Abstract
Analysis of L-type amino acid transport expression of hepatocellular carcinoma cells (HCCs) of the dog was performed. The leucine transport activity of canine HCCs was 0.628 ± 0.018 nmol/mg protein/min. The inhibitor of LAT 2-aminobicyclo[2.2.1]heptane-2-carboxylic acid (BCH) reduced 90% of the activity at 1 mM. The deduced amino acid sequences of canine LAT2, LAT3 and LAT4 were well conserved in mammalians, exhibiting 89, 88 and 77% homology, respectively. RT-PCR revealed distinct LAT1 expression compared with normal hepatocytes. Western blotting analysis confirmed the potent LAT1 expression in canine HCCs but not hepatocytes, and real-time RT-PCR analysis indicated that canine HCCs possessed 28 times higher LAT1 expression than hepatocytes. These results indicated that the leucine transport activity of canine HCCs was due to LAT1.
Keywords: canine, hepatoma, LAT, transporter
Many amino acid transport systems have been distinguished based on differences in their substrate-selectivity, ion-dependence, pH sensitivity, kinetics and regulatory properties by using membrane vesicle preparations or cultured cells [5, 6]. Among them, system L is an amino acid transport system that mediates sodium-independent transport of neutral amino acids. It was first described in Ehrlich ascites tumor cells as a sodium-independent transport system for neutral amino acids that is specifically inhibited by a bicyclic amino acid, 2-aminobicyclo[2.2.1]heptane-2-carboxylic acid (BCH) [28, 29, 37]. Later, 2 subtypes for system L with distinct characteristics in substrate affinity, selectivity, and transport properties were reported and named systems L1 and L2 [37]. To date, 4 isoforms of system L have been identified at the molecular level. The first cloned transporters with system L activity were LAT1 and LAT2, which are members of the SLC (solute carrier) 7 family of transporters [17, 21, 34, 36]. They mediate sodium-independent amino acid exchange and recognize a wide range of large neutral amino acids as substrates [35], expanding to small neutral amino acids in the case of LAT2 [30, 31, 33]. These proteins form heteromeric complexes via a disulfide bond with the heavy chain of 4F2 antigen (4F2hc, SLC3A2), a single transmembrane domain protein essential for the functional expression of LAT1 and LAT2.
Later, other transporters were identified by expression cloning [1, 3], and it was reported that they exhibited Na-independent, BCH-sensitive neutral amino acid transport activity. In spite of possessing the properties of system L, these transporters (LAT3 and LAT4) were structurally different from system L1 (LAT1 and LAT2), and additionally, they did not require co-expression with 4F2hc to elicit transport activity at the plasma membrane. LAT3 and LAT4 transport activities show specific properties: (i) preferential substrate specificity [6], (ii) complex kinetics [6], which are compatible with 2 simultaneous apparent affinities and (iii) inactivation by the thiol reagent N-ethylmaleimide [25]. Northern blot analysis indicated the tissue distribution of system L2 was different between LAT3 and LAT4. Potent expression of LAT3 was found in the placenta and kidney, and that of LAT4 was found in the pancreas and skeletal muscle [1, 3]. Previously, we reported the cDNA sequence and distribution of canine LAT1 in the healthy dog and in several tumor cells [27]. The accumulating data indicated the close relationship between tumor malignancy and LAT1 expressions in human tumor tissues [15, 16]. Notably, the expression of LAT1 has been described to be a significant factor indicating a poor outcome in various human cancers [2, 12, 14, 22, 32]. Hepatocellular carcinoma is the most common primary hepatic tumor in dogs and arises from the uncontrolled proliferation of hepatocytes. Viral infections have been associated with hepatocellular carcinoma in humans, but no causal link with hepatocellular carcinoma in dogs has yet been established. While Fukumoto et al. reported the LAT1 expression in the canine mammary gland tumor and melanoma in the veterinary clinic [9,10,11], little information is available concerning LAT expression in hepatocellular carcinoma cells (HCCs) of dog. In this study, we examined functional analysis of LAT activity, determined the molecular structure of canine LAT3 and LAT4 and investigated the expression of the 4 isotypes of LATs by RT-PCR and Western blot analysis.
MATERIALS AND METHODS
Animal and cells samples: All experiments were performed according to the guidelines of the Laboratory Animal Care Committee of Azabu University, and were in compliance with the Fundamental Guideline for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions. A tumor was removed from an 11-year female Yorkshire Terrier with hepatocellular carcinoma and diagnosed histologically. Tissues samples were fixed in 20% buffered formalin, embedded in paraffin, sectioned at 4 µm and stained with hematoxylin and eosin (HE).A cell line was established after transplantation to a nude mouse, cultured through >100 passages and maintained in Eagle’s MEM (Gibco, Carlsbad, CA, U.S.A.) supplemented with 10% fetal bovine serum, 6.6 mg/l spectinomycin and 2 mM glutamine in a 60 mm dish (BioCoat Collagen I 60 mm Dish, Corning, Tokyo, Japan) 37°C in a 5% CO2 humidified incubator. Tissue samples were also obtained from a healthy 5-year-old male Shiba dog.
Determination of the cDNA sequence of canine LATs: Determination of the cDNA sequence was carried out as described previously [26]. Total RNA was isolated from tissues of the healthy dog using an RNA extraction solution (Isogen, Nippon Gene, Tokyo, Japan). The primers used in this study were prepared according to the conserved sequence region between the human and mouse. The DDBJ accession numbers of the human and mouse LATs used in this study were as follows: human LAT2, AF171669; mouse LAT2, AF171668; human LAT3, AB103033; mouse LAT3, AB103034; human LAT4, AB120364; mouse LAT4, AB120363). RT-PCR amplification was performed by employing a SuperScript III first-Strand Synthesis System kit (Invitrogen, Carlsbad, CA, U.S.A.) with Hot start Ex Taq DNA polymerase (Takara Bio, Kyoto, Japan). The band was excised from the agarose gel and purified using a Wizard SV gel clean-up system (Promega, Tokyo, Japan). The extracted and purified DNA was cloned into a pCR II-TOPO cloning vector (Invitrogen) and sequenced with a BigDye Terminator kit ver.3.1 Cycle Sequencing Kit (Applied Biosystems, Carlsbad, CA, U.S.A.). The nucleotide sequence obtained exhibited high similarities (>85%) to human LATs cDNA sequences. In order to determine the 3′ and 5′ regions of cDNA, RACE methods were carried out using a SMARTer RACE cDNA Amplification Kit (Clontech, Mountain View, CA, U.S.A.) and a set of canine LATs sequences obtained.
Measurement of leucine transport activity: Radioactive (3H-) L-leucine was purchased from American Radiolabeled Chemicals (St. Louis, MO, U.S.A.). Na-independent leucine uptake was measured as described elsewhere [20]. Simply, all buffers containing Na were replaced with N-methyl-D-glucamine. The cells were plated 5 × 105 cells/6-well plate 24 hr before the experiment. After washing the cells, the medium containing 10 µM leucine with a radioisotope was added and incubated at 37°C. To study the effect of BCH, the inhibitor of LAT, was included in an incubation medium containing radiolabeled leucine. Uptake was terminated by washing with ice-cold phosphate-buffered saline. After solubilizing the cells with 1% SDS, the radioactivity was measured with a liquid scintillation counter, and the protein content was determined by the Micro BCA method.
RT-PCR analysis of the 4 isotypes of LAT expression in hepatocarcinoma and normal hepatocytes: In order to examine the expression of LATs, we performed RT-PCR using the primers specific to each LAT (Table 1). RT-PCR conditions were as follows: 25 cycles of three steps, 94°C for 15 sec, 68°C for 15 sec and 72°C for 20 sec.
Table 1. Sequences of oligonucleotides used in this study.
| Primer | Sequence (5′-3′) | Product (bp) | |
|---|---|---|---|
| Oligonucleotides for RT-PCR | |||
| Transcript | |||
| LAT1 | Sense | TGTACGGCTCGCTGCCCGCCTTCCT | 468 |
| (AB636469) | Antisense | GATGGCCAGGGGCAGGTTTCTGTAGGG | |
| LAT2 | Sense | GGTGGGCCACCCGGGTTCAAGACATTT | 609 |
| (AB923978) | Antisense | TGTCGCTGGTGACCAGCATCAGCAGGG | |
| LAT3 | Sense | GGTCGGGACTGGCCTGTCTCATCTTTC | 624 |
| (AB924118) | Antisense | AGCGTGGTCTGGCAGACTTGGTGGCCG | |
| LAT4 | Sense | GCCATCACCCTGCCTCTAGGCATCGTC | 578 |
| (AB924119) | Antisense | TTATGGCCCTCCTGCAGTGCCGCTTGC | |
| GAPDH | Sense | ATC ACC ATC TTC CAG GAG CGA GA | 192 |
| (AB038240) | Antisense | GTC TTC TGG GTG GCA GTG ATG G | |
| Oligonucleotides for real-time RT-PCR | |||
| LAT1 | sense | CCTGGTGTACGTGCTGACGAA | 106 |
| (AB636469) | Antisense | TCCCAGGTGATAGGTCCCAAAG | |
| RP19 | sense | CCTTCCTCAAAAAGTCTGGG | 95 |
| (XM538673) | Antisense | CTTCTCATCGTAGGGACGAAG | |
Accession numbers are indicated in parentheses.
Real-time RT-PCR evaluation of LAT1: The housekeeping gene RP19 was used as a reference gene. The primer set for LAT1 used for real-time PCR was designed and purchased from Takara (Table 1). Real-time quantitative PCR was performed using a Thermal Cycler Dice® Real Time System II (Takara). Samples (final volume of 25 µl) were run in duplicate and contained the following: X1 SYBR® Premix Ex Taq™ II (Takara), 1 µl 10 µM of each primer and 2 µl cDNA template. Amplification was conducted using the following protocol: initial denaturation phase at 95°C for 10 sec and then 40 cycles at 95°C for 5 sec for denaturation and 60°C for 20 sec for annealing and extension. The qRT-PCR results are presented as the gene expression of the target gene (LAT1) relative to that of the housekeeping gene (RP19), and LAT1 gene expression levels were obtained using the standard method of quantification.
Western blot analysis of LAT: The anti-canine LAT1 serum was prepared as described previously [26]. The cell membrane of the tissues for Western blot analysis was prepared as reported by Denker et al. [7]. In brief, the cells were homogenized at 4°C in the buffer containing 0.1 M KCl, 5 mM Na2HPO4, pH 7.5, 0.75 mM Na-EGTA, pH 7.5, 1 mM DDT, 5 mM MgCl2, 200 µg/ml phenylmethylsulfonyl fluoride and 4 µg/ml leupeptin. Homogenates were centrifuged for 10 min to remove debris. The 1 ml supernatant was laid over a 5 ml sucrose solution containing 0.8 mM sucrose and 2 mM Na-EGTA, and was centrifuged at 32,000 g for 40 min. Protein concentration of the pellet was determined by the BCA method and was used for Western blot analysis. The membranes were simply solubilized, electrophoresed into 12% polyacrylamide gels and immunoblotted with a chemiluminescence autoradiograph. The membrane was then treated with a primary antibody against the C-terminus of canine LAT1, followed by a secondary antibody (anti-rabbit IgG (H+L) goat IgG Fab’ HRP, ×100,000, Seikagaku Corp., Tokyo, Japan). The LAT1 protein was detected with an ECL Plus chemiluminescence detection system (GE Healthcare Bioscience, Chalfont, UK) and exposed to x-ray film.
RESULTS
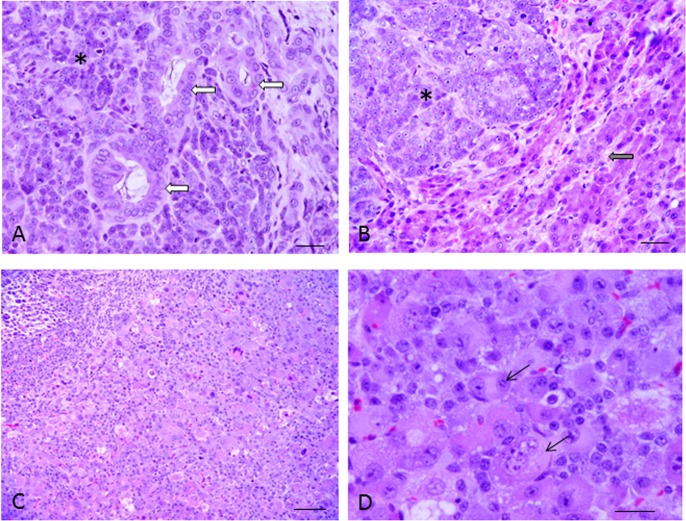
Histologic sections from the specimen of hepatocellular carcinoma from the dog are shown in Fig. 1 (A, B). The tumor was composed of 2 dissimilar forms, hepatocellular carcinoma and cholangiocellular carcinoma. The tumor cells contained various-sized cord-like structures and conspicuous nucleoli. Histologic sections of a nude mouse with a transplanted tumor are also shown in Fig. 1 (C, D). Mitosis was observed, but cord-like structures disappeared. The tumor cytoplasm was abundant and eosinophilic.
Fig. 1.
Histologic section from the specimen from the liver of the dog. HE stain (A, B). Hepatocellular carcinoma (*) and cholangiocellular carcinoma (arrow) (A). In part, hepatic cells with a normal pattern were observed (arrow). The tumor cells contained various-sized cord-like structures and conspicuous nucleoli (*). Bar=20 µm. (B). Histologic section of the nude mouse with a transplanted tumor. HE stain (C, D). Bar=100 µm (C). The cells of 2 nuclei were observed in various-sized and inequality (arrow). Bar=20 µm (D).
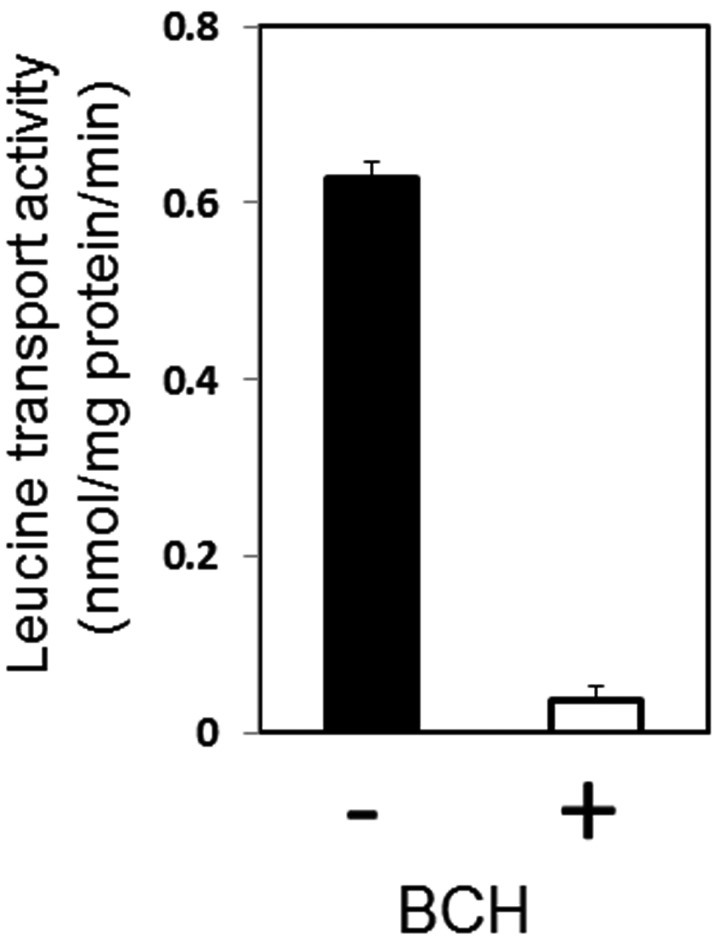
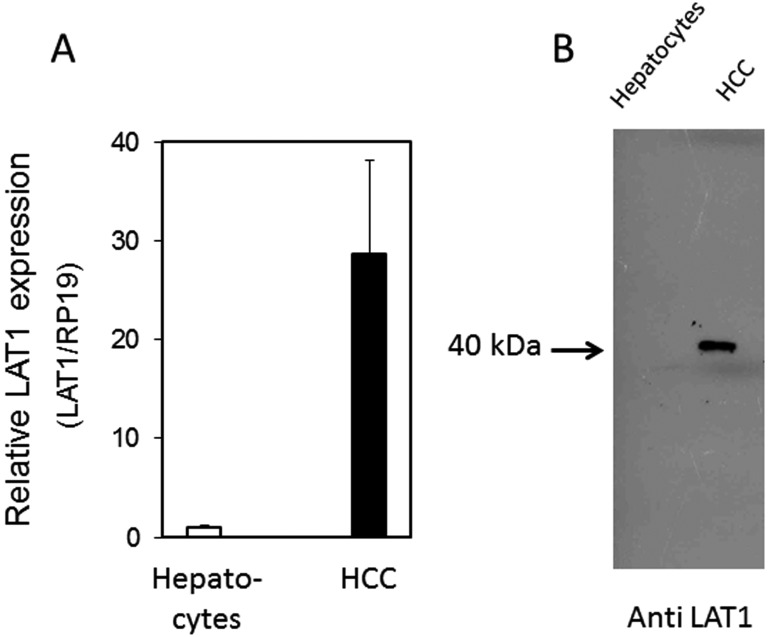
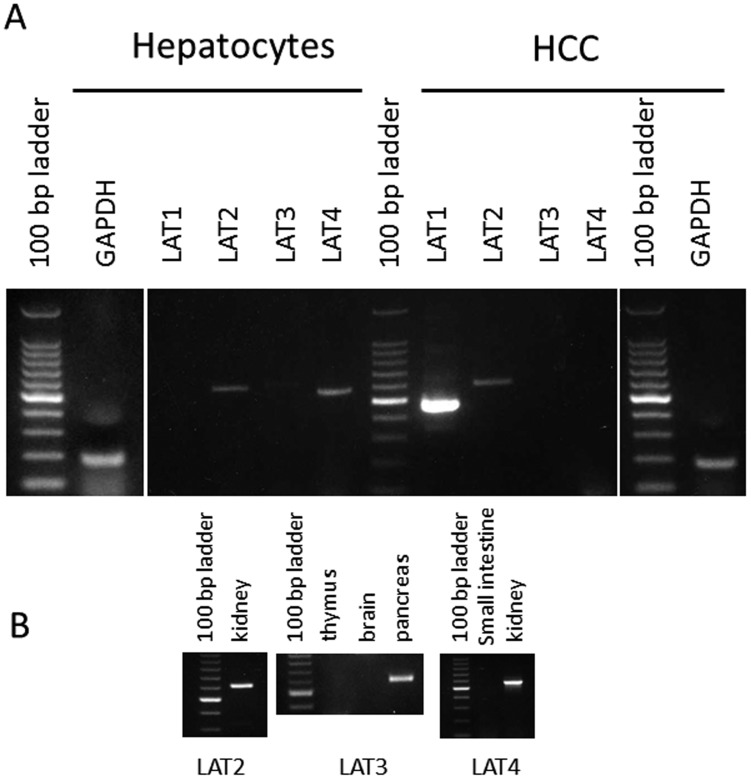
The leucine transport activity of canine HCCs is indicated in Fig. 2. The transport activity was 0.628 ± 0.018 nmol/mg protein/min, while in the presence of 1 mM BCH, the specific inhibitor of carnitine transporter, the transport activity was reduced by 90%. Figures 2, 3, 4 show the deduced amino acid sequences of canine LAT2–LAT4 and a comparison with those of other mammalians. Canine LAT2 was 1 amino acid longer than that of the mouse, and 3 amino acids shorter than that of the human (Fig. 8). Canine LAT3 was 1 amino acid shorter than that of the human. Mouse LAT3 possessed 5 and 14 amino acid insertions in the same location compared with those of the dog and human (Fig. 4). Canine LAT4 consisted of the same number of amino acid resides as human LAT4, while mouse LAT4 possessed 4 amino acid insertions (Fig. 5). Canine LAT2, LAT3 and LAT4 exhibited 89, 88% and 77% homology among mammalians, respectively. The homology of canine LAT1 and LAT2 was 54%, and LAT3 and LAT4 showed 58% identity (data not shown). On the other hand, significant homology was not observed among LAT1–LAT4 (Fig. 6). Figure 7 shows the RT-PCR analysis of LAT expression in HCCs and hepatocytes from the healthy dog. A potent signal was observed only in LAT1 in the hepatocarcinoma, while LAT1 was not observed in hepatocytes. Signals of LAT3 (weakly) and LAT4 were detected in hepatocytes, while LAT3 and LAT4 were not observed in HCCs. As a control experiment, the expressions of LAT2 and LAT4 were confirmed in the kidney, and that of LAT3 was confirmed in the pancreas (Fig. 7B). Detailed evaluation of LAT1 expression in the hepatocarcinoma and healthy liver was investigated by real-time RT-PCR analysis. Expression of LAT1 in the hepatocarcinoma was 28 times higher than that in the healthy liver (Fig. 8A). Western blotting analysis using anti-LAT1 serum confirmed the certain signal in the hepatocellular carcinoma (Fig. 8B). RT-PCR analysis indicated no LAT3 and LAT4 expression in canine HCCs, while hepatocytes possessed weak expression.
Fig. 2.
Leucine transport activity of canine HCCs in the presence or absence of 1 mM BCH. The values represent the means and SD of 4 individual experiments.
Fig. 3.
Amino acid sequences of canine LAT4 were compared with those of the human and mouse. Multiple sequence alignments were performed using the GENETYX program (ver. 10). Asterisks and dots indicate identical residues and conservative substitutions, respectively. The cDNA sequences data of canine LAT2 were deposited in the DNA Data Bank of Japan (accession numbers AB923978).
Fig. 4.
Amino acid sequences of canine LAT3 were compared with those of the human and mouse. Multiple sequence alignments were performed using the GENETYX program (ver. 10). Asterisks and dots indicate identical residues and conservative substitutions, respectively. The cDNA sequences data of canine LAT3 were deposited in the DNA Data Bank of Japan (accession numbers AB924118).
Fig. 8.
Real-time RT-PCR analysis of mRNA of canine LAT1 in canine HCCs and normal hepatocytes (A) and Western blot analysis of LAT1 expression using antiserum against the C-terminus of canine LAT1 peptides (B).
Fig. 5.
Amino acid sequences of canine LAT4 were compared with those of the human and mouse. Multiple sequence alignments were performed using the GENETYX program (ver. 10). Asterisks and dots indicate identical residues and conservative substitutions, respectively. The cDNA sequences data of canine LAT4 were deposited in the DNA Data Bank of Japan (accession numbers AB924119).
Fig. 6.
Amino acid sequences of the four isotypes of canine LATs were compared. Multiple sequence alignments were performed using the GENETYX program (ver. 10). Asterisks and dots indicate identical residues and conservative substitutions, respectively.
Fig. 7.
RT-PCR analysis of the four isotypes of LATs in canine HCCs and normal hepatocytes. Integrity of RNA was examined by using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (A). Control experiments of LAT2-LAT4 were also examined.
DISCUSSION
In this study, we investigated the leucine transport activity and confirmed potent BCH-sensitive, Na-independent transport activity in canine HCCs. To evaluate the contribution of 4 LAT isoforms, we determined the cDNA sequences of LAT2, LAT3 and LAT4, and the expression of the 4 isotypes of LATs was examined by RT-PCR analysis. Distinct LAT1 expression in canine HCCs was observed, while other signals were either weak or not detected.
Real-time RT-PCR evaluation of LAT1 indicated that the expression of LAT1 was 28 times higher in HCCs than that in hepatocytes. Western blotting analysis using anti-LAT1 serum supported these results. Therefore, potent BCH-sensitive, Na-independent leucine transport activity occurred due to LAT1. LAT1 has been closely associated with cancerous or proliferative cells, and previous studies have shown it to be highly expressed in proliferating tissues, many tumor cell lines and primary human tumors [18, 24, 38]. The expression of LAT1 has been described to be a significant factor indicating a poor outcome in various human cancers [12, 16, 22, 33]. Cancer cells require considerable nourishment to meet their demands for rapid growth and cell division. Therefore, upregulation of amino acid transporters, especially the system L amino acid transporters that supply large neutral amino acids including many essential amino acids, has been reported [8]. Notably, downregulation of LAT1 by silencing LAT1 shRNA introduction or BCH treatment suppressed invasion and migration of cholangiocarcinoma [13]. Hepatocellular carcinoma is the most common primary hepatic tumor in dogs. Canine hepatocellular carcinoma arises from the uncontrolled proliferation of hepatocytes. The pathogenesis of hepatocellular carcinoma is a multistep process involving sequential events, such as chronic inflammation, hyperplasia, dysplasia, and ultimately, malignant transformation [4]. Several epigenetic and genetic alterations are involved in hepatocellular carcinoma, which ultimately lead to alterations of molecular pathways. Upregulation of LAT1 was reported to be associated with a poor prognosis in malignant tumors [19, 23]. Potent expression of LAT1 may involve the alternation of amino acid metabolism in hepatocellular carcinoma. Therefore, alteration of LAT1 function could possibly be a therapeutic target for canine hepatocellular carcinoma. Although we confirmed that LAT1 played an important role in leucine transport in canine HCC in this study, only one cell line was investigated. We will next investigate more cell lines of canine hepatocellular carcinoma or other tumor cell lines. The data clarified in this paper may facilitate the study on understanding of the pathophysiology of canine hepatocellular carcinoma.
Acknowledgments
This study was in part supported by a research project grant awarded by the Azabu University Research Services Division.
REFERENCES
- 1.Babu E., Kanai Y., Chairoungdua A., Kim D. K., Iribe Y., Tangtrongsup S., Jutabha P., Li Y., Ahmed N., Sakamoto S., Anzai N., Nagamori S., Endou H.2003. Identification of a novel system L amino acid transporter structurally distinct from heterodimeric amino acid transporters. J. Biol. Chem. 278: 43838–43845. doi: 10.1074/jbc.M305221200 [DOI] [PubMed] [Google Scholar]
- 2.Betsunoh H., Fukuda H., Anzai N., Nishihara D., Mizuno T., Yuki H., Masuda A., Yamaguchi Y., Abe H., Yashi M., Fukabori Y., Yoshida K., Kamai T.2013. Increased expression of system large amino acid transporter (LAT)-1 mRNA is associated with invasive potential and unfavorable prognosis of human clear cell renal cell carcinoma. BMC Cancer 13: 509. doi: 10.1186/1471-2407-13-509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodoy S., Martín L., Zorzano A. M., Estévez R., Bertran J.2005. Identification of LAT4, a novel amino acid transporter with system L activity. J. Biol. Chem. 280: 12002–12011. doi: 10.1074/jbc.M408638200 [DOI] [PubMed] [Google Scholar]
- 4.Bartlett D. L., DiBisceglie A. M., Dawson L. A.2011. Cancer of the liver. pp. 997–1018. In: Cancer: Principles and Practice of Oncology, 9th ed. (De-Vita, V. T., Lawrence, T. S. and Rosenberg, S. A. eds.), Lippincott Williams & Wilkins, Philadelphia. [Google Scholar]
- 5.Christensen H. N., Handlogten M. E., Lam I., Tager H. S., Zand R.1969. A bicyclic amino acid to improve discriminations among transport systems. J. Biol. Chem. 244: 1510–1520. [PubMed] [Google Scholar]
- 6.Christensen H. N.1990. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol. Rev. 70: 43–77. [DOI] [PubMed] [Google Scholar]
- 7.Denker B. M., Smith B. L., Kuhajda F. P., Agre P.1988. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J. Biol. Chem. 263: 15634–15642. [PubMed] [Google Scholar]
- 8.Fuchs B. C., Bode B. P.2005. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin. Cancer Biol. 15: 254–266. doi: 10.1016/j.semcancer.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 9.Fukumoto S., Hanazono K., Fu D. R., Endo Y., Kadosawa T., Iwano H., Uchide T. A.2013. New treatment for human malignant melanoma targeting L-type amino acid transporter 1 (LAT1): a pilot study in a canine model. Biochem. Biophys. Res. Commun. 439: 103–108. doi: 10.1016/j.bbrc.2013.08.020 [DOI] [PubMed] [Google Scholar]
- 10.Fukumoto S., Hanazono K., Komatsu T., Iwano H., Kadosawa T., Uchide T.2013. L-type amino acid transporter 1 (LAT1) expression in canine mammary gland tumors. J. Vet. Med. Sci. 75: 431–437. doi: 10.1292/jvms.12-0356 [DOI] [PubMed] [Google Scholar]
- 11.Fukumoto S., Hanazono K., Komatsu T., Ueno H., Kadosawa T., Iwano H., Uchide T.2013. L-type amino acid transporter 1 (LAT1): a new therapeutic target for canine mammary gland tumour. Vet. J. 198: 164–169. doi: 10.1016/j.tvjl.2013.06.016 [DOI] [PubMed] [Google Scholar]
- 12.Ichinoe M., Mikami T., Yoshida T., Igawa I., Tsuruta T., Nakada N., Anzai N., Suzuki Y., Endou H., Okayasu I.2011. High expression of L-type amino-acid transporter 1 (LAT1) in gastric carcinomas: comparison with non-cancerous lesions. Pathol. Int. 61: 281–289. doi: 10.1111/j.1440-1827.2011.02650.x [DOI] [PubMed] [Google Scholar]
- 13.Janpipatkul K., Suksen K., Borwornpinyo S., Jearawiriyapaisarn N., Hongeng S., Piyachaturawat P., Chairoungdua A.2014. Downregulation of LAT1 expression suppresses cholangiocarcinoma cell invasion and migration. Cell. Signal. 26: 1668–1679. doi: 10.1016/j.cellsig.2014.04.002 [DOI] [PubMed] [Google Scholar]
- 14.Kaira K., Oriuchi N., Imai H., Shimizu K., Yanagitani N., Sunaga N., Hisada T., Tanaka S., Ishizuka T., Kanai Y., Endou H., Nakajima T., Mori M.2008. Prognostic significance of L-type amino acid transporter 1 expression in resectable stage I-III nonsmall cell lung cancer. Br. J. Cancer 98: 742–748. doi: 10.1038/sj.bjc.6604235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaira K., Oriuchi N., Takahashi T., Nakagawa K., Ohde Y., Okumura T., Murakami H., Shukuya T., Kenmotsu H., Naito T., Kanai Y., Endo M., Kondo H., Nakajima T., Yamamoto N.2011. LAT1 expression is closely associated with hypoxic markers and mTOR in resected non-small cell lung cancer. Am. J. Transl. Res. 3: 468–478. [PMC free article] [PubMed] [Google Scholar]
- 16.Kaira K., Oriuchi N., Takahashi T., Nakagawa K., Ohde Y., Okumura T., Murakami H., Shukuya T., Kenmotsu H., Naito T., Kanai Y., Endo M., Kondo H., Nakajima T., Yamamoto N.2011. L-type amino acid transporter 1 (LAT1) expression in malignant pleural mesothelioma. Anticancer Res. 31: 4075–4082. [PubMed] [Google Scholar]
- 17.Kanai Y., Segawa H., Miyamoto K., Uchino H., Takeda E., Endou H.1998. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J. Biol. Chem. 273: 23629–23632. doi: 10.1074/jbc.273.37.23629 [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi H., Ishii Y., Takayama T.2005. Expression of L-type amino acid transporter 1 (LAT1) in esophageal carcinoma. J. Surg. Oncol. 90: 233–238. doi: 10.1002/jso.20257 [DOI] [PubMed] [Google Scholar]
- 19.Li J., Qiang J., Chen S. F., Wang X., Fu J., Chen Y.2013. The impact of L-type amino acid transporter 1 (LAT1) in human hepatocellular carcinoma. Tumour Biol. 34: 2977–2981. doi: 10.1007/s13277-013-0861-5 [DOI] [PubMed] [Google Scholar]
- 20.Maruo T., Kanemaki N., Onda K., Sato R., Ochiai H.2014. Canine amino acid transport system xc-: cDNA sequence, distribution and cystine transport activity in lens epithelial cells. J. Vet. Med. Sci. 76: 523–530. doi: 10.1292/jvms.13-0170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mastroberardino L., Spindler B., Pfeiffer R., Skelly P. J., Loffing J., Shoemaker C. B., Verrey F.1998. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature 395: 288–291. doi: 10.1038/26246 [DOI] [PubMed] [Google Scholar]
- 22.Nakanishi K., Ogata S., Matsuo H., Kanai Y., Endou H., Hiroi S., Tominaga S., Aida S., Kasamatsu H., Kawai T.2007. Expression of LAT1 predicts risk of progression of transitional cell carcinoma of the upper urinary tract. Virchows Arch. 451: 681–690. doi: 10.1007/s00428-007-0457-9 [DOI] [PubMed] [Google Scholar]
- 23.Namikawa M., Kakizaki S., Kaira K., Tojima H., Yamazaki Y., Horiguchi N., Sato K., Oriuchi N., Tominaga H., Sunose Y., Nagamori S., Kanai Y., Oyama T., Takeyoshi I., Yamada M.Expression of amino acid transporters (LAT1, ASCT2 and xCT) as clinical significance in hepatocellular carcinoma. Hepatol. Res. (in press). [DOI] [PubMed] [Google Scholar]
- 24.Nawashiro H., Otani N., Shinomiya N., Fukui S., Ooigawa H., Shima K., Matsuo H., Kanai Y., Endou H.2006. L-type amino acid transporter 1 as a potential molecular target in human astrocytic tumors. Int. J. Cancer 119: 484–492. doi: 10.1002/ijc.21866 [DOI] [PubMed] [Google Scholar]
- 25.Novak D. A., Kilberg M. S., Beveridge M. J.1994. Ontogeny and plasma- membrane domain localization of amino acid transport system L in rat liver. Biochem. J. 301: 671–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochiai H., Kanemaki N., Kamoshida S., Murakami M., Ichihara N., Asari M., Nishita T.2009. Determination of full-length cDNA nucleotide sequence of equine carbonic anhydrase VI and its expression in various tissues. J. Vet. Med. Sci. 71: 1233–1237. doi: 10.1292/jvms.71.1233 [DOI] [PubMed] [Google Scholar]
- 27.Ochiai H., Morishita T., Onda K., Sugiyama H., Maruo T.2012. Canine Lat1: molecular structure, distribution and its expression in cancer samples. J. Vet. Med. Sci. 74: 917–922. doi: 10.1292/jvms.11-0353 [DOI] [PubMed] [Google Scholar]
- 28.Oxender D. L., Christensen H. N.1963. Evidence for two types of mediation of neutral amin-acid transport in Ehrlich cells. Nature 197: 765–767. doi: 10.1038/197765a0 [DOI] [PubMed] [Google Scholar]
- 29.Oxender D. L., Christensen H. N.1963. Distinct mediating systems for the transport of neural amino acids by the Ehrlich cell. J. Biol. Chem. 238: 3686–3699. [PubMed] [Google Scholar]
- 30.Pineda M., Fernandez E., Torrents D., Estevez R., Lopez C., Camps M., Lloberas J., Zorzano A., Palacin M.1999. Identification of a membrane protein, LAT-2, that co-epresses with 4F2 heavy chain, an L-type amino acid transport with broard specificity for small and large zwitterinic acids. J. Biol. Chem. 274: 19738–19744. doi: 10.1074/jbc.274.28.19738 [DOI] [PubMed] [Google Scholar]
- 31.Rossier G., Meier C., Bauch C., Summa V., Sordat B., Verrey F., Kuhn L. C.1999. LAT2, a new basolateral 4F2hc/CD98-associated amino acid transporter of kidney and intestine. J. Biol. Chem. 274: 34948–34954. doi: 10.1074/jbc.274.49.34948 [DOI] [PubMed] [Google Scholar]
- 32.Sakata T., Ferdous G., Tsuruta T., Satoh T., Baba S., Muto T., Ueno A., Kanai Y., Endou H., Okayasu I.2009. L-type amino acid transporter 1 as a novel biomarker for high-grade malignancy in prostate cancer. Pathol. Int. 59: 7–18. doi: 10.1111/j.1440-1827.2008.02319.x [DOI] [PubMed] [Google Scholar]
- 33.Segawa H., Fukasawa Y., Miyamoto K., Takeda E., Endou H., Kanai Y.1999. Identification and functional characteization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J. Biol. Chem. 274: 19745–19751. doi: 10.1074/jbc.274.28.19745 [DOI] [PubMed] [Google Scholar]
- 34.Torrents D., Estevez R., Pineda M., Fernandez E., Lloberas J., Shi Y. B., Zorzano A., Palacin M.1998. Identification and characterization of a membrane protein (y+L amino acid transporter-1) that associates with 4F2hc to encodes the amino acid transport activity y-L. A candidate gene for lysiuric protein in intolerance. J. Biol. Chem. 273: 32437–32445. doi: 10.1074/jbc.273.49.32437 [DOI] [PubMed] [Google Scholar]
- 35.Uchino H., Kanai Y., Kim D. K., Wempe M. F., Chairoungdua A., Morimoto E., Anders M. W., Endou H.2002. Transport of amino acid-related compounds mediated by L-type amino acid transporter 1 (LAT1): insights into the mechanisms of substrate recognition. Mol. Pharmacol. 61: 729–737. doi: 10.1124/mol.61.4.729 [DOI] [PubMed] [Google Scholar]
- 36.Verrey F.2003. System L: heteromeric exchanger of large, neutral amino acids involved in directional transport. Pflugers Arch. 445: 529–533. [DOI] [PubMed] [Google Scholar]
- 37.Weissbach L., Handlogten M. E., Christensen H. N., Kilberg M. S.1982. Amino acid activation of amino acid transport System N early in primary cultures of rat hepatocytes. J. Biol. Chem. 257: 12006–12011. [PubMed] [Google Scholar]
- 38.Yanagida O., Kanai Y., Chairoungdua A., Kim D. K., Segawa H., Nii T., Cha S. H., Matsuo H., Fukushima J., Fukasawa Y., Tani Y., Taketani Y., Uchino H., Kim J. Y., Inatomi J., Okayasu I., Miyamoto K., Takeda E., Goya T., Endou H.2001. Human L-type amino acid transporter 1 (LAT 1): characterization of function and expression in tumor cell lines. Biochim. Biophys. Acta 1514: 291–302. doi: 10.1016/S0005-2736(01)00384-4 [DOI] [PubMed] [Google Scholar]