Abstract.
Optogenetics has revolutionized neuroscience by enabling precise control of neural excitation. The development of similar optogenetics strategies in the heart is just emerging and mainly focused on pacing with light activation of channelrhodopsin-2. Here, we aimed to develop an optogenetic approach to suppress local cardiac electrical activity by using engineered cell-grafts (HEK293-cells) transfected to express the light-sensitive hyperpolarizing proton-pump archaerhodopsin-3 (Arch3). To evaluate the ability of the engineered cells to couple and modulate the electrical activity of cardiomyocytes, we co-cultured the Arch3-HEK293 cells with neonatal rat cardiomyocytes (NRCMs) or human embryonic stem cells derived cardiomyocytes (hESC-CMs). The co-cultures’ conduction and chronotropic properties were evaluated prior, during, and following application of focused monochromatic light (590 nm) using a multielectrode array mapping system. Application of focused illumination completely silenced electrical activity at the illuminated area in all NRCM co-cultures, leading to development of localized functional conduction blocks. Similarly, illumination significantly slowed spontaneous beating-rate in the hESCs-CMs co-cultures (from to , ). Interestingly, a transient acceleration in beating-rate was noted immediately postillumination. In conclusion, a combined gene and cell therapy approach, using light-sensitive hyperpolarizing proteins, could be used to modulate conduction and automaticity in cardiomyocyte cultures, opening the way for future optogenetic treatments for cardiac tachyarrhythmias.
Keywords: optogenetics, molecular ablation, archaerhodopsin, cardiomyocytes, cardiac suppression
1. Introduction
Cardiac arrhythmias are defined as any deviation from the normal pattern or rate of the cardiac electrical activation and are a major cause of worldwide morbidity and mortality. Traditionally, cardiac arrhythmias can be classified into rhythm disorders that result in abnormally low heart rate (bradyarrhythmias) requiring the implantation of an electronic pacemaker, or those that produce an abnormally fast and uncoordinated beating rate (tachyarrhythmias). Cardiac tachyarrhythmias usually stem from abnormalities in the myocardial electrophysiological or structural substrate, with a clinical presentation ranging from symptomatic palpitations to sudden cardiac death.
Conventional treatments for tachyarrhythmias include implantable cardiac defibrillators, radiofrequency catheter ablation, and the use of antiarrhythmic drugs. The latter are limited by systemic side effects, may have proarrhythmic influence, and lacks significant effect on survival.1–3 Similarly, radiofrequency ablation is associated with the destruction of cardiac tissue, which is undesirable, for example, in patients with heart failure. Finally, although implanted defibrillators were found to prolong life, they are also associated with decreased quality of life, especially in the presence of inappropriate shocks.4 Therefore, innovative approaches are warranted for the treatment and prevention of tachyarrhythmias.
Optogenetics, using light to activate specific light-sensitive proteins, has revolutionized neuroscience by enabling precise control (activation or suppression) of neural circuits both in vitro and in vivo with high spatial and temporal precision. Efficient suppression of cell excitation with light was first reported in 2007 using the light-sensitive chloride-pump halorhodopsin (Halo)5 and its use for neural suppression was demonstrated in various models and species.6–8 In 2010, a potentially more potent light-controlled hyperpolarizing protein was described for suppression of neuronal activity: a light-sensitive proton-pump termed archaerhodopsin-3 (Arch3).9
Until recently, most optogenetics studies were devoted to neuroscience research. Only a few proof-of-concept reports on using optogenetics in the cardiac field were published. It was demonstrated that channelrhodopsin-2 (ChR2), a light-sensitive depolarizing protein, could be used to stimulate cardiac cells cultures.10,11 Arrenberg et al. demonstrated that zebrafish cardiomyocytes could be both stimulated and silenced by expressing both depolarizing and hyperpolarizing light sensitive proteins.12 Abilez reported on inhibition of beating-induced pluripotent stem cells colony virally infected to express Halo for up to 2 s by illumination with an orange light.13 Bruegmann et al. created a transgenic mice expressing ChR2, and were able to demonstrate cardiac pacing in vivo.14 Recently, we showed that fibroblasts could be transfected with ChR2 and that these cells could couple with neighboring neonatal rat cardiomyocytes (NRCMs) in co-culture studies and used for single- or multisite optogenetic pacing.11 In further feasibility experiments in the same study,11 the fibroblasts were transfected to also express the light-sensitive protein Arch-T and were demonstrated to be able to also suppress activity in the co-cultures.
Here, we aimed to expand on these previous observations and to evaluate the ability of engineered cell grafts, expressing the light-sensitive hyperpolarization proton pump Arch3, to suppress electrical activity in two different in vitro models of cardiomyocyte cultures [using either NRCMs or human embryonic stem cells derived cardiomyocytes (hESC-CMs)]. We hypothesized that the engineered cells (Arch3-expressing HEK293 cells) will be able to couple functionally with neighboring cardiomyocytes and to suppress their electrical activity upon appropriate illumination through gap junction-mediated electrotonic interactions. To this end, we aimed to evaluate the ability of the engineered cells to create localized functional conduction blocks following focal illumination (“molecular-ablation” strategy) in the NRCM monolayers as well as to suppress automaticity in the hESC-CMs co-cultures. Finally, dose–response studies were performed to evaluate the spatial and temporal magnitudes of the electrophysiological effects.
2. Materials and Methods
2.1. Preparation of Genetically Modified Human Embryonic Kidney Cells
The plasmid FCK-Arch-GFP (Plasmid No. 22217) was acquired from the Addgene Corporation. Stable transfection was achieved in HEK293 cells by the jetPEI transfection reagent (PolyPlus transection Inc). Transfected cells were selected according to their fluorescence level by using repeated fluorescence-activated cell sorting.
2.2. Preparation of Neonatal Rat Cardiomyocytes Monolayers and Co-Cultures
Primary cultures of 0- to 1-day-old neonatal rat (Sprague-Dawley) ventricular cardiomyocytes (NRCM) were extracted as previously described.11,15 The experimental protocol was approved by the Animal Study Committee of the Technion Faculty of Medicine. In brief, the excised ventricular tissue was suspended in a culture medium (F-10, 5% FCS, 5% horse serum, penicillin, streptomycin), and cardiomyocytes were extracted enzymatically with RDB (IIBR, Ness-Ziona, Israel). 5-bromo-2’-deoxyuridine was used during the preparation of the cultures to reduce the replication of nonmyocytes within the NRCM cultures. Cells were then cultured on a microelectrode array (MEA) culture plates (Multichannels System, Reutlingen, Germany) at a density of . To create the co-culture, transfected Arch3-expressing HEK293 cells were added to the cultures with a ratio of human embryonic kidney (HEK) to cardiomyocyte cells of 1:6–16 cells.
2.3. Immunostaining of the NRCM Co-Cultures
NRCM-HEK co-cultures were prepared as described previously by seeding the NRCMs and Arch3-HEK cells (expressing eGFP) at ratio of 10:1 on fibronectin-coated cover slips. Specimens were fixed 3 days later with 4%-paraformaldehyde, permeabilized with 1%-Triton X-100, blocked with 5%-DHS, and incubated overnight at 4°C with mouse anti-sarcomeric--actinin (Sigma, 1:200). The preparations were incubated with secondary antibodies (1:200, Jackson). Nuclei were counterstained with DAPI. Preparations were examined using confocal microscopy (Zeiss LSM-510-PASCAL).
2.4. Preparation of Human Embryonic Stem Cells Derived Cardiomyocytes and Co-Culture
Undifferentiated hESCs (H9.2 clone) were cultivated in suspension for 7 to 10 days as embryoid bodies (EBs) as previously described.16,17 Beating areas, identified within the EBs after plating, were dissected and plated on 60 microelectrode array MEA plates (1–2 EBs per MEA plate) as reported elsewhere.18 One to two days later, Arch3-transfected HEK cells were added and co-cultured for 4–7 days until the beating EB was surrounded with an abundant layer of HEK cells.
2.5. Multielectrode Array Mapping
Extracellular recordings from the cultured NRCM and from the hESC-CMs were recorded and analyzed by an MEA data acquisition system (Multichannel systems, Reutlingen, Germany).19 The MEA consists of a matrix of 252 () or 60 () electrodes with an interelectrode distance of and a sampling rate of 20 kHz. Temperature was kept at during measurements. Extracellular electrograms were recorded 3–5 days following NRCM seeding after development of synchronized contraction. Measurement of electrical activity from the beating EBs was conducted 4–7 days following co-culturing with Arch3-transfected HEK cells.
2.6. Optogenetics Illumination
Illuminations were conducted with fiber-coupled 1.0A monochromic light-emitting diode (LED) (590 nm, Item# M590F1, Thorlab Inc.) connected to high-power LED driver (Item# LEDD1B, Thorlab Inc.). Light was transferred to the co-cultures using a 1.0-mm optical fiber. A 30-s-long basal recording at darkened environment was followed with 30-s-long focused beam illumination, and by another 30-s recording period postillumination. Measurements were conducted at different LED output currents (1000, 666, and 333 mA) resulting in irradiance levels of 11.61, 10.14, and as measured via the MEA glass.
2.7. Data Analysis
The 252-channel data from the MEA recording were analyzed by custom-made MATLAB®-based software. Local activation time (LAT) was calculated at each electrode by determining the timing of the maximal negative slope of the extracellular signal as described.15,18 The LAT was detected only in regions where the “peak-to-trough” amplitude of the electrical depolarization complex was larger than six standard deviations of the filtered signal. Contraction rate was measured with the peak detector utility of the MC_Rack (version 4.3.5; Multi Channel Systems). Measurement of the NRCM mean beating-rate was calculated during 30 s at complete darkness prior to illumination, during 30-s-long illumination, and for 30 s following the termination of illumination. Beating rates for the contracting hESC-derived cardiomyocyte cell-aggregates were measured as the mean rate during 30 s prior to illumination, according to the first beat during illumination (i.e., 60,000/first interbeat-interval [ms]), as the mean rate during the entire illumination period, and for the first beat after termination of illumination.
2.8. Statistical Analysis
Data were analyzed using JMP Pro version 10.0 (SAS Institute, Cary, North Carolina). Results are presented as . Distribution analysis was performed using the Shapiro-Wilk W test. Matched pairs were compared with the paired test for normally distributed parameters and by nonparametric Wilcoxon/Kruskal–Wallis test for non-normally distributed parameters. Comparisons of the effects of different irradiance levels on the culture’s beating rate were computed only for cultures which maintained an unchanged baseline contraction rate (standard deviation of mean baseline contractions rates of the different illumination studies lower than 8.5% of the mean contraction rate of all measurements regardless of the illumination intensity).
3. Results
3.1. Focused Illumination to Suppress Local Electrical Activity in the NRCM Cultures
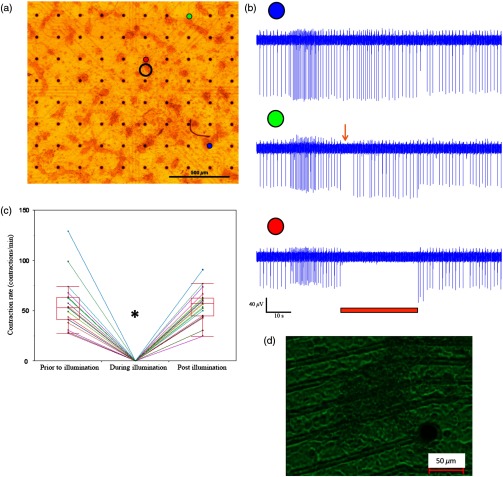
To evaluate the ability to modulate cardiomyocyte excitability with the proposed optogenetics strategy, the Arch3-expressing HEK cells were co-cultured in a diffuse manner with primary NRCMs on top of MEA culture plates [Fig. 1(a)]. Following the development of synchronized electrical activity in the co-cultures (with a spontaneous beating-rate of ), ), we utilized an LED-coupled optical-fiber to apply focused illumination in an attempt to activate the Arch3-HEK cells at a highly localized area within the co-cultures [black circle in the example in Fig. 1(a)].
Fig. 1.
Application of focused illumination led to complete suppression of the localized electrical activity at the illuminated area in all co-cultures studied (). This can be appreciated by the cessation of mechanical contraction in the illuminated area [Video 1, as illustrated in Fig. 1(d)] as well as by the silencing of the electrical activity in the same region. Note in the example depicted in Fig. 1(b), the complete termination of electrical activity during the illumination period in the recordings in the relevant electrode located at the illuminated area (highlighted in red). Electrical activity resumed at this region immediately following the termination of illumination. In contrast, electrical activity was unaffected and continued throughout the experiment in remote nonilluminated areas, as can be appreciated in the recordings from the electrode highlighted in blue in Fig. 1(b).
To confirm that the observed optogenetics-based electrophysiological suppression effect was not unique to specific locations within the co-cultures, we also tested the effects of shifting the location of the focal illumination area to additional zones in the same culture. Figure 2 shows an example of shifting the illumination sites between two different areas within the same co-culture (targeting either the green or red electrodes in the example provided). Note the successful termination of the electrical activity at each case at the illuminated area [Figs. 2(b)–2(c)].
Fig. 2.
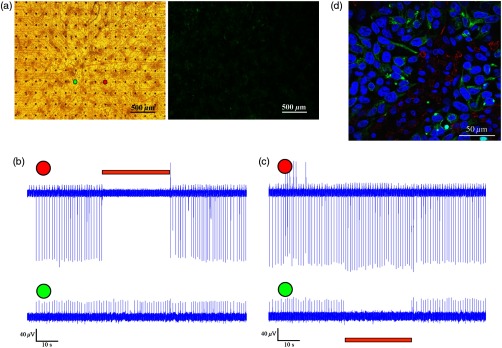
Optogenetics suppression of electrical activity at different co-culture sites. (a) The NRCMs were co-cultured with Arch3-expressing HEK cells (identified by their eGFP fluorescence, right panel). (b,c) Extracellular potentials recordings from two microelectrode array (MEA) electrodes [highlighted in red and green in (a)] during targeted illumination (590-nm monochromatic light) focusing on either the culture area surrounding the red (b) or green (c) electrodes. (d) Immunostaining of the co-cultures. The NRCMs were identified by staining with anti-sarcomeric--actinin antibodies (red) and the Arch-HEK cells were identified by their eGFP fluorescence (green).
Finally, to quantify the cell ratio between the NRCM and Arch3-HEK cells within the co-cultures required to achieve the optogenetic effect, we performed immunostaining analysis of co-cultures prepared in a similar manner on fibronectin-coated cover-slips and studied 3 days later [Fig. 2(d)]. The cardiomyocytes in the co-cultures were identified by staining with anti-sarcomeric -actinin antibodies (red), while the Arch3-HEK cells were identified by their eGFP fluorescence (green). As shown in Fig. 2(d), the two cell types developed tight structural interactions within the co-cultures. It was found that the mean number of NRCM within a field of view of () was compared with HEK cells, resulting in an average NRCM/HEK cell ratio of 1:1.4.
3.1.1. Spatial and temporal extents of the optogenetics effects
During the experiments, we noted an interesting phenomenon regarding the spatial extensions of the effects induced by illumination. Thus, termination of electrical activity was dependent on the distance from the focused illumination source. Complete silencing of the electrical activity was observed throughout the entire irradiation period in cardiomyocytes directly underlying the center of the illuminated area as observed in the recordings from electrodes located within this zone [Fig. 1(b) (red-electrode) and Fig. 1(c)]. A transient electrical suppression effect was recorded in electrodes surrounding the illuminated area. Note for example in Fig. 1(b), the transient suppression of electrical activity (during the initial beats following the beginning of illumination, arrow) at an electrode located 0.56 mm away from the light source (green electrode). In contrast, no changes were noted in the extracellular potential recordings made by electrodes located further away such as in electrograms recorded from the electrode highlighted in blue in Fig. 1(b), located 1 mm away from the light source.
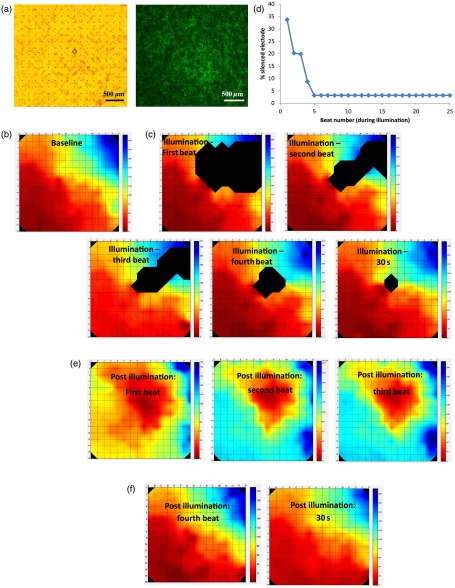
To follow the spatial and temporal effects induced by Arch3 activation by focal illumination [black circle in Fig. 3(a)] of the co-culture, we constructed detailed activation (conduction) maps during several beats occurring before [Fig. 3(b)], during [Figs. 3(c)], and following the termination [Figs. 3(e)–3(f)] of illumination. At each beat, the electrograms recorded from all MEA electrodes were analyzed to determine the local activation time at each electrode. This information was then used to construct color-coded activation maps (with red representing the earliest activity in the culture and blue the latest). Electrodes where electrical activity could not be recorded were represented by black areas in the maps.
Fig. 3.
Activation mapping of the NRCM co-cultures: optogenetics effects. (a) The NRCMs were co-cultured with Arch3-expressing HEK cells (identified by their eGFP fluorescence, right). The black circle represents the illuminated area. (b, c) Activation maps of the co-cultures derived during spontaneous contraction prior to illumination (b) and during illumination (c). Shown are the first, second, third, and fourth beats during illuminations as well as the activation map following 30 s of illumination. (d) A plot depicting the extent of the “electrically silent” area (defined as the percentage of the total number of recording electrodes) as function of the number of contraction-cycles after the beginning of the illumination period. (e, f) Activation maps of the co-cultures derived following the termination of illumination. The first three beats postillumination showed a shift in the pacemaker location (red area) to the site of illumination (e). Normal pacemaker activity was restored from the fourth beat postillumination (f).
As can be appreciated in the different maps constructed during the 30-s illumination period [Figs. 3(c)], areas of functional conduction block were generated as a consequence of the focal illumination, with the activation wave-front propagating around these affected areas of complete electrical silencing (black areas in the maps). Notably, the size of the functional block area was largest at the onset of illumination [Fig. 3(c); first beat] involving a zone (black area) that was larger than the targeted illuminated area. This area of functional block gradually decreased in size with time [Fig. 3(c); second, third, and fourth beats] until it was minimal and only present at the center of the illuminated area [Fig. 3(c); 30 s following initiation of illumination]. This phenomenon can also be appreciated in the plot depicting the extent of the “electrically silent” area (defined as the percentage of the total number of recording electrodes) over time [Fig. 3(d)].
Interestingly, in the first beats occurring immediately following the termination of illumination, we noted a shift in the pacemaker area with the activation originating at the area that was previously illuminated [Fig. 3(e)]. This activity was rather transient and only involved three contraction cycles [Fig. 3(e)]. At the fourth beat, we noted an abrupt change in the location of the pacemaker, with the earliest activation shifting back to the original area prior to illumination [Fig. 3(f)]. The restored activation pattern was then continuously maintained as shown in the activation map generated 30 s later [Fig. 3(f)]. A similar pattern was observed in all cultures studied ( in which the location of the pacemaker at baseline recordings was away from the illuminated area) with the number of contraction cycles originating from the irradiated area following the termination of illumination averaging cycles.
3.1.2. Optogenetic-based functional conduction block for electrical isolation
We next evaluated the ability to completely electrically isolate a specific area with the NRCM monolayer from the rest of the culture. This electrical isolation may be relevant, for example, for clinical procedures designed to treat atrial fibrillation by isolating the pulmonary veins.20 To this end, we applied a greater illumination area to a specific zone within the co-culture attempting to functionally isolate a quadrant of the co-culture studied. Figure 4 depicts a successful example of such an experiment. Note that initially the co-culture displayed synchronized electrical propagation [as evident by the recordings made from the green, red, and blue electrodes, Fig. 4(b)]. Following the application of targeted illumination to the area surrounding the red electrode, a functional line of conduction block developed leading to silencing of electrical activity in this area [red electrode tracing in Fig. 4(c)] and to complete isolation of the lower right quadrant of the co-culture, as manifested by the complete dissociation between the activities recorded in the green and blue electrodes [Fig. 4(c)]. Following the termination of illumination, synchronization of the electrical activity in the culture activity was restored as well as the electrical activity in the red electrode [Fig. 4(d)].
Fig. 4.
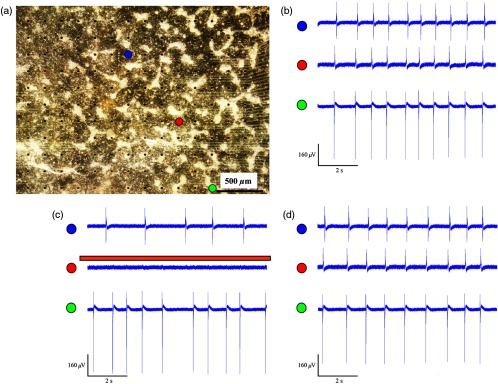
Optogenetics for creation of functional conduction blocks and electrical isolation. (a) NRCM and Arch3-HEK cells were co-cultured on top of the MEA culture plate. Illumination was focused on the area surrounding the red electrode in an attempt to electrically isolate the lower right quadrant of the co-culture. (b–d) Electrograms recorded (b) prior, (c) during, and (d) after illumination from three representative electrodes marked by red (area of illumination), green (isolated quadrant), and blue circles. Note that during illumination, a complete suppression of electrical activity was recorded from the red electrode as well the development of transient conduction block and electrical isolation of lower right quadrant manifested by dissociation of electrical activity between the green and blue electrodes.
3.2. Optogenetics Suppression of Automaticity in the hESC-Derived Cardiomyocyte Co-Cultures
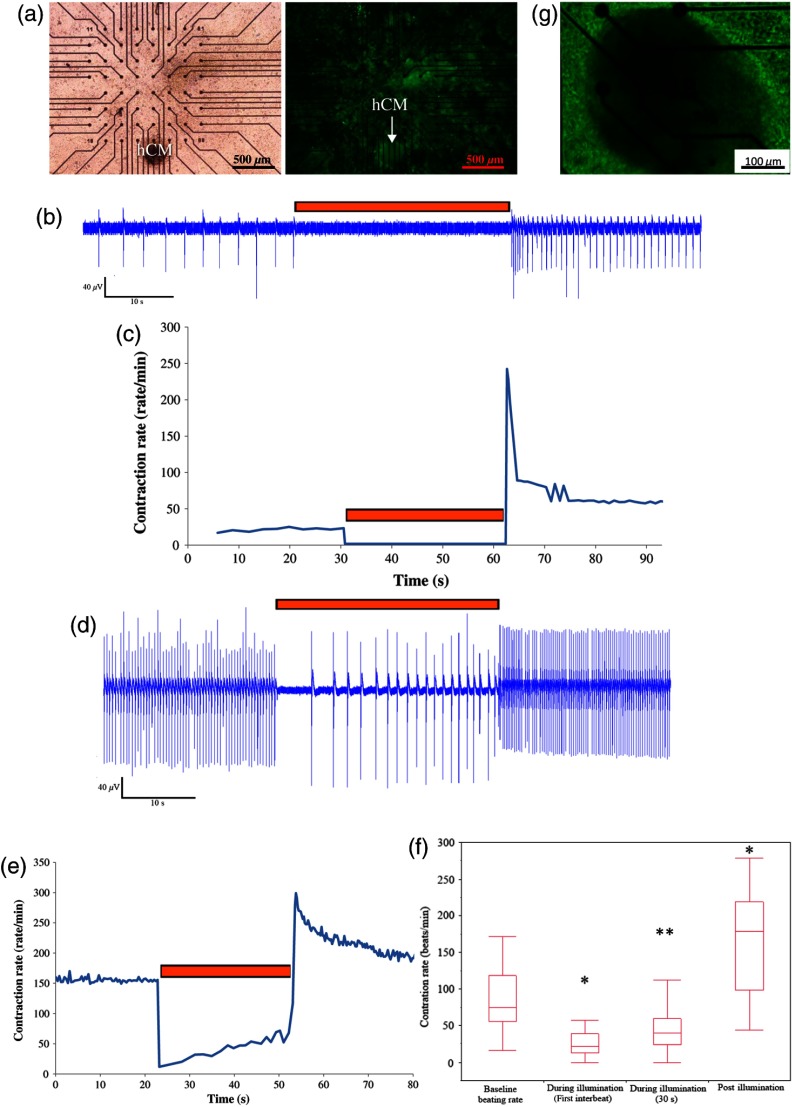
We next evaluated the ability of the Arch3-expressing HEK cells to also suppress electrical activity of human cardiomyocytes. To this end, we co-cultured the Arch3-HEK cells with small hESC-derived three-dimensional cardiomyocyte cell-clusters [Fig. 5(a) and Video 2; illustrated in Fig. 5(g)]. Upon illumination and activation of Arch3 within the HEK cells, we observed a significant suppression of automaticity in the hESC-derived cardiomyocyte cell-aggregates. In the minority of cases (), this was manifested by complete cessation of all electrical activity in the hESC-derived cardiomyocytes during the illumination period [Figs. 5(b) and 5(c)]. This can also be appreciated in Video 2, where the illumination period leads to complete cessation of contraction of the hESC-derived cardiomyocyte cell-aggregate.
Fig. 5.
In the majority of cases (), complete cessation of electrical activity was not observed, however, during the 30-s-long illumination, but rather illumination led to a significant decrease in the beating-rate of the coupled hESC-derived cardiomyocyte cell-aggregates [Figs. 5(d)–5(f)]. As can be viewed in the example in Fig. 5(e), the reduction in beating-rate during illumination was maximal during the initial beats and the magnitude of the suppressive effect decreased somewhat with time. Thus, the mean baseline beating-rate of the hESC-derived cardiomyocyte cell-aggregates prior to illumination was [; Fig. 5(f)]. Illumination caused significant suppression of automaticity [Fig. 5(f)] that was maximal during the initial beats (decreasing to at the first illuminated beat; ) and persisted, albeit to a lower extent, throughout the entire illumination period (average contraction-rate decreasing to ; ).
Interestingly, in all cases we could note a significant acceleration in the beating rate of the hESC-derived cardiomyocyte cell-aggregates immediately following the termination of illumination [Figs. 5(b)–5(f)]. Thus, the beating-rate following the termination of illumination increased to [ when compared to rates at baseline and during illumination; Fig. 5(f)].
3.3. Dose–Response Studies
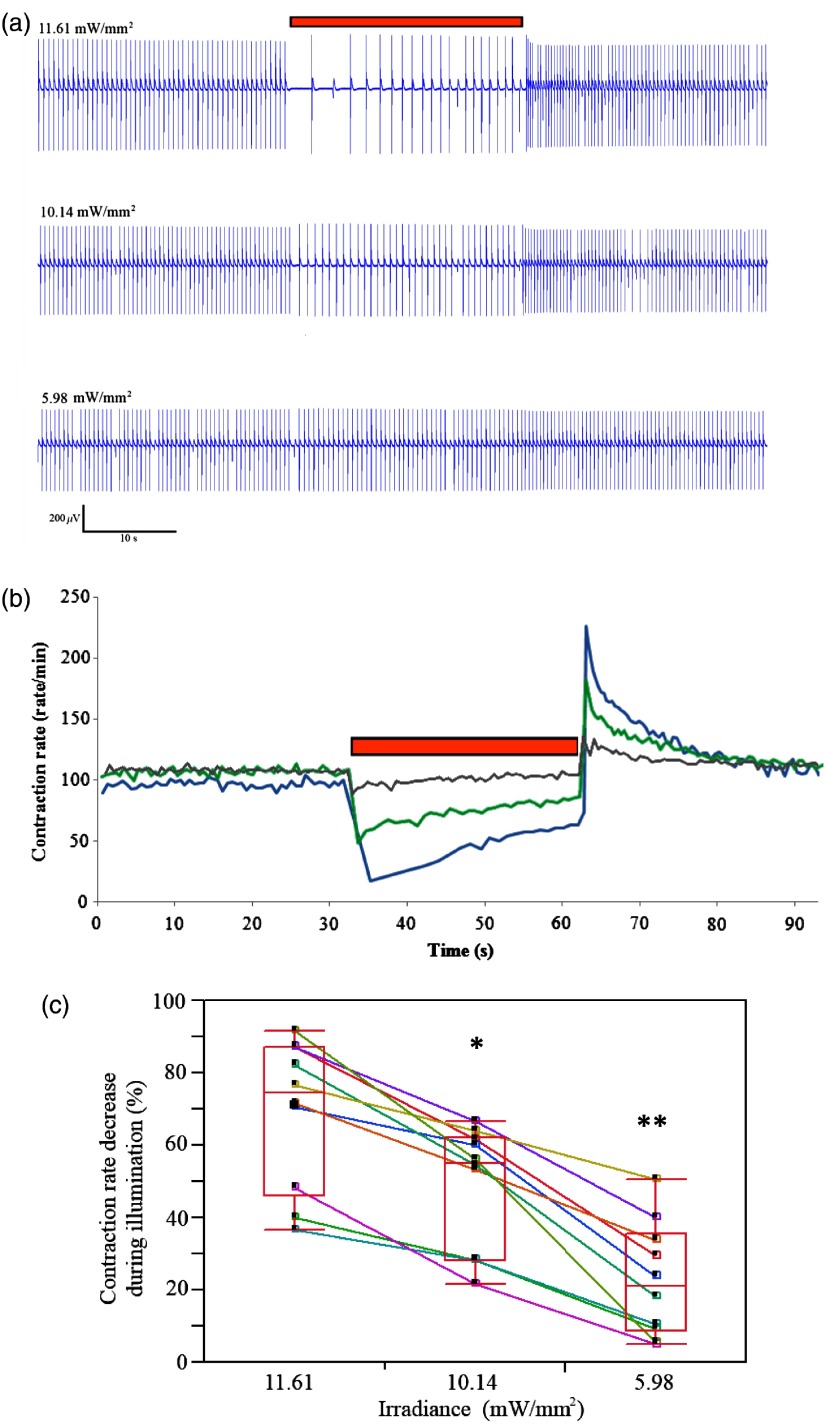
We next performed dose–response studies aiming to evaluate the relationship between light intensity and the resulting functional outcome. To this end, we evaluated the effects of varying the irradiance intensity during the illumination period in the Arch3-HEK/hESC-derived cardiomyocyte co-cultures. Figures 6(a) and 6(b) present the examples of such an experiment that tested the effects of application of the monochromatic light at three different intensities: 11.61, 10.14, and . Note in both the electrogram tracing [Fig. 6(a)] and in the plot summarizing the co-culture contraction rate over time [Fig. 6(b)], the direct relationship between illumination intensity and the functional outcome. Thus, while an irradiance level of led to the most pronounced suppression of automaticity, application of resulted in a less pronounced slowing of beating rate, and delivery of had only modest effects on the chronotropic properties of the cardiomyocyte cell-aggregates.
Fig. 6.
Dose–response characterization of the optogenetic effects. (a, b) The association between different illumination intensities (11.61, 10.14, and ) and the resulting effects on the electrical activity of the hESC-derived cardiomyocyte cell-clusters. Shown are the different electrical recordings (a) and analysis of the beating-rate over-time (b). (c) Box-plots presentation of the association between light intensity and the magnitude of the reduction in beating-rate (relative to baseline value) of the hESC-derived cardiomyocyte cell-aggregates during illumination (). Reduction in the beating rate was at , at ( when compared with illumination intensity of ), and at ( when compared with 11.61 and ).
The significant correlation between light intensity and the magnitude of the functional effect was observed in all co-cultures studied [only those that maintained a constant baseline beating-rate were chosen for the study; ; Fig. 6(c)]. Hence, the reductions in the beating rates of the hESC-derived cardiomyocyte cultures (according to the first interbeat interval during illumination) were at ; at ( when compared with illumination intensity of ), and at [ compared with 11.61 and ; Fig. 6(c)].
As noted previously, following the termination of illumination, we observed a significant acceleration in the beating rates of the hESC-derived cardiomyocyte cell-aggregates. Interestingly, the degree of this increase in contraction rate was also dependent on the preceding illumination intensity. Note in the example provided in Figs. 6(a) and 6(b) that the highest increase in postillumination beating rate was noted after illumination with an irradiation level of with lower rates observed after applications of 10.14 and . This effect was noted in all cultures studied. Thus, when compared to baseline values contraction rate, the beating-rate following the termination of illumination increased by at , by at (), and by at ( when compared with 11.6 and ; for cultures that maintained a stable baseline beating rate).
4. Discussion
Optogenetics is an exciting new method of controlling cell excitation in living mammals without the use of electrical discharge. For suppression of neuronal activity, one of the most commonly utilized optogenetics approach is the induction of neuronal hyperpolarization through the activation of the light-sensitive chloride-pump Halo.6–8 The isolation of the light-sensitive proton-pump Arch3 from Halobacterium Sodomense and its application for neural silencing was first reported in 2010.9 It was found that upon illumination with orange wavelength, Arch3 produces larger photocurrents than those generated by Halo, resulting in a high efficacy in silencing neural activity.9
Surprisingly, there are only a few reports on utilizing optogenetics in the cardiac field in general10–14 and even less data exist regarding its potential use for suppression of cardiac activity.11–13 In the current study, we aimed to expand on these previous observations and to further evaluate the potential use of optogenetics for targeted modulation (suppression) of the localized electrical activity of the cardiac syncytium. To this end, we engineered HEK293 cells, capable of forming functional gap junctions with cardiomyocytes,21 to express the Arch3 transgene. By using hESC-derived or neonatal rat cardiomyocyte co-culture models, we were able to demonstrate the ability of this combined cell and gene therapy strategy to modulate the culture’s electrophysiological activity through electrotonic interactions with neighboring cardiomyocytes. This was manifested either by complete termination of the localized electrical activity in the NRCM co-cultures by targeted illumination leading to development of functional conduction blocks or by the significant slowing of the beating rate in the hESC-derived cardiomyocyte co-cultures.
Our study also provides important insights into both the spatial and temporal extents of the electrophysiological suppression effects induced by the optogenetics strategy. An interesting observation was initially noted with respect to the spatial dimensions of the effects induced by the targeted illumination in the NRCM monolayers. Not surprisingly, focal illumination-induced complete electrical silencing of areas of the NRCM monolayers directly underlying the illumination beam. This complete electrical suppression was continuous during the entire 30-s illumination period. Interestingly, the extent of the optogenetics effect was larger than just at the center of the illuminated area. Thus, some degree of electrical suppression was also noted at the periphery of the illuminated area and even extended to areas outside the illuminated zone, with electrodes as far as 0.6 mm being affected. The extent of the aforementioned affected areas varied somewhat between the co-cultures and we also noted some directional variability in the light-induced suppressive effect. This variability may stem from technical issues (such as slight changes in the angle of the illuminated beam), from spatial inhomogeneities within the co-cultures (such as in the ratio between the number of Arch3-HEK cells and cardiomyocytes and in the degree of electrical coupling between the cells), or from variations in the location of the pacemaker and the activation patterns in the co-cultures.
The aforementioned electrophysiological effects, noted at some distance away from the illumination area, are probably the result of hyperpolarizing electrotonic currents originating at the HEK-cardiomyocyte interface and then spreading through gap junctions to closely coupled cardiomyocytes. The spatial dimensions of the optogenetics effect in the current study correlate with previous reports in the literature regarding the extent of electrotonic interactions in similar co-cultures models. Yankelson et al., for example, noted that fibroblasts expressing voltage-sensitive potassium channels were able to modulate the electrophysiological properties of similar NRCM co-cultures at a distance of up to .15 Interestingly, in contrast to the latter report, which identified a fixed electrophysiological modulation effect over time, we observed in the current study a temporal decay in the optogenetics effect. Thus, the optogenetics effects observed in remote (nonilluminated) areas at the beginning of illumination were lost over time, suggesting either a reduction in the magnitude of the hyperpolarizing current at the source (HEK–cardiomyocyte interface) or a reduction in gap junction conductance. Both phenomena may result from the metabolic changes that can be induced as the result of the continuous activation of the Arch3 proton-pump.
Similar metabolic changes may also underlie another interesting phenomenon noted in the current study. It was found, both in the NRCM and human cardiomyocyte co-culture studies, that the area undergoing hyperpolarization and electrical suppression during illumination become hyperactive upon termination of illumination, resulting in either transient transformation of this zone into a new pacemaker region (for up to three beats in the NRCM co-culture studies) or in transient acceleration of the existing pacemaker firing rate (in the hESC-derived cardiomyocyte cell-clusters co-culture studies). These transient chronotropic effects might be explained by hyperpolarization-activated currents,22 by posthyperpolarization recruitment of larger amounts of recovered sodium channels, or by the metabolic changes induced by chronic activation of the proton-pump. A potential role for the former mechanism and activation of hyperpolarization-activated cationic channels may be supported by the finding of a higher postillumination rate found in the hESC-derived cardiomyocytes known to robustly express such channels23 when compared to the NRCM co-culture studies. Interestingly, a similar transient hyperexcitable state postillumination was also noted in optogenetic studies in Arch-expressing neurons.9
Finally, the effect of Arch3 in the co-cultures was found to be dependent on the intensity of illumination, as it has been reported that higher output illumination yields higher photocurrents.9 This was manifested by the degree of chronotropic changes induced in the hESC-derived cardiomyocyte cell-aggregates that directly correlated with irradiance intensity. Interestingly, the irradiance level also affected the magnitude of the acceleration in the beating rate in the postillumination period, again stressing the role of the induced photocurrents in underlying the observed chronotropic changes. Finally, the fact that in the vast majority of co-cultures, we could induce significant slowing of the spontaneous beating-rate of the hESC-derived cell-clusters but not completely silence their activity probably stems from the structural properties of these cell-aggregates. Since the Arch3-HEK cells are probably in direct contact only with the outer cell layer of the human cell-clusters, the light-induced hyperpolarizing electrotonic interactions generated are, therefore, maximal at the interface between the two cell types and are reduced in magnitude at deeper layers within the cell-clusters. The resulting electrotonic currents affecting the pacemaker cells may therefore not be large enough to completely silence pacemaker activity but are sufficient to hyperpolarize the resting membrane potential and reduced automaticity.
The results of the current proof-of-concept study suggest a number of conceptual approaches for optogenetic suppression of excitability, which could form the basis for future attempts to develop therapeutic strategies for cardiac arrhythmias. These include the ability to generate localized functional conduction blocks that may be useful for the treatment of re-entrant arrhythmias, the ability to suppress the automaticity of ectopic pacemaker (“molecular ablation” approaches), the ability to transiently silence electrical activity diffusely (for potential painless “molecular defibrillation”), and the ability to electrically isolate cardiac tissue that could prove useful for the treatment of paroxysmal atrial fibrillation.
Nevertheless, while providing proof-of-concepts evidence for the ability to achieve optogenetic suppression of cardiac excitability, the results of the current study were limited to in vitro setting using co-cultures of NRCM monolayers and hESC-derived cardiomyocyte cell-aggregates. Translating these findings to the in vivo settings, first to small and large animal models and eventually to the clinic would require overcoming major challenges. These include the need to shift from the use of an immortalized transfected HEK cell line in an in vitro model to the utilization of primary autologous cells in more clinically relevant in vivo models. This would also necessitate prolonged survival of the transplanted cells, long-lasting expression of the Arch transgene in the engrafted cells, and the efficient coupling of the engineered cells with host cardiomyocytes also in the intact heart. Moreover, it is not clear whether the electrotonic currents generated, which were sufficient to suppress excitability in the monolayers or cell-clusters, will also be large enough to affect cardiac activity also in vivo. Another potential caveat to clinical translation may be the transient hyperexcitable state noted postillumination at the treated region that may be proarrhythmogenic. Finally, it is not clear whether the monochromatic light sources used to activate the hyperpolarizing light-sensitive proteins can sufficiently penetrate through cardiac muscle or blood to efficiently influence the transduced cells that may be embedded deep within the myocardium.
5. Conclusion
In the current study, we described a combined cell and gene approach toward controlling cardiac hyperpolarization and modulation of cardiac excitability. Specifically, we demonstrated the potential of this approach to generate local conduction block, silence electrical activity, and modulate automaticity in neonatal rat and hESC-derived cardiomyocyte cultures, opening the way for development of future optogenetic treatments for cardiac arrhythmias. Nevertheless, translation of these initial in vitro results in co-culture studied into the clinics would require significant technological developments and overcoming significant challenges.
Acknowledgments
This study was supported in part by the Israel Science Foundation (1609/14) by the Nancy & Stephen Grand Philanthropic Fund and by the Randy and Melvin Berlin Family Regenerative Medicine Research Grant.
References
- 1.Huikuri H. V., Castellanos A., Myerburg R. J., “Sudden death due to cardiac arrhythmias,” N. Engl. J. Med. 345, 1473–1482 (2001). 10.1056/NEJMra000650 [DOI] [PubMed] [Google Scholar]
- 2.Koyak Z., et al. , “Sudden cardiac death in adult congenital heart disease,” Circulation 126, 1944–1954 (2012). 10.1161/CIRCULATIONAHA.112.104786 [DOI] [PubMed] [Google Scholar]
- 3.Napolitano C., et al. , “Sudden cardiac death and genetic ion channelopathies: long QT, Brugada, short QT, catecholaminergic polymorphic ventricular tachycardia, and idiopathic ventricular fibrillation,” Circulation 125, 2027–2034 (2012). 10.1161/CIRCULATIONAHA.111.055947 [DOI] [PubMed] [Google Scholar]
- 4.Czosek R. J., et al. , “Impact of cardiac devices on the quality of life in pediatric patients,” Circ. Arrhythm. Electrophysiol. 5, 1064–1072 (2012). 10.1161/CIRCEP.112.973032 [DOI] [PubMed] [Google Scholar]
- 5.Han X., Boyden E. S., “Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution,” PLoS One 2, e299 (2007). 10.1371/journal.pone.0000299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arrenberg A. B., Del Bene F., Baier H., “Optical control of zebrafish behavior with halorhodopsin,” Proc. Natl. Acad. Sci. U. S. A. 106, 17968–17973 (2009). 10.1073/pnas.0906252106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inada K., et al. , “Optical dissection of neural circuits responsible for Drosophila larval locomotion with halorhodopsin,” PLoS One 6, e29019 (2011). 10.1371/journal.pone.0029019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsunematsu T., et al. , “Acute optogenetic silencing of orexin/hypocretin neurons induces slow-wave sleep in mice,” J. Neurosci. 31, 10529–10539 (2011). 10.1523/JNEUROSCI.0784-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow B. Y., et al. , “High-performance genetically targetable optical neural silencing by light-driven proton pumps,” Nature 463, 98–102 (2010). 10.1038/nature08652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia Z., et al. , “Stimulating cardiac muscle by light: cardiac optogenetics by cell delivery,” Circ. Arrhythm Electrophysiol. 4, 753–760 (2011). 10.1161/CIRCEP.111.964247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nussinovitch U., Shinnawi R., Gepstein L., “Modulation of cardiac tissue electrophysiological properties with light-sensitive proteins,” Cardiovasc. Res. 102, 176–187 (2014). 10.1093/cvr/cvu037 [DOI] [PubMed] [Google Scholar]
- 12.Arrenberg A. B., et al. , “Optogenetic control of cardiac function,” Science 330, 971–974 (2010). 10.1126/science.1195929 [DOI] [PubMed] [Google Scholar]
- 13.Abilez O. J., “Cardiac optogenetics,” Conf. Proc. IEEE Eng. Med. Biol. Soc. 2012, 1386–1389 (2012). 10.1109/EMBC.2012.6346197 [DOI] [PubMed] [Google Scholar]
- 14.Bruegmann T., et al. , “Optogenetic control of heart muscle in vitro and in vivo,” Nat. Methods 7, 897–900 (2010). 10.1038/nmeth.1512 [DOI] [PubMed] [Google Scholar]
- 15.Yankelson L., et al. , “Cell therapy for modification of the myocardial electrophysiological substrate,” Circulation 117, 720–731 (2008). 10.1161/CIRCULATIONAHA.106.671776 [DOI] [PubMed] [Google Scholar]
- 16.Kehat I., et al. , “Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes,” J. Clin. Invest. 108, 407–414 (2001). 10.1172/JCI200112131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lev S., Kehat I., Gepstein L., “Differentiation pathways in human embryonic stem cell-derived cardiomyocytes,” Ann. N. Y. Acad. Sci. 1047,50–65 (2005). 10.1196/annals.1341.005 [DOI] [PubMed] [Google Scholar]
- 18.Kehat I., et al. , “High-resolution electrophysiological assessment of human embryonic stem cell-derived cardiomyocytes: a novel in vitro model for the study of conduction,” Circ. Res. 91, 659–661 (2002). 10.1161/01.RES.0000039084.30342.9B [DOI] [PubMed] [Google Scholar]
- 19.Feld Y., et al. , “Electrophysiological modulation of cardiomyocytic tissue by transfected fibroblasts expressing potassium channels: a novel strategy to manipulate excitability,” Circulation 105, 522–529 (2002). 10.1161/hc0402.102661 [DOI] [PubMed] [Google Scholar]
- 20.Santangeli P., Marchlinski F. E., “Pulmonary vein isolation for atrial fibrillation: forever young,” J. Am. Coll. Cardiol. 64, 2468–2470 (2014). 10.1016/j.jacc.2014.09.054 [DOI] [PubMed] [Google Scholar]
- 21.Hofshi A., et al. , “A combined gene and cell therapy approach for restoration of conduction,” Heart Rhythm 8, 121–130 (2011). 10.1016/j.hrthm.2010.10.011 [DOI] [PubMed] [Google Scholar]
- 22.Kurata Y., et al. , “Roles of hyperpolarization-activated current If in sinoatrial node pacemaking: insights from bifurcation analysis of mathematical models,” Am. J. Physiol. Heart Circ. Physiol. 298, H1748–1760 (2010). 10.1152/ajpheart.00729.2009 [DOI] [PubMed] [Google Scholar]
- 23.Satin J., et al. , “Mechanism of spontaneous excitability in human embryonic stem cell derived cardiomyocytes,” J. Physiol. 559(Pt.2), 477–494 (2004). 10.1113/jphysiol.2004.068213 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.