Abstract.
The current study aimed to explore the neural substrate for atomoxetine effects on attentional control in school-aged children with attention deficit hyperactivity disorder (ADHD) using functional near-infrared spectroscopy (fNIRS), which can be applied to young children with ADHD more easily than conventional neuroimaging modalities. Using fNIRS, we monitored the oxy-hemoglobin signal changes of 15 ADHD children (6 to 14 years old) performing an oddball task before and 1.5 h after atomoxetine or placebo administration, in a randomized, double-blind, placebo-controlled, crossover design. Fifteen age-, gender-, and intelligence quotient-matched normal controls without atomoxetine administration were also monitored. In the control subjects, the oddball task recruited the right prefrontal and inferior parietal cortices. The right prefrontal and parietal activation was normalized after atomoxetine administration in ADHD children. This was in contrast to our previous study using a similar protocol showing methylphenidate-induced normalization of only the right prefrontal function. fNIRS allows the detection of differential neuropharmacological profiles of both substances in the attentional network: the neuropharmacological effects of atomoxetine to upregulate the noradrenergic system reflected in the right prefrontal and inferior parietal activations and those of methylphenidate to upregulate the dopamine system reflected in the prefrontal cortex activation.
Keywords: serotonin, dorsolateral prefrontal cortex, optical topography, ventrolateral prefrontal cortex, target detection, autism spectrum disorder
1. Introduction
Attention deficit hyperactivity disorder (ADHD) is the most prevalent psychiatric disorder of childhood, with a prevalence rate estimated to be between 3% and 7%.1,2 ADHD is characterized with heterogeneous phenotypes, including age-inappropriate inattention, impulsivity, and hyperactivity. ADHD symptoms are most often identified during early elementary school years.3–6 ADHD patients often suffer from academic difficulties and develop antisocial behaviors that result from emotional and social problems associated with ADHD-related core symptoms.7 The disorder persists into adolescence and adulthood in 65% to 85% of cases, leads to impaired educational and vocational performance, and increases the risk of developing antisocial behaviors that are not directly related to ADHD.8 Furthermore, ADHD during childhood can robustly predict later depression and suicidal risk in adolescence and adulthood.9 Therefore, early identification with appropriate treatment of ADHD in childhood is essential to maintain a sufficient quality of life over a long duration of time.10,11
The stimulant drug methylphenidate (MPH) and the non-stimulant drug atomoxetine (ATX) have been the most frequently prescribed medications to treat inhibition- and attention-related dysfunctions in ADHD children.12–16 Both substances act as monoamine agonists and increase the release of dopamine (DA) and noradrenaline (NA).17,18 More specifically, MPH inhibits catecholamine reuptake by blocking their transporters.17,18 However, ATX and MPH affect the DA and NA systems to different degrees. Indeed, the dissociation constant value, or K(i), of MPH to DA is 34 nM, while that to NA is 339 nM: MPH has a higher affinity to DA than to NA by 10-fold.19 Given these biochemical characteristics, the major target of MPH is considered to be the DA system involving prefrontal and striatal regions.20 On the other hand, ATX, an approved nonstimulant medication for ADHD, is a highly selective NA reuptake inhibitor.21 The affinities of ATX to DA and NA transporters in terms of K(i) are 1451 and 5 nM, respectively: ATX has a 300-fold higher specificity to NA than to DA.19 Thus, ATX is considered to have a far larger effect on the NA system involving the prefrontal, parietal, and coeruleus areas.22 However, physiological examination of NA transmission has been limited to animal models, and no studies are available for humans because of the lack of suitable radiotracers with adequate binding specificity characteristics.23,24
One promising tool for clarifying the differences in neuronal substrate for both substances would be neurofunctional examination using functional near-infrared spectroscopy (fNIRS). fNIRS, which is convenient and robust, is suitable for the functional monitoring of children with ADHD who have difficulty performing active cognitive tasks in the enclosed environments of other imaging modalities. In fact, the cumulative postscan exclusion rate of our previous fNIRS studies (all studies)25–28 was of a total of 66 right-handed ADHD children and 54 control subjects including six year olds.25–28 This is far lower than the rejection rate of functional magnetic resonance imaging (fMRI) studies, which is typically 50% for ADHD subjects six years old and older and 30% for the corresponding normal controls.29 This is mainly due to motion and lack of compliance.30
In order to clarify the differences in the neuronal substrate for both substances, our previous studies have aimed to assess the pharmacological neuromodulation of inhibitory control (using go/no-go tasks) by MPH25,26 and ATX,28 and of attention control (using oddball tasks) by MPH,27 using a randomized, double-blind, placebo-controlled, crossover design, with fNIRS measurement. In summary, we first detected brain activation in the right middle frontal gyrus/inferior frontal gyrus (MFG/IFG) during go/no-go tasks25–28 and in the right MFG/IFG and inferior parietal lobe (IPL) during oddball tasks27 in healthy control subjects. These activation patterns were consistent with the results of previous fMRI studies and were considered especially important for inhibitory control18 and attention control.31,32
Subsequently, we examined the neuroactivation of ADHD children. In premedicated ADHD children, the right prefrontal and/or parietal activations associated with attentional control and inhibition were missing. On the other hand, in post-MPH and ATX administration ADHD groups, functional normalization was observed, using fNIRS, in the right MFG/IFG during go/no-go tasks.25,26,28 These activation patterns were consistent with the results of previous fMRI studies.33,34 In essence, the right prefrontal dysfunction in ADHD children was normalized by MPH for both inhibitory and attentional controls, and by ATX for inhibitory control.
One final piece is missing here: the effect of ATX on attentional control. We can anticipate two possibilities. First, as in the case of MPH studies on attentional control, the right IFG/MFG dysfunction could be normalized. This possibility is supported by the results of our previous ATX study on inhibitory control showing the normalization of the right IFG/MFG but not of parietal dysfunction.27 However, we must consider that ATX predominantly affects the NA system where parietal as well as frontal contributions are important.22 It is possible that the ATX-mediated right prefrontal functional normalization is particular to inhibitory control and may not be extended to attentional control. If this is the case, ATX should lead to normalization of both right prefrontal and parietal dysfunctions.
In order to ascertain which of these possibilities is applicable, we conducted a randomized, double-blind, placebo-controlled study employing an oddball task to evaluate ATX-related specific neuroactivation in ADHD children using fNIRS analysis. To our knowledge, this is the first neuropharmacological examination of ATX effects on attention control in ADHD children,23 including fMRI and fNIRS studies.
2. Methods
2.1. Subjects
Fifteen clinically referred, right-handed Japanese children who met the Diagnostic and Statistical Manual of Mental Disorders-IV criteria for ADHD with a mean age of 9.9 years (SD 2.1, range 6 to 14 years) participated in the study (Table 1). The Wechsler Intelligence Scale of Children-Third Edition full intelligence quotient (IQ) scores of subjects were all (mean 97.1, SD 9.3, range 84 to 110). We describe demographic and clinical characteristics of the patients in Table 1. The subjects had been taking ATX (5 to ) for between 2 months and 3.5 years. All patients were premedicated with ATX as part of their regular medication regimen. Specific acute doses were the same as the patient’s daily dose as described in Table 1.
Table 1.
Demographic and clinical profiles for attention deficit hyperactivity disorder (ADHD) subjects.
| ID | Age (years) | Sex | ADHD subtype | Complication | ATX (mg) | WISC-III full IQ | Duration of ATX exposure (months) | Other medications |
|---|---|---|---|---|---|---|---|---|
| 1 | 6 | M | Combined | ASD | 10 | 84 | 2 | None |
| 2 | 9 | M | Combined | ASD | 10 | 97 | 7 | None |
| 3 | 10 | F | Inattentive | None | 25 | 79 | 3 | None |
| 4 | 7 | M | Combined | None | 10 | 96 | 11 | Risperidone |
| 5 | 12 | M | Combined | None | 15 | 92 | 6 | None |
| 6 | 11 | M | Inattentive | ASD | 40 | 110 | 17 | None |
| 7 | 11 | M | Combined | ASD | 40 | 95 | 17 | None |
| 8 | 8 | M | Combined | ASD | 20 | 96 | 19 | None |
| 9 | 11 | M | Combined | ASD | 50 | 109 | 26 | None |
| 10 | 9 | F | Combined | None | 20 | 102 | 7 | None |
| 11 | 14 | M | Inattentive | None | 50 | 109 | 41 | None |
| 12 | 12 | M | Inattentive | ASD | 50 | 90 | 36 | None |
| 13 | 9 | M | Combined | ASD | 5 | 107 | 11 | None |
| 14 | 10 | F | Inattentive | ASD | 25 | 101 | 33 | None |
| 15 | 10 | M | Combined | None | 35 | 90 | 5 | None |
| Mean | 9.9 | 97.1 | 16.1 | |||||
| SD | 2.1 | 9.3 | 12.2 |
Note: ATX, atomoxetine; WISC-III, Wechsler Intelligence Scale of Children-third edition; IQ, intelligence quotient; SD, standard deviation; ASD, autism spectrum disorders.
Fifteen right-handed control subjects were matched with the ADHD subjects according to gender (12 boys and 3 girls), age (mean 10.1 years, SD 1.7, range 7 to 13 years), and IQ (mean 104.1, SD 10.9, range 85 to 121). All children and their parents gave oral consent for participation in the study, and written consent was obtained from the subjects’ parents. The study was authorized by the Ethics Committees of Jichi Medical University Hospital and the International University of Health and Welfare, and designed in accordance with the latest version of the Declaration of Helsinki. We registered this study with the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR; UMIN000014036) as “Clinically-oriented monitoring of acute effects of Atomoxetine (ATX) on cerebral hemodynamics in ADHD children: an exploratory fNIRS study using a target detection task.”
2.2. Experimental Design
We adopted a visual-based oddball task that represents measures of attention. The task consists of detection of and response to infrequent (oddball) target events included in a series of repetitive events. An oddball task is often referred to as a response selection task. The effects of ATX while the subjects performed oddball tasks were examined in a randomized, double-blind, placebo-controlled, crossover study. We summarize the experimental procedure in Fig. 1.
Fig. 1.
Experimental design.
ADHD subjects were examined twice (the times of day for both measurements were scheduled to be as close as possible), at least two days apart, but within 30 days. Control subjects underwent only one examination and did not receive medication. ADHD subjects performed two sessions on each examination day, one before medication (ATX or placebo) administration, and the other at 1.5 h after medication administration. Subjects were permitted to take off the probe during the resting period between the first and second measurements. After ADHD subjects underwent the first session, either ATX (Strattera, Eli Lilly and Co., Indianapolis, Indiana, USA) or a placebo was administered orally. Specific acute doses were the same as the patient’s daily dose as described in Table 1.
The experimental design was as previously described.27 During the session, subjects viewed a series of pictures once every second and responded by pressing a key for every picture. Each session consisted of six block sets, each containing alternating baseline and oddball blocks. In the baseline block, we presented subjects with one picture and asked them to press a red button on a response box for that picture. Following the baseline block, we presented tiger (standard stimulus, 80% of trials) or elephant (target stimulus, 20% of trials) pictures sequentially for 200 ms with an interstimulus interval of 800 ms. A target versus standard ratio of 20:80 was selected so as to maintain consistency with former neuroimaging studies.35–42 The total number of trials, presented in a single run, was 325. Participants were instructed to respond to the standard stimuli (tiger) by pressing a red button and to target stimuli (elephant) by pressing a blue button on the response box. The blue button was located next to the red button on the response box. Specific instructions (in Japanese) were as follows: “For this task, you will be shown tigers and elephants on the computer screen. You should press the red button for tigers and press the blue button for elephants, as quickly as possible. Don’t forget that you want to be prompt but also accurate, so do not go too fast.” Participants responded using the forefinger of their right hand.
Each session consisted of six block sets, each containing alternating baseline and oddball blocks. Each block lasted 25 s and was preceded by instructions displayed for 3 s to inform the subject of the new block, giving an overall block-set time of 56 s and a total session time of 6.0 min. Each subject performed a practice block before any measurements to ensure their understanding of the instructions.
To reduce habituation, we made four versions with different picture and button combinations: red button in baseline blocks and elephant red button/tiger blue button (target stimulus/standard stimulus) (version 1) or tiger red button/elephant blue button (version 2) in oddball blocks, blue button in baseline blocks and elephant red button/tiger blue button (version 3) or tiger red button/elephant blue button (version 4) in oddball blocks. These four versions were randomly allotted for ATX and placebo conditions.
We generated stimuli and collected responses using E-Prime (version 2.0; Psychology Software Tools, Inc., Sharpsburg, Pennsylvania). Stimuli were presented to the subject on a 17-in. desktop computer screen. The distance between the subject’s eyes and the screen was .
2.3. Behavioral Data Analysis
The average reaction times (RT) for correct trials, commission error rates, and omission error rates were calculated, respectively, for target and standard trials in the oddball block for control and ADHD subjects. We calculated the mean RT for each participant by averaging the RT for correct target trials. We calculated commission error rates by dividing the number of commission errors (i.e., subjects pushed the incorrect button) by the total number of target trials. We computed omission error rates by dividing the number of omission errors (i.e., subjects failed to push any button) by the total number of target trials. The statistical threshold was set at .
2.4. fNIRS Measurements
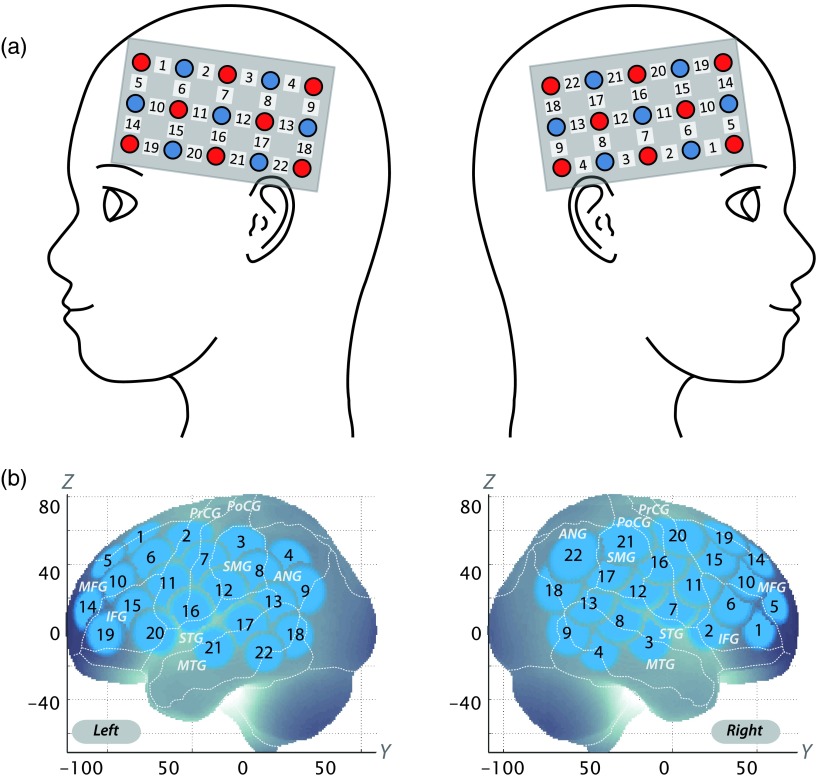
We utilized the multichannel fNIRS system ETG-4000 (Hitachi Medical Corporation, Kashiwa, Japan) with two wavelengths of near-infrared light (695 and 830 nm). Optical data based on the modified Beer-Lambert law43 as previously described was analyzed.44 Signals reflecting the oxygenated hemoglobin (oxy-Hb), deoxygenated hemoglobin (deoxy-Hb), and total hemoglobin (total-Hb) signal changes were computed in units of millimolar-millimeter (mM-mm).44 The fNIRS probes were set to cover the lateral prefrontal cortices and inferior parietal lobe in reference to previous studies.45–49 Specifically, we used two sets of multichannel probe holders, consisting of eight illuminating and seven detecting probes installed alternately at an interprobe distance of 3 cm. This resulted in 22 channels (CH) per set (Fig. 2).
Fig. 2.
Spatial profiles of functional near-infrared spectroscopy channels.
The midpoint of a pair of illuminating and detecting probes was defined as a channel location. We placed the bilateral probe holders in the following manner: (1) their upper anterior corners, where the left and right probe holders were connected by a belt, were symmetrically placed across the sagittal midline; (2) the lower anterior corners of the probe holder were placed over the supraorbital prominence; and (3) the lower edges of the probe holders were attached at the upper part of the auricles (Fig. 2).
For spatial profiling of fNIRS data, we adopted the probabilistic registration method50,51 to register fNIRS data to Montreal Neurological Institute (MNI) standard brain space. Specifically, the positions for channels and reference points, which included the Nz (nasion), Cz (midline central), and left and right preauricular points, were measured using a three-dimensional digitizer in real-world (RW) space. We affine-transformed each RW reference point to the corresponding MRI-database reference point and then replaced them to MNI space. Adopting the same transformation parameters allowed us to obtain the MNI coordinate values for the fNIRS channels in order to obtain the most likely estimate of the location of given channels for the group of subjects, and the spatial variability associated with the estimation.52 Finally, the estimated locations were anatomically labeled using a MATLAB® function that reads anatomical labeling information coded in a macroanatomical brain atlas [LBPA40 (Ref. 53) and Brodmann54].
2.5. Analysis of fNIRS Data
The individual timeline data for the oxy-Hb and deoxy-Hb signals of each channel were preprocessed with a first-degree polynomial fitting, a 0.01-Hz high-pass filter to exclude baseline drift, and a 0.8-Hz low-pass filter to exclude heartbeat pulsations. Hb signals thus obtained do not directly represent cortical Hb concentration changes, but include an unknown optical path length that cannot be measured. Direct comparison of Hb signals among different channels and regions should be avoided because the optical path length is known to vary among cortical regions.55 Therefore, channel-wise statistical analyses were performed in the current study. For the six oddball blocks, the motion of the subjects was monitored and the blocks with sudden, obvious, discontinuous noise were removed. Channel-wise and subject-wise contrasts were obtained by computing the intertrial mean of differences between the peak Hb signals (4 to 25 s after trial onset) and baseline (0 to 10 s before trial onset) periods from the preprocessed time series data. These contrasts were subjected to second-level, random effects group analyses.
2.6. Statistical Analyses
We performed channel-wise statistical analyses of oxy-Hb signals. Specifically, for control subjects who underwent measurement only once, the oddball versus baseline contrast of the session was generated. For ADHD subjects, the following contrasts were generated: (1) premedication contrasts: oddball versus baseline contrast of the premedication conditions (either placebo or ATX administration); (2) postmedication contrasts: oddball versus baseline contrast of the postplacebo and post-ATX conditions; (3) intramedication contrasts: difference between post- and premedication contrasts for each medication (i.e., and contrasts); and (4) intermedication contrast: difference between and contrasts.
Based on our previous observations of control subjects exhibiting right prefrontal and parietal activations in the oddball versus baseline contrast, we set two channels (right CH 10 and 22) as regions of interest (ROI). We first confirmed this assumption in the current set of control subjects and analyzed oddball versus baseline contrasts using a paired t test (two-tails). We set the statistical threshold at 0.05 with the Bonferroni method for family-wise error correction.
For the thus-confirmed ROI, we compared the following three ADHD contrasts with the control: (1) premedication, (2) postplacebo, and (3) post-ATX. Independent two-sample t tests (two tails) with a statistical threshold of were performed.
To examine the medication effects on ADHD subjects, we compared intra-ATX with intraplacebo contrasts (i.e., intermedication contrast) for the ROI using a paired t test (two-tails) with a statistical threshold of .
For all statistical analyses, we used the SPSS software package (version 18 for Windows; SPSS Inc., Chicago, Illinois, USA).
3. Results
3.1. Behavioral Performance
We have summarized the averages of RT, omission error rates, and commission error rates for target trials in the oddball block for control and ADHD subjects as well as ADHD intermedication ( versus ) comparisons in Tables 2 and 3.
Table 2.
Oddball task performance data for control and ADHD subjects.
| Control | Premedication versus control (mean of preplacebo and pre-ATX) | Postplacebo versus control | Post-ATX versus control | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Sig | ES | Mean | SD | Sig | ES | Mean | SD | Sig | ES | |||||||
| RT for correct trials (ms) | 489.3 | 39.6 | 577.4 | 58.4 | 4.833 | 0.000 | * | 1.765 | 548.5 | 67.3 | 2.937 | 0.007 | * | 1.072 | 545.8 | 60.8 | 3.020 | 0.005 | * | 1.103 |
| Commission errors (%) | 16.4 | 16.4 | 28.3 | 18.0 | 1.889 | 0.069 | ns | 0.690 | 33.3 | 20.4 | 2.494 | 0.019 | † | 0.911 | 31.1 | 15.2 | 2.540 | 0.017 | † | 0.927 |
| Omission errors (%) | 0.7 | 1.4 | 5.8 | 8.2 | 2.387 | 0.024 | † | 0.871 | 5.1 | 4.0 | 4.107 | 0.000 | * | 1.500 | 3.8 | 5.3 | 2.190 | 0.037 | † | 0.800 |
Note: Performance data [reaction times (RT) for correct trials, commission errors, and omission errors] are displayed for target trials for oddball blocks. For ADHD subjects, data for premedication (mean of preplacebo and pre-ATX) and postmedications (placebo and ATX) are indicated. values, values, and statistical significance were the results of t tests between control and each ADHD condition. values are presented as uncorrected values. Statistical significances are presented as follows: †, (uncorrected); *, (Bonferroni-corrected); ns, not significant. SD, standard deviation; , value; , value; Sig, statistical significance; ES, effect size (Cohen’s ).
Table 3.
Oddball task performance data for ADHD intermedication ( versus ) comparison.
| minus | versus | |||
|---|---|---|---|---|
| Mean | SD | |||
| RT for correct trials (ms) | 50.6 | 0.898ns | ||
| Commission errors (%) | 14.9 | 0.692ns | ||
| Omission errors (%) | 0.0 | 12.8 | 0.000 | 1.000ns |
Note: Performance data (RT for correct trials, commission errors, and omission errors) are presented for target trials for oddball blocks. Data for intermedication comparisons (i.e., versus ) are shown for ADHD subjects. Mean values were calculated by first subtracting the values of from those of for each subject and then averaging the resulting values across subjects. SD was calculated similarly. values, values, and statistical significance were the results of two-sample t tests between and . , the difference between post- and pre-ATX; , the difference between post and preplacebo; SD, standard deviation; , value; , value. Statistical significances are presented as follows: *; **; and ns, not significant.
Control-subject RT for correct trials were significantly shorter than those of premedicated (paired t test, , Bonferroni-corrected, Cohen’s ), postplacebo (paired t test, , Bonferroni-corrected, Cohen’s ), and post-ATX (paired t test, , Bonferroni-corrected, Cohen’s ) ADHD subjects. These results suggest that while RT for correct trials represented the slower behavioral performance of premedicated ADHD children well, it was not normalized by administration of ATX.
Control-subject commission error rates for target trials were not significantly lower than those of premedicated (paired t test, , uncorrected) ADHD subjects, and were marginally lower than those of postplacebo (paired t test, , uncorrected, Cohen’s ) and post-ATX (paired t test, , uncorrected, Cohen’s ) ADHD subjects. These results suggest that commission errors for target trials failed to represent presumably lower behavioral performances in premedicated ADHD children and, thus, do not provide any relevant information about the normalization effect of ATX.
Control-subject omission error rates for target trials were marginally lower than those for premedicated (paired t test, , uncorrected, Cohen’s ) and post-ATX (paired t test, , uncorrected, Cohen’s ) ADHD subjects, and significantly lower than those for postplacebo (paired t test, , Bonferroni-corrected, Cohen’s ) ADHD subjects. These results suggest that omission error rates partially reflected the less-correct behavioral performance of premedicated ADHD children, but failed to indicate normalization by ATX administration (Table 2).
Within-ADHD-subject analysis revealed no significant differences in the intermedication contrast comparing the effect of ATX with that of the placebo (Table 3). Namely, no parameters indicated behavioral improvement due to ATX over the placebo.
3.2. fNIRS Analyses
Confirmation of ROI was performed in control subjects of the current study. As assumed, significant oxy-Hb increase was found in the right CH 10 (mean 0.065, SD 0.056, , Bonferroni-corrected, Cohen’s ) and the right CH 22 (mean 0.067, SD 0.062, , Bonferroni-corrected, Cohen’s ). Thus, the right CH 10 and 22 were confirmed as ROI for the rest of the study (Table 4).
Table 4.
Functional data for control and ADHD subjects in oddball task.
| Control | ADHD | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Premedication versus control (mean of preplacebo and pre-ATX) | Postplacebo versus control | Post-ATX versus control | ||||||||||||||||||
| Oxy-Hb | Mean | SD | Mean | SD | Sig | ES | Mean | SD | Sig | ES | Mean | SD | Sig | ES | ||||||
| R CH 10 () | 0.065 | 0.056 | 0.005 | 0.051 | 3.099 | 0.004 | * | 1.132 | 0.079 | 3.103 | 0.004 | * | 1.133 | 0.074 | 0.080 | 0.329 | 0.745 | ns | 0.120 | |
| R CH 22 () | 0.067 | 0.062 | 0.009 | 0.089 | 2.070 | 0.048 | † | 0.756 | 0.048 | 0.113 | 0.572 | 0.572 | ns | 0.209 | 0.050 | 0.077 | 0.659 | 0.515 | ns | 0.241 |
Note: Oxy-Hb data include right CH 10 and 22. For ADHD subjects, data for premedication (mean of preplacebo and pre-ATX) and postmedications (placebo and ATX) are indicated. values, values, and statistical significance were the results of t tests between control and each ADHD condition. values are presented as uncorrected values. Statistical significances are presented as follows: †, (uncorrected); *, (Bonferroni-corrected); ns, not significant. SD, standard deviation; , value; , value; Sig, statistical significance; ES, effect size (Cohen’s ); R, right hemisphere; and CH, channel.
The right CH 10 was located in the border region between the right MFG and IFG (MNI coordinates , , (SD): 45,44,31 (15), MFG 78%, IFG 22%; Table 5), and the right CH 22 was located in the border region between the right angular gyrus and the right supramarginal gyrus (MNI coordinates , , (SD): 57, , 46 (19), angular gyrus 96%, supramarginal gyrus 4%; Table 5) with reference to macroanatomical brain atlases (Table 5).54,56
Table 5.
Spatial profiles of the channels screened for involvement with oddball tasks.
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| CH | , , (SD) | Macroanatomy | Prob | Brodmann area | Prob | |
| R 10 | 45, 44, 31 (15) | R middle frontal gyrus | 0.78 | 45 | Pars triangularis (Broca’s area) | 0.50 |
| R inferior frontal gyrus | 0.22 | 46 | Dorsolateral prefrontal cortex | 0.37 | ||
| 9 | Dorsolateral prefrontal cortex | 0.12 | ||||
| 44 | Pars opercularis (Broca’s area) | 0.00 | ||||
| R 22 | 57, , 46 (19) | R angular gyrus | 0.96 | 39 | Angular gyrus (Wernicke’s area) | 0.48 |
| R supramarginal gyrus | 0.04 | 40 | Supramarginal gyrus (Wernicke’s area) | 0.48 | ||
| 22 | Superior temporal gyrus | 0.02 | ||||
| 7 | Somatosensory association cortex | 0.01 | ||||
Note: Data for CH 10 and CH 22 of the right hemisphere. For Montreal Neurological Institute (MNI) coordinates, the most likely values are submitted with standard deviation in units of millimeter. Macroanatomical estimation is based on LBPA40. Brodmann area estimation is based on MRIcro. SD, standard deviation; Prob, probability.
Effects of medications were investigated between control and premedicated ADHD subjects, between control and postplacebo ADHD subjects, and between control and post-ATX ADHD subjects (Table 4). In the right CH 10, oxy-Hb signal in control subjects was significantly higher than that in premedicated (paired t test, , Bonferroni-corrected, Cohen’s ) and postplacebo (paired t test, , Bonferroni-corrected, Cohen’s ) ADHD subjects, whereas no significant difference was found in control and post-ATX ADHD subjects (paired t test, , Cohen’s ; Table 4). This indicates that the impaired right prefrontal activation was normalized by ATX administration. In the right CH 22, oxy-Hb signal in control subjects was marginally higher than that in premedicated ADHD subjects (paired t test, ,uncorrected, Cohen’s ), but no significant difference was found among control, postplacebo ADHD (paired t test, , Cohen’s ), and post-ATX ADHD subjects (paired t test, , Cohen’s ; Table 4). This suggests that right parietal activation was normalized by the effect of ATX, while a placebo effect was also present.
Finally, we examined whether there were ATX- or placebo-induced right prefrontal and parietal activations in ADHD subjects. In the intermedication contrast, we found significant activations in the right CH 10 (paired t test, , Cohen’s ) and the right CH 22 (paired t test, , Cohen’s ; Table 6). These results suggests that (1) the oxy-Hb signal increase during oddball tasks in CH 10 was induced by ATX but not by the placebo and (2) a signal increase in CH 22 was induced by both ATX and the placebo but the effect of ATX was greater than that of the placebo.
Table 6.
Functional data for ADHD intermedication ( versus ) comparison.
| minus | versus | |||
|---|---|---|---|---|
| Mean | SD | |||
| Right CH 10 () | 0.127 | 0.123 | 3.998 | 0.001** |
| Right CH 22 () | 0.048 | 0.075 | 2.452 | 0.028* |
Note: Functional data for intermedication comparisons (i.e., versus ) are shown for ADHD subjects. Mean values were calculated by first subtracting the values of from those of for each subject and then averaging the resulting values across subjects. SD was calculated similarly. values, values, and statistical significance were the results of two-sample t tests between and . , the difference between post- and pre-ATX; , the difference between post and preplacebo; SD, standard deviation; , value; , value; CH, channel. Statistical significances are presented as follows: *; **; and ns, not significant.
3.3. Oxy-Hb Timeline Data
The grand-average waveforms for all 15 control subjects and 15 ADHD subjects can be seen in Fig. 3. For ADHD, the oxy-Hb and deoxy-Hb signals for pre-/postplacebo and pre-/post-ATX conditions for CH 10 and CH 22 of the right hemisphere are displayed. While task-related changes were stably observed in oxy-Hb signals, they were insufficiently visible in deoxy-Hb signals, demonstrating the robustness of oxy-Hb signals for our experimental conditions. Oxy-Hb increases in the right CH 10 and 22 were clearly observed for control subjects and for post-ATX ADHD subjects in the grand-average waveforms.
Fig. 3.
Waveforms of oxy-Hb (red lines) and deoxy-Hb (blue lines) signals for right CH 10 and right CH 22.
4. Discussion
4.1. Overview
The current study used fNIRS to explore the neural substrate for the effects of ATX medication on attentional control in school-aged ADHD children. First, as in our previous studies, we confirmed that healthy control subjects performing an oddball task reflecting attentional functions recruited the right IFG/MFG and IPL. Second, ATX administration normalized the right IFG/MFG and IPL activation in age-, gender-, and IQ-matched ADHD children. The intermedication comparison further validated this normalization effect. These results confer a clear answer to our experimental question: in attentional control, ATX-mediated normalization is not limited to right prefrontal dysfunctions, but also extends to parietal dysfunctions.
4.2. Behavioral Performance for Oddball Task
An oddball task is commonly used to assess attentional function. It generally requires subjects to extract an infrequently presented target (oddball) out of a sequence of frequently presented standard stimuli.57 Oddball tasks are considered to involve top-down attention drawn to standard stimuli as well as additional selective attention targeted toward deviant events that may interfere with ongoing focused attention. However, the deviation is normally transient, and top-down attention is restored for ongoing stimuli after evaluation of the deviant event.
Various parameters available in the oddball paradigm enable the evaluation of detailed facets of attentional controls.58–65 Inattention is reflected in omission errors (failure to respond to the target). On the other hand, impulsivity is reflected in commission errors (failure to respond appropriately to the nontarget), as well as in RT for nontarget responses.
However, in the current study, we did not detect normalization or upregulation of all behavioral parameters (average RT for correct trials, commission error rates, and omission error rates for target trials; see Tables 2 and 3). Thus far, we have observed inconsistency in behavioral data for ADHD children: our previous studies showed performance impairment in ADHD children compared with control subjects.27 However, our fNIRS studies have consistently exhibited hypoactivation in the right MFG/IFG and right IPL in premedicated ADHD children without corresponding behavioral effects.
Taken together, these results suggest that although behavioral parameters may often well reflect specific cognitive aspects of ADHD symptoms or ATX effects on those symptoms, consistent confirmation of the ATX effect cannot be made with behavioral parameters. Since, to date, there are no studies employing ATX-related neuroimaging during oddball tasks, further investigation is needed in order to gauge the reliability of behavioral parameters in neuroimaging examinations.
4.3. fNIRS Examination of Oddball Task and ATX Effects
A wealth of studies using different modalities, including fMRI, electroencephalography (EEG), and event-related potential (ERP), have explored the neural correlates of attentional control using oddball tasks.57 Generally, examinations of healthy subjects have demonstrated that oddball tasks elicit several brain activation points in a network involving the bilateral superior, inferior, and dorsolateral prefrontal cortices, the supplementary motor area, the anterior cingulate gyrus, the parietal and temporal lobes, the caudate nucleus, and the amygdala (e.g., Refs. 66 and 67).
Among these regions, our fNIRS measurements detected cortical activations during an oddball task at the border of the right MFG, IFG (BA 9/44/45/46), and angular gyrus (BA 39) in control subjects. Previous studies have demonstrated that the MFG, IFG, and angular gyrus constitute the attentional system and have extensive reciprocal connections.68,69 These networks are thought to play an important part in the executive control needed to guide goal-directed and stimulus-driven attention.70 There are also many recent fMRI and ERP studies of healthy adults, providing experimental evidence for involvement of the prefrontal and parietal networks using oddball tasks.57,67,71–78 Thus, we can conclude that our current fNIRS-based study appropriately detected activations in the attentional network between the prefrontal and parietal cortices in control subjects.
On the other hand, an fMRI study of ADHD children in comparison to control children has indicated reduced functional connectivity among the IFC, basal ganglia, parietal cortices, and cerebellum during sustained attention,79 which may indicate that dysfunctions are not limited to specific brain regions, but involve the whole fronto-striato-parieto-cerebellar network underlying sustained attention processes.
In the current study, we confirmed the absence of right fronto-parietal activations in premedicated ADHD children, as in our previous study.27 These observations coincide with the results of an fMRI study80 by indicating reduced activation and functional interconnectivity in the bilateral fronto-striato-parieto-cerebellar networks during a continuous performance task under a placebo condition for ADHD children. Thus, our results add to the experimental evidence for dysfunction of the attention-associated regions in ADHD children.
The impaired right prefrontal and inferior parietal activations were significantly normalized by ATX administration in ADHD children. Although a placebo effect was found in the parietal normalization, we confirmed that the effects of ATX significantly surpassed those of the placebo as revealed by the intermedication contrasts both in the right IFG/MFG and IPL channels (Table 4). Thus, the current results suggest that ATX administration leads to functional normalization of right prefrontal and inferior parietal activations of the attention network in ADHD children.
By comparison, in our previous study using the same protocol, MPH-induced functional normalization of attentional control in ADHD children was associated solely with right prefrontal activation, but did not extend to the inferior parietal cortex to activate wider components of the attention network. In addition, the failure of MPH to affect the inferior parietal regions is not limited to our former study. Shafritz et al. (2004) reported that the reduced middle temporal activation in ADHD adolescents compared to control subjects was not normalized by MPH administration.81
The differences between ATX- and MPH-induced normalization appear relevant when considering the pharmacological effects of both substances. ATX, an approved nonstimulant medication used to treat ADHD, is a selective NA reuptake inhibitor21 with a predominantly higher affinity to DA than to NA transporters.19 Given the prevalence of NA neurons in the right IFG/MFG and IPL innervating from the locus coeruleus,22,27 it appears relevant that normalization of cortical activation in ADHD children occurs in the right IPL with ATX administration.
On the other hand, MPH is known to affect both DA and NA systems,82,83 but has a 10-fold higher affinity to DA than to NA receptors,19 resulting in by far larger effects on the DA system. Given the predominant distribution of DA neurons in the right IFG/MFG reflecting the fronto-striatal DA system but not in the right IPL,20,22 it is reasonable that MPH-induced normalization of the cortical activation in ADHD children occurs solely in the right IFG/MFG.
We could conclude that, when taken together, the current fNIRS results illustrate the specific neuropharmacological mechanism of ATX underlying the functional normalization of the attention network components. Furthermore, these results extend the conclusions of our previous fNIRS studies revealing ATX- and MPH-induced normalization in the right MFG/IFG:25,26,28 fNIRS-based measurement can distinguish between neural substrates of the DA and NA systems differentially involved in inhibitory and attentional controls.
4.4. Placebo Effect in the Right IPL
Interestingly, we observed a placebo effect in the right IPL. In the right CH 22, while the oxy-Hb signal in control subjects was marginally higher than that in premedicated (paired t test, , uncorrected, Cohen’s ), no significant difference was found either between control and postplacebo ADHD (paired t test, , Cohen’s ) or between control and post-ATX ADHD subjects (paired t test, , Cohen’s ) (Table 4). In addition, the effect sizes (ES: Cohen’s ) were examined. For the postplacebo contrast, oddball versus baseline in the right CH 22 had an ES of 0.428 (right CH 10: ), and for the intramedication contrasts, post- versus preplacebo in the right CH 22 had an ES of 0.140 (right CH 10: ). However, the effects of ATX were greater than those of the placebo, and thus, confirmed the effect of ATX, a placebo effect was present.
This observation of a placebo effect was novel within the series of our studies employing double-blind, placebo-controlled, crossover designs.26–28 Also, former fMRI studies with similar experimental designs have not observed placebo effects. However, EEG studies on psychostimulants84 and antidepressants85 have demonstrated that the placebo effect is present in of patients. Although elucidation of the specific reason for placebo-induced neuroactivation is beyond the scope of the current study, the current results lead us to believe that when exploring pharmacological effects using neuroimaging modalities, double-blinded, placebo-controlled studies should be adopted to exclude placebo effects.
5. Conclusion
The current study examining the effects of ATX administration on the attentional control of ADHD children using a double-blind, placebo-controlled, crossover design provided the following major findings: (1) Impaired right IFG/MFG and IPL activation was acutely normalized after ATX administration in ADHD subjects. (2) Compared to placebo-induced activation, ATX-induced right IFG/MFG and IPL activations were significantly greater. These experimental results are consistent with the neuropharmacological effects of ATX to upregulate the NA system in the locus coeruleus noradrenergic system associated with attentional function in the right IFG/MFG and IPL. This is in contrast to the effects of MPH on attentional control, which induced only right prefrontal activation and may reflect upregulated functions of the DA system in the right prefrontal cortex. These findings led us to conclude that fNIRS-based measurement is sufficiently sensitive to dissociate the neuropharmacological functional differences of ATX and MPH during different cognitive operations, at least at a group level. Furthermore, activation in the right IFG/MFG and IPL could serve as an objective neurofunctional biomarker to indicate the effects of ATX, as well as MPH in the case of IFG/MFG activation, on attentional and inhibitory controls in ADHD children. Further exploration with individual-level analysis will strengthen the clinical utility of fNIRS-based measurement for the functional, neuropharmacological monitoring of ADHD children.
Acknowledgments
We appreciate ELCS for English proofreading. We thank Illpop (http://illpop.com/animal_top01.htm) for kindly providing source pictures for experimental materials. We appreciate Qualicaps Co., Ltd. for providing capsules for making placebos. This work was supported in part by the Grant-in-Aid for Scientific Research from the Japan Society for Promotion of Science (24300105 and 25282243 to I.D., 23390354 to E.W., 23700885 to H.D., 80382951 to Y.M., and 70438662 to M.N.), and Health and Labor Sciences Research Grants, Research on Psychiatric and Neurological Diseases and Mental Health (to I.D.).
Biographies
Masako Nagashima received her medical degree from Japan in 2004. She was a resident from 2004 to 2009 and has been a research associate since 2009 in the Department of Pediatrics, Jichi Medical University in Tochigi, Japan. Also, she was a chief of inpatient medicine at the International University of Health and Welfare, Japan, from 2012 to 2013. Her research focuses on neuroimaging studies of neurodevelopmental disorders.
Yukifumi Monden received his medical degree from Japan in 2002 and his PhD from Jichi Medical University, Japan, in 2013. His major professional achievements include being the chief intern at the U.S. Naval Hospital, Okinawa, Japan, from 2006 to 2007 and a clinical associate from 2009 to 2012. He is currently an assistant professor in the Department of Pediatrics, Jichi Medical University in Tochigi, Japan. His research focuses on neuroimaging studies of neurodevelopmental disorders.
Ippeita Dan graduated from International Christian University in 1993 and received his PhD from the University of Tokyo, Japan, in 2002. He was a senior research fellow at the National Food Research Institute and an associate professor at Jichi Medical University, Japan. He was appointed as a professor at Chuo University, Tokyo, Japan. He has authored 70 peer-reviewed articles attracting over 2500 citations. His main research missions lie in clinical application of functional near-infrared spectroscopy (fNIRS), methodological development of fNIRS data analyses, and application of psychometrics for marketing in food-related business.
Haruka Dan received her MA in 2001 from International Christian University and her PhD in 2005 from Tohoku University, Japan. She was a postdoctoral fellow at the National Food Research Institute and Jichi Medical University, Japan. She is now a visiting assistant professor of Research and Development Initiatives at Chuo University, Japan. She has authored 35 peer-reviewed scientific articles, including 20 top-authored contributions over various topics ranging from plant physiology, behavioral food science, and cognitive neuroscience.
Tsutomu Mizutani received his BA in psychology in 2006 from Rissho University, his MA in education in 2008 from Ibaraki University, and his PhD in psychology in 2012 from Rissho University, Japan. He is a postdoctoral fellow (2012 to 2014) at Jichi Medical University, Japan. He is currently involved in studies of fNIRS-based examination of children with neurodevelopmental disorders, and patients with epilepsy, as well as presurgical functional examination of various brain disorders.
Daisuke Tsuzuki received his BA in 2000 and his MA in 2002 from Nihon University, and his PhD in 2012 from the University of Tsukuba, Japan. He was a research resident (2011 to 2012) and postdoctoral fellow (2012 to 2013) at Jichi Medical University, Japan. He is currently an assistant professor in the Research and Development Initiatives, Chuo University in Tokyo, Japan. His research interests include studies on computer science, information visualization, and spatial analysis of brain function.
Yasushi Kyutoku received his BA in 2001 from the University of Texas at San Antonio, and his MSc in 2007 and his PhD in 2008 from the University of Texas at Arlington, TX, USA. He was a postdoctoral fellow in Japan at the National Food Research Institute from 2009 to 2010 and at Jichi Medical University from 2010 to 2012. Currently, he is an associate professor at Research and Development Initiative, Chuo University, Tokyo, Japan. His research focuses on psychometrics and their applications.
Yuji Gunji received his medical degree from Japan in 1985 and his PhD from Jichi Medical University, Japan, in 1992. He was a research fellow at the Molecular/Cancer Biology Laboratory, Haartman Institute, Republic of Finland, from 1996 to 1999. He was an assistant professor from 1994 to 2006 and is currently an associate professor in the Department of Pediatrics, Jichi Medical University (2006 to 2014). He is also a professor at the International University of Health and Welfare, Japan (since 2008). His research interests include studies on the role of angiogenesis in the pathogenesis of cancer.
Daisuke Hirano graduated from the International University of Health and Welfare, Japan, in 2004. He received his doctor of philosophy in health sciences from Japan in 2009. He was an occupational therapist at Nasu Institute for Developmental Disabilities from 2004 to 2010 and is currently an associate professor in the Department of Pediatrics, International University of Health and Welfare (since 2010). His research interests include studies on fNIRS studies of individuals with severe intellectual motor disabilities.
Takamichi Taniguchi graduated from National Hospital of Tokyo School of Rehabilitation, Japan, in 1988. He is an occupational therapist and received the degree of doctor of engineering from Nihon University, Japan, in 2000. He is currently a professor and chair in the Department of Occupational Therapy School of Health Science, International University of Health and Welfare in Tochigi, Japan. His specialty is occupational therapy of developmental disabilities.
Hideo Shimoizumi received his medical degree from Japan in 1982 and his PhD from Jichi Medical University, Japan, 1992. He is currently a director in International Medical and Welfare Rehabilitation Center and a professor in International University of Health and Welfare in Tochigi, Japan. His research focuses on rehabilitation of developmental disabilities.
Mariko Y. Momoi received her medical degree from Japan in 1973 and her PhD from Tokyo University, Japan, in 1982. Her professional achievements include being research associate in the Department of Neurology, Mayo Clinic, from 1980 to 1982 and professor in the Department of Pediatrics, Jichi Medical University, from 1994 to 2013. She is currently vice president of the International University of Health and Welfare, Japan, and her research interest is the molecular neuroscience of autism spectrum disorder.
Takanori Yamagata received his medical degree from Japan in 1986 and his PhD from Jichi Medical University, Japan, in 1993. He was a research associate in the Department of Molecular and Human Genetics, Baylor College of Medicine, USA, from 1997 to 2000. He is currently a professor and the chairman of the Department of Pediatrics, Jichi Medical University, Japan. His research interests include studies on the molecular pathogenesis of autism spectrum disorder, a neurodevelopmental disorder.
Eiju Watanabe received his MD in 1976 and his PhD in 1984 from the University of Tokyo, Japan. His major career achievements include being assistant professor in the Department of Neurosurgery at the University of Tokyo, research fellow at the University of Erlangen, West Germany, and chairman of the Department of Neurosurgery at Tokyo Metropolitan Police Hospital. He is currently a professor and the chairman of the Department of Neurosurgery at Jichi Medical University, Japan. He is known for his work as an inventor of a neuronavigation system and an initiator of fNIRS imaging known as optical topography.
Appendix.
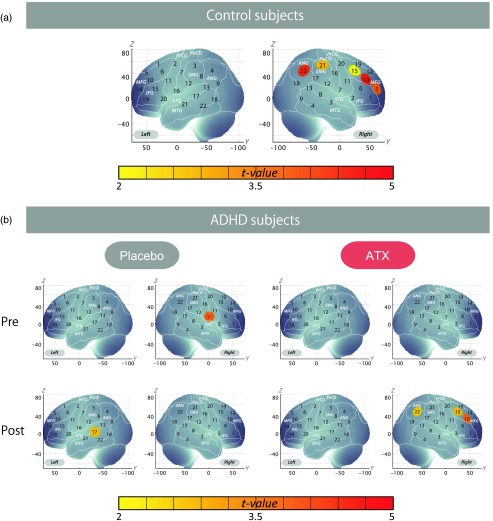
Figure 4 demonstrates the cortical activation pattern of control subjects and ADHD subjects (uncorrected). Table 7 shows the spatial profiles of the channels screened for involvement with oddball tasks (uncorrected).
Fig. 4.
Cortical activation patterns of control subjects and attention deficit hyperactivity disorder subjects (uncorrected). (a) Control subjects. (b) ADHD subjects.
Table 7.
Spatial profiles of the channels screened for involvement with oddball tasks (uncorrected).
| Montreal Neurological Institute coordinates | ||||||
|---|---|---|---|---|---|---|
| CH | , , (SD) | Macroanatomy | Prob | Brodmann area | Prob | |
| L 17 | , , 4 (14) | L middle temporal gyrus | 0.51 | 22 | Superior temporal gyrus | 0.53 |
| L superior temporal gyrus | 0.49 | 21 | Middle temporal gyrus | 0.43 | ||
| 20 | Inferior temporal gyrus | 0.04 | ||||
| 42 | Primary and auditory association cortex | 0.00 | ||||
| R 5 | 39, 61, 17 (13) | R middle frontal gyrus | 0.83 | 46 | Dorsolateral prefrontal cortex | 0.52 |
| R inferior frontal gyrus | 0.18 | 10 | Frontopolar area | 0.48 | ||
| 45 | pars triangularis Broca’s area | 0.00 | ||||
| R 7 | 68, 1, 16 (16) | R postcentral gyrus | 0.4 | 43 | Subcentral area | 0.3 |
| R precentral gyrus | 0.4 | 6 | Premotor and supplementary motor cortex | 0.3 | ||
| R superior temporal gyrus | 0.2 | 48 | Retrosubicular area | 0.2 | ||
| 22 | Superior temporal gyrus | 0.1 | ||||
| 44 | pars opercularis, part of Broca’s area | 0.0 | ||||
| 4 | Primary motor cortex | 0.0 | ||||
| 21 | Middle temporal gyrus | 0.0 | ||||
| R 10 | 46, 44, 31 (15) | R middle frontal gyrus | 0.78 | 45 | pars triangularis Broca’s area | 0.50 |
| R inferior frontal gyrus | 0.22 | 46 | Dorsolateral prefrontal cortex | 0.37 | ||
| 9 | Dorsolateral prefrontal cortex | 0.12 | ||||
| 44 | pars opercularis, part of Broca’s area | 0.00 | ||||
| R 15 | 49, 25, 46 (17) | R middle frontal gyrus | 0.88 | 9 | Dorsolateral prefrontal cortex | 0.53 |
| R precentral gyrus | 0.09 | 44 | pars opercularis, part of Broca’s area | 0.27 | ||
| R inferior frontal gyrus | 0.04 | 45 | pars triangularis Broca’s area | 0.11 | ||
| 6 | Premotor and supplementary motor cortex | 0.05 | ||||
| 46 | Dorsolateral prefrontal cortex | 0.03 | ||||
| 8 | Includes frontal eye fields | 0.01 | ||||
| R 21 | 59, , 55 (19) | R supramarginal gyrus | 0.71 | 40 | Supramarginal gyrus part of Wernicke’s area | 0.32 |
| R postcentral gyrus | 0.18 | 1 | Primary somatosensory cortex | 0.26 | ||
| R angular gyrus | 0.09 | 3 | Primary somatosensory cortex | 0.21 | ||
| R superior parietal gyrus | 0.02 | 4 | Primary motor cortex | 0.12 | ||
| 2 | Primary somatosensory cortex | 0.08 | ||||
| 6 | Premotor and supplementary motor cortex | 0.01 | ||||
| R 22 | 57, , 46 (19) | R angular gyrus | 0.96 | 39 | Angular gyrus, part of Wernicke’s area | 0.48 |
| R supramarginal gyrus | 0.04 | 40 | Supramarginal gyrus part of Wernicke’s area | 0.48 | ||
| 22 | Superior temporal gyrus | 0.02 | ||||
| 7 | Somatosensory association cortex | 0.01 | ||||
Note: Data for CH 17 of the left hemisphere and for CH 5, 10, 15, 21, and 22 of the right hemisphere. For MNI coordinates, the most likely values are presented with standard deviation in units of millimeter. Macroanatomical estimation is based on LBPA40. Brodmann area estimation is based on MRIcro. CH, channel; SD, standard deviation; Prob, probability; L, left hemisphere; R, right hemisphere.
Cortical activation patterns of control subjects [Fig. 4(a)] and ADHD subjects [Fig. 4(b)] are shown as maps of oxy-Hb signal, with significant values (one-sample t test, uncorrected) being shown according to the color bar.
We screened for any channels involved in the oddball task for the control subjects. A significant increase was found in five channels on the right hemisphere (CH 5, 10, 15, 21, and 22). Among these channels, the right CH 10 in the right prefrontal and CH 22 in the right parietal cortices exhibited the most significant activation and were the only channels remaining after family-wise error correction (CH 10, mean 0.074, SD 0.080, , Bonferroni-corrected, Cohen’s and CH 22, mean 0.050, SD 0.077, , Bonferroni-corrected, Cohen’s ). Therefore, the right CH 10 and CH 22 were determined as the channels of interest in the main analyses. In ADHD subjects, significant ATX effects on the oxy-Hb increase were found in three channels on the right hemisphere (CH 10, 15, and 22). We found that no channels were activated in the pre-ATX condition, but that the right post- and precentral gyri (CH 7, , uncorrected) were activated in the preplacebo condition. In addition, the left middle and superior temporal gyri were significantly activated in the postplacebo condition (CH 17, , uncorrected), while significant activation was found in the right prefrontal and parietal regions in the post-ATX condition (CH 10, 15, and 22, , uncorrected). The marginal activations in the right inferior and middle temporal gyri in the pre-/postplacebo condition might reflect compensatory or placebo activations specific to ADHD children, but further studies are necessary to examine the reproducibility and underlying functional mechanisms of these results.
References
- 1.Dittmann R. W., et al. , “Atomoxetine treatment and ADHD-related difficulties as assessed by adolescent patients, their parents and physicians,” Child Adolesc. Psychiatry Ment. Health 3(1), 21 (2009). 10.1186/1753-2000-3-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merikangas K. R., et al. , “Prevalence and treatment of mental disorders among US children in the 2001–2004 NHANES,” Pediatrics 125(1), 75–81 (2010). 10.1542/peds.2008-2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biederman J., et al. , “Young adult outcome of attention deficit hyperactivity disorder: a controlled 10-year follow-up study,” Psychol. Med. 36(2), 167–179 (2006). 10.1017/S0033291705006410 [DOI] [PubMed] [Google Scholar]
- 4.Barkley R. A., et al. , “The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder,” J. Abnorm. Psychol. 111(2), 279–289 (2002). 10.1037/0021-843X.111.2.279 [DOI] [PubMed] [Google Scholar]
- 5.Lahey B. B., et al. , “The structure of child and adolescent psychopathology: generating new hypotheses,” J. Abnorm. Psychol. 113(3), 358–385 (2004). 10.1037/0021-843X.113.3.358 [DOI] [PubMed] [Google Scholar]
- 6.Drechsler R., et al. , “The course of neuropsychological functions in children with attention deficit hyperactivity disorder from late childhood to early adolescence,” J. Child Psychol. Psychiatry 46(8), 824–836 (2005). 10.1111/j.1469-7610.2004.00384.x [DOI] [PubMed] [Google Scholar]
- 7.Classi P., et al. , “Social and emotional difficulties in children with ADHD and the impact on school attendance and healthcare utilization,” Child Adolesc. Psychiatry Ment. Health 6(1), 33 (2012). 10.1186/1753-2000-6-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mannuzza S., et al. , “Educational and occupational outcome of hyperactive boys grown up,” J. Am. Acad. Child Adolesc. Psychiatry 36(9), 1222–1227 (1997). 10.1097/00004583-199709000-00014 [DOI] [PubMed] [Google Scholar]
- 9.Chronis-Tuscano A., et al. , “Very early predictors of adolescent depression and suicide attempts in children with attention-deficit/hyperactivity disorder,” Arch. Gen. Psychiatry 67(10), 1044–1051 (2010). 10.1001/archgenpsychiatry.2010.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Power T. J., Shapiro E. S., DuPaul G. J., “Preparing psychologists to link systems of care in managing and preventing children’s health problems,” J. Pediatr. Psychol. 28(2), 147–155 (2003). 10.1093/jpepsy/28.2.147 [DOI] [PubMed] [Google Scholar]
- 11.Hodgkins P., et al. , “Amfetamine and methylphenidate medications for attention-deficit/hyperactivity disorder: complementary treatment options,” Eur. Child Adolesc. Psychiatry 21(9), 477–492 (2012). 10.1007/s00787-012-0286-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cubillo A., et al. , “Shared and drug-specific effects of atomoxetine and methylphenidate on inhibitory brain dysfunction in medication-naive ADHD boys,” Cereb. Cortex 24(1), 174–185 (2014). 10.1093/cercor/bhs296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faraone S. V., Buitelaar J., “Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis,” Eur. Child Adolesc. Psychiatry 19(4), 353–364 (2010). 10.1007/s00787-009-0054-3 [DOI] [PubMed] [Google Scholar]
- 14.Faraone S. V., Wigal S. B., Hodgkins P., “Forecasting three-month outcomes in a laboratory school comparison of mixed amphetamine salts extended release (Adderall XR) and atomoxetine (Strattera) in school-aged children with ADHD,” J. Atten. Disord. 11(1), 74–82 (2007). 10.1177/1087054706292196 [DOI] [PubMed] [Google Scholar]
- 15.Newcorn J. H., et al. , “Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response,” Am. J. Psychiatry 165(6), 721–730 (2008). 10.1176/appi.ajp.2007.05091676 [DOI] [PubMed] [Google Scholar]
- 16.Sallee F. R., et al. , “Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder,” J. Child Adolesc. Psychopharmacol. 19(3), 215–226 (2009). 10.1089/cap.2008.0080 [DOI] [PubMed] [Google Scholar]
- 17.Gatley S. J., et al. , “Affinities of methylphenidate derivatives for dopamine, norepinephrine and serotonin transporters,” Life Sci. 58(12), 231–239 (1996). 10.1016/0024-3205(96)00052-5 [DOI] [PubMed] [Google Scholar]
- 18.Aron A. R., Poldrack R. A., “The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder,” Biol. Psychiatry 57(11), 1285–1292 (2005). 10.1016/j.biopsych.2004.10.026 [DOI] [PubMed] [Google Scholar]
- 19.Bymaster F. P., et al. , “Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder,” Neuropsychopharmacology 27(5), 699–711 (2002). 10.1016/S0893-133X(02)00346-9 [DOI] [PubMed] [Google Scholar]
- 20.Faraone S. V., Biederman J., “Neurobiology of attention-deficit hyperactivity disorder,” Biol. Psychiatry 44(10), 951–958 (1998). 10.1016/S0006-3223(98)00240-6 [DOI] [PubMed] [Google Scholar]
- 21.Bolden-Watson C., Richelson E., “Blockade by newly-developed antidepressants of biogenic amine uptake into rat brain synaptosomes,” Life Sci. 52(12), 1023–1029 (1993). 10.1016/0024-3205(93)90194-8 [DOI] [PubMed] [Google Scholar]
- 22.Singh-Curry V., Husain M., “The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy,” Neuropsychologia 47(6), 1434–1448 (2009). 10.1016/j.neuropsychologia.2008.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Logan J., et al. , “Imaging the norepinephrine transporter in humans with (S,S)-[11C]O-methyl reboxetine and PET: problems and progress,” Nucl. Med. Biol. 34(6), 667–679 (2007). 10.1016/j.nucmedbio.2007.03.013 [DOI] [PubMed] [Google Scholar]
- 24.Johnston B. A., et al. , “Brainstem abnormalities in attention deficit hyperactivity disorder support high accuracy individual diagnostic classification,” Hum. Brain Mapp. 35(10), 5179–5189 (2014). 10.1002/hbm.22542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monden Y., et al. , “Clinically-oriented monitoring of acute effects of methylphenidate on cerebral hemodynamics in ADHD children using fNIRS,” Clin. Neurophysiol. 123(6), 1147–1157 (2012). 10.1016/j.clinph.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 26.Monden Y., et al. , “Right prefrontal activation as a neuro-functional biomarker for monitoring acute effects of methylphenidate in ADHD children: an fNIRS study,” NeuroImage Clin. 1(1), 131–140 (2012). 10.1016/j.nicl.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagashima M., et al. , “Neuropharmacological effect of methylphenidate on attention network in children with attention deficit hyperactivity disorder during oddball paradigms as assessed using functional near-infrared spectroscopy,” Neurophotonics 1(1), 015001 (2014). 10.1117/1.NPh.1.1.015001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagashima M., et al. , “Acute neuropharmacological effects of atomoxetine on inhibitory control in ADHD children: an fNIRS study,” NeuroImage Clin. 6, 192–201 (2014). 10.1016/j.nicl.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durston S., et al. , “Differential patterns of striatal activation in young children with and without ADHD,” Biol. Psychiatry 53(10), 871–878 (2003). 10.1016/S0006-3223(02)01904-2 [DOI] [PubMed] [Google Scholar]
- 30.Yerys B. E., et al. , “The fMRI success rate of children and adolescents: typical development, epilepsy, attention deficit/hyperactivity disorder, and autism spectrum disorders,” Hum. Brain Mapp. 30(10), 3426–3435 (2009). 10.1002/hbm.v30:10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weissman D. H., Mangun G. R., Woldorff M. G., “A role for top-down attentional orienting during interference between global and local aspects of hierarchical stimuli,” NeuroImage 17(3), 1266–1276 (2002). 10.1006/nimg.2002.1284 [DOI] [PubMed] [Google Scholar]
- 32.Kiehl K. A., et al. , “An adaptive reflexive processing model of neurocognitive function: supporting evidence from a large scale () fMRI study of an auditory oddball task,” NeuroImage 25(3), 899–915 (2005). 10.1016/j.neuroimage.2004.12.035 [DOI] [PubMed] [Google Scholar]
- 33.Cubillo A., et al. , “Shared and drug-specific effects of atomoxetine and methylphenidate on inhibitory brain dysfunction in medication-naive ADHD boys,” Cereb. Cortex 24(1), 174–185 (2014). 10.1093/cercor/bhs296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulz K. P., et al. , “Common and unique therapeutic mechanisms of stimulant and nonstimulant treatments for attention-deficit/hyperactivity disorder,” Arch. Gen. Psychiatry 69(9), 952–961 (2012). 10.1001/archgenpsychiatry.2011.2053 [DOI] [PubMed] [Google Scholar]
- 35.Barry R. J., et al. ,” Event-related potentials in adults with attention-deficit/hyperactivity disorder: an investigation using an inter-modal auditory/visual oddball task,” Int. J. Psychophysiol. 71(2), 124–131 (2009). 10.1016/j.ijpsycho.2008.09.009 [DOI] [PubMed] [Google Scholar]
- 36.Bernat E., Shevrin H., Snodgrass M., “Subliminal visual oddball stimuli evoke a P300 component,” Clin. Neurophysiol. 112(1), 159–171 (2001). 10.1016/S1388-2457(00)00445-4 [DOI] [PubMed] [Google Scholar]
- 37.Guntekin B., Saatci E., Yener G., “Decrease of evoked delta, theta and alpha coherences in Alzheimer patients during a visual oddball paradigm,” Brain Res. 1235, 109–116 (2008). 10.1016/j.brainres.2008.06.028 [DOI] [PubMed] [Google Scholar]
- 38.Houlihan M. E., Pritchard W. S., Robinson J. H., “Faster P300 latency after smoking in visual but not auditory oddball tasks,” Psychopharmacology 123(3), 231–238 (1996). 10.1007/BF02246577 [DOI] [PubMed] [Google Scholar]
- 39.Kayser J., Tenke C. E., Bruder G. E., “Dissociation of brain ERP topographies for tonal and phonetic oddball tasks,” Psychophysiology 35(5), 576–590 (1998). 10.1017/S0048577298970214 [DOI] [PubMed] [Google Scholar]
- 40.Paller K. A., et al. , “Potentials evoked in human and monkey medial temporal lobe during auditory and visual oddball paradigms,” Electroencephalogr. Clin. Neurophysiol. 84(3), 269–279 (1992). 10.1016/0168-5597(92)90008-Y [DOI] [PubMed] [Google Scholar]
- 41.Struber D., Polich J., “P300 and slow wave from oddball and single-stimulus visual tasks: inter-stimulus interval effects,” Int. J. Psychophysiol. 45(3), 187–196 (2002). 10.1016/S0167-8760(02)00071-5 [DOI] [PubMed] [Google Scholar]
- 42.Wang W., Schoenen J., Timsit-Berthier M., “Cognitive functions in migraine without aura between attacks: a psychophysiological approach using the ‘oddball’ paradigm,” Neurophysiol. Clin. 25(1), 3–11 (1995). 10.1016/0987-7053(96)81029-X [DOI] [PubMed] [Google Scholar]
- 43.Cope M., et al. , “Methods of quantitating cerebral near infrared spectroscopy data,” Adv. Exp. Med. Biol. 222, 183–189 (1988). 10.1007/978-1-4615-9510-6 [DOI] [PubMed] [Google Scholar]
- 44.Maki A., et al. , “Spatial and temporal analysis of human motor activity using noninvasive NIR topography,” Med. Phys. 22(12), 1997–2005 (1995). 10.1118/1.597496 [DOI] [PubMed] [Google Scholar]
- 45.Garavan H., Ross T. J., Stein E. A., “Right hemispheric dominance of inhibitory control: an event-related functional MRI study,” Proc. Natl. Acad. Sci. USA 96(14), 8301–8306 (1999). 10.1073/pnas.96.14.8301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herrmann M. J., et al. , “Optical topography during a Go-NoGo task assessed with multi-channel near-infrared spectroscopy,” Behav. Brain Res. 160(1), 135–140 (2005). 10.1016/j.bbr.2004.11.032 [DOI] [PubMed] [Google Scholar]
- 47.Herrmann M. J., Ehlis A. C., Fallgatter A. J., “Bilaterally reduced frontal activation during a verbal fluency task in depressed patients as measured by near-infrared spectroscopy,” J. Neuropsychiatry Clin. Neurosci. 16(2), 170–175 (2004). 10.1176/appi.neuropsych.16.2.170 [DOI] [PubMed] [Google Scholar]
- 48.Liddle P. F., Kiehl K. A., Smith A. M., “Event-related fMRI study of response inhibition,” Hum. Brain Mapp. 12(2), 100–109 (2001). 10.1002/(ISSN)1097-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubia K., et al. , “Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection,” NeuroImage 20(1), 351–358 (2003). 10.1016/S1053-8119(03)00275-1 [DOI] [PubMed] [Google Scholar]
- 50.Tsuzuki D., et al. , “Virtual spatial registration of stand-alone fNIRS data to MNI space,” NeuroImage 34(4), 1506–1518 (2007). 10.1016/j.neuroimage.2006.10.043 [DOI] [PubMed] [Google Scholar]
- 51.Tsuzuki D., Dan I., “Spatial registration for functional near-infrared spectroscopy: from channel position on the scalp to cortical location in individual and group analyses,” NeuroImage 85(Pt 1), 92–103 (2014). 10.1016/j.neuroimage.2013.07.025 [DOI] [PubMed] [Google Scholar]
- 52.Singh A. K., Dan I., “Exploring the false discovery rate in multichannel NIRS,” NeuroImage 33(2), 542–549 (2006). 10.1016/j.neuroimage.2006.06.047 [DOI] [PubMed] [Google Scholar]
- 53.Shattuck D. W., et al. , “Construction of a 3D probabilistic atlas of human cortical structures,” NeuroImage 39(3), 1064–1080 (2008). 10.1016/j.neuroimage.2007.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rorden C., Brett M., “Stereotaxic display of brain lesions,” Behav. Neurol. 12(4), 191–200 (2000). 10.1155/2000/421719 [DOI] [PubMed] [Google Scholar]
- 55.Katagiri A., et al. , “Mapping of optical pathlength of human adult head at multi-wavelengths in near infrared spectroscopy,” Adv. Exp. Med. Biol. 662, 205–212 (2010). 10.1007/978-1-4419-1241-1 [DOI] [PubMed] [Google Scholar]
- 56.Schecklmann M., et al. , “Diminished prefrontal oxygenation with normal and above-average verbal fluency performance in adult ADHD,” J. Psychiatr. Res. 43(2), 98–106 (2008). 10.1016/j.jpsychires.2008.02.005 [DOI] [PubMed] [Google Scholar]
- 57.Bledowski C., et al. , “Attentional systems in target and distractor processing: a combined ERP and fMRI study,” Neuroimage 22(2), 530–540 (2004). 10.1016/j.neuroimage.2003.12.034 [DOI] [PubMed] [Google Scholar]
- 58.Epstein J. N., et al. , “Relations between continuous performance test performance measures and ADHD behaviors,” J. Abnorm. Child Psychol. 31(5), 543–554 (2003). 10.1023/A:1025405216339 [DOI] [PubMed] [Google Scholar]
- 59.Nichols S. L., Waschbusch D. A., “A review of the validity of laboratory cognitive tasks used to assess symptoms of ADHD,” Child Psychiatry Hum. Dev. 34(4), 297–315 (2004). 10.1023/B:CHUD.0000020681.06865.97 [DOI] [PubMed] [Google Scholar]
- 60.Coghill D. R., et al. , “Effects of methylphenidate on cognitive functions in children and adolescents with attention-deficit/hyperactivity disorder: evidence from a systematic review and a meta-analysis,” Biol. Psychiatry S0006-3223(13), 00911–00916 (2013). 10.1016/j.biopsych.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 61.Riccio C. A., et al. , “The continuous performance test: a window on the neural substrates for attention?,” Arch. Clin. Neuropsychol. 17(3), 235–272 (2002). 10.1016/S0887-6177(01)00111-1 [DOI] [PubMed] [Google Scholar]
- 62.Riccio C. A., et al. , “Effects of stimulants on the continuous performance test (CPT): implications for CPT use and interpretation,” J. Neuropsychiatry Clin. Neurosci. 13(3), 326–335 (2001). 10.1176/appi.neuropsych.13.3.326 [DOI] [PubMed] [Google Scholar]
- 63.Newcorn J. H., et al. , “Symptom profiles in children with ADHD: effects of comorbidity and gender,” J. Am. Acad. Child Adolesc. Psychiatry 40(2), 137–146 (2001). 10.1097/00004583-200102000-00008 [DOI] [PubMed] [Google Scholar]
- 64.Barkley R. A., “The ecological validity of laboratory and analogue assessment methods of ADHD symptoms,” J. Abnorm. Child Psychol. 19(2), 149–178 (1991). 10.1007/BF00909976 [DOI] [PubMed] [Google Scholar]
- 65.Park S., et al. , “Possible effect of norepinephrine transporter polymorphisms on methylphenidate-induced changes in neuropsychological function in attention-deficit hyperactivity disorder,” Behav. Brain Funct. 8(22), 1–8 (2012). 10.1186/1744-9081-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weissman D., Mangun G., Woldorff M., “A role for top-down attentional orienting during interference between global and local aspects of hierarchical stimuli,” Neuroimage 17(3), 1266–1276 (2002). 10.1006/nimg.2002.1284 [DOI] [PubMed] [Google Scholar]
- 67.Kiehl K. A., et al. , “An adaptive reflexive processing model of neurocognitive function: supporting evidence from a large scale () fMRI study of an auditory oddball task,” Neuroimage 25(3), 899–915 (2005). 10.1016/j.neuroimage.2004.12.035 [DOI] [PubMed] [Google Scholar]
- 68.Petrides M., Pandya D. N., “Projections to the frontal cortex from the posterior parietal region in the rhesus monkey,” J. Comp. Neurol. 228(1), 105–116 (1984). 10.1002/(ISSN)1096-9861 [DOI] [PubMed] [Google Scholar]
- 69.Rushworth M. F., Passingham R. E., Nobre A. C., “Components of attentional set-switching,” Exp. Psychol. 52(2), 83–98 (2005). 10.1027/1618-3169.52.2.83 [DOI] [PubMed] [Google Scholar]
- 70.Corbetta M., Shulman G. L., “Control of goal-directed and stimulus-driven attention in the brain,” Nat. Rev. Neurosci. 3(3), 201–215 (2002). 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- 71.Adler C. M., et al. , “Changes in neuronal activation with increasing attention demand in healthy volunteers: an fMRI study,” Synapse 42(4), 266–272 (2001). 10.1002/(ISSN)1098-2396 [DOI] [PubMed] [Google Scholar]
- 72.Ardekani B. A., et al. , “Functional magnetic resonance imaging of brain activity in the visual oddball task,” Cognit. Brain Res. 14(3), 347–356 (2002). 10.1016/S0926-6410(02)00137-4 [DOI] [PubMed] [Google Scholar]
- 73.Clark V. P., et al. , “Responses to rare visual target and distractor stimuli using event-related fMRI,” J. Neurophysiol. 83(5), 3133–3139 (2000). [DOI] [PubMed] [Google Scholar]
- 74.Gur R. C., et al. , “Hemodynamic responses in neural circuitries for detection of visual target and novelty: an event-related fMRI study,” Hum. Brain Mapp. 28(4), 263–274 (2007). 10.1002/(ISSN)1097-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kiehl K. A., et al. , “An event-related fMRI study of visual and auditory oddball tasks,” J. Psychophysiol. 15(4), 221–240 (2001). 10.1027//0269-8803.15.4.221 [DOI] [Google Scholar]
- 76.Kiehl K. A., et al. , “Neural sources involved in auditory target detection and novelty processing: an event‐related fMRI study,” Psychophysiology 38(1), 133–142 (2001). 10.1111/psyp.2001.38.issue-1 [DOI] [PubMed] [Google Scholar]
- 77.Mccarthy G., et al. , “Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI,” J. Neurophysiol. 77(3), 1630–1634 (1997). [DOI] [PubMed] [Google Scholar]
- 78.Stevens A. A., et al. , “Event-related fMRI of auditory and visual oddball tasks,” Magn. Reson. Imaging 18(5), 495–502 (2000). 10.1016/S0730-725X(00)00128-4 [DOI] [PubMed] [Google Scholar]
- 79.Rubia K., et al. , “Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task,” Neuropharmacology 57(7–8), 640–652 (2009). 10.1016/j.neuropharm.2009.08.013 [DOI] [PubMed] [Google Scholar]
- 80.Rubia K., et al. , “Shared and disorder-specific prefrontal abnormalities in boys with pure attention-deficit/hyperactivity disorder compared to boys with pure CD during interference inhibition and attention allocation,” J. Child Psychol. Psychiatry 50(6), 669–678 (2009). 10.1111/j.1469-7610.2008.02022.x [DOI] [PubMed] [Google Scholar]
- 81.Shafritz K. M., et al. , “The effects of methylphenidate on neural systems of attention in attention deficit hyperactivity disorder,” Am. J. Psychiatry 161(11), 1990–1997 (2004). [DOI] [PubMed] [Google Scholar]
- 82.Arnsten A. F., Li B. M., “Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions,” Biol. Psychiatry 57(11), 1377–1384 (2005). 10.1016/j.biopsych.2004.08.019 [DOI] [PubMed] [Google Scholar]
- 83.Volkow N. D., et al. , “Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction,” J. Neurosci. 25(15), 3932–3939 (2005). 10.1523/JNEUROSCI.0433-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saletu B., Anderer P., Saletu-Zyhlarz G. M., “EEG topography and tomography (LORETA) in the classification and evaluation of the pharmacodynamics of psychotropic drugs,” Clin. EEG Neurosci. 37(2), 66–80 (2006). 10.1177/155005940603700205 [DOI] [PubMed] [Google Scholar]
- 85.Stassen H. H., et al. , “Is there a common resilience mechanism underlying antidepressant drug response? Evidence from 2848 patients,” J. Clin. Psychiatry 68(8), 1195–1205 (2007). 10.4088/JCP.v68n0805 [DOI] [PubMed] [Google Scholar]