The endosymbionts of tsetse flies: manipulating host parasite interactions. Int J Parasitol. 2001;31:628–631. doi: 10.1016/s0020-7519(01)00151-5. [DOI] [PubMed] [Google Scholar]
- De Vooght L, Caljon G, Stijlemans B, De Baetselier P, Coosemans M, Van Den Abbeele J. Expression and extracellular release of a functional anti-trypanosome Nanobody in Sodalis glossinidius, a bacterial symbiont of the tsetse fly. Microb Cell Fact. 2012;11:23. doi: 10.1186/1475-2859-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean GJ, Paget J, Williamson BR. Survival and reproduction of Glossina morsitans Westw. in different types of cages exposed to variable and constant climatic conditions. Bull Entomol Res. 1969;58:773–785. doi: 10.1017/s0007485300056017. [DOI] [PubMed] [Google Scholar]
- Dobson SL, Fox C, Jiggins FM. The effect of Wolbachia-induced cytoplasmic incompatibility on host population size in natural and manipulated systems. Proc Biol Sci. 2002;269:437–445. doi: 10.1098/rspb.2001.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Manfredini F, Dimopoulos G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009;5:e1000423. doi: 10.1371/journal.ppat.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudoumis V, Tsiamis G, Wamwiri F, Brelsfoard C, Alam U, Aksoy A, et al. Detection and characterization of Wolbachia infections in laboratory and natural populations of different species of tsetse flies (genus Glossina) BMC Microbiol. 2012;12 (Suppl 1:S3. doi: 10.1186/1471-2180-12-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukes P, Kaukas A, Hudson KM, Asonganyi T, Gashumba JK. A new method for isolating Trypanosoma brucei gambiense from sleeping sickness patients. Trans R Soc Trop Med Hyg. 1989;83:636–639. doi: 10.1016/0035-9203(89)90379-9. [DOI] [PubMed] [Google Scholar]
- Dumas M, Bouteille B. Human African trypanosomiasis. C R Seances Soc Biol Fil. 1996;190:395–408. [PubMed] [Google Scholar]
- Engel P, Moran NA. The gut microbiota of insects—diversity in structure and function. FEMS Microbiol Rev. 2013;37:699–735. doi: 10.1111/1574-6976.12025. [DOI] [PubMed] [Google Scholar]
- Farikou O, Njiokou F, Mbida Mbida JA, Njitchouang GR, Djeunga HN, Asonganyi T, et al. Tripartite interactions between tsetse flies, Sodalis glossinidius and trypanosomes—an epidemiological approach in two historical human African trypanosomiasis foci in Cameroon. Infect Genet Evol. 2010;10:115–121. doi: 10.1016/j.meegid.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Farikou O, Njiokou F, Simo G, Asonganyi T, Cuny G, Geiger A. Tsetse fly blood meal modification and trypanosome identification in two sleeping sickness foci in the forest of southern Cameroon. Acta Trop. 2010;116:81–88. doi: 10.1016/j.actatropica.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Farikou O, Thevenon S, Njiokou F, Allal F, Cuny G, Geiger A. Genetic diversity and population structure of the secondary symbiont of tsetse flies, Sodalis glossinidius, in sleeping sickness foci in Cameroon. PLoS Negl Trop Dis. 2011;5:e1281. doi: 10.1371/journal.pntd.0001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco JR, Simarro PP, Diarra A, Jannin JG. Epidemiology of human African trypanosomiasis. Clin Epidemiol. 2014;6:257–275. doi: 10.2147/CLEP.S39728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Hase K.2014Commensal microbiota regulates T cell fate decision in the gut Semin Immunopatholdoi: 10.1007/s00281-014-0455-3 [DOI] [PubMed]
- Gavriel S, Jurkevitch E, Gazit Y, Yuval B. Bacterially enriched diet improves sexual performance of sterile male Mediterranean fruit flies. J Appl Entomol. 2011;135:564–573. [Google Scholar]
- Geiger A, Fardeau ML, Falsen E, Ollivier B, Cuny G. Serratia glossinae sp. nov., isolated from the midgut of the tsetse fly Glossina palpalis gambiensis. Int J Syst Evol Microbiol. 2010;60:1261–1265. doi: 10.1099/ijs.0.013441-0. [DOI] [PubMed] [Google Scholar]
- Geiger A, Fardeau ML, Grebaut P, Vatunga G, Josénando T, Herder S, et al. First isolation of Enterobacter, Enterococcus, and Acinetobacter spp. as inhabitants of the tsetse fly (Glossina palpalis palpalis) midgut. Infect Genet Evol. 2009;9:1364–1370. doi: 10.1016/j.meegid.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Geiger A, Fardeau ML, Njiokou F, Joseph M, Asonganyi T, Ollivier B, et al. Bacterial diversity associated with populations of Glossina spp. from cameroon and distribution within the campo sleeping sickness focus. Microb Ecol. 2011;62:632–643. doi: 10.1007/s00248-011-9830-y. [DOI] [PubMed] [Google Scholar]
- Geiger A, Ravel S, Mateille T, Janelle J, Patrel D, Cuny G, et al. Vector competence of Glossina palpalis gambiensis for Trypanosoma brucei s.l. and genetic diversity of the symbiont Sodalis glossinidius. Mol Biol Evol. 2007;24:102–109. doi: 10.1093/molbev/msl135. [DOI] [PubMed] [Google Scholar]
- Haines LR, Lehane SM, Pearson TW, Lehane MJ. Tsetse EP protein protects the fly midgut from trypanosome establishment. PLoS Pathog. 2010;6:e1000793. doi: 10.1371/journal.ppat.1000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidou Soumana I, Loriod B, Ravel S, Tchicaya B, Simo G, Rihet P, et al. The transcriptional signatures of Sodalis glossinidius in the Glossina palpalis gambiensis flies negative for Trypanosoma brucei gambiense contrast with those of this symbiont in tsetse flies positive for the parasite: possible involvement of a Sodalis-hosted prophage in fly Trypanosoma refractoriness. Infect Genet Evol. 2014;24C:41–56. doi: 10.1016/j.meegid.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Hao Z, Kasumba I, Lehane MJ, Gibson WC, Kwon J, Aksoy S. Tsetse immune responses and trypanosome transmission: implications for the development of tsetse- based strategies to reduce trypanosomiasis. Proc Natl Acad Sci USA. 2001;98:12648–12653. doi: 10.1073/pnas.221363798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques C, Sanchez MA, Tryon R, Landfear SM. Molecular and functional characterization of the first nucleobase transporter gene from African trypanosomes. Mol Biochem Parasitol. 2003;31:101–110. doi: 10.1016/s0166-6851(03)00167-1. [DOI] [PubMed] [Google Scholar]
- Hoare CA. The Trypanosomes of Mammals, A Zoological Monograph. Blackwell Scientific Publications: Oxford; 1972. [Google Scholar]
- Hoffman AA, Hercus M, Dagher H. Population dynamics of the Wolbachia infection causing cytoplasmic incompatibility in Drosophila melanogaster. Genetics. 1998;148:221–231. doi: 10.1093/genetics/148.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Fenwick A, Savioli L, Molyneux DH. Rescuing the bottom billion through control of neglected tropical diseases. Lancet. 2009;373:1570–1575. doi: 10.1016/S0140-6736(09)60233-6. [DOI] [PubMed] [Google Scholar]
- Hu C, Aksoy S. Innate immune responses regulate trypanosome parasite infection of the tsetse fly Glossina morsitans morsitans. Mol Microbiol. 2006;60:1194–1204. doi: 10.1111/j.1365-2958.2006.05180.x. [DOI] [PubMed] [Google Scholar]
- International Glossina Genome Initiative Genome sequence of the tsetse fly (Glossina morsitans): vector of African trypanosomiasis. Science. 2014;344:380–386. doi: 10.1126/science.1249656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MA, Mott TM, Tapley EC, Lewis EE, Luckhart S. Insulin regulates aging and oxidative stress in Anopheles stephensi. J Exp Biol. 2008;211:741–748. doi: 10.1242/jeb.012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazadi JML. Interactions Between Vector and Trypanosome in Determining the Vectorial Competence of Tsetse Flies [D. V. Sc. thesis] University of Liège: Liège (Belgium); 2000. [Google Scholar]
- Kennedy PG. The continuing problem of human African trypanosomiasis (sleeping sickness) Ann Neurol. 2008;64:116–126. doi: 10.1002/ana.21429. [DOI] [PubMed] [Google Scholar]
- Kubi C, Van den Abbeele J, De Deken R, Marcotty T, Dorny P, van den Bossche P. The effect of starvation on the susceptibility of teneral and non-teneral tsetse flies to trypanosome infection. Med Vet Entomol. 2006;20:388–392. doi: 10.1111/j.1365-2915.2006.00644.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Molina-Cruz A, Gupta L, Rodrigues J, Barillas-Mury C. A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science. 2010;327:1644–1648. doi: 10.1126/science.1184008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro JE, Nitcheu J, Predicala RZ, Mangalindan GC, Nesslany F, Marzin D, et al. Heptyl prodigiosin, a bacterial metabolite is antimalarial in vivo and non-mutagenic in vitro. J Nat Toxins. 2002;11:367–377. [PubMed] [Google Scholar]
- Lehane MJ, Aksoy S, Gibson W, Kerhornou A, Berriman M, Hamilton J, et al. Adult midgut expressed sequence tags from the tsetse fly Glossina morsitans morsitans and expression analysis of putative immune response genes. Genome Biol. 2003;4:R63. doi: 10.1186/gb-2003-4-10-r63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindh JM, Lehane MJ. The tsetse fly Glossina fuscipes fuscipes (Diptera: Glossina) harbours a surprising diversity of bacteria other than symbionts. Antonie Van Leeuwenhoek. 2011;99:711–720. doi: 10.1007/s10482-010-9546-x. [DOI] [PubMed] [Google Scholar]
- Luckhart S, Riehle MA. The insulin signaling cascade from nematodes to mammals: Insights into innate immunity of Anopheles mosquitoes to malaria parasite infection. Dev Comp Immunol. 2007;31:647–656. doi: 10.1016/j.dci.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod ET, Darby AC, Maudlin I, Welburn SC. Factors affecting trypanosome maturation in tsetse flies. PLoS ONE. 2007;2:e239. doi: 10.1371/journal.pone.0000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod ET, Maudlin I, Darby AC, Welburn SC. Antioxidants promote establishment of trypanosome infections in tsetse. Parasitology. 2007;134:827–831. doi: 10.1017/S0031182007002247. [DOI] [PubMed] [Google Scholar]
- Masurekar PS. Nutritional and engineering aspects of microbial process development. Prog Drug Res. 2008;291:293–328. doi: 10.1007/978-3-7643-8117-2_8. [DOI] [PubMed] [Google Scholar]
- Maudlin I, Ellis DS. Association between intracellular rickettsial-like infections of midgut cells and susceptibility to trypanosome infection in Glossina spp. Z Parasitenkd. 1985;71:683–687. doi: 10.1007/BF00925601. [DOI] [PubMed] [Google Scholar]
- Maudlin I, Welburn SC. Maturation of trypanosome infections in tsetse. Exp Parasitol. 1994;79:202–205. doi: 10.1006/expr.1994.1081. [DOI] [PubMed] [Google Scholar]
- Medlock J, Atkins KE, Thomas DN, Aksoy S, Galvani AP. Evaluating paratransgenesis as a potential control strategy for African trypanosomiasis. PLoS Negl Trop Dis. 2013;7:e2374. doi: 10.1371/journal.pntd.0002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloo SK, Asonganyi T, Jenni L. Cyclical development of Trypanosoma brucei gambiense from cattle and goats in Glossina. Acta Trop. 1986;43:407–408. [PubMed] [Google Scholar]
- Moore S, Shrestha S, Tomlinson KW, Vuong H. Predicting the effect of climate change on African trypanosomiasis: integrating epidemiology with parasite and vector biology. J R Soc Interface. 2012;9:817–830. doi: 10.1098/rsif.2011.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukabana WR, Takken W, Knols BG. Analysis of arthropod blood meals using molecular genetic markers. Trends Parasitol. 2002;18:505–509. doi: 10.1016/s1471-4922(02)02364-4. [DOI] [PubMed] [Google Scholar]
- Muturi CN, Ouma JO, Malele I, Ngure RM, Rutto JJ, Mithöfer KM, et al. Tracking the feeding patterns of tsetse flies Glossina Genus by analysis of bloodmeals using mitochondrial cytochromes genes. PLoS ONE. 2011;6:e17284. doi: 10.1371/journal.pone.0017284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayduch D, Aksoy S. Refractoriness in tsetse flies (Diptera: Glossinidae) may be a matter of timing. J Med Entomol. 2007;44:660–665. doi: 10.1603/0022-2585(2007)44[660:ritfdg]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ndegwa PN, Irungu LW, Moloo SK. Effect of puparia incubation temperature: increased infection rates of Trypanosoma congolense in Glossina morsitans centralis, G. fuscipes fuscipes and G. brevipalpis. Med Vet Entomol. 1992;6:127–130. doi: 10.1111/j.1365-2915.1992.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Njiokou F, Laveissière C, Simo G, Nkinin S, Grébaut P, Cuny G, et al. Wild fauna as probable animal reservoir for Trypanosoma brucei gambiense in Cameroon. Infect Genet Evol. 2006;6:147–153. doi: 10.1016/j.meegid.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Nogge G. Significance of symbionts for the maintenance of an optional nutritional state for successful reproduction in hematophagous arthropods. Parasitology. 1982;82:299–304. [Google Scholar]
- Nyeko JPH, Ole-Moi-Yoi OK, Majiwa PAO, Otieno LH, Ociba PM. Characterization of trypanosome isolates from cattle in Uganda using species DNA probes reveals predominance of mixed infections. Insect Sci Appl. 1990;11:271–280. [Google Scholar]
- Odiit M, Shaw A, Welburn SC, Fèvre EM, Coleman PG, McDermott JJ. Assessing the patterns of health-seeking behaviour and awareness among sleeping-sickness patients in eastern Uganda. Ann Trop Med Parasitol. 2004;98:339–348. doi: 10.1179/000349804225003389. [DOI] [PubMed] [Google Scholar]
- Oliveira JHM, Gonçalves RL, Lara FA, Dias FA, Gandara AC, Menna-Barreto RF, et al. Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathog. 2011;7:e1001320. doi: 10.1371/journal.ppat.1001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omolo MO, Hassanali A, Mpiana S, Esterhuizen J, Lindh J, Lehane MJ, et al. Prospects for developing odour baits to control Glossina fuscipes spp., the major vector of human African Trypanosomiasis. PLoS Negl Trop Dis. 2009;3:e435. doi: 10.1371/journal.pntd.0000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill SL, Gooding RH, Aksoy S. Phylogenetically distant symbiotic microorganisms reside in Glossina midgut and ovary tissues. Med Vet Entomol. 1993;7:377–383. doi: 10.1111/j.1365-2915.1993.tb00709.x. [DOI] [PubMed] [Google Scholar]
- Pagabeleguem S, Sangaré M, Bengaly Z, Akoudjin M, Belem AM, Bouyer J. Climate, cattle rearing systems and African Animal Trypanosomosis risk in Burkina Faso. PLoS One. 2012;7:e49762. doi: 10.1371/journal.pone.0049762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakpour N, Akman-Anderson L, Vodovotz Y, Luckhart S. The effects of ingested mammalian blood factors on vector arthropod immunity and physiology. Microbes Infect. 2013;15:243–254. doi: 10.1016/j.micinf.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinar GO, Jr, Wassink HJ, Leegwater-van der Linden ME, van der Geest LP. Serratia marcescens as a pathogen of tsetse flies. Acta Trop. 1979;36:223–227. [PubMed] [Google Scholar]
- Ponton F, Wilson K, Cotter SC, Raubenheimer D, Simpson SJ. Nutritional immunology: a multi-dimensional approach. PLoS Pathog. 2011;7:e1002223. doi: 10.1371/journal.ppat.1002223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponton F, Wilson K, Holmes AJ, Cotter SC, Raubenheimer D, Simpson SJ. Integrating nutrition and immunology: a new frontier. J Insect Physiol. 2013;59:130–137. doi: 10.1016/j.jinsphys.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Reifenberg JM, Solano P, Duvallet G, Cuissance D, Simpore J, Cuny G. Molecular characterization of trypanosome isolates from naturally infected domestic animals in Burkina, Faso. Vet Parasitol. 1997;71:251–262. doi: 10.1016/s0304-4017(97)00011-3. [DOI] [PubMed] [Google Scholar]
- Reinhardt E.2002. Travailler ensemble: la mouche tsé-tsé et la pauvreté rurale. [Internet]. Available from www.un.org/french/pubs/chronique/2002/numero2/0202p17_la_mouche_tsetse.html Chronique ONU; ONU Editor-September 02.
- Rio RVM, Symula RE, Wang J, Lohs C, Wu YN, Snyder AK, et al. Insight into the transmission biology and species-specific functional capabilities of tsetse (Diptera: Glossinidae) obligate symbiont Wigglesworthia. mBio. 2012;3:e00240-11. doi: 10.1128/mBio.00240-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DJ, Randolph SE. Climate change and vector-borne diseases. Adv Parasitol. 2006;62:345–381. doi: 10.1016/S0065-308X(05)62010-6. [DOI] [PubMed] [Google Scholar]
- Rueda LM, Patel KJ, Axtell RC, Stinner RE. Temperature-dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae) J Med Entomol. 1990;27:892–898. doi: 10.1093/jmedent/27.5.892. [DOI] [PubMed] [Google Scholar]
- Savage AF, Cerqueira GC, Regmi S, Wu Y, El Sayed NM, Aksoy S. Transcript expression analysis of putative Trypanosoma brucei GPI-anchored surface proteins during development in the tsetse and mammalian hosts. PLoS Negl Trop Dis. 2012;6:e1708. doi: 10.1371/journal.pntd.0001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbicego S, Vassella E, Kurath U, Blum B, Roditi I. The use of transgenic Trypanosoma brucei to identify compounds inducing the differentiation of bloodstream forms to procyclic forms. Mol Biochem Parasitol. 1999;104:311–322. doi: 10.1016/s0166-6851(99)00157-7. [DOI] [PubMed] [Google Scholar]
- Simarro PP, Diarra A, Ruiz Postigo JA, Franco JR, Jannin JG. The human African trypanosomiasis control and surveillance programme of the World Health Organization 2000-2009: the way forward. PLoS Negl Trop Dis. 2011;5:e1007. doi: 10.1371/journal.pntd.0001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simarro PP, Jannin J, Cattand P. Eliminating human African trypanosomiasis: where do we stand and what comes next. PLoS Med. 2008;5:e55. doi: 10.1371/journal.pmed.0050055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo G, Asonganyi T, Nkinin SW, Njiokou F, Herder S. High prevalence of Trypanosoma brucei gambiense group 1 in pigs from the Fontem sleeping sickness focus in Cameroon. Vet Parasitol. 2006;139:57–66. doi: 10.1016/j.vetpar.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Simo G, Herder S, Cuny G, Hoheisel J. Identification of subspecies specific genes differentially expressed in procyclic forms of Trypanosoma brucei subspecies. Infect Genet Evol. 2010;10:229–237. doi: 10.1016/j.meegid.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Simo G, Njiokou F, Mbida Mbida JA, Njitchouang GR, Herder S, Asonganyi T, et al. Tsetse fly host preference from sleeping sickness foci in Cameroon: epidemiological implications. Infect Genet Evol. 2008;8:34–39. doi: 10.1016/j.meegid.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Solano P, Michel JF, Lefrançois T, La Rocque S, Sidibé I, Zoungrana A, et al. Polymerase chain reaction as diagnosis tool for detecting trypanosomes in naturally infected cattle in Burkina Faso. Vet Parasitol. 1999;86:95–103. doi: 10.1016/s0304-4017(99)00137-5. [DOI] [PubMed] [Google Scholar]
- Staak C, Kämpe U, Korkowski G. Species identification of blood meals from tsetse flies (Glossinidae): results 1979-1985. Trop Med Parasitol. 1986;37:59–60. [PubMed] [Google Scholar]
- Stecher B, Hardt WD. Mechanisms controlling pathogen colonization of the gut. Curr Opin Microbiol. 2011;14:82–91. doi: 10.1016/j.mib.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Terblanche JS, Clusella-Trullas S, Deere JA, Chown SL. Thermal tolerance in a south-east African population of the tsetse fly Glossina pallidipes (Diptera, Glossinidae): implications for forecasting climate change impacts. J Insect Physiol. 2008;54:114–127. doi: 10.1016/j.jinsphys.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Toh H, Weiss BL, Perkin SA, Yamashita A, Oshima K, Hattori M, et al. Massive genome erosion and functional adaptations provide insights into the symbiotic lifestyle of Sodalis glossinidius in the tsetse host. Genome Res. 2006;16:149–156. doi: 10.1101/gr.4106106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefson J. Seven billion and counting. Nature. 2011;478:300. doi: 10.1038/478300a. [DOI] [PubMed] [Google Scholar]
- Van den Abbeele J, Claes Y, van Bockstaele D, Le Ray D, Coosemans M. Trypanosoma brucei spp. development in the tsetse fly: characterization of the post-mesocyclic stages in the foregut and proboscis. Parasitology. 1999;118:469–478. doi: 10.1017/s0031182099004217. [DOI] [PubMed] [Google Scholar]
- Van den Bossche P, de La Rocque S, Hendrickx G, Bouyer J. A changing environment and the epidemiology of tsetse-transmitted livestock trypanosomiasis. Trends Parasitol. 2010;26:236–243. doi: 10.1016/j.pt.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Vreysen MJ, Saleh KM, Ali MY, Abdulla AM, Zhu ZR, Juma KG, et al. Glossina austeni (Diptera: Glossinidae) eradicated on the island of Unguja, Zanzibar, using the sterile insect technique. J Econ Entomol. 2000;93:123–135. doi: 10.1603/0022-0493-93.1.123. [DOI] [PubMed] [Google Scholar]
- Wang J, Brelsfoard C, Wu Y, Aksoy S. Intercommunity effects on microbiome and GpSGHV density regulation in tsetse flies. J Invertebr Pathol. 2013;112:S32–S39. doi: 10.1016/j.jip.2012.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Weiss BL, Aksoy S. Tsetse fly microbiota: form and function. Front Cell Infect Microbiol. 2013;3:69. doi: 10.3389/fcimb.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wu Y, Yang G, Aksoy S. Interactions between mutualist Wigglesworthia and tsetse peptidoglycan recognition protein (PGRP-LB) influence trypanosome transmission. Proc Natl Acad Sci USA. 2009;106:12133–12138. doi: 10.1073/pnas.0901226106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gilbreath TM, 3rd, Kukutla P, Yan G, Xu J. Dynamic gut microbiome across life history of the Malaria Mosquito Anopheles gambiae in Kenya. PLoS One. 2011;6:e24767. doi: 10.1371/journal.pone.0024767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss BL, Wang J, Aksoy S. Tsetse immune system maturation requires the presence of obligate symbionts in larvae. PLoS Biol. 2011;9:e1000619. doi: 10.1371/journal.pbio.1000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welburn SC, Arnold K, Maudlin I, Gooday GW. Rickettsia-like organisms and chitinase production in relation to transmission of trypanosomes by tsetse flies. Parasitology. 1993;107:141–145. doi: 10.1017/s003118200006724x. [DOI] [PubMed] [Google Scholar]
- Welburn SC, Maudlin I. Tsetse-trypanosome interactions: rites of passage. Parasitol Today. 1999;15:399–403. doi: 10.1016/s0169-4758(99)01512-4. [DOI] [PubMed] [Google Scholar]
- Welburn SC, Maudlin I, Simarro PP. Controlling sleeping sickness-a review. Parasitology. 2009;136:1943–1949. doi: 10.1017/S0031182009006416. [DOI] [PubMed] [Google Scholar]
- Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]