Abstract
Cigarette smoke (CS)-induced cellular senescence is involved in the pathogenesis of chronic obstructive pulmonary disease (COPD). The molecular mechanism by which CS induces cellular senescence is unknown. Here, we show that CS stress (exposure of primary lung cells to CS extract 0.2–0.75% with a half-maximal inhibitory concentration of ∼0.5%) led to impaired mitophagy and perinuclear accumulation of damaged mitochondria associated with cellular senescence in both human lung fibroblasts and small airway epithelial cells (SAECs). Impaired mitophagy was attributed to reduced Parkin translocation to damaged mitochondria, which was due to CS-induced cytoplasmic p53 accumulation and its interaction with Parkin. Impaired Parkin translocation to damaged mitochondria was also observed in mouse lungs with emphysema (6 months CS exposure, 100 mg TPM/m3) as well as in lungs of chronic smokers and patients with COPD. Primary SAECs from patients with COPD also exhibited impaired mitophagy and increased cellular senescence via suborganellar signaling. Mitochondria-targeted antioxidant (Mito-Tempo) restored impaired mitophagy, decreased mitochondrial mass accumulation, and delayed cellular senescence in Parkin-overexpressing cells. In conclusion, defective mitophagy leads to CS stress-induced lung cellular senescence, and restoring mitophagy delays cellular senescence, which provides a promising therapeutic intervention in chronic airway diseases.—Ahmad, T., Sundar, I. K., Lerner, C. A., Gerloff, J., Tormos, A. M., Yao, H., Rahman, I. Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: implications for chronic obstructive pulmonary disease.
Keywords: Parkin, Pink1, reactive oxygen species, perinuclear mitochondrial clustering, DNA damage
Cigarette smoke (CS)-induced cellular senescence is involved in the pathogenesis of chronic obstructive pulmonary disease (COPD)/emphysema, a disease characterized by premature lung aging (1–3). Lung airway structural cells (epithelium and fibroblasts) show increased cellular senescence (irreversible growth arrest) induced by CS in vitro and in vivo (4–6). However, the molecular mechanism by which CS induces cellular senescence is unknown. Cellular senescence is characterized by the activation of p16, p21, and p53, along with increased activity of senescence-associated β-galactosidase (SA-β-gal) (6, 7). We have recently shown that CS leads to increased cellular senescence associated with increased reactive oxygen species (ROS) production in lung cells (3, 6, 8). Most of the cellular ROS are predominantly generated by mitochondria, and there is accumulating evidence suggesting a critical role of mitochondrial dysfunction in airway diseases (9–12).
Mitochondria are highly dynamic organelles, which continuously fuse and divide in cells. Mitochondrial fission highly correlates with cell apoptosis and mitophagy (selective degradation of damaged mitochondria), whereas mitochondrial fusion has the opposite role (13). Cigarette smoke extract (CSE) induces mitochondrial fission in human smooth muscle cells, which may contribute to cell death during the pathogenesis of COPD/emphysema (14). Similarly, elongated mitochondria with increased mitochondrial fusion activity occur during long-term CSE treatment in a human lung epithelial cell line (15). This suggests that mitochondrial structural changes or its dysfunction is induced by CS stress. However, the molecular mechanism underlying these phenomena and their impact during cellular senescence is not known.
Damaged or dysfunctional mitochondria are cleared from the cells by a process called mitophagy (16–18). Mitophagy is activated by mitochondrial membrane depolarization, followed by stabilization of a phosphatase and tensin homolog-induced putative kinase 1 (Pink1) on mitochondrial outer membrane, which then recruits an E3 ubiquitin ligase (Parkin) from the cytosol (17–19). Once recruited to mitochondria, Parkin mediates degradation of mitofusin-2 (Mfn2; a core protein involved in mitochondrial fusion) and hence prevents mitochondrial fusion. This initiates the localization of damaged mitochondria to membranes containing microtubule-associated protein light-chain 3 (LC3) leading to the formation of autophagosomes (20, 21). These autophagosomes containing the damaged mitochondria fuse with lysosomes which are cleared from the cells (16–21). Accumulation of damaged or dysfunctional mitochondria, due to impaired mitophagy, has been associated with many pathologic conditions (22–24). Defective mitophagy has also been linked with premature aging and many age-associated disorders, such as Alzheimer’s and Parkinson’s diseases (25–28). However, it is not clear whether CS stress-induced lung cellular senescence is due to mitochondrial dysfunction via defective mitophagy. In light of this, we hypothesized that CS causes persistent mitochondrial dysfunction, which leads to defective mitophagy and accumulation of damaged mitochondria resulting in cellular senescence. We show that CSE causes persistent mitochondrial dysfunction leading to defective mitophagy by altering Parkin levels and translocation. We further show that CSE-induced perinuclear mitochondrial accumulation results in DNA damage and hence cellular senescence. Impaired mitophagy was also seen in both smokers and patients with COPD as well as in a mouse model of chronic CS-induced emphysema.
MATERIALS AND METHODS
Cells and treatments
Human and mouse lung fibroblasts as well as human primary small airway epithelial cells (SAECs) were used in this study. Primary mouse lung fibroblasts were isolated as previously described (29). Briefly, mouse lungs were isolated and digested with Liberase (Roche, Indianapolis, IN, USA) for 1 hour at room temperature. Cells were washed using Roswell Park Memorial Institute medium and initially grown in DMEM-F12 with fetal bovine serum (FBS) (10%) for 10 days with media changed on alternate days under 5% CO2 and low oxygen concentration (3%). After 10 days, cells were gown in Eagle’s minimum essential medium with 10% FBS. Human lung fibroblast (HLF) WI-38 stably expressing LC3-green fluorescent protein (GFP) and human fetal lung fibroblasts (HFLs-1) were grown in DMEM-F12 with FBS (10%) under low oxygen concentration (3%). Human SAECs from healthy subjects (nonsmokers) and patients with COPD were obtained from Lonza (Walkersville, MD, USA) and grown as per the instructions. CSE preparation was performed as described earlier (30). Different concentrations of CSE (0.2–0.75%) were used, and finally, CSE (0.5%) was chosen in most of the treatments (unless specified) for induction of cellular senescence in HFL1 cells, whereas CSE (0.25%) was used to induce cellular senescence in mouse lung fibroblasts. For human SAECs, 0.2% of CSE was used to induce cellular senescence. The dose of CSE was chosen on the basis of dose response curve generated, which induced substantial cellular senescence with minimal apoptosis for a period of 10 or 15 days with alternate days of CSE treatment. Media were changed every alternate day before CSE treatments.
Cells were treated with different concentrations of Mdivi-1 (1–10 μM, catalog number M0199; Sigma-Aldrich, St. Louis, MO, USA) or bafilomycin (10 nM, catalog number B1793; Sigma-Aldrich) for 15 days, whereas bafilomycin was used for 10 days with alternate day treatments. Mito-Tempo (MitoT) was used at different concentrations (100 nM, 500 nM, 1 µM, and 2 µM), and a dose (1 µM) that induced substantial reduction in mitochondrial reactive oxygen species (mtROS) was used for further experiments. MitoT treatment was performed at 2 hours before every CSE treatment, which was found to be more effective compared to post-CSE treatment. Rotenone (catalog number R8875; Sigma-Aldrich) was used at concentrations of 10 and 100 nM unless specified. Nocodazole (catalog number M1404; Sigma-Aldrich) was used at a concentration of 50 nM as described earlier (31). Carbonyl cyanide m-chlorophenyl hydrazine (CCCP; Life Technologies, Carlsbad, CA, USA) treatment was given for 2 hours at a concentration of 10 μM (32–34).
SA-β-gal activity in lung fibroblasts and SAECs
SA-β-gal activity was measured in human and mouse lung fibroblasts or SAECs as per the protocol (Cell Signaling Technology, Danvers, MA, USA) (6). Briefly, cells were fixed with 1× paraformaldehyde for 10 minutes, washed with PBS, and stained with 1× staining solution. After 16 hours, images were taken using a bright-field microscope (Olympus CKX41; Tokyo, Japan).
Constructs and transfections
LC3-GFP, mCherry-Parkin, and yellow fluorescent protein (YFP)-Parkin were used as described earlier (32–34). Cells were seeded with or without cover glasses in 6-well plates for imaging and flow cytometry. Cells were transfected with Lipofectamine (Invitrogen, Life Technologies) for 24 hours, and the media were changed the next day followed by CSE or the drug treatment. For the measurement of perinuclear mitochondrial accumulation, DNA damage, mitochondrial mass, and cellular senescence, HFL1 cells were transfected with mCherry-Parkin at 24 hours before CSE treatment. For the measurement of mtROS, cells were transfected with YFP-Parkin. Similarly, SAECs were transfected with mCherry-Parkin or YFP-Parkin at 24 hours before CSE treatment. Lipofectamine was used as a transfection reagent in all the experiments unless specified, and the transfection protocol was followed as per the instructions.
Immunofluorescence
For immunofluorescence, an equal number of cells were seeded on the cover glass. Cells were fixed with 2% paraformaldehyde for 10 minutes followed by washing with PBS. Glycine buffer was used for blocking (0.3 M glycine, 5% goat sera, and 0.1 Triton X-100) for 1 hour at room temperature. Primary antibody incubations were performed overnight, whereas secondary antibody incubations were carried out for 45–60 minutes at room temperature. Images were taken by either a fluorescent microscope (Eclipse Ni; Nikon, Tokyo, Japan) or a confocal microscope (Olympus FV1000).
Cellular senescence was measured in cells using a fluorogenic senescent marker: C12FDG (5-dodecanoylaminofluorescein di-β-d-galactopyranoside) (catalog number D-2893; Life Technologies). In brief, cells were treated with C12FDG (25 μM) for 2 hours before performing fluorescence-activated cell sorting (FACS) or confocal imaging. Images were analyzed using ImageJ (National Institutes of Health, Bethesda, MD, USA) and MetaMorph software (version 7.7.0.0; Molecular Devices, Sunnyvale, CA, USA). 3-Dimensional (3D) reconstruction was done by ImageJ. Tissue immunofluorescence was performed using formalin-fixed and paraffin-embedded lung tissue sections from mice as described earlier (6, 8). Lung homogenates were used to prepare total cell proteins as described earlier (6, 8).
Mitochondrial assays and flow cytometry
Mitochondrial imaging was performed using cells stained with MitoTracker Red (catalog number M7512; Life Technologies). Cells were treated with MitoTracker Red (25 nM) for 10 minutes before doing the imaging. Mitochondrial mass was measured by MitoTracker Green staining (catalog number M-7514; Life Technologies). Cells were treated with MitoGreen (25 nM; Life Technologies) for 10 minutes before the FACS measurement. mtROS was measured by MitoSOX Red (5 μM, catalog number M36008; Life Technologies). Cells were stained with MitoSOX Red for 15 minutes before performing the FACS or imaging as described earlier (35, 36). Mitochondrial membrane potential (ΔΨm) was measured using tetramethylrhodamine ethyl ester (TMRM; catalog number T-668; Life Technologies) as described earlier (35, 36). Cells were incubated in TMRM (1 μM) for 15 minutes before performing the FACS analysis. CCCP was used to induce mitochondrial depolarization at a concentration of 10 µM for 2 hours before processing samples for imaging. Cellular senescence was measured using a fluorescent senescence marker, C12FDG, as described earlier (37). Mitochondrial ATP was measured as per the protocol (Life Technologies) and as described earlier (36). Briefly, ATP was measured in a 96-well plate with an equal number of cells using the SpectraMax M2 microplate reader (Molecular Devices) with an emission maximum of ∼560 nm at pH 7.8.
Electron microscopy
For the measurement of mitochondrial structural changes, electron microscopy studies were carried out in HFL1 cells fixed with a combination of paraformaldehyde and glutaraldehyde. After fixation, cells were processed and sectioned with a diamond knife on copper grids. Grids were examined with a Hitachi (Tokyo, Japan) 7100 electron microscope, and images were captured using a MegaView III digital camera (Soft Imaging System, Lakewood, CO, USA).
Perinuclear mitochondrial measurements
Tom 20 [catalog numbers ab23707 and ab56783 (Abcam, Cambridge, MA, USA) and/or catalog number 13929S (Cell Signaling Technology)] was used to label the mitochondria as described above in the Immunofluorescence section, and DAPI for nuclear staining. Perinuclear mitochondrial accumulation was measured as described earlier (31) with some modifications. Circles (3) of equal width and height were drawn around the nuclei, and average intensity of each individual ring was calculated. Average intensities of the first 2 circles were merged, which was found to be more consistent compared to using the average intensities of all the 3 circles. Final data were shown as the average intensity rather than the percentage of perinuclear mitochondrial mass as described earlier (31), and instead of total percentage, average intensity values were plotted, which were found to be more consistent. All the intensity measurements were done using MetaMorph software.
Measurement of DNA damage foci and cell ROS
For the measurement of DNA damage foci, phospho-Histone H2A.X on Ser139 (γ-H2AX, 20E3 Rabbit mAb, catalog number 9718S; Cell Signaling Technology) staining was carried out as described above in the Immunofluorescence section. DNA damage foci were calculated by counting >100 cells per slide. The number of foci for each cell was counted individually, and data were plotted as the number of foci per cell (38–40). For correlation studies with perinuclear mitochondria mass accumulation, the average fluorescence intensities of DNA damage foci were plotted against the average intensities of perinuclear mitochondrial mass. Same cells were used for the measurement of DNA damage foci and perinuclear mitochondrial mass. For dual immunofluorescence, the secondary antibodies used were as follows: Alexa Fluor 546 secondary antibody was used for mitochondrial Tom 20, whereas Alexa Fluor 488 secondary antibody [A-21206 and A-1108 (Life Technologies) and 711-546-152 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA)] was used for γ-H2AX.
Total cell ROS was measured by CellROX (catalog number C10444; Life Technologies). Briefly, the cells were treated with CellROX at a final concentration of 5 μM and incubated for 20 minutes. Cells were washed with PBS before imaging. Nuclear ROS was measured by counting the pixel intensity of CellROX inside the nucleus (stained with DAPI), using MetaMorph software.
CS exposure to mice
Eight-week-old C57BL/6J mice were used for CS exposure as described previously (6, 8). 3R4F cigarettes were used to generate a mixture of sidestream smoke (89%) and mainstream smoke (11%) by a Teague smoking machine (Model TE-10; Teague Enterprises, Woodland, CA, USA) at a concentration of ∼100 mg/m3 total particulate matter (6). Each smoldering cigarette was puffed for 2 seconds, once every minute for a total of 8 puffs, at a flow rate of 1.05 L/minute, to provide a standard puff of 35 cm3. Mice received 5-hour exposures per day, 5 days per week for 6 months, and were killed at 24 hours after the last CS exposure. All animal protocols described in this study were approved by the University Committee on Animal Resources of the University of Rochester.
Preparation of whole-cell lysate and mitochondrial isolation from cells and lung tissue
The preparation of whole-cell lysate was carried out as described previously (6, 8). Briefly, lung tissue (100 mg) was mechanically homogenized with 0.5 ml RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 0.25% deoxycholate, 1 mM Na3VO4, 1 mM NaF, 1 mg/L leupeptin, 1 mg/L aprotinin, and 1 mM PMSF), and the tissue homogenates were kept on ice for 45 minutes to allow total cell lysis. Similarly, lung fibroblasts or SAECs were lysed with RIPA buffer for 30 minutes on ice and then vortexed for 15 seconds. Following centrifugation at 13,000 g in an Eppendorf tube for 5 minutes, the supernatant was collected as whole-cell lysate. Mitochondria were isolated from HLFs-1 or mouse or human lung tissues as per the protocol (Abcam). Briefly, cells were seeded in 10 cm dishes in triplicate, and after appropriate treatment, cell pellets were collected with a scrapper. Cell pellets were resuspended in Reagent A, and cells were homogenized with a dounce homogenizer. Cells were centrifuged at 1000 g for 10 minutes at 4°C. The pellet was resuspended in Reagent B and centrifuged again. The supernatants of both the centrifugation steps were combined and further centrifuged at 12,000 g for 15 minutes. The final pellet was resuspended in Reagent C and stored at −80°C until use. All the instructions were followed as per the kit protocol. A similar protocol was used for the tissue samples. Protein estimation was done by the bicinchoninic acid method as described earlier (6, 8).
Western blot
Western blotting was performed as described earlier (6, 8). Primary antibody incubations were performed overnight with 1:1000 dilutions, followed by secondary incubation for 1 hour at room temperature (1:5000 dilutions). ECL (Bio-Rad, Hercules, CA, USA) was used for detection, and images were taken with the Bio-Rad ChemiDoc MP, Imaging System.
Human samples
The lung tissue specimens from normal, lifelong nonsmokers and patients with COPD were collected by the Department of Medicine and Pathology, Helsinki University Central Hospital (Helsinki, Finland) (6). The clinical characteristics of the patients used were described in detail previously (6).
Statistical analysis
Statistical analysis of significance was calculated using 1-way ANOVA for multigroup comparisons using GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA). The results were shown as the means ± sem. Values of P < 0.05, P < 0.01, and P < 0.001 were considered as statistically significant.
RESULTS
CS-induced lung cellular senescence is associated with mitochondrial dysfunction
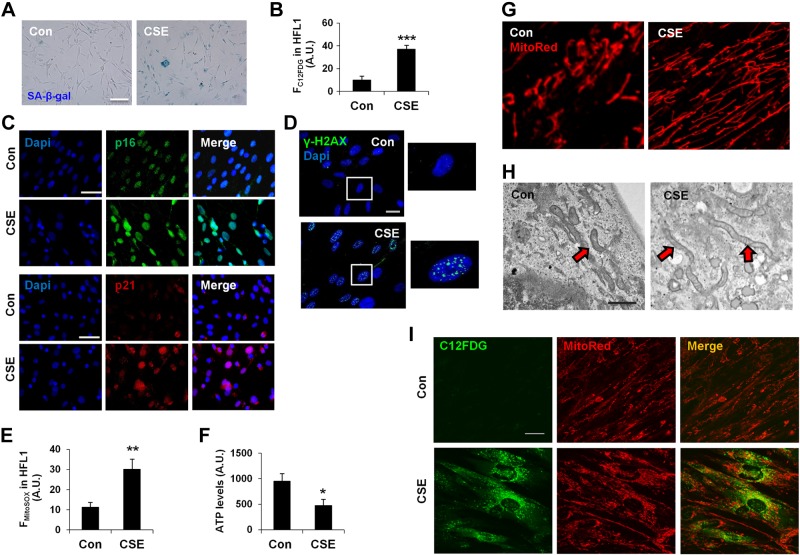
Mitochondrial functional alterations are involved in CS stress-induced suborganellar signaling, which may lead to cellular senescence. To determine the mitochondrial functional changes during CS stress-induced cellular senescence, we utilized a CSE-induced cell culture model of cellular senescence. A time-dependent increase in cellular senescence was observed in HFL1 and mouse primary lung fibroblasts, which was characterized by increased SA-β-gal activity (Fig. 1A and Supplemental Fig. S1A, B). Using a fluorogenic senescence marker (C12FDG), we quantified the degree of cellular senescence by flow cytometry, which showed an increase in cellular senescence by CSE (Fig. 1B). Increased levels of prosenescence genes, p16 and p21, as well as DNA damage are other hallmarks for cellular senescence (6, 7, 41). Our results show increased p16 and p21 levels (Fig. 1C), as well as DNA damage, measured in terms of γ-H2AX foci formation [catalog number Ab2893 (Abcam) and catalog number 05-636 (EMD Millipore, Billerica, MA, USA)] in CSE-treated HFL1 cells (Fig. 1D), which confirmed the establishment of cellular senescence in an in vitro model. Furthermore, CSE treatment led to increased mtROS and decreased ATP levels in HFL1 cells with senescence (Fig. 1E, F). Immunofluorescence and electron microscopy were employed to determine the mitochondrial structural changes after CSE treatment. Confocal microscopy imaging revealed an increase in mitochondrial mass and formation of highly elongated mitochondria after CSE treatment (Fig. 1G), which was further confirmed by electron microscopy (Fig. 1H). Using C12FDG to label the senescent cells and MitoTracker Red for mitochondria, we found that elongated mitochondria were mostly present in highly senescent cells after CSE treatment for 15 days (Fig. 1I).
Figure 1.
CSE causes cellular senescence and mitochondrial dysfunction in lung fibroblasts. A) Representative images of SA-β-gal activity in HLFs (HFL1) with and without CSE. Con, control. B) C12FDG fluorescence in HFL1 cells was measured by FACS and plotted as average fluorescent intensity (FC12FDG in HFL1), which showed the degree of cellular senescence in arbitrary units (A.U.). ***P < 0.001 vs. control. C) Representative images of p16 and p21 immunofluorescence in HFL1 cells with and without CSE (as above). D) Representative images of HFL1 cells stained with γ-H2AX (green), whereas DAPI (blue) was used to label nucleus. Areas in squares are enlarged as shown on right panel. E) mtROS measurement by FACS and plotted as mean fluorescence intensity (FMitoSOX) in HFL1. **P < 0.01 vs. control. F) ATP level measurement in cells with equal cell numbers. *P < 0.05 vs. control. G) Representative images show mitochondrial morphology in HFL1 cells stained with MitoTracker Red (MitoRed) using confocal microscopy, and the images are digitally zoomed. H) Electron microscopy images of HFL1 cells showing mitochondrial morphologic changes. I) Representative images of mitochondrial morphologic changes in senescent HFL1 cells as shown by colocalization of MitoTracker Red with a senescence marker, C12FDG (green). CSE was used at 0.5% with alternate day treatment for 15 days. Data are shown as the means ± sem (n = 3–4). Scale bars, 10 µm (A), 20 µm (C, D, and I), and 0.5 μm (H).
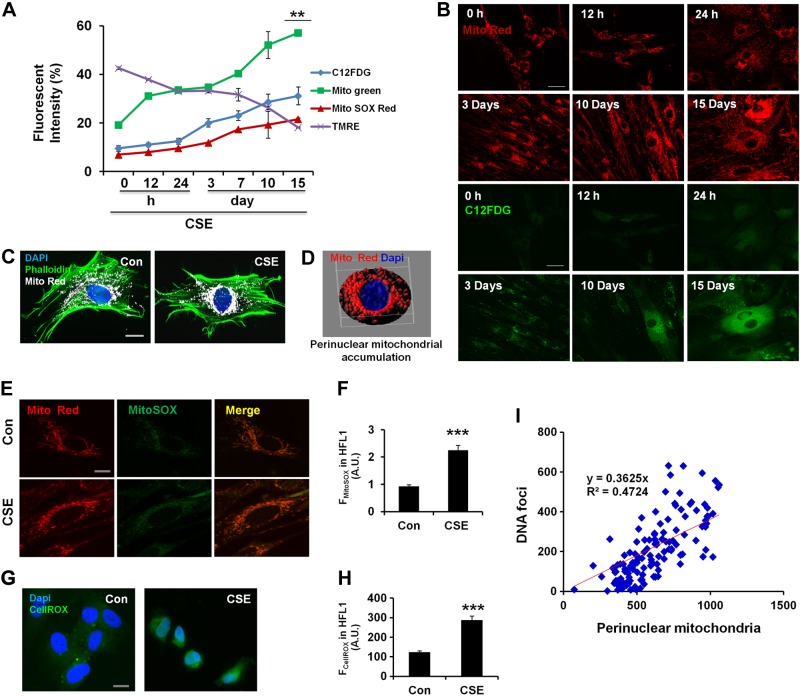
To further determine any correlation between mitochondrial functional and structural changes with cellular senescence, we performed time kinetic experiments in CSE-treated HFL1 cells during the establishment of cellular senescence. Time kinetic data revealed a close correlation among mitochondrial mass accumulation, mtROS, ΔΨm, and cellular senescence during CSE treatment (Fig. 2A). Immunofluorescence staining revealed that mitochondria become highly elongated during the establishment of cellular senescence and increase in mass (Fig. 2B). These data suggest that CS stress-induced lung cellular senescence is associated with mitochondrial dysfunction and elongation. This was concurred by immunofluorescence, which revealed that mitochondria become highly elongated during the establishment of cellular senescence and increase in mass, whereas at earlier time points, more mitochondria showed a perinuclear accumulation (Fig. 2B). From these results, we infer that CSE treatments lead to mitochondrial dysfunction, and mitochondria become highly interconnected and accumulated in senescent cells. Furthermore, during earlier time points, CSE stress results in increased perinuclear mitochondrial clustering, which may be the mechanism whereby dysfunctional mitochondria trigger the cellular senescence process.
Figure 2.
CSE induces perinuclear mitochondrial accumulation and DNA damage in lung fibroblasts. A) Time kinetic experiment shows correlation among cellular senescence (C12FDG), mtROS (MitoSOX Red), ΔΨm (TMRM), and mitochondrial mass (MitoGreen) in HFL1 cells. **P < 0.01 vs. 0 hours. B) Representative images show mitochondrial structural changes during cellular senescence in HFL1 cells treated with 0.5% CSE at indicated time points. HFL1 cells were stained with MitoTracker Red (Mito Red), and C12FDG (green) was used to label senescent cells. C) Representative images show perinuclear mitochondrial accumulation in HFL1 cells treated with CSE (0.75%) for 24 hours. Mitochondria were stained with MitoTracker Red (white), phalloidin (catalog number A12380; Life Technologies) for actin (green), and DAPI for nucleus (blue). Con, control. D) 3D representation of perinuclear mitochondrial clustering during CSE treatment (mitochondria as red and nucleus as blue) using ImageJ. E) Representative images of MitoSOX red (green) and mitochondria stained with MitoTracker Red. Cells were treated with CSE (0.75%) for 24 hours before imaging. F) Data were plotted as average fluorescent intensity (FMitoSOX) of MitoSOX, which was measured by FACS in HFL1. A.U., arbitrary units. ***P < 0.001 vs. control. G) Representative images of CellROX for the measurement of ROS. HFL1 cells were treated with or without CSE (0.75%) for 24 hours and stained with CellROX (green) and DAPI (blue) to visualize the ROS in the nucleus. H) Average fluorescent intensity (FCellROX) of CellROX as measured in HFL1 cells using the MetaMorph software. ***P < 0.001 vs. control. I) Correlation data between perinuclear mitochondrial accumulation and DNA foci formation. HFL1 cells were stained with Tom 20 for mitochondria and DAPI for nucleus to visualize perinuclear mitochondria, along with γ-H2AX for DNA damage foci. Data are shown as the means ± sem (n = 3–4). Scale bars, 20 μm (B, C, E, and G).
CSE-mediated perinuclear mitochondrial accumulation leads to increased ROS and DNA damage foci formation
During mitophagy, damaged mitochondria form a perinuclear cluster, which is subsequently eliminated from the cell (17–21). Our results showed that during earlier time points, CSE treatment increased perinuclear mitochondrial accumulation, which is spared from degradation, as evident at later time points. In context of this, we then determined the role of perinuclear mitochondrial accumulation at earlier time points with CSE treatment in HFL1 cells. After 24 hours, CSE treatment induced a robust perinuclear mitochondrial accumulation (Fig. 2C, D), with increased mtROS, particularly in the perinuclear region (Fig. 2E, F). ROS are highly diffusible species, and after observing the clustering of mtROS near the nuclear periphery, we next determined whether mtROS leads to any changes in the pattern of nuclear ROS. We used CellROX, an ROS-measuring dye, to determine the changes in nuclear ROS by CSE. Imaging studies revealed an increased nuclear ROS with CSE treatment, although most of the ROS showed a cytoplasmic localization in control untreated cells (Fig. 2G, H). Increased ROS is known to induce DNA damage, and a persistent increase in ROS is associated with cellular senescence (42). We next asked whether CSE stress-induced perinuclear mitochondrial accumulation and increased nuclear ROS can induce formation of DNA damage foci in lung cells. CSE-induced perinuclear mitochondrial accumulation was associated with increased γ-H2AX foci formation, and a close correlation between the 2 was observed (Fig. 2I). We next used nocodazole to inhibit microtubule polymerization and hence mitochondrial movement to determine whether inhibition of perinuclear mitochondrial accumulation reduces nuclear ROS and/or γ-H2AX foci formation in response to CSE in lung cells. We found that nocodazole treatment inhibited perinuclear mitochondrial accumulation (Supplemental Fig. S1C, D) and decreased γ-H2AX foci formation after 24 hours in CSE-treated cells (Supplemental Fig. S1E) but caused cell death later (Supplemental Fig. S1F). Altogether, CSE stress-induced perinuclear mitochondrial accumulation was associated with nuclear ROS and DNA damage. Furthermore, long-term CSE treatment was associated with accumulation of damaged mitochondria, which would have repercussions on mitophagy.
CS impairs mitophagy during cellular senescence
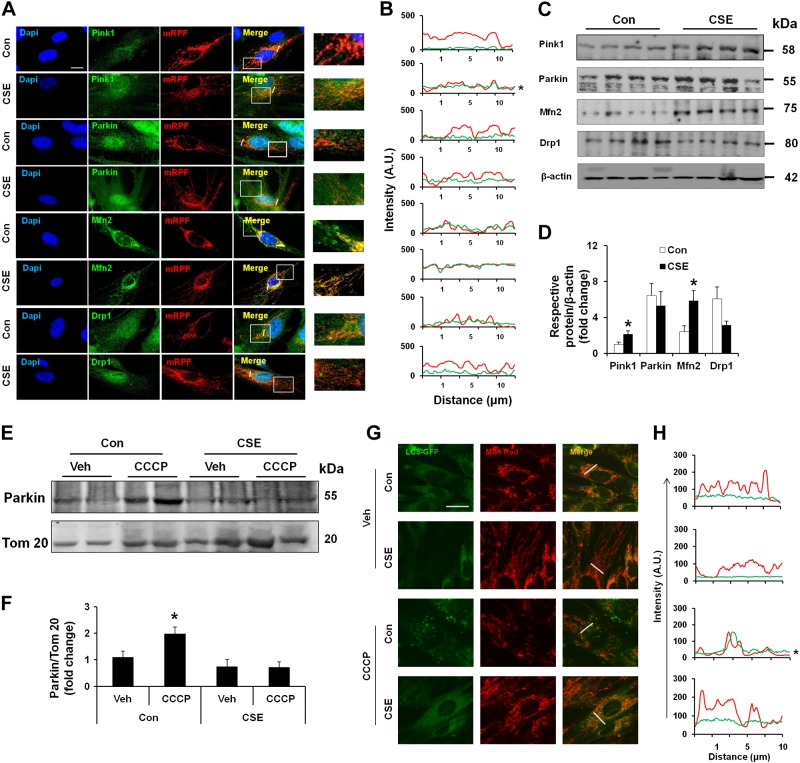
Perinuclear mitochondrial accumulation is a critical step during mitophagy, whereby damaged or dysfunctional mitochondria are cleared from the cells (17–21). Damaged mitochondria, which have decreased ΔΨm, are cleared from the cells via a Pink1 and Parkin-dependent manner, involving autophagosome formation (18–21). We asked whether CSE treatment leads to any changes in mitophagy pathway-associated proteins. Both Western blot and immunofluorescence approaches were used to determine the integral mitophagy pathway-associated proteins during CSE-induced cellular senescence. CSE treatment resulted in a decrease in ΔΨm (Fig. 2A) and showed increased stabilization of Pink1 (catalog number 6946S; Cell Signaling Technology) on mitochondria. However, Parkin (catalog number 2132S; Cell Signaling Technology) mitochondrial translocation was highly impaired in HFL1 cells by CSE (Fig. 3A, B). Furthermore, levels of Mfn2 (catalog number 11925; Cell Signaling Technology), a mitochondrial fusion protein, were increased and showed more mitochondrial localization, whereas the mitochondrial fission protein dynamin-related protein-1 (Drp1; catalog number ab56788; Abcam) showed more cytoplasmic localization, although slightly decreased (Fig. 3A, B). Immunoblot results revealed increased Pink1 and Mfn2 levels, whereas Drp1 showed a slight decrease without statistical significance after CSE treatment (Fig. 3C, D). Parkin levels were not significantly changed in the whole-cell extracts between control and CSE-treated cells (Fig. 3B). In isolated mitochondria, CCCP (a known Parkin mitochondrial translocation agent) treatments resulted in increased Parkin mitochondrial translocation in control cells, whereas CCCP-induced Parkin mitochondrial translocation was highly reduced in CSE-treated HFL1 cells (Fig. 3E, F), indicating impaired mitophagy. This is in agreement with the findings that mitochondrial localization to autophagosomes was highly impaired in CSE-treated human LC3-GFP-expressing fibroblast cells, as reflected by decreased mitochondrial localization to LC3-GFP (Supplemental Fig. S2A). Although there was not much difference in the overall LC3-GFP levels (Supplemental Fig. S2B), the localization of LC3-GFP with elongated mitochondria was highly reduced in CSE-treated cells (Supplemental Fig. S2A, C, D). Treatment of cells with CCCP induced a Parkin-dependent mitophagy, as evident by LC3-GFP colocalization in control cells, but not in CSE-treated cells with cellular senescence (Fig. 3G, H). Cytosolic p53 (catalog number 2524; Cell Signaling Technology) can interact with Parkin, which inhibits Parkin mitochondrial translocation in aged cardiomyocytes (27). We next asked whether CSE treatment inhibits Parkin mitochondrial translocation by increasing its interaction with cytosolic p53. CSE treatment for 15 days induced increased cytosolic p53 levels and its association with Parkin, which was reduced by nocodazole treatment (Supplemental Fig. S2E, F, H, I). These data suggest that increased cytosolic p53 has a role in inhibiting Parkin mitochondrial translocation. Overall, CS impairs mitophagy, which is associated with reduced Parkin mitochondrial translocation via p53.
Figure 3.
CSE treatment leads to mitophagy impairment in lung fibroblasts. A) Representative images of Pink1, Parkin, Mfn2, and Drp1 (green) in HFL1 cells treated with or without CSE. Cells were transfected with mitochondria-targeted red fluorescent protein (mRPF; red) (CellLight Mitochondria-RFP, BacMam, catalog number C10505; Cell Technologies) for 24 hours before staining with respective antibodies (green) and DAPI (blue). Areas in squares are enlarged as shown on right panel. Slanting lines on images indicate the areas assessed for fluorescence intensity. Con, control. B) Line scan data of fluorescence intensity in the corresponding images to show the degree of colocalization between mRFP (mitochondria) and the respective proteins. A.U., arbitrary units. *P < 0.05 vs. control. C) Western blots of Pink1, Parkin, Mfn2, and Drp1 in whole-cell extracts prepared from HFL1 cells treated with or without CSE. β-Actin (catalog number R-22 sc-130657; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as a loading control. Representative housekeeping loading control is shown. D) Densitometry of the respective blots. *P < 0.05 vs. control. E) Western blot of Parkin in mitochondrial extracts from HFL1 cells with or without CSE treatment. CCCP was used as a positive control to induce Parkin mitochondrial translocation. Tom 20 was used as a loading control. Veh, vehicle. F) Densitometry of the respective blots. *P < 0.05 vs. vehicle. G) Representative images of LC3-GFP (CellLight Mitochondria-GFP, BacMam, catalog number C10600; Life Technologies) expressing HFL1 cells stained with MitoTracker Red. Cells were treated with CSE (0.5%) for 15 days followed by CCCP treatment for 2 hours. Images show the colocalization of LC3-GFP with mitochondria (Mito Red) after CCCP treatment. Slanting lines on images indicate the areas assessed for fluorescence intensity. H) Corresponding line scan of fluorescence intensity shows the colocalization of LC3-GFP with mitochondria (red). *P < 0.05 vs. control. CSE was used at 0.5% with alternate day treatment for 15 days. Data are shown as the means ± sem (n = 3–4). Scale bars, 20 μm (A and G).
Mitophagy inhibition enhances CSE stress-induced cellular senescence
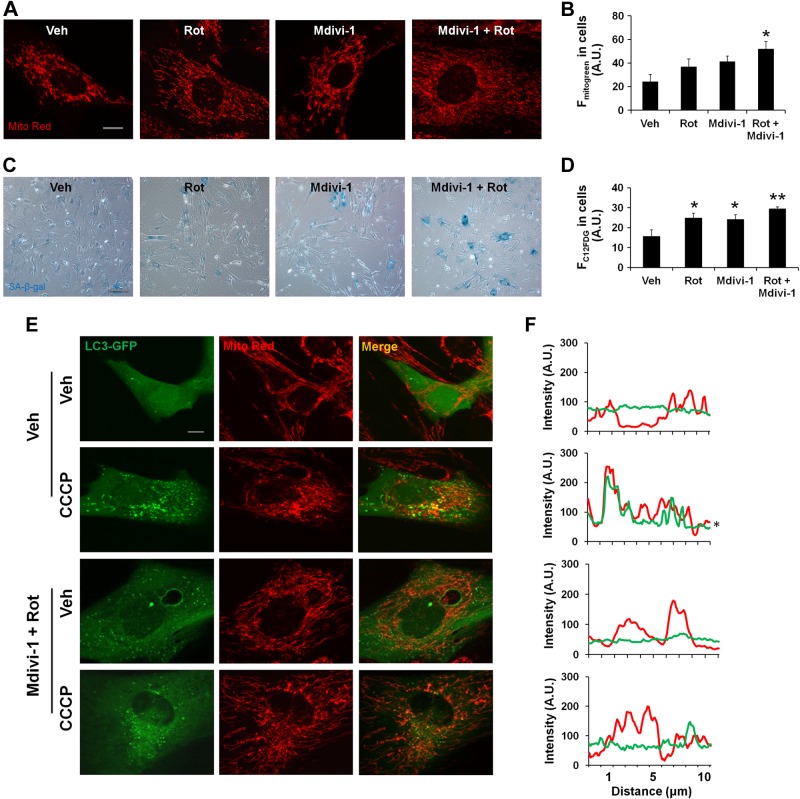
Impaired mitophagy is associated with cellular perturbations in a number of pathological conditions (42–45). To determine whether impaired mitophagy is one of the important prerequisite factors to induce cellular senescence, mouse lung fibroblasts were treated with a general mitophagy inhibitor (Mdivi-1), a mitochondrial complex I inhibitor (rotenone), and a general autophagy inhibitor (bafilomycin) to inhibit mitophagy and induce mitochondrial dysfunction and mitochondrial mass accumulation. Treatment of cells with Mdivi-1 led to mitochondrial elongation, mitochondrial mass accumulation (Fig. 4A, B), and an increase in cellular senescence (Fig. 4C), whereas bafilomycin treatment induced more mitochondria fragmentation, and long-term bafilomycin treatment led to cell death rather than cellular senescence (Supplemental Fig. S2G, J). This further strengthens the results that mitochondrial elongation is a prerequisite for impaired mitophagy during cellular senescence.
Figure 4.
Accumulation of damaged mitochondria leads to cellular senescence in lung fibroblasts. A) Representative images of mouse lung fibroblasts show mitochondrial elongation and accumulation of mitochondrial mass with rotenone (Rot) and Mdivi-1 treatment. Rotenone (10 nM), Mdivi-1 (1 µM), and their combination for 15 days with alternate day treatment are shown. Veh, vehicle. B) Average fluorescent intensity of MitoTracker Green (Fmitogreen) reflects mitochondrial mass, measured by FACS in mouse lung fibroblasts. A.U., arbitrary units. *P < 0.05 vs. vehicle. C) Representative images of SA-β-gal activity in mouse lung fibroblasts with and without rotenone (10 nM), Mdivi-1 (1 µM), and combination treatment. D) C12FDG fluorescence in mouse lung fibroblasts was measured by FACS and plotted as average fluorescent intensity (FC12FDG in cells), which showed the degree of cellular senescence in arbitrary units. *P < 0.05 and **P < 0.01 vs. vehicle. E) Representative images of mouse lung fibroblasts show colocalization of LC3-GFP with mitochondria (Mito Red). Vehicle or rotenone- and Mdivi-1-treated cells were further treated with or without CCCP for 2 hours before imaging. F) Line scan of fluorescence intensity in the corresponding images. *P < 0.05 vs. control. Data are shown as the means ± sem (n = 3–4). Scale bars, 10 μm (A and E) and 100 μm (C).
We next asked whether increased mtROS can also induce cellular senescence and mitochondrial elongation. Rotenone resulted in increased mtROS (Supplemental Fig. S2K) and mitochondrial mass accumulation, as well as an increase in cellular senescence after 15 days of CSE treatment (Fig. 4A–D). Interestingly, a combination of rotenone and Mdivi-1 induced a robust mitochondrial elongation, increased mitochondrial mass, and cellular senescence (Fig. 4A–D). An additive effect of rotenone and Mdivi-1 was also evident on LC3-GFP, which showed a reduced colocalization with mitochondria, indicating impaired mitophagy. CCCP (catalog number C2759; Sigma-Aldrich) treatment could not induce LC3-GFP association with mitochondria in cells treated with a combination of rotenone and Mdivi-1 (Fig. 4E, F). These results confirm the notion that mitochondrial dysfunction and impaired mitophagy are some of the important prerequisite factors to cause cellular senescence.
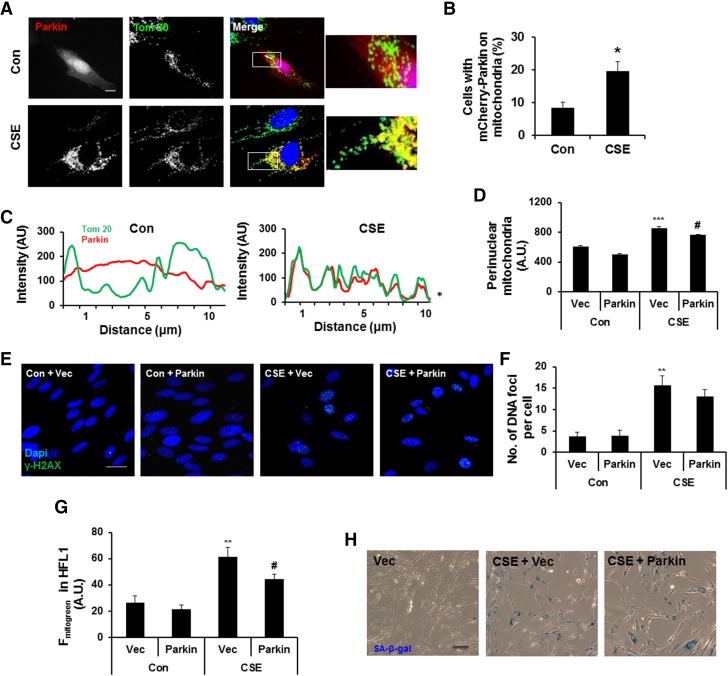
Parkin overexpression restores impaired mitophagy during CSE treatment in HFL1 cells
Mitochondrial elongation induced by either Drp1 inhibition and/or Mfn2 overexpression is regulated by coordinated action of Pink1 and Parkin during mitophagy (17–21). Parkin causes ubiquitination of Mfn2 followed by its degradation in a Pink1-dependent manner (21), leading to the formation of small fragmented mitochondria and hence mitophagy. Thus, Parkin activation is one of the important determining factors to induce mitophagy. As shown by our results, mitochondria became highly elongated during CSE treatment, which was associated with decreased Parkin mitochondrial translocation by CCCP (Fig. 3E, F). We next asked whether Parkin overexpression overcomes the inhibitory effects of endogenous Parkin mitochondrial translocation by CSE. To address this, we overexpressed Parkin in HFL1 cells followed by CSE treatment. Parkin overexpression during CSE treatment restored mitophagy, as shown by recruitment of Parkin to mitochondria (Fig. 5A–C), which resulted in decreased perinuclear mitochondrial accumulation (Fig. 5D), and a slight decrease in γ-H2AX foci formation, but not much change in mtROS levels (Fig. 5E, F, and Supplemental Fig. S3A, B). Furthermore, using Parkin overexpression in HFL1 cells, we observed a decrease in mitochondrial mass accumulation (Fig. 5G), but not much change in cellular senescence after 15 days of CSE treatment (Fig. 5H). These results show that Parkin overexpression can restore mitochondrial mass accumulation but does not have a significant impact on cellular senescence when CS already establishes the cellular senescence.
Figure 5.
Parkin overexpression reduces CS-induced mitophagy impairment in lung fibroblasts. A) Representative images of HFL1 cells transfected with mCherry-Parkin. Cells were treated with CSE (0.75%) for 24 hours and stained with Tom 20 for mitochondria (green). Con, control. Areas in squares are zoomed as shown on right panel. Slanting lines on images indicate the areas assessed for fluorescence intensity. B) Percentage of cells with mCherry-Parkin on mitochondria. *P < 0.05 vs. control. C) Line scan of the corresponding images. AU, arbitrary units. *P < 0.05 vs. control. D) Perinuclear mitochondrial accumulation in HFL1 cells treated with or without CSE (0.75%) for 24 hours. ***P < 0.001 vs. vector (Vec); #P < 0.05 vs. CSE plus vector. E) Representative images of DNA damage foci (reflected by γH2AX foci) in vector or Parkin-overexpressing HFL1 cells (Parkin), treated with or without CSE. DAPI (blue) was used to label nucleus and γ-H2AX for DNA damage foci (green). F) Average number of DNA damage foci per cell. G) Average fluorescence intensity of MitoTracker Green (Fmitogreen), which was measured by FACS in HFL1. **P < 0.01 vs. vector; #P < 0.05 vs. CSE plus vector. H) Representative images of SA-β-gal activity in vector or Parkin-overexpressing HFL1 cells treated with or without alternate day of CSE for 15 days (0.5%). Vec represents the cells transfected with vector only, whereas Parkin represents the cells transfected with mCherry-Parkin plasmid. Data are shown as the means ± sem (n = 3–4). Scale bars, 20 μm (A and E) and 100 μm (H).
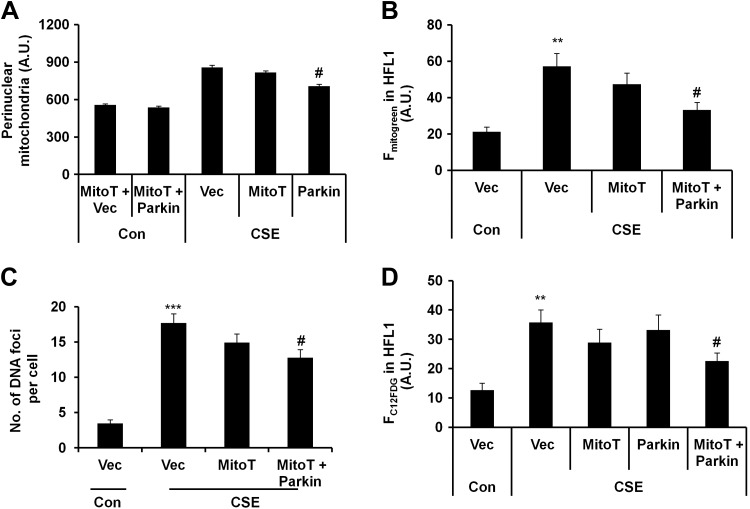
Mitochondria-targeted antioxidant ameliorates cellular senescence in Parkin-overexpressing HFL1 cells
Because perinuclear mitochondria accumulation was associated with increased nuclear ROS and DNA damage foci formation, Parkin overexpression could not rescue the cells after the establishment of cellular senescence. We next asked whether a mitochondria-targeted antioxidant can rescue these changes in HFL1 cells. We treated HFL1 cells with different concentrations of mitochondria-targeted antioxidant (MitoT; 100 nM to 1 μM, for 24 hours) and measured mtROS levels. MitoT treatment led to mtROS reduction in CSE-treated cells (Supplemental Fig. S3A, B). In addition, MitoT treatment resulted in a slight but insignificant decrease in perinuclear mitochondrial accumulation (Fig. 6A), mitochondrial mass (Fig. 6B), and DNA damage foci (reflected by γH2AX foci) formation (Fig. 6C). There was also a slight decrease in cellular senescence after MitoT treatment (Fig. 6D). However, MitoT treatment could not restore impaired mitophagy (Fig. 6A, B). These results indicate that MitoT reduced mtROS but was unable to rescue impaired mitophagy or cellular senescence.
Figure 6.
Mitochondria-targeted antioxidant attenuates mitochondrial mass accumulation and cellular senescence in Parkin-overexpressing HFL1 cells. A) Perinuclear mitochondrial accumulation was measured by FACS in HFL1 cells overexpressing Parkin (Parkin), which were treated with or without CSE (0.75%) for 24 hours. Average intensity of perinuclear mitochondrial accumulation was plotted. A.U., arbitrary units; Con, control. #P < 0.001 vs. CSE plus vector (Vec). B) Average fluorescent intensity of MitoTracker Green (Fmitogreen) was measured by FACS in HFL1 cells treated with or without CSE (0. 5%) for 15 days. **P < 0.01 vs. control vector; #P < 0.05 vs. CSE plus vector. C) Data show number of DNA damage foci per cell in γ-H2AX-stained HFL1 cells that were treated with or without CSE (0.75%) for 24 hours. ***P < 0.001 vs. control vector; #P < 0.05 vs. CSE plus vector. Average number of DNA damage foci per cell was plotted by counting >50 cells. D) FACS data show degree of cellular senescence in cells treated with or without CSE (0.5%) for 15 days. Average fluorescent intensity of C12FDG (FC12FDG in HFL1) was plotted. **P < 0.001 vs. control vector; #P < 0.05 vs. CSE plus vector. Vec represents the cells transfected with vector only, whereas Parkin represents the cells transfected with mCherry-Parkin plasmid, and MitoT represents the cells treated with Mito-Temp. Data are shown as the means ± sem (n = 4).
Next, we overexpressed Parkin in HFL1 cells and treated these cells with MitoT at 2 hours before every CSE treatment (for 24 hours). As expected, MitoT reduced mtROS levels (Supplemental Fig. S3B) and decreased perinuclear mitochondrial accumulation in Parkin-overexpressing cells (Fig. 6A). Moreover, Parkin overexpression along with MitoT significantly reduced mitochondrial mass (Fig. 6B), DNA foci formation (Fig. 6C), and cellular senescence (Fig. 6D). These results show that restoring mitophagy and attenuating mtROS can prevent the progression of cellular senescence in primary lung fibroblasts.
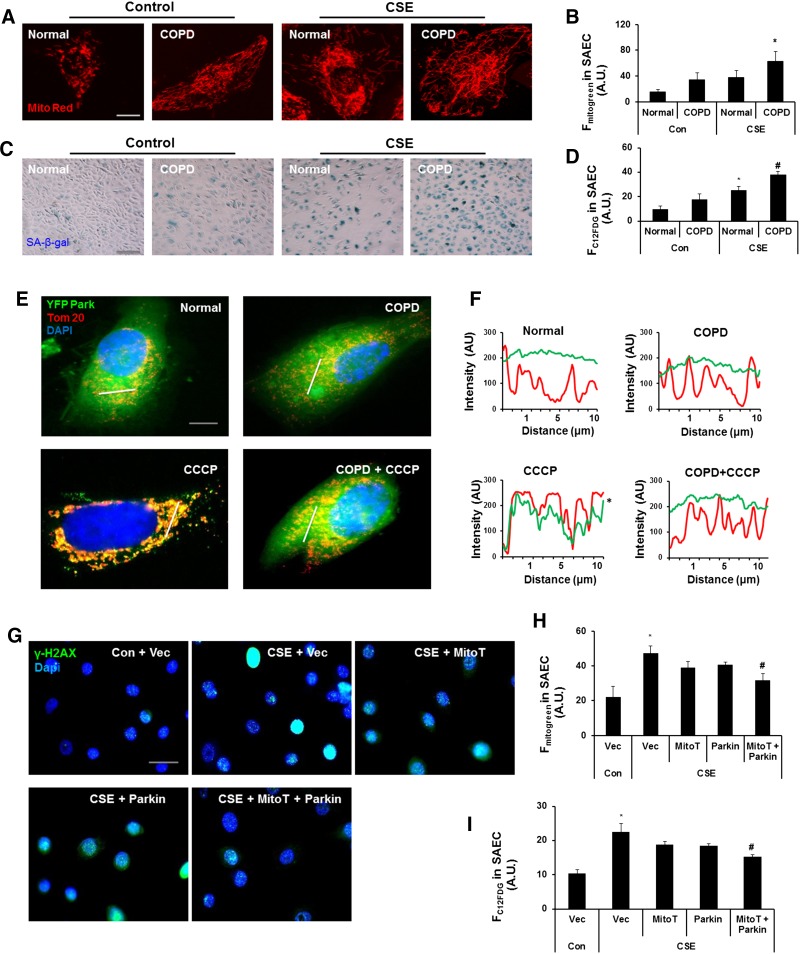
MitoT and Parkin overexpression delays cellular senescence in CSE-treated SAECs
Recent findings along with our data suggest an important role of cellular senescence in both lung fibroblasts and epithelial cells (particularly SAECs) in the pathogenesis of COPD/emphysema (1, 6). To determine the effect of CSE on mitophagy in primary SAECs, we used the cells from normal subjects and patients with COPD. Human COPD SAECs showed increased mitochondrial mass (Fig. 7A, B) and cellular senescence (Fig. 7C, D), which was further augmented by CSE treatment (Fig. 7A–D). SAECs from patients with COPD also displayed reduced Parkin mitochondrial translocation in the presence of CCCP compared to cells from normal subjects, implicating impaired mitophagy (Fig. 7E, F). Furthermore, MitoT along with Parkin overexpression reduced γ-H2AX foci formation, mitochondrial mass accumulation, and mtROS along with partial reduction in cellular senescence after 15 days of CSE treatment (Fig. 7G–I and Supplemental Fig. S3C). These results show that mitophagy plays a critical role during the process of cellular senescence, whereas decreased mitophagy augments CSE stress-induced senescence, and Parkin overexpression along with mitochondria-targeted antioxidants delays cellular senescence in human SAECs.
Figure 7.
Parkin overexpression and MitoT rescue defective mitophagy and protect human primary SAECs against CS-induced cellular senescence. A) Representative images of cells show mitochondrial morphology in human primary SAECs treated with or without CSE (0.2%) for 10 days with alternate day treatment. Cells were stained with MitoTracker Red for mitochondria. B) Average MitoTracker Green fluorescence (Fmitogreen) was measured by FACS in normal and COPD SAECs treated with or without CSE (0. 2%) for 10 days. A.U., arbitrary units. *P < 0.05 vs. Normal. C) Representative images show SA-β-gal activity in SAECs treated with or without CSE. Cells were treated for 10 days followed by staining with SA-β-gal. D) Average fluorescent intensity of C12FDG (FC12FDG) was measured by FACS in SAECs. Con, control. *P < 0.05 vs. Con-Normal; #P < 0.05 vs. Con-COPD. E) Representative images of normal and COPD cells that were transfected with YFP-Parkin and treated with CCCP (10 µM) for 2 hours. SAECs were stained with Tom 20 (red) and DAPI (blue). Slanting lines on images indicate the areas assessed for fluorescence intensity. F) Corresponding line scan of fluorescence intensity shows the colocalization of YFP-Parkin with mitochondria. *P < 0.05 vs. Normal. G) Representative images of SAECs from normal subjects stained with γ-H2AX (green); DAPI was used to label nucleus (blue). Vec, vector. H) Average MitoTracker Green fluorescence (Fmitogreen) was measured by FACS in SAECs treated with or without CSE (0. 2%) for 15 days. *P < 0.05 vs. control vector; #P < 0.05 vs. CSE plus vector. I) Average fluorescent intensity of C12FDG (FC12FDG) was measured by FACS in SAECs. *P < 0.05 vs. control vector; #P < 0.05 vs. CSE plus vector. Vec is a vector, MitoT represents cells treated with MitoT, and Parkin represents cells transfected with mCherry-Parkin, whereas MitoT + Parkin represents cells transfected with Parkin and treated with MitoT with or without CSE (0.2%) treatment for 15 days. Data are shown as the means ± sem (n = 3). Scale bars, 10 μm (A and E), 100 μm (C), and 20 μm (G).
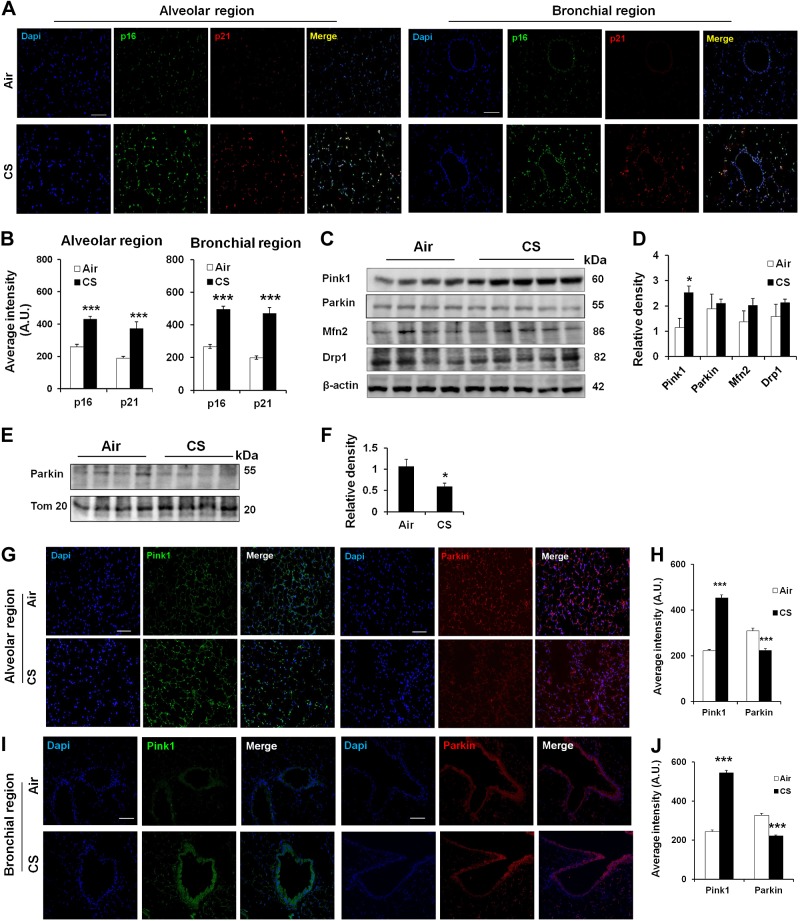
Alteration of mitophagy in mouse lungs with emphysema as well as in lungs of smokers and patients with COPD
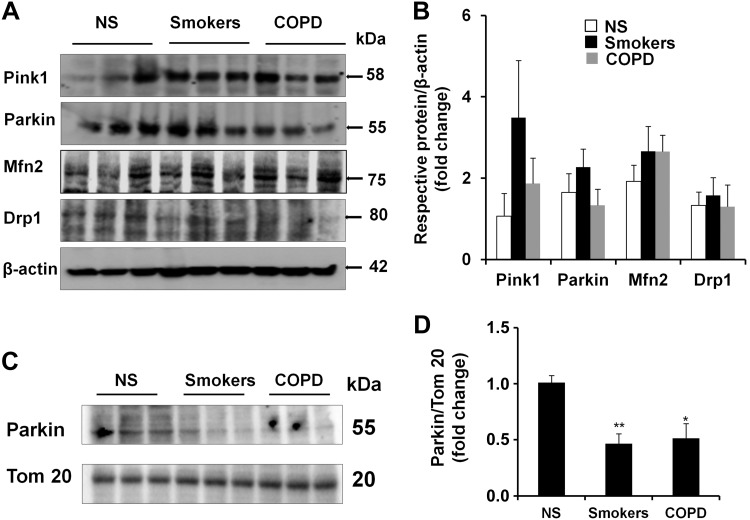
Based on our results showing that CSE stress induced mitophagy impairment and hence cellular senescence in both human and mouse lung fibroblasts, we next asked whether mitophagy-associated proteins are altered in the lungs of smokers and patients with COPD, as well as in CS-exposed mouse lungs, where cellular senescence occurs. Consistent with our previous studies (6), increased p16 and p21 levels were seen in mouse lung structural cells after 6 mo of CS exposure (Fig. 8A, B). We have also previously shown that human COPD and smoker lungs are associated with increased p16 and p21 levels, as well as SA-β-gal activity (6). The levels of mitophagy-regulating proteins, such as Pink1, Parkin, Mfn2, and Drp1, were measured in chronic CS-exposed mouse lungs (Fig. 8C, D), as well as in the lungs of smokers and patients with COPD (Fig. 9A, B). CS-exposed mice lungs showed increased levels of Pink1, whereas Parkin expression did not show any differences (Fig. 8C, D). Studies in the isolated mitochondria showed impaired Parkin translocation to mitochondria in CS-exposed mouse lungs, as well as in the lungs of human smokers and patients with COPD (Figs. 8E, F, and 9C, D). Immunofluorescence results revealed increased expression of Pink1, whereas Parkin levels were decreased in both alveolar and bronchial epithelial cells in mice during chronic CS exposure (Fig. 8G–J). These results provide evidence that mitophagy is highly impaired during CS exposure in mouse lungs as well as in the lungs of smokers and patients with COPD, which may be a contributing factor for cellular senescence during the pathogenesis of COPD.
Figure 8.
Chronic CS induces impaired mitophagy in mouse lungs with emphysema. A) Representative images of alveolar and bronchial regions show p16 and p21 staining in mouse lungs exposed to CS for 6 months. Tissues were stained with p16 (green), p21 (red), and DAPI (blue). B) Average intensity of p16 and p21 was calculated using MetaMorph software. A.U., arbitrary units. ***P < 0.001 vs. Air. C) Western blot of Pink1, Parkin, Mfn2, and Drp1 in whole-cell extracts prepared from mouse lungs, and β-actin was used as a loading control. Representative housekeeping loading control is shown. D) Densitometry of the respective blots. *P < 0.05 vs. Air. E) Western blot of Parkin and Tom 20 in mitochondrial extracts prepared from lungs of mice exposed to CS for 6 months. Representative loading control is shown. F) Densitometry of the respective blots. *P < 0.05 vs. Air. G and I) Representative images of alveolar and bronchial regions show Pink1 and Parkin staining in mouse lungs. Air is the control group, and CS represents the mice exposed to CS for 6 months. Tissues were stained with Pink1 (green), Parkin (red), and DAPI (blue). H and J) Average intensity of Pink1 and Parkin staining was calculated using MetaMorph software. ***P < 0.001 vs. Air. Data are shown as the means ± sem (n = 3–4). Scale bars, 50 μm (A, G, and I).
Figure 9.
Impaired mitophagy in lungs of smokers and patients with COPD. A) Western blots of Pink1, Parkin, Mfn2, and Drp1 in whole-cell extracts prepared from human lungs are shown, whereas β-actin was used as a loading control. Representative housekeeping loading control is shown. NS, nonsmokers. B) Densitometry of the respective blots. C) Western blots of Parkin and Tom 20 in mitochondrial extracts prepared from lungs of nonsmokers, smokers, and patients with COPD. Representative loading control is shown. D) Densitometry of the respective blots. *P < 0.05 and **P < 0.01 vs. nonsmokers. Data are represented as the means ± sem (n = 3–4).
DISCUSSION
Cigarette smoking is the most formidable cause of COPD. CS is shown to induce cellular senescence in lung structural cells, including SAECs, bronchial epithelial cells, and fibroblasts (1, 2, 5, 6). Senescence of these cells plays direct (impaired re-epithelialization) and indirect (increased release of inflammatory mediators) contributing roles in the pathogenesis of COPD/emphysema (1–3, 46). Although a well-appreciated phenomenon, the mechanisms and the signaling pathways associated with CS stress-induced cellular senescence and COPD per se are not known. One of the major contributing factors of CS-induced COPD is oxidative stress. In cells, most of the ROS is produced by mitochondria, and CS is known to cause mitochondrial dysfunction (9–12, 47). We proposed that mitochondrial dysfunction is a critical and prerequisite cause for CS stress-induced cellular senescence in the pathogenesis of COPD. We have shown here a previously unknown molecular pathway for CS stress-induced cellular senescence via mitochondrial dysfunction in vitro in human lung fibroblasts and human primary SAECs, and in vivo in mouse lungs as well as ex vivo in the lungs of patients with COPD.
Our study aimed at deciphering the molecular pathways associated with mitochondrial function and mitochondrial quality control during CS stress-induced cellular senescence in the pathogenesis of COPD. We first developed a cell culture model of CSE stress-induced cellular senescence, where we showed that CSE treatment induced mitochondrial dysfunction and increased mtROS. At earlier time points, CSE treatment was associated with increased perinuclear mitochondrial accumulation, which was highly correlated with the DNA damage foci formation. Perinuclear mitochondrial clustering associated with increased mtROS led to an increase in nuclear ROS, which may be due to the dysfunctional mitochondria itself. Persistent increase in ROS levels is a well-known factor mediating DNA damage and cellular senescence (42, 48), where mitochondria may play a critical role. Long-term CSE-treated cells show increased mtROS and mitochondrial mass accumulation as well as mitophagy impairment. Chronic CSE treatment induced mitochondrial elongation, which prevented mitochondrial clearance via mitophagy. These results are in agreement with the previous reports showing impaired mitophagy occurring during mitochondrial elongation (49). Therefore, CS causes mitophagy impairment and mtROS generation, which may drive DNA damage and cellular senescence.
Parkin is a core mitophagy-regulating protein, which is recruited to damaged or depolarized mitochondria to induce mitochondrial clearance (16–19). Both Pink1 and Parkin knockout mice exhibit increased ROS levels and mitochondrial dysfunction (43, 44), and persistent increased ROS is one of the important factors in accelerating cellular senescence (42, 45). Parkin overexpression has been shown to increase the life span in flies and reduces oxidative stress by activating mitophagy (50, 51). In our study, although the total Parkin levels were not significantly altered between control and CSE-treated cells, CCCP-mediated Parkin translocation to mitochondria was reduced by CSE, suggesting impaired clearance of damaged mitochondria. A recent study has shown that increased p53 cytosolic localization prevents Parkin translocation to mitochondria during organismal aging (27). We found increased p53 expression in the cytoplasm with CSE treatment. Additionally, p53 interaction with Parkin was highly increased during CSE-induced cellular senescence. Inhibition of perinuclear mitochondrial accumulation by nocodazole decreased DNA damage/γH2AX foci, p53 cytosolic levels, and p53’s association with Parkin in response to CSE treatment. This indicates that cytosolic translocation of p53 was partly mediated by perinuclear mitochondrial accumulation, followed by DNA damage foci formation. Although long-term nocodazole treatment led to cell death, we were therefore unable to directly evaluate the effect of perinuclear mitochondrial accumulation on cellular senescence. Nevertheless, these findings provide convincing evidence that perinuclear mitochondrial clustering is a critical step to induce DNA damage during the pathogenesis of COPD/emphysema after chronic CS exposures. Further research is needed to dissect the role of perinuclear mitochondrial clustering in CS stress-induced cellular senescence.
In order to rescue impaired mitophagy, we used an alternate approach by overexpressing Parkin during CSE-treated conditions. Parkin overexpression is shown to delay aging in flies (47, 48). We found that Parkin overexpression restored mitophagy in CSE-treated cells. Parkin overexpression also reduced mitochondrial mass accumulation as well as caused a slight decrease in γ-H2AX foci formation and cellular senescence. This mild effect of Parkin overexpression in attenuating cellular senescence can be explained as follows. First, Parkin was transiently overexpressed in HFL1 cells, whereas cells were continuously treated for 15 days to induce cellular senescence. Second, Parkin overexpression did not have any effect on mtROS levels, which is one of the important factors in inducing cellular senescence. Further experiments using stable Parkin-overexpressing cells or Parkin knockout mice will reveal its role in CS stress-induced cellular senescence. Interestingly, Parkin-overexpressing cells treated with MitoT showed a significant decrease in DNA damage foci formation, perinuclear mitochondrial accumulation, mtROS, and delayed cellular senescence. This is corroborated by our findings that either Mdivi-1 (mitophagy inhibitor) (49) or rotenone (increasing mtROS generation) (35) induced perinuclear accumulation of damaged mitochondria and cellular senescence. It is interesting to note that MitoT treatment alone did not show any significant effect on cellular senescence, which may be due to its insufficient concentration and duration used. mtROS has been shown as a key inducer for premature aging, and mtROS inhibitor reduces senescence in bone marrow mesenchymal stem cells (52, 53). Further study using global and mitochondria-specific antioxidants and mtROS scavengers will define the roles of mtROS in CS-induced cellular senescence. Altogether, both mitophagy impairment and mtROS generation contribute to CS-induced cellular senescence.
We next extrapolated the in vitro findings into mouse lungs with emphysema and lung tissues of patients with COPD. As expected, increased expression of senescence markers (p16 and p21) was observed in both lung epithelia (alveolar and bronchial) and fibroblasts of mice with emphysema (6). This was associated with defective mitophagy and decreased mitochondrial function. Similarly, the lungs from both patients with COPD and smokers exhibited defective mitophagy. Furthermore, primary SAECs from patients with COPD exhibited increased cellular senescence and decreased mitophagy, which was further augmented by CSE treatment. Parkin overexpression along with MitoT treatment showed a significant restoration of mitophagy, decreased DNA damage foci formation, and delayed cellular senescence in SAECs. The use of primary SAECs in these experiments was to expand our study of CS-induced cellular senescence into other lung cell types besides lung fibroblasts. It is not clear whether CS impairs transmitophagy between lung fibroblasts and epithelial cells during the development of COPD/emphysema (54). Further in vivo study using mice treated with MitoT will reveal the role of mtROS in causing DNA damage, cellular senescence, and emphysema by CS. Recent studies have also shown that smooth muscle and transformed lung epithelial cells are equally sensitive to CS-induced cellular senescence (14, 15). It remains unknown whether mitophagy is impaired in these cells in response to CS exposure.
There is a study showing elongated mitochondria with increased mitochondrial fusion activity during long-term CSE treatment in a human lung epithelial cell line (15), which is in agreement with our findings. However, there are conflicting reports relating the role of CSE in mitochondrial structural changes (14, 48). The discrepancies among these studies may be due to different cell culture conditions and the concentration and duration of CSE used (14, 15, 48). A recent study has shown increased mitophagy with acute CSE treatment in cultured lung epithelial cells, whereas mitochondria were highly fragmented in CS-exposed mouse lung epithelial cells (55). This study has focused on necroptosis contributing to airspace enlargement, which may be the ultimate fate of these cells. Although we have also observed increased mitochondrial fragmentation with high concentration of CSE treatment (data not shown), the objective of this study was to elucidate the role of mitochondrial quality control during cellular senescence (using low concentration of CSE treatment), which is one of the critical factors for COPD. Pink1 deficiency leads to mitochondrial damage and hence promotes lung fibrosis (56), whereas a decreased expression of mitochondrial fission proteins Drp1 and mitochondrial fission 1 protein induces cellular senescence via a Pink1-dependent manner (57). We showed increased translocation of Pink1, but not Drp1, into mitochondria in CSE-treated cells, CS-exposed mouse lung with emphysema, and the lung of patients with COPD. Further study is required to determine their roles in CS-induced cellular senescence in COPD/emphysema and associated pulmonary fibrosis (58–60).
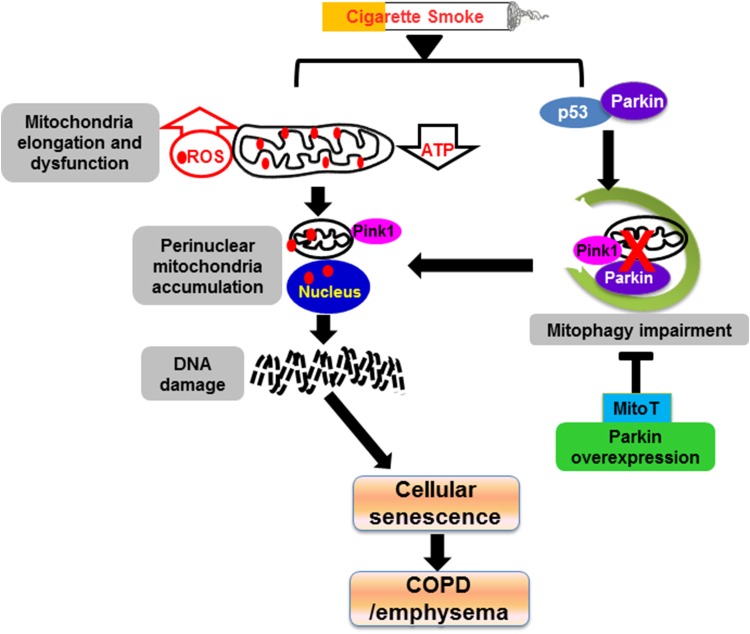
In summary, we show here for the first time that the mechanisms of defective mitophagy lead to accumulation of dysfunctional and damaged mitochondria and, hence, augmented cellular senescence during CS exposure in vitro in lung epithelial cells and fibroblasts, mouse lungs, as well as human smokers and patients with COPD (Fig. 10). CS stress induces mitochondrial structural and functional changes by interfering with mitochondrial quality control machinery. This is associated with a reduction of Parkin-mediated mitophagy and an increase in mtROS-induced DNA damage. A combination of Parkin overexpression and MitoT shows promising results in restoring the defective mitophagy and hence delaying cellular senescence. Overall, these findings unravel new insights into the molecular mechanisms of mitophagy regulating cellular senescence during CS stress-induced lung pathophysiologic responses. Furthermore, the restoration of Parkin-mediated mitophagy and scavenging of dysfunctional mitochondria-derived mtROS may prove to be therapeutic avenues to protect mitochondria damage and DNA damage-mediated cellular senescence in airway diseases.
Figure 10.
Schematic diagram shows that CS induces mitochondrial dysfunction and mitophagy impairment leading to cellular senescence via suborganellar signaling in COPD. CS stress causes mitochondrial elongation and dysfunction (i.e., ATP reduction and increased ROS release), leading to perinuclear accumulation of damaged mitochondria and DNA damage-initiated cellular senescence via suborganellar signaling during the development of COPD. CS exposure also increases the interaction of p53 with Parkin, which impairs Parkin-dependent mitophagy and further augments perinuclear mitochondrial clustering. Parkin overexpression along with MitoT treatment reduces mitophagy impairment and cellular senescence. Red dots indicate ROS.
Supplementary Material
Acknowledgments
The authors thank Dr. Christian Sell for providing the WI-38 cells expressing microtubule-associated protein light-chain 3-green fluorescent protein. They also thank Dr. Thomas L. Schwarz from the Department of Neuroscience, Harvard Medical School (Boston, MA, USA), for providing the yellow fluorescent protein-Parkin, mCherry-Parkin, and FLAG-Pink1 constructs. The authors thank the late Dr. Vuokko L. Kinnula for providing the human tissue samples from smokers and patients with chronic obstructive pulmonary disease, which were previously used in our published studies. This study was supported by the U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute Grants 2R01HL085613 and 1R01HL092842 (to I.R.), American Lung Association Grants RG-266456-N (to H.Y.) and RG-305393 (to I.K.S.), and NIH National Institute of Environmental Health Sciences Grant P30-ES01247. T.A. contributed to the experiment design, data analysis, and manuscript writing. T.A., I.K.S., C.L., J.G., H.Y., and A.M.T. performed the experiments. H.Y. analyzed the data and participated in manuscript writing/editing. I.R. contributed significantly to the experimental design and manuscript writing/editing. The authors declare no conflicts of interest.
Glossary
- ΔΨm
mitochondrial membrane potential
- 3D
3-dimensional
- C12FDG
5-dodecanoylaminofluorescein di-β-d-galactopyranoside
- CCCP
carbonyl cyanide m-chlorophenyl hydrazine
- COPD
chronic obstructive pulmonary disease
- CS
cigarette smoke
- CSE
cigarette smoke extract
- Drp1
dynamin-related protein-1
- FACS
fluorescence-activated cell sorting
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- HLF
human lung fibroblast
- HLF-1
human fetal lung firbroblast
- LC3
microtubule-associated protein light-chain 3
- Mfn2
mitofusin-2
- MitoT
Mito-Tempo
- mtROS
mitochondrial reactive oxygen species
- Pink1
phosphatase and tensin homolog-induced putative kinase 1
- ROS
reactive oxygen species
- SA-β-gal
senescence-associated β-galactosidase
- SAEC
small airway epithelial cell
- TMRM
tetramethylrhodamine ethyl ester
- YFP
yellow fluorescent protein
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Nyunoya T., Monick M. M., Klingelhutz A., Yarovinsky T. O., Cagley J. R., Hunninghake G. W. (2006) Cigarette smoke induces cellular senescence. Am. J. Respir. Cell Mol. Biol. 35, 681–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nyunoya T., Monick M. M., Klingelhutz A. L., Glaser H., Cagley J. R., Brown C. O., Matsumoto E., Aykin-Burns N., Spitz D. R., Oshima J., Hunninghake G. W. (2009) Cigarette smoke induces cellular senescence via Werner’s syndrome protein down-regulation. Am. J. Respir. Crit. Care Med. 179, 279–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang J. W., Yao H., Caito S., Sundar I. K., Rahman I. (2013) Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic. Biol. Med. 61, 95–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hara H., Araya J., Takasaka N., Fujii S., Kojima J., Yumino Y., Shimizu K., Ishikawa T., Numata T., Kawaishi M., Saito K., Hirano J., Odaka M., Morikawa T., Hano H., Nakayama K., Kuwano K. (2012) Involvement of creatine kinase B in cigarette smoke-induced bronchial epithelial cell senescence. Am. J. Respir. Cell Mol. Biol. 46, 306–312 [DOI] [PubMed] [Google Scholar]
- 5.Nyunoya T., Mebratu Y., Contreras A., Delgado M., Chand H. S., Tesfaigzi Y. (2014) Molecular processes that drive cigarette smoke-induced epithelial cell fate of the lung. Am. J. Respir. Cell Mol. Biol. 50, 471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao H., Chung S., Hwang J. W., Rajendrasozhan S., Sundar I. K., Dean D. A., McBurney M. W., Guarente L., Gu W., Rönty M., Kinnula V. L., Rahman I. (2012) SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J. Clin. Invest. 122, 2032–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodier F., Campisi J. (2011) Four faces of cellular senescence. J. Cell Biol. 192, 547–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao H., Sundar I. K., Ahmad T., Lerner C., Gerloff J., Friedman A. E., Phipps R. P., Sime P. J., McBurney M. W., Guarente L., Rahman I. (2014) SIRT1 protects against cigarette smoke-induced lung oxidative stress via a FOXO3-dependent mechanism. Am. J. Physiol. Lung Cell. Mol. Physiol. 306, L816–L828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tait S. W., Green D. R. (2012) Mitochondria and cell signalling. J. Cell Sci. 125, 807–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia L., Liu Z., Sun L., Miller S. S., Ames B. N., Cotman C. W., Liu J. (2007) Acrolein, a toxicant in cigarette smoke, causes oxidative damage and mitochondrial dysfunction in RPE cells: protection by (R)-alpha-lipoic acid. Invest. Ophthalmol. Vis. Sci. 48, 339–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo L., Li L., Wang W., Pan Z., Zhou Q., Wu Z. (2012) Mitochondrial reactive oxygen species mediates nicotine-induced hypoxia-inducible factor-1α expression in human non-small cell lung cancer cells. Biochim. Biophys. Acta 1822, 852–861 [DOI] [PubMed] [Google Scholar]
- 12.Zuckerbraun B. S., Chin B. Y., Bilban M., d’Avila J. C., Rao J., Billiar T. R., Otterbein L. E. (2007) Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 21, 1099–1106 [DOI] [PubMed] [Google Scholar]
- 13.Youle R. J., van der Bliek A. M. (2012) Mitochondrial fission, fusion, and stress. Science 337, 1062–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aravamudan B., Kiel A., Freeman M., Delmotte P., Thompson M., Vassallo R., Sieck G. C., Pabelick C. M., Prakash Y. S. (2014) Cigarette smoke-induced mitochondrial fragmentation and dysfunction in human airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 306, L840–L854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann R. F., Zarrintan S., Brandenburg S. M., Kol A., de Bruin H. G., Jafari S., Dijk F., Kalicharan D., Kelders M., Gosker H. R., Ten Hacken N. H., van der Want J. J., van Oosterhout A. J., Heijink I. H. (2013) Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respir. Res. 14, 97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashrafi G., Schwarz T. L. (2013) The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 20, 31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youle R. J., Narendra D. P. (2011) Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 12, 9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bingol B., Tea J. S., Phu L., Reichelt M., Bakalarski C. E., Song Q., Foreman O., Kirkpatrick D. S., Sheng M. (2014) The mitochondrial deubiquitinase USP30 opposes parkin-mediated mitophagy. Nature 510, 370–375 [DOI] [PubMed] [Google Scholar]
- 19.Vincow E. S., Merrihew G., Thomas R. E., Shulman N. J., Beyer R. P., MacCoss M. J., Pallanck L. J. (2013) The PINK1-Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo. Proc. Natl. Acad. Sci. USA 110, 6400–6405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamano K., Fogel A. I., Wang C., van der Bliek A. M., Youle R. J. (2014) Mitochondrial Rab GAPs govern autophagosome biogenesis during mitophagy. eLife 3, e01612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y., Dorn G. W. II (2013) PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 340, 471–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osellame L. D., Duchen M. R. (2013) Defective quality control mechanisms and accumulation of damaged mitochondria link Gaucher and Parkinson diseases. Autophagy 9, 1633–1635 [DOI] [PubMed] [Google Scholar]
- 23.Heeman B., Van den Haute C., Aelvoet S. A., Valsecchi F., Rodenburg R. J., Reumers V., Debyser Z., Callewaert G., Koopman W. J., Willems P. H., Baekelandt V. (2011) Depletion of PINK1 affects mitochondrial metabolism, calcium homeostasis and energy maintenance. J. Cell Sci. 124, 1115–1125 [DOI] [PubMed] [Google Scholar]
- 24.Fang E. F., Scheibye-Knudsen M., Brace L. E., Kassahun H., SenGupta T., Nilsen H., Mitchell J. R., Croteau D. L., Bohr V. A. (2014) Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell 157, 882–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chistiakov D. A., Sobenin I. A., Revin V. V., Orekhov A. N., Bobryshev Y. V. (2014) Mitochondrial aging and age-related dysfunction of mitochondria. Biomed Res. Int. 2014, 238463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bratic A., Larsson N. G. (2013) The role of mitochondria in aging. J. Clin. Invest. 123, 951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoshino A., Mita Y., Okawa Y., Ariyoshi M., Iwai-Kanai E., Ueyama T., Ikeda K., Ogata T., Matoba S. (2013) Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat. Commun. 4, 2308 [DOI] [PubMed] [Google Scholar]
- 28.Bernhardt D., Müller M., Reichert A. S., Osiewacz H. D. (2015) Simultaneous impairment of mitochondrial fission and fusion reduces mitophagy and shortens replicative lifespan. Sci. Rep. 5, 7885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seluanov A., Vaidya A., Gorbunova V. (2010) Establishing primary adult fibroblast cultures from rodents. J. Vis. Exp. (44) 2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao H., Hwang J. W., Moscat J., Diaz-Meco M. T., Leitges M., Kishore N., Li X., Rahman I. (2010) Protein kinase C zeta mediates cigarette smoke/aldehyde- and lipopolysaccharide-induced lung inflammation and histone modifications. J. Biol. Chem. 285, 5405–5416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Mehdi A. B., Pastukh V. M., Swiger B. M., Reed D. J., Patel M. R., Bardwell G. C., Pastukh V. V., Alexeyev M. F., Gillespie M. N. (2012) Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci. Signal. 5, ra47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X., Winter D., Ashrafi G., Schlehe J., Wong Y. L., Selkoe D., Rice S., Steen J., LaVoie M. J., Schwarz T. L. (2011) PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell 147, 893–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narendra D., Kane L. A., Hauser D. N., Fearnley I. M., Youle R. J. (2010) p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy 6, 1090–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narendra D. P., Wang C., Youle R. J., Walker J. E. (2013) PINK1 rendered temperature sensitive by disease-associated and engineered mutations. Hum. Mol. Genet. 22, 2572–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmad T., Aggarwal K., Pattnaik B., Mukherjee S., Sethi T., Tiwari B. K., Kumar M., Micheal A., Mabalirajan U., Ghosh B., Sinha Roy S., Agrawal A. (2013) Computational classification of mitochondrial shapes reflects stress and redox state. Cell Death Dis. 4, e461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmad T., Mukherjee S., Pattnaik B., Kumar M., Singh S., Kumar M., Rehman R., Tiwari B. K., Jha K. A., Barhanpurkar A. P., Wani M. R., Roy S. S., Mabalirajan U., Ghosh B., Agrawal A. (2014) Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 33, 994–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Debacq-Chainiaux F., Erusalimsky J. D., Campisi J., Toussaint O. (2009) Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat. Protoc. 4, 1798–1806 [DOI] [PubMed] [Google Scholar]
- 38.Wu J., Clingen P. H., Spanswick V. J., Mellinas-Gomez M., Meyer T., Puzanov I., Jodrell D., Hochhauser D., Hartley J. A. (2013) γ-H2AX foci formation as a pharmacodynamic marker of DNA damage produced by DNA cross-linking agents: results from 2 phase I clinical trials of SJG-136 (SG2000). Clin. Cancer Res. 19, 721–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trainor C., Butterworth K. T., McGarry C. K., McMahon S. J., O’Sullivan J. M., Hounsell A. R., Prise K. M. (2012) DNA damage responses following exposure to modulated radiation fields. PLoS One 7, e43326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodríguez-Vargas J. M., Ruiz-Magaña M. J., Ruiz-Ruiz C., Majuelos-Melguizo J., Peralta-Leal A., Rodríguez M. I., Muñoz-Gámez J. A., de Almodóvar M. R., Siles E., Rivas A. L., Jäättela M., Oliver F. J. (2012) ROS-induced DNA damage and PARP-1 are required for optimal induction of starvation-induced autophagy. Cell Res. 22, 1181–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao H., Sundar I. K., Gorbunova V., Rahman I. (2013) P21-PARP-1 pathway is involved in cigarette smoke-induced lung DNA damage and cellular senescence. PLoS One 8, e80007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muñoz-Espín D., Serrano M. (2014) Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol. 15, 482–496 [DOI] [PubMed] [Google Scholar]
- 43.Palacino J. J., Sagi D., Goldberg M. S., Krauss S., Motz C., Wacker M., Klose J., Shen J. (2004) Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J. Biol. Chem. 279, 18614–18622 [DOI] [PubMed] [Google Scholar]
- 44.Haque M. E., Mount M. P., Safarpour F., Abdel-Messih E., Callaghan S., Mazerolle C., Kitada T., Slack R. S., Wallace V., Shen J., Anisman H., Park D. S. (2012) Inactivation of Pink1 gene in vivo sensitizes dopamine-producing neurons to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and can be rescued by autosomal recessive Parkinson disease genes, Parkin or DJ-1. J. Biol. Chem. 287, 23162–23170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vigneron A., Vousden K. H. (2010) p53, ROS and senescence in the control of aging. Aging (Albany, N.Y. Online) 2, 471–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dagouassat M., Gagliolo J. M., Chrusciel S., Bourin M. C., Duprez C., Caramelle P., Boyer L., Hue S., Stern J. B., Validire P., Longrois D., Norel X., Dubois-Randé J. L., Le Gouvello S., Adnot S., Boczkowski J. (2013) The cyclooxygenase-2-prostaglandin E2 pathway maintains senescence of chronic obstructive pulmonary disease fibroblasts. Am. J. Respir. Crit. Care Med. 187, 703–714 [DOI] [PubMed] [Google Scholar]
- 47.Ballweg K., Mutze K., Königshoff M., Eickelberg O., Meiners S. (2014) Cigarette smoke extract affects mitochondrial function in alveolar epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 307, L895–L907 [DOI] [PubMed] [Google Scholar]
- 48.Hara H., Araya J., Ito S., Kobayashi K., Takasaka N., Yoshii Y., Wakui H., Kojima J., Shimizu K., Numata T., Kawaishi M., Kamiya N., Odaka M., Morikawa T., Kaneko Y., Nakayama K., Kuwano K. (2013) Mitochondrial fragmentation in cigarette smoke-induced bronchial epithelial cell senescence. Am. J. Physiol. Lung Cell. Mol. Physiol. 305, L737–L746 [DOI] [PubMed] [Google Scholar]
- 49.Gomes L. C., Di Benedetto G., Scorrano L. (2011) During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 13, 589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rana A., Rera M., Walker D. W. (2013) Parkin overexpression during aging reduces proteotoxicity, alters mitochondrial dynamics, and extends lifespan. Proc. Natl. Acad. Sci. USA 110, 8638–8643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saini N., Georgiev O., Schaffner W. (2011) The parkin mutant phenotype in the fly is largely rescued by metal-responsive transcription factor (MTF-1). Mol. Cell. Biol. 31, 2151–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu J., Niu J., Li X., Wang X., Guo Z., Zhang F. (2014) TGF-β1 induces senescence of bone marrow mesenchymal stem cells via increase of mitochondrial ROS production. BMC Dev. Biol. 14, 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tao R., Vassilopoulos A., Parisiadou L., Yan Y., Gius D. (2014) Regulation of MnSOD enzymatic activity by Sirt3 connects the mitochondrial acetylome signaling networks to aging and carcinogenesis. Antioxid. Redox Signal. 20, 1646–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis C. H., Marsh-Armstrong N. (2014) Discovery and implications of transcellular mitophagy. Autophagy 10, 2383–2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mizumura K., Cloonan S. M., Nakahira K., Bhashyam A. R., Cervo M., Kitada T., Glass K., Owen C. A., Mahmood A., Washko G. R., Hashimoto S., Ryter S. W., Choi A. M. (2014) Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J. Clin. Invest. 124, 3987–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bueno M., Lai Y. C., Romero Y., Brands J., St Croix C. M., Kamga C., Corey C., Herazo-Maya J. D., Sembrat J., Lee J. S., Duncan S. R., Rojas M., Shiva S., Chu C. T., Mora A. L. (2015) PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J. Clin. Invest. 125, 521–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mai S., Klinkenberg M., Auburger G., Bereiter-Hahn J., Jendrach M. (2010) Decreased expression of Drp1 and Fis1 mediates mitochondrial elongation in senescent cells and enhances resistance to oxidative stress through PINK1. J. Cell Sci. 123, 917–926 [DOI] [PubMed] [Google Scholar]
- 58.Morse D., Rosas I. O. (2014) Tobacco smoke-induced lung fibrosis and emphysema. Annu. Rev. Physiol. 76, 493–513 [DOI] [PubMed] [Google Scholar]
- 59.Samara K. D., Margaritopoulos G., Wells A. U., Siafakas N. M., Antoniou K. M. (2011) Smoking and pulmonary fibrosis: novel insights. Pulm. Med. 2011, 461439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hackett T. L., Shaheen F., Zhou S., Wright J. L., Churg A. (2014) Fibroblast signal transducer and activator of transcription 4 drives cigarette smoke-induced airway fibrosis. Am. J. Respir. Cell Mol. Biol. 51, 830–839 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.