Abstract
Although color Doppler ultrasonography has been used to evaluate testicular blood flow in many species, very little has been done in goat. Eight male Shiba goats were exposed to a single intramuscular injection of either gonadotropin-releasing hormone (GnRH group; 1 µg/kg BW) or human chorionic gonadotropin (hCG group; 25 IU/kg BW). Plasma testosterone (T), estradiol (E2) and inhibin (INH) were measured just before (0 hr) and at different intervals post injection by radioimmunoassay. Testis volume (TV) and Doppler indices, such as resistive index (RI) and pulsatility index (PI) of the supratesticular artery, were measured by B-mode and color Doppler ultrasonography, respectively. The results indicated an increase in testicular blood flow in both groups, as RI and PI decreased significantly (P<0.05), but this increase was significant higher and earlier in hCG group (1 hr) than in the GnRH group (2 hr). A high correlation was found for RI and PI with both T (RI, r= −0.862; PI, r= −0.707) and INH in the GnRH group (RI, r=0.661; PI, r=0.701). However, a significant (P<0.05) correlation was found between E2 and both RI (r= −0.610) and PI (r= −0.763) in hCG group. In addition, TV significantly increased and was highly correlated with RI in both groups (GnRH, r= −0.718; hCG, r= −0.779). In conclusion, hCG and GnRH may improve testicular blood flow and TV in Shiba goats.
Keywords: blood supply, goat, testis
Testicular blood flow is highly important for organ function and may be particularly critical for the testis, because the seminiferous tubules compromise 70% to 80% of the testicular mass with a very low oxygen concentration [36, 37]. Therefore, any reduction of testicular blood flow causes ischemic damage and deterioration of spermatogenesis [13]. Herwig et al. [10] demonstrated that sperm quality is depending on blood perfusion within the testicle. Recent technical advances in Doppler applications have enabled new aspects in structural and functional analyses of testicular tissue and, in turn, extended the field of ultrasonographic imaging from an anatomical to a physiological basis [5]. Therefore, color Doppler ultrasonography is considered a useful noninvasive method of assessing and rapidly determining the presence and direction of testicular blood flow [1].
Unlike human medicine, Doppler ultrasonography has not often been used in veterinary practice. In dogs, color Doppler sonography has been used not only for demonstrating and measuring the blood flow of the testicular arteries, but also as a potential marker of semen quality in dog [44]. In alpaca, testicular blood flow measured by Doppler ultrasonography has been used to discriminate male fertility [15]. In the stallion, Doppler ultrasonography has been used to characterize blood flow in the testicular artery and to evaluate some pathological problems, such as testicular varicocele [25, 27]. To the best of the authors’ knowledge, the available data to date concerning the usefulness of color pulsed Doppler ultrasonography in small ruminants are limited and concerned mainly female reproduction during pregnancy [22, 35]. Generally, various treatments aimed at improving testicular blood flow and, in turn, increasing testicular function should be of great value for male fertility. Gonadotropin- releasing hormone (GnRH) and human chorionic gonadotropin (hCG) are considered important hormones used in reproduction and valuable tools for testing whether the function of the male reproductive endocrine system is optimal [4, 24]. However, there has been a lack of knowledge concerning the effect of GnRH and hCG on testicular blood flow, especially in ruminants. So, the objectives of the present study were to examine whether GnRH or hCG has different effects on testicular blood flow and to examine testicular volume in male Shiba goats. Furthermore, we aimed to investigate whether there are associative relationships between testicular hormones and Doppler parameters.
MATERIALS AND METHODS
This study was conducted on male Shiba goats. The Shiba goat (Capra hircus), a Japanese miniature goat, is small in size with less food consumption. The Shiba goat is a nonseasonal breeder under natural daylight, reaches puberty at 3.5 months and is considered a useful model animal for studying the physiology of ruminants [12].
Animals and management: Eight 10- to 12- month- old postpubertal male Shiba bucks (Capra hircus), weighing 18 to 25 kg and housed under natural daylight, were used in this study during May and December 2013. Each animal received a maintenance diet of 400 g of hay cubes twice a day. Clean water and mineralized salt licks were available ad libitum. Each male underwent complete andrological and ultrasonographic examinations to ensure absence of any abnormalities related to the reproductive tract before beginning the research. The animals were vaccinated and dewormed, and none of them had evidence of disease. All procedures were carried out in accordance with guidelines established by the Tokyo University of Agriculture and Technology, Japan, for the use of animals.
GnRH and hCG administrations: Male Shiba goats were exposed to a single intramuscular injection of either GnRH (GnRH group; 1 µg/kg BW; fertirelin acetate 100 µg; Conceral; Nagase Pharmaceuticals Co., Ltd., Osaka, Japan) or human chorionic gonadotropin (hCG group; 25 IU/kg BW; Pubergen; Yell Pharmaceutical Co., Ltd., Tokyo, Japan). The dose of GnRH or hCG was chosen depending on previous studies in goats [30, 32]. Both hCG and GnRH administrations were started at a fixed time (between 08:00 and 09:00 hr) to avoid any effects of circadian rhythmicity on blood flow [44]. Additionally, to minimize individual variation, animals that had been treated with hCG in the first trial were subjected to GnRH treatment in the second trial and vice versa. So, all goats (n=8) were injected either with GnRH or hCG in 2 trials with a 3- week interval. The time interval between the 2 trials was based on previous reports [26, 40].
Blood sampling: A venous blood sample was collected from a jugular vein into an evacuated heparinized tube (Venoject II, Terumo, Tokyo, Japan) just before injection of either hCG or GnRH (basal or 0 hr) and at 1, 2, 3, 6, 24, 48, 72, 96, 120, 144 and 168 hr after injection in both groups. The blood samples were centrifuged at 3,200 rpm for 15 min at 4°C and stored at −20°C until hormonal analysis. These times were selected based on previous studies and the pharmacokinetics of hCG in goats [2, 32, 40].
Hormonal analysis: All hormonal assays were performed in triplicates by a double antibody radioimmunoassay system using I125 labeled radioligands. Plasma concentrations of testosterone (T) and estradiol (E2) were measured as described by Taya et al. [38], and immunoreactive inhibin (INH) was measured as described by Hamada et al. [9].
Ultrasound examinations: All ultrasonographic measurements were carried out by the same ultrasonographer just after blood sampling. All examinations were performed using ultrasound scanner (EUB-7500, Hitachi Medical Corporation, Tokyo, Japan) equipped with a linear array transducer (6.5 MHz; Model EUP-L65; Hitachi Medical Corporation).
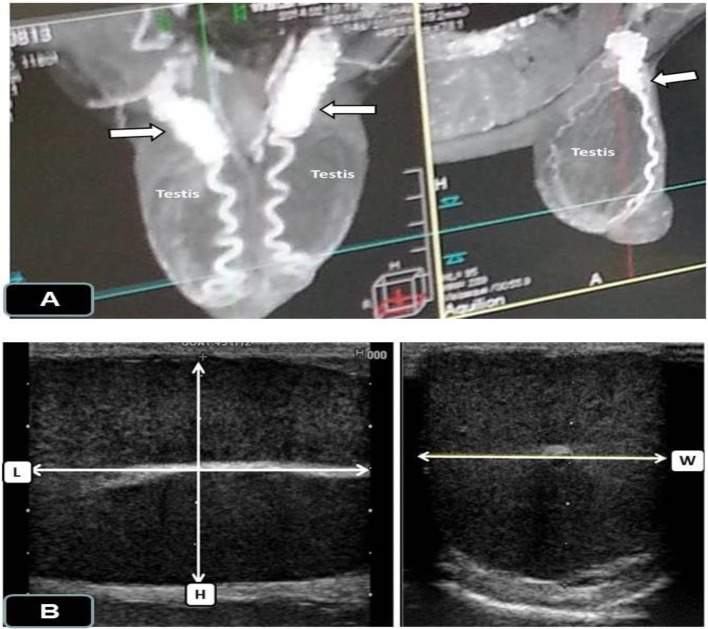
Doppler examination: Preparation for examination: The bucks were simply restrained without tranquilization or sedation. To eliminate the presence of air spaces, the hairs on both sides of the scrotum were shaved well, and the transducer was covered with a copious amount of gel to facilitate ultrasonographic imaging. In goats, the testicular artery on approaching the testis convolutes in order to form a convoluted or coiled part known as the supratesticular artery (STA) (Fig. 1A). The testicular veins collect to surround the artery. However, for distinguishing between a testicular artery and vein by Doppler analysis, an artery, for example, will typically have a waveform on the spectral graph corresponding to the arterial pulse in each cardiac cycle (systole and diastole), while the flow in veins be almost constant, that is, without a pulse.
Fig. 1.
Testis of a male Shiba goat imaged by computerized tomography (A) showing the supratesticular artery (white arrows) and by B-mode ultrasonography (B) for measurement of testis volume (TV) using the following formula: TV=length (L) × width (W) × height (H).
Doppler analysis was performed by identifying all the vascular structures using gray-scale ultrasonography and locating the largest and possibly a longitudinal or oblique section of the supratesticular artery (STA). The angle between the Doppler beam and the long axis of the vessel never was over 60 in the direction of blood flow with the high-pass filter set at 50 Hz. The Doppler gate was kept constant at 1.5–2.5 mm [8, 27].
Doppler parameters of the STA: After appearance of the spectral pattern of the STA, the Doppler indices studied were resistive index (RI = (maximum velocity − minimum velocity) ⁄ maximum velocity) and pulsatility index (PI = (maximum velocity − minimum velocity) ⁄ mean velocity) [7].
Three to 5 measurements were taken for each parameter in different locations along the path of the STA. Furthermore, the waveforms of STA were characterized as either “resistive” or “non-resistive”. Resistive waveforms are characterized by great differences between systolic and diastolic velocity of blood flow with high values of RI, while non-resistive waveforms are the opposites [27]. To minimize the variations in recording, the ultrasound settings (focus, gains, brightness and contrast) were standardized, fixed and used equally for all examinations. In this study, all examinations were recorded digitally on videotape for subsequent analysis.
Testicular volume (TV) measurement by B-mode ultrasonography: At least 3 separate transverse and longitudinal images of each testis were recorded, and the testicular length (L), width (W) and height (H) were measured using electronic calipers without inclusion of the epididymis (Fig. 1B). The testis volume was measured using this formula: TV (ml)=L * W * H * 0.71 [21].
Statistical analysis: All data are presented as the mean ± SEM. The statistical significance of hormonal changes and ultrasonic findings in both groups was evaluated by repeated measures analysis of variance (ANOVA). A Student’s t- test was used to determine the significance at each time point in each group considering 0 hr as the control. An unpaired t-test was used for comparisons between the 2 groups at each time point. Moreover, the relationships between plasma concentrations of hormones and ultrasonographic results were evaluated using Pearson’s correlation coefficient. All statistical analyses were performed by using SPSS version 20. P values less than 0.05 were considered significant.
RESULTS
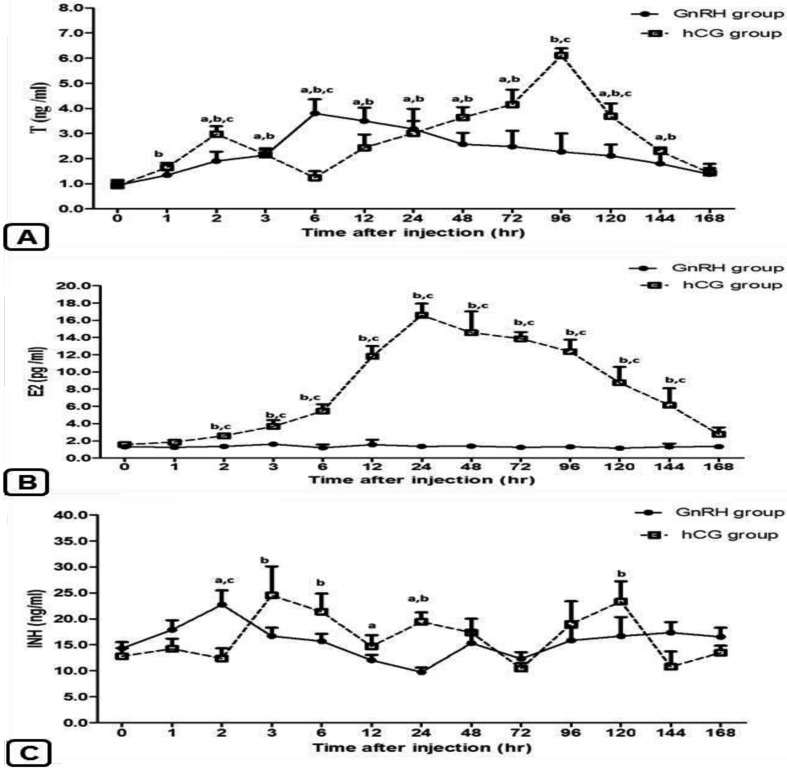
Hormonal results: Testosterone (T): Plasma concentrations of T significantly (P<0.05) changed in both groups. A monophasic peak (3.80 ± 0.56 ng/ml) was observed at 6 hr post injection in the GnRH group, while biphasic peaks were expressed at 2 hr (2.98 ± 0.31 ng/ml) and 96 hr (6.11 ± 0.28 ng/ml) in the hCG group (Fig. 2A).
Fig. 2.
Changes in plasma concentrations of testosterone (T; A), estradiol (E2; B) and inhibin (INH; C) in male Shiba goats after injection of GnRH or hCG. Results are presented as mean ± SEM. a, b Values are significantly different (P<0.05) compared with 0 hr in the GnRH and hCG groups, respectively. c Values are significantly different (P<0.05) between the groups at the indicated times during the study.
Estradiol (E2.): Unlike the GnRH group, a significant change (P<0.05) in E2 level was observed in the hCG group. The level increased gradually, and the maximum values were observed at 24 hr (16.58 ± 1.36 pg/ml) post hCG injection followed by gradual decreases (Fig. 2B). Additionally, a strong positive correlation was found between the T and E2 profiles (r=0.672, P=0.012) in this group.
Inhibin (INH): In both groups, the plasma levels of INH significantly changed (Fig. 2C). However, significant differences between the 2 groups were not observed except at 2 hr (GnRH, 22.74 ± 2.73 ng/ml; hCG, 12.38 ± 1.96 ng/ml) and 24 hr (GnRH, 9.75 ± 0.89 ng/ml; hCG, 19.41 ± 1.84 ng/ml).
Ultrasonographic results: Doppler parameters of the STA as well as TV measurement showed no significant difference between the right and left testes (P>0.05). So, the mean ± SEM was used.
Testicular blood flow by color Doppler ultrasonography: Color pulsed Doppler ultrasonography of the STA in the spermatic cord was easily performed (Fig. 3; left side). Arterial pulsation of blood in this vessel was clearly visible during real-time scanning. Doppler ultrasonography of the STA revealed a spectral pattern with a wave-like pattern. It showed monophasic (1 systolic peak) non-resistive waveforms (Fig. 3; right side).
Fig. 3.
Ultrasonographic scan of the goat testis using color pulsed-wave Doppler ultrasonography showing blood flow within the supratesticular artery (convoluted part of the testicular artery) (left side with red colors). Blood flow within the supratesticular artery revealed a spectral pattern with a wave-like pattern that appeared as monophasic non-resistive waveforms (right side).
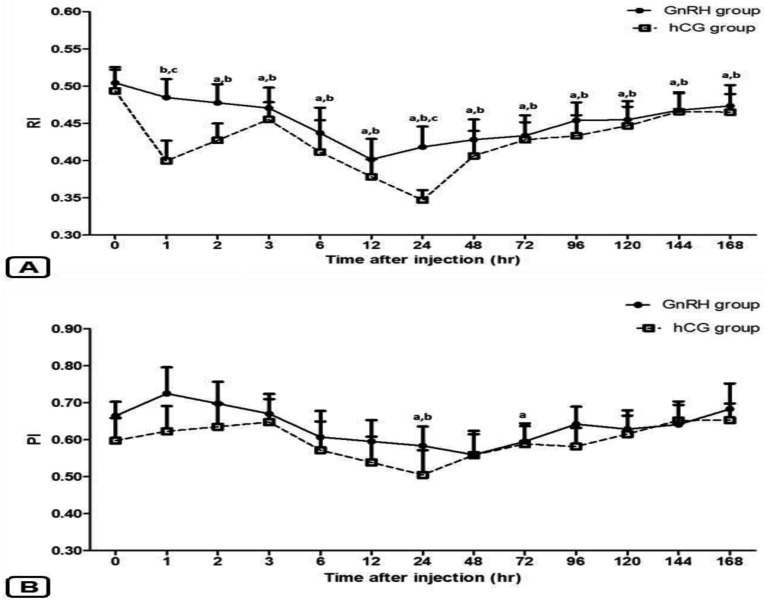
Although the resistive index (RI) of the STA significantly decreased (P<0.05) in both groups, a faster decrease in RI was observed in the hCG group (1 hr) compared with the GnRH group (2 hr) (Fig. 4A). For comparison between the 2 groups, the RI values decreased significantly (P<0.05) in the hCG group at 1 hr (0.40 ± 0.03) and 24 hr (0.35 ± 0.01) post injection compared with the GnRH group (0.48 ± 0.02, and 0.42 ± 0.03; respectively) (Fig. 4A).
Fig. 4.
Doppler results for testicular blood flow in male Shiba goats showing the changes in the resistive index (RI; A) and pulsatility index (PI; B) of the supratesticular artery after injection of GnRH or hCG. Results are presented by mean ± SEM. a, b Values are significantly different (P<0.05) compared with 0 hr in the GnRH and hCG groups, respectively. c Values are significantly different (P<0.05) between the groups at the indicated times during the study. (Note: the RI showed significant differences between the groups at 1 hr and 24 hr post injection without any significant difference between the groups in term of PI.)
Regarding to the PI, although significant (P<0.05) changes in PI values were observed in the GnRH and hCG groups, there were no significant differences (P>0.05) between the groups at any time during the period of a study (Fig. 4B).
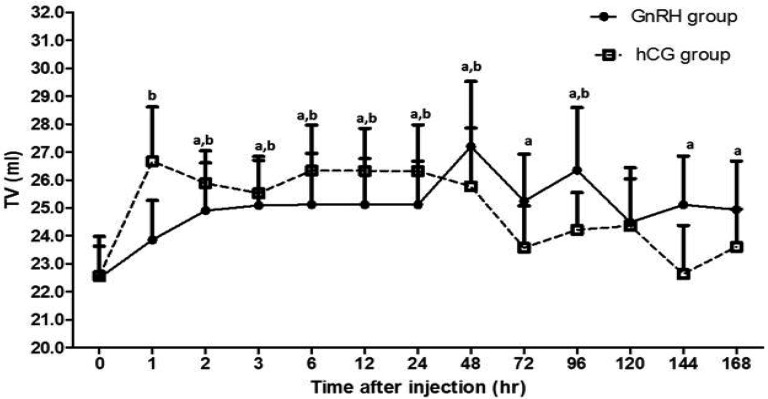
Testicular volume (TV) measurements by B-mode ultrasonography: Both the GnRH and hCG groups showed significant changes in TV compared with 0 hr. However, we did not find any significant difference (P>0.05) between them at any time during the period of study. Similar to the RI changes, a faster increase of TV was observed in the hCG group (I hr) compared with in the GnRH group (2 hr) (Fig. 5).
Fig. 5.
The results of ultrasonographic examination of male Shiba goats showed changes in testis volume (TV) after injection of GnRH or hCG. Results are presented as the mean ± SEM. a, b Values are significantly different (P<0.05) compared with 0 hr in the GnRH and hCG groups, respectively. (Note: No significant difference was found between the groups at any time during the study.)
Correlations between hormonal changes, Doppler parameters and TV changes: In the GnRH group, a strong negative correlation was found between the T level and both the RI (r= −0.862, P=0.001) and PI (r= −0.707, P=0.007) of the STA. Additionally, this group showed a high positive correlation between INH and both the RI (r=0.661, P=0.014) and PI (r=0.701, P=0.008) of the STA. Controversially, high negative correlations were found between E2 and both the RI (r= −0.610, P=0.027) and PI (r= −0.763, P=0.002) of the STA in the hCG group. Moreover, high negative correlations were found between TV and the RI of the STA in the GnRH group (r= −0.607, P=0.028) as well as the hCG group (r= −0.799, P=0.001).
DISCUSSION
A single injection of either GnRH or hCG in male Shiba goats evoked series of changes in three different parameters: hormonal, testicular blood flow, and TV changes.
Although the dose of GnRH analogue was the same, the maximum T response recorded in the current study (6 hr) did not occur as earlier as those reported in 3- to 6- month-old male Shiba goats (2 hr) [30]. The different response might be attributed to the different route of injection and/or the age effect [16]. In the current study, GnRH injected intramuscularly resulted in lower absorption than when injected intravenously in the previous study. The biphasic T response observed in the hCG group (at 2 hr and 96 hr) was in agreement with those reported in equine (1–3 hr and 24–72 hr) [2, 29].
Unlike the GnRH group, the faster T response and biphasic peaks recorded in hCG group might be attributed to various factors. Firstly, T response was secondary to LH release in the GnRH group, while hCG had a direct effect [17]. Secondly, the hormones have different half-lives: that of LH is short, while that of hCG is prolonged [41, 43]. Finally, binding to the receptor is reversible for LH, while it is not for hCG [3, 20].
The nonsignificant changes in the level of E2 observed in the GnRH group were consistent with a previous published study in stallions [33]. However, the significant increase of E2 observed in the hCG group might be related to the pattern of the plasma levels: pulsatile for LH-induced GnRH and sustained for hCG, which resulted in a prolonged stimulation of Leydig cell steroidogenesis [3, 39]. Moreover, the strong positive correlation found between T and E2 responses after hCG injection in the current study was consistent with those reported in stallions and might be attributable to transformation of T into E2 by the aromatase enzyme in Leydig cells [2, 29, 45].
Blood flow is important to testis function, because any defect in the arterial blood flow to the testis could cause impaired spermatogenesis secondary to defective energy metabolism at the mitochondrial level [11]. The non-resistive monophasic waveforms of the testicular blood flow observed in the current study were consistent with those reported in the human testis [19] and dog testis [7, 8]. On the other hand, Pozor and McDonnell [27] found high resistive waveforms of testicular blood flow in stallions. Although color Doppler ultrasonography has been used to evaluate testicular blood flow after hCG administration [2, 26], the present study was the first to evaluate the effect of GnRH compared with hCG on testicular hemodynamic.
An earlier study in bulls [4] and a recent study in rams [40] found an increase of the scrotal surface temperature measured with a laser thermometer, representing changes of testicular blood flow after GnRH administration.
The RI of the testicular artery is considered a valuable indicator of the sperm production rate score in the human testis [1] and semen quality in dogs [44]. It was reported that RI has a negative correlation with vascular perfusion of tissue downstream from the sample gate [5]. In the current study, because the RI and PI of the supratesticular artery significantly (P<0.05) decreased after the injections in both groups, it can be said that hCG or GnRH injection could induce an improvement of testicular blood flow. However, this improvement occurred significantly earlier and was greater in the hCG group (1 hr) than in the GnRH group (2 hr). A possible explanation for this may be related to the direct effect of hCG compared with the indirect effect of GnRH [34].
In addition, one of the main objectives of this study was to evaluate how testicular hormone levels affect testicular blood flow. In the current study, although an increase in testicular blood flow (represented by decreases in the RI and PI of the STA) was observed in both groups, there were discrepancies in terms of T and E2 correlations with Doppler indices. In the hCG group, E2 was highly negatively correlated with the RI and PI of the STA without any correlation with T, and the opposite was observed in the GnRH group. These results were consistent with previously published studies revealing that hCG treatment induces an increase in testicular blood flow in parallel with an increase in E2 level without any correlation with T levels [2, 6]. However, another study found a strong correlation between testicular blood flow and T after hCG treatment [26]. Indeed, the strong correlations between E2 and Doppler indices in the current study might be related to the strong vasodilatory effect of estrogen and its role in testicular perfusion [2, 28]. Importantly, the involvement of T and E2 in the present study only indicated an associative relationship between these hormones and Doppler indices and did not elucidate the mechanism; however, verification of this hypothesis requires a further study at the testicular level.
Interestingly, the strong positive correlations found between INH and the RI and PI of the STA post GnRH injection were novel data obtained in the present study. In humans, both the peripheral INH level [18] and the RI of the testicular artery [1] were significantly related to the sperm production rate score and good predictors of spermatogenesis. Therefore, a further study is needed to clarify the role of inhibin in testicular blood flow in male Shiba goats.
B-mode ultrasonography is considered to be a more accurate, precise and reproducible method for measurement of TV in assessing testicular function and pubertal development [21, 31, 37]. An increase of TV recorded in this study after hCG injection was similar to those observed in men [14] as well as in rats [23].
In both groups, the strong negative correlations found between TV and the RI and PI of the STA might provide us with an explanation for the TV increases. Decreased values of the RI and PI of the STA might result in an increase of testicular blood perfusion and leads to an increase in testicular vascular permeability and interstitial fluid (IF). Similarly, in hCG-treated rats, dose- and time-dependent covariation was found between the increase in testicular IF volume and the increase in testicular microcirculation [42]. Moreover, it was recently reported that administration of GnRH triggers a rapid increase in testicular fluid content in rams [40]. Collectively, the increase in TV observed in this study might be attributed to increases in testicular blood flow resulting in increases in IF.
In conclusion, the results of the present study show that the administration of a single dose of GnRH or hCG may have a beneficial effect on testicular blood flow in male Shiba goats as well as on TV, but that hCG has a faster effect on testicular blood flow compared with GnRH. These beneficial effects may result in an increase in male fertility through positive influences on testicular blood flow and, in turn, on spermatogenesis. Moreover, the involvement of T, E2 and INH in testicular hemodynamics in male Shiba goats requires a further study to test this hypothesis.
Acknowledgments
We are grateful to Dr. G.D. Niswender (Animal Reproduction and Biotechnology Laboratory, Colorado State University, Fort Collins, CO, U.S.A.) for providing antisera to estradiol-17β (GDN 244) and testosterone (GDN 250).
REFERENCES
- 1.Biagiotti G., Cavallini G., Modenini G., Vitali G., Gianoroli L.2002. Spermatogenesis and spectral echo-colour Doppler traces from the main testicular artery. BJU Int. 90: 903–908. doi: 10.1046/j.1464-410X.2002.03033.x [DOI] [PubMed] [Google Scholar]
- 2.Bollwein H., Schulze J. J., Miyamoto A., Sieme H.2008. Testicular blood flow and plasma concentrations of testosterone and total estrogen in the stallion after the administration of human chorionic gonadotropin. J. Reprod. Dev. 54: 335–339. doi: 10.1262/jrd.20014 [DOI] [PubMed] [Google Scholar]
- 3.Dufau M. L., Catt K. J.1978. Gonadotropin receptors and regulation of steroidogenesis in testis and ovary. Vitam. Horm. 36: 461–592. doi: 10.1016/S0083-6729(08)60989-9 [DOI] [PubMed] [Google Scholar]
- 4.Gábor G., Sasser R. G., Kastelic J. P., Coulter G. H., Everson D. O., Falkay G., Mezes M., Bozo S., Cook R. B., Volgyi Csil J., Barany I., Szasz F., Jr1998. Endocrine and thermal responses to GnRH treatment and prediction of sperm output and viability in Holstein -Friesian breeding bulls. Theriogenology 50: 177–183. doi: 10.1016/S0093-691X(98)00124-1 [DOI] [PubMed] [Google Scholar]
- 5.Ginther O. J. (ed.). 2007. Ultrasonic Imaging and Animal Reproduction: Color- Doppler Ultrasonography. Cross Plains, WI: Equiservices Publishing. [Google Scholar]
- 6.Gonzalvo V., Calvo M. A., Navalon P., Cejalvo D., Ramada F. J., Blasco J. E., Donderis C., Lloris J. M.1993. Role of testosterone in the testicular microcirculatory changes produced in the rat by the administration of high doses of human chorionic gonadotrophin. Arch. Esp. Urol. 46: 669–672(Abstract; Article in Spanish). [PubMed] [Google Scholar]
- 7.Gumbsch P., Gabler C., Holzmann A.2002. Colour-coded duplex sonography of the testes of dogs. Vet. Rec. 151: 140–144. doi: 10.1136/vr.151.5.140 [DOI] [PubMed] [Google Scholar]
- 8.Günzel-Apel A. R., Mohrke C., Poulsen Nautrup C.2001. Colour-coded and pulsed Doppler sonography of the canine testis, epididymis and prostate gland: physiological and pathological findings. Reprod. Domest. Anim. 36: 236–240. doi: 10.1046/j.1439-0531.2001.00288.x [DOI] [PubMed] [Google Scholar]
- 9.Hamada T., Watanabe G., Kokuho T., Taya K., Sasamoto S., Hasegawa Y., Miyamoto K., Igarashi M.1989. Radioimmunoassay of inhibin in various mammals. J. Endocrinol. 122: 697–704. doi: 10.1677/joe.0.1220697 [DOI] [PubMed] [Google Scholar]
- 10.Herwig R., Tosun K., Pinggera G. M., Soelder E., Moeller K. T., Pallwein L., Frauscher E., Bartsch G., Wildt L., Illmensee K.2004. Tissue perfusion essential for spermatogenesis and outcome of testicular sperm extraction (TESE) for assisted reproduction. J. Assist. Reprod. Genet. 21: 175–180. doi: 10.1023/B:JARG.0000031251.57848.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu H. S., Chang L. S., Chen M. T., Wei Y. H.1994. Decreased blood flow and defective energy metabolism in the varicocele-bearing testicles of rats. Eur. Urol. 25: 71–75. [DOI] [PubMed] [Google Scholar]
- 12.Kano Y., Sawasaki T., Oyama T.1977. Biological characteristics of miniature “Shiba” goats. Exp. Anim. 26: 239–246(Article in Japanese). [PubMed] [Google Scholar]
- 13.Kay G. W., Grobbelaar J. A., Hattingh J.1992. Effect of surgical restriction of growth of the testicular artery on testis size and histology in bulls. J. Reprod. Fertil. 96: 549–553. doi: 10.1530/jrf.0.0960549 [DOI] [PubMed] [Google Scholar]
- 14.Kim S. O., Ryu K. H., Hwang I. S., Jung S. I., Oh K. J., Park K.2011. Penile growth in response to human chorionic gonadotropin (HCG) treatment in patients with idiopathic hypogonadotrophic hypogonadism. Chonnam Med. J. 47: 39–42. doi: 10.4068/cmj.2011.47.1.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutzler M., Tyson R., Grimes M., Timm K.2011. Determination of testicular blood flow in Camelids Using vascular casting and colour pulsed-wave Doppler ultrasonography. Vet. Med. Int. 2011: . doi: 10.4061/2011/638602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Y. C., Fukaya T., Rikihisa Y.1985. Effects of age and luteinizing hormone antiserum on human chorionic gonadotropin-stimulated testosterone secretion in young male rats. Life Sci. 37: 481–488. doi: 10.1016/0024-3205(85)90411-4 [DOI] [PubMed] [Google Scholar]
- 17.McKinnon A. O., Voss J. L.1993. Equine Reproduction, Lea & Febiger, Philadelphia. [Google Scholar]
- 18.Meachem S. J., Nieschlag E., Simoni M.2001. Inhibin B in male reproduction: pathophysiology and clinical relevance. Eur. J. Endocrinol. 145: 561–571. doi: 10.1530/eje.0.1450561 [DOI] [PubMed] [Google Scholar]
- 19.Middleton W. D., Thorne D. A., Melson G. L.1989. Color Doppler ultrasound of the normal testis. AJR Am. J. Roentgenol. 152: 293–297. doi: 10.2214/ajr.152.2.293 [DOI] [PubMed] [Google Scholar]
- 20.Padrón R. S., Wischusen J., Hudson B., Burger H. G., de Kretzer D. M.1980. Prolonged biphasic response of plasma testosterone to single intramuscular injections of human chorionic gonadotropin. J. Clin. Endocrinol. Metab. 50: 1100–1104. doi: 10.1210/jcem-50-6-1100 [DOI] [PubMed] [Google Scholar]
- 21.Paltiel H. J., Diamond D. A., Di Canzio J., Zurakowski D., Borer J. G., Atala A.2002. Testicular volume: comparison of orchidometer and US measurements in dogs. Radiology 222: 114–119. doi: 10.1148/radiol.2221001385 [DOI] [PubMed] [Google Scholar]
- 22.Panarace M., Garnil C., Cane L., Rodriguez E., Medina M.2008. EchoDoppler ultrasonographic assessment of resistance and velocity of blood flow in the ductus venosus throughout gestation in fetal lambs. Theriogenology 70: 648–654. doi: 10.1016/j.theriogenology.2008.04.027 [DOI] [PubMed] [Google Scholar]
- 23.Papparella A., Nino F., Noviello C., Romano M., Papparella S., Paciello O., Sinisi A. A.2013. Morphologic changes due to human chorionic gonadotropin in the rat testis: Role of vascular endothelial growth factor. Open J. Pediatrics 3: 85–91. doi: 10.4236/ojped.2013.32016 [DOI] [Google Scholar]
- 24.Parlevliet J. M., Bevers M. M., van de Broek J., Colenbrander B.2001. Effect of GnRH and hCG administration on plasma LH and testosterone concentrations in normal stallions, aged stallions and stallions with lack of libido. Vet. Q. 23: 84–87. doi: 10.1080/01652176.2001.9695088 [DOI] [PubMed] [Google Scholar]
- 25.Pozor M. A.2007. Evaluation of Testicular Vasculature in Stallions. Clin. Tech. Equine Pract. 6: 271–277. doi: 10.1053/j.ctep.2007.09.007 [DOI] [Google Scholar]
- 26.Pozor M. A., Macpherson M. L., Troedsson M. H. T., Verstegen J.2006. Effect of a single administration of human chorionic gonadotropin (hCG) on testicular blood flow in stallions. Anim. Reprod. Sci. 94: 146–147. doi: 10.1016/j.anireprosci.2006.03.092 [DOI] [Google Scholar]
- 27.Pozor M. A., McDonnell S. M.2004. Color Doppler ultrasound evaluation of testicular blood flow in stallions. Theriogenology 61: 799–810. doi: 10.1016/S0093-691X(03)00227-9 [DOI] [PubMed] [Google Scholar]
- 28.Rosenfeld C. R., Roy T., Cox B. E.2002. Mechanisms modulating estrogen-induced uterine vasodilation. Vascul. Pharmacol. 38: 115–125. doi: 10.1016/S0306-3623(02)00135-0 [DOI] [PubMed] [Google Scholar]
- 29.Roser J. F.1995. Endocrine profiles in fertile, subfertile, and infertile stallions: testicular response to human chorionic gonadotropin in infertile stallions. Biol. Reprod. Mono. 1: 661–669. [Google Scholar]
- 30.Saito H., Sawada T., Yaegashi T., Goto Y., Jin J., Sawai K., Hashizume T.2012. Kisspeptin-10 stimulates the release of luteinizing hormone and testosterone in pre- and post-pubertal male goats. Anim. Sci. J. 83: 487–492. doi: 10.1111/j.1740-0929.2011.00978.x [DOI] [PubMed] [Google Scholar]
- 31.Sakamoto H., Ogawa Y., Yoshida H.2008. Relationship between testicular volume and testicular function: comparison of the Prader orchidometric and ultrasonographic measurements in patients with infertility. Asian J. Androl. 10: 319–324. doi: 10.1111/j.1745-7262.2008.00340.x [DOI] [PubMed] [Google Scholar]
- 32.Saleh M., Shahin M., Wuttke W., Gauly M., Holtz W.2012. Pharmacokinetics of human chorionic gonadotropin after i.m. administration in goats (Capra hircus). Reproduction 144: 77–81. doi: 10.1530/REP-12-0093 [DOI] [PubMed] [Google Scholar]
- 33.Seamans M. C., Roser J. F., Linford R. L., Liu I. K., Hughes J. P.1991. Gonadotrophin and steroid concentrations in jugular and testicular venous plasma in stallions before and after GnRH injection. J. Reprod. Fertil. 44Suppl.: 57–67. [PubMed] [Google Scholar]
- 34.Seguin B. E., Oxender W. D., Britt J. H.1977. Effect of human chorionic gonadotropin and gonadotropin-releasing hormone on corpus luteum function and oestrous cycle duration in dairy heifers. Am. J. Vet. Res. 38: 1153–1156. [PubMed] [Google Scholar]
- 35.Serin G., Gokdal O., Tarımcılar T., Atay O.2010. Umbilical artery Doppler sonography in Saanen goat fetuses during singleton and multiple pregnancies. Theriogenology 74: 1082–1087. doi: 10.1016/j.theriogenology.2010.05.005 [DOI] [PubMed] [Google Scholar]
- 36.Setchell B. P.1990. Local control of testicular fluids. Reprod. Fertil. Dev. 2: 291–309. doi: 10.1071/RD9900291 [DOI] [PubMed] [Google Scholar]
- 37.Takihara H., Sakatoku J., Fujii M., Nasu T., Cosentino M. J., Cockett A. T. K.1983. Significance of testicular size measurement in andrology. A new orchidometer and its clinical application. Fertil. Steril. 39: 836–840. [DOI] [PubMed] [Google Scholar]
- 38.Taya K., Watanabe G., Sasamoto S.1985. Radioimmunoassay for progesterone, testosterone and estradiol 17 b using 125I- Iodohistamine radioligands. Jpn. J. Anim. Reprod. 31: 186–197. doi: 10.1262/jrd1977.31.186 [DOI] [Google Scholar]
- 39.Trinchard-Lugan I., Khan A., Porchet H. C., Munfano A.2002. Pharmacokinetics and pharmacodynamics of recombinant human chorionic gonadotrophin in healthy male and female volunteers. Reprod. Biomed. Online 4: 106–115. doi: 10.1016/S1472-6483(10)61927-X [DOI] [PubMed] [Google Scholar]
- 40.Ungerfeld R., Fila D.2011. Testicular fluid content evaluated by ultrasound image computer-assisted analysis increases with small-dose multiple GnRH injections in rams. Reprod. Domest. Anim. 46: 720–723. doi: 10.1111/j.1439-0531.2010.01735.x [DOI] [PubMed] [Google Scholar]
- 41.Veldhuis J. D., Fraioli F., Rogol A. D., Dufau M. L.1986. Metabolic clearance of biologically active luteinizing hormone in man. J. Clin. Invest. 77: 1122–1128. doi: 10.1172/JCI112411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Widmark A., Damber J. E., Bergh A.1989. High and low doses of luteinizing hormone induce different changes in testicular microcirculation. Acta Endocrinol. (Copenh.) 121: 621–627. [DOI] [PubMed] [Google Scholar]
- 43.Yen S. S., Iberena O., Little B., Pearson O. H.1968. Disappearance rates of endogenous luteinizing hormone and chorionic gonadotropin in man. J. Clin. Endocrinol. Metab. 28: 1763–1767. doi: 10.1210/jcem-28-12-1763 [DOI] [PubMed] [Google Scholar]
- 44.Zelli R., Troisi A., Elad Ngonput A., Cardinali L., Polisca A.2013. Evaluation of testicular artery blood flow by Doppler ultrasonography as a predictor of spermatogenesis in the dog. Res. Vet. Sci. 95: 632–637. doi: 10.1016/j.rvsc.2013.04.023 [DOI] [PubMed] [Google Scholar]
- 45.Zwain I., Gaillard J. L., Dintinger T., Silberzahn P.1989. Down-regulation of testicular aromatization in the horse. Biol. Reprod. 40: 503–510. doi: 10.1095/biolreprod40.3.503 [DOI] [PubMed] [Google Scholar]