Abstract
Currently, there are no generally applicable noninvasive methods for defining the relationship between atherosclerotic vascular damage and risk of focal thrombosis. Herein, we demonstrate methods to delineate the progression and regression of vascular damage in response to an atherogenic diet by quantifying the in vivo accumulation of semipermeable 200–300 nm perfluorocarbon core nanoparticles (PFC-NP) in ApoE null mouse plaques with [19F] magnetic resonance spectroscopy (MRS). Permeability to PFC-NP remained minimal until 12 weeks on diet, then increased rapidly following 12 weeks, but regressed to baseline within 8 weeks after diet normalization. Markedly accelerated clotting (53.3% decrease in clotting time) was observed in carotid artery preparations of fat-fed mice subjected to photochemical injury as defined by the time to flow cessation. For all mice on and off diet, an inverse linear relationship was observed between the permeability to PFC-NP and accelerated thrombosis (P = 0.02). Translational feasibility for quantifying plaque permeability and vascular damage in vivo was demonstrated with clinical 3 T MRI of PFC-NP accumulating in plaques of atherosclerotic rabbits. These observations suggest that excessive permeability to PFC-NP may indicate prothrombotic risk in damaged atherosclerotic vasculature, which resolves within weeks after dietary therapy.—Palekar, R. U., Jallouk, A. P., Goette, M. J., Chen, J., Myerson, J. W., Allen, J. S., Akk, A., Yang, L., Tu, Y., Miller, M. J., Pham, C. T. N., Wickline, S. A., Pan, H. Quantifying progression and regression of thrombotic risk in experimental atherosclerosis.
Keywords: nanoparticles, endothelium, thrombosis, MRI
Atherosclerosis is the leading cause of death in the developed world, manifesting high morbidity and mortality as a consequence of recurrent acute vascular events that are essentially unpredictable in individuals and frequent despite maximal medical therapy (1, 2). Recent focus on the pathophysiology of atherosclerosis has shifted to the panoply of inflammatory cell types and necrotic debris that engage a host of prothrombotic signaling events, resulting in acute focal clotting and vascular obstruction, unstable angina, and infarction (3, 4). In territories prone to plaque development, early lesion formation initiates with the development of a proinflammatory endothelium, characterized by weakened tight junctions (<20 nm) that permit the passage of small molecule dyes (e.g., Evans blue) (5, 6) and albumin (7–9). Noninvasive delineation of these very early pathophysiological features that emerge well before clinical events arise has been available for years with application of numerous imaging techniques that appear to presage an increased incidence of events, at least in study populations that harbor traditional coronary risk factors (10, 11).
We recently reported an MRI and MRS approach for delineating the severity of vascular endothelial damage in atherosclerotic vessels by measuring the passive permeation of PFC-NP into plaques of fat-fed rabbits after only 60–120 minutes of circulation in vivo (12). Endothelial apoptosis, erosions, and fibrin deposition were observed in these plaques after 6 months on a high cholesterol diet. Ex vivo fluorine ([19F]) MRI and MRS depicted the intimal localization and quantity of PFC-NP in rabbit atherosclerotic lesions that appeared after 6 months on diet and also in diseased human carotid endarterectomy samples that were incubated ex vivo with the PFC-NP. However, the related clinically relevant question as to whether increased vascular permeability to PFC-NP in damaged vessels is correlated with focal prothrombotic risk in genetically prone models of vascular disease has not been examined.
Accordingly, we sought to answer the following questions: 1) whether prolonged feeding of Western diet induces a state of increased vascular permeability in ApoE null mice that can be detected and quantified with the use of MRS imaging methods; 2) whether the permeability of the endothelium to PFC-NP resolves following cessation of a Western diet and if such phenomena can be tracked and quantified with PFC-NP imaging methods, and 3) whether vessel thrombotic risk is related directly to diet-induced vascular permeability and how quickly accelerated thrombosis might resolve following Western diet cessation.
MATERIALS AND METHODS
NP formulation
Fluorescent PFC-NP were formulated according to previously established emulsification techniques (12). Briefly, the PFC-NP consisted of a 20% (vol/vol) perfluoro-15-crown-5-ether (PFCE) core, 2% (wt/vol) surfactant, 1.7% (wt/vol) glycerin, and water. The surfactant consisted of 98.8 mol% egg phosphatidylcholine (Lipoid, Newark, NJ, USA), 1 mol% 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (Avanti Polar Lipids, Alabaster, AL, USA) and 0.2 mol% 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (Avanti Polar Lipids). The emulsion was modified to replace the crown ether core with perfluorooctylbromide (PFOB) for use as a [19F] MRS reference standard for quantification of nanoparticle (NP) concentration in plaques. For in vivo [19F] MRI studies in rabbits, the emulsion was modified to increase the perfluorocarbon and surfactant content to include 4% (wt/vol) surfactant and 40% (vol/vol) PFCE.
Animal experimental model
Mouse feeding regimen
To produce aortic plaques, groups of male ApoE-null mice 4- to 6-week-old were fed either normal chow or Western diet (TD-88137, Harlan Laboratories, Madison, WI, USA) for 2, 3, 4, 5, and 6 months. Two other sets of mice were fed with Western diet for 4 months and then switched to normal chow for either 1 or 2 months, thus serving as experimental off-diet time points age-matched with mice continued on Western diet for 5 or 6 months. At each selected time point, a 1 ml/kg bolus dose of NPs was administered via tail vein injection and allowed to circulate for 2 hours (12) to allow for saturation of NP accumulation in plaques.
Carotid artery thrombosis procedure
Following 2 hours of in vivo NP circulation, mice were anesthetized with a cocktail of ketamine and xylazine (87 mg/kg and 37 mg/kg, respectively) and subjected to photochemical injury of the carotid artery (13–15) to determine time to vessel occlusion. The right common carotid artery was exposed through a midline cervical incision and blood flow rate was monitored using an ultrasonic Doppler probe (Transonic Systems Inc., Ithaca, NY, USA). The intended injury site was illuminated with a 1.5 mW 540 nm HeNe laser, followed by injection of 50 mg/kg Rose Bengal (Sigma-Aldrich, St. Louis, MO, USA) in saline to initiation of thrombus growth. The experiment was concluded upon achieving a >85% decrease in carotid blood flow rate maintained for >5 minutes. Time to occlusion was recorded and used to evaluate coagulability. Mice were then sacrificed and the aortas were cleaned and removed for [19F] spectroscopic evaluation of NP accumulation.
Rabbit feeding regimen
Male New Zealand White rabbits were maintained on a 0.25% cholesterol feed (#9433, TestDiet, St. Louis, MO, USA) for 9 months. For control images without plaque development, young rabbits were fed normal chow. Both control and atherosclerotic rabbits were anesthetized and given a 1 ml/kg intravenous bolus of NPs 2–3 hours prior to [19F] MRI.
[19F] MRS
[19F] MRS was utilized to quantify NP accumulation in aortic plaques ex vivo. [19F] MRS was performed on an 11.7 T Varian magnetic resonance scanner (Varian Medical Systems, Palo Alto, CA, USA) with a custom-built single-turn solenoid radiofrequency coil, with the following parameters: repetition time (TR) = 2.5 seconds, 1024 signal averages, with a scan time of ∼42 minutes. A fluorine reference standard of 0.1% PFOB emulsion was included with each aorta sample. To quantify plaque accumulation of crown ether NPs, crown ether signal was compared with the distinct signal of the known PFOB standard. The amount of crown ether NPs was normalized to the weight of the aorta sample.
Aortic perfusion of PFC-NP and [19F] MRI ex vivo
Aortas from rabbits fed a cholesterol diet for 8 months were excised and cannulated with stub adapters for attachment to a custom-built perfusion system. The perfusion system consisted of a variable flow rate perfusion pump (Cole Parmer, Barrington, IL, USA) with Masterflex tubing connected to stub adapters at either end of the vessel. A 50 ml conical tube served as a reservoir for the perfusate, containing a 1:20 dilution of PFC-NP in saline. The diluted PFC-NP mixture was perfused through the arteries at a flow rate of 30 ml/min for 4 hours. Following perfusion, the isolated aortas were rinsed and fixed in 10% formalin for 24–48 hours.
Ex vivo [19F] spectroscopy of isolated aorta was performed on an 11.7 T Varian scanner. Prior to ex vivo [19F] imaging, the vessel lumen was filled with 2% agarose gel and stored in saline. Upon MRI, aortas were placed in a 1 cm tube filled with saline. A capillary tube containing diluted (1:200) PFCE NPs were attached to the tube wall to serve as the external signal standard. All images were acquired using a solenoid radiofrequency coil and a standard fast-spin echo sequence. The coil was first tuned to [1H] frequency to acquire a set of multislice [1H] images. Imaging parameters were: field of view, 2 × 2 cm2; image matrix, 128 × 128; slice thickness, 2 mm; number of slices, 11; TR, 2 seconds; echo time (TE), 20 ms, echo train length (ETL), 8; number of averages,4; imaging time, 2 minutes. The coil was then tuned for [19F] MRI. A set of [19F] images was acquired at the same location as [1H] images. Imaging parameters were: field of view, 2 × 2 cm2; image matrix, 32 × 32; slice thickness, 2 mm; TR, 1.2 s; TE, 20 ms, etl, 8; number of averages, 4096; imaging time, 5 hours, 28 minutes. The acquired [19F] images (matrix size = 32 × 32) were interpolated to 128 × 128 and overlaid on corresponding [1H] images to localize [19F] signal of PFC-NP.
In vivo [19F] MRI
To image the aortic wall for detection of NPs permeating into the plaque intima, we employed a 3 T clinical whole-body scanner (Achieva, Philips Healthcare, Best, The Netherlands), outfitted with a dual [19F]/[1H] spectrometer system (16). A dual-resonant [19F]/[1H] surface RF coil was used (15 × 15 cm), which can either transmit or receive at both resonance frequencies simultaneously (17). Imaging was performed ∼2 hours postinjection of 1.0 ml/kg PFC-NP with PFCE (C10F20O5) core as previously described. To avoid signal contamination from inhaled fluorinated anesthesia, a xylazine (10 mg/kg)/ketamine (85 mg/kg) intramuscular injection was used for anesthesia induction, which was maintained with a ketamine intravenous infusion (18 mg/kg/h). A 2-dimensional simultaneous [19F]/[1H] gradient echo (fast field echo) sequence was used with the following parameters: field of view (FOV)= 128 × 128 mm, matrix = 96 × 96, slice thickness = 20 mm, voxel size = 1.33 × 1.33 × 20 mm, α = 25°, excitation bandwidth = 5 kHz centered on single PFCE peak, pixel bandwidth = 500 Hz, TR/TE = 14/1.72 ms, number of signal averages (NSA) = 1000, and a scanning time of 33 minutes. Saturation bands proximal and distal to the imaging slice were applied to eliminate [19F] signal from the blood pool. The imaging slice was centered on the abdominal aorta, located 2–3 cm distal to the renal artery via an angiogram consisting of a multi-2-dimensional time-of-flight gradient echo sequence with the following parameters: FOV = 100 × 78 mm, matrix = 112 × 112, slice thickness = 2 mm, α = 60°, TR/TE = 13.54/4.06 ms, 4 NSA, and a scanning time of 3 minutes. After anatomic colocalization of [19F] signal was confirmed with the simultaneously acquired [1H] image, a high-resolution gradient echo [1H] image was used to display the overlaid [19F] signal with the following parameters: FOV = 128 × 128 mm, matrix = 256 × 256, slice thickness = 4 mm, voxel size = 0.5 × 0.5 × 4 mm, α = 35°, TR/TE = 25.16/7.02 ms, 23 NSA, and a scanning time of 2.5 minutes.
Scanning electron microscopy
Freshly harvested aortas from ApoE−/− mice fed a high-fat diet for 6 months were speed vacuum dried overnight to allow visualization of superficial cholesterol crystals (18). Dried tissues were then mounted for imaging. Scanning electron microscope images were acquired with Hitachi S-2600H (Hitachi, Schaumburg, IL, USA) and Nova Nano 2300 (FEI, Hillsboro, OR, USA).
Histology
For immunofluorescent evaluation of CD31 and thrombin deposition, mouse aortas were harvested and embedded in optical cutting temperature (OCT) medium. Aortas were sectioned and stained with either an anti-CD31 antibody (ab28364, Abcam Incorporated, Cambridge, MA, USA) or an anti-thrombin antibody (ab92621, Abcam Incorporated). For Oil Red O staining of rabbit aortas, tissue was embedded in OCT medium and sectioned. Slides were fixed in formalin, and then stained in freshly prepared Oil Red O solution followed by rinsing with 60% isopropanol. Sections were stained with hematoxylin, rinsed, and then mounted for microscopy.
Two-photon imaging
Aortas were removed from both the normal chow and high-cholesterol fed rabbits and incubated en face for 15 minutes with 500 kD FITC-dextran to allow for ex vivo penetration of the FITC-dextran into plaques. The imaging of the aorta samples was performed on a custom-built video rate 2-photon microscope (19) equipped with a Chameleon Vision II Ti:sapphire laser (Coherent, Santa Clara, CA, USA) in the Washington University in St. Louis School of Medicine In Vivo Imaging core. Fluorescence emission was passed through 480 nm and 560 nm dichroic mirrors placed in series and detected as red (>560 nm), green (480–560 nm), and blue (<480 nm) channels by 3 head-on multialkali photomultiplier tubes.
Fluorescence-activated cell sorting analysis of PFC-NP uptake by peripheral blood leukocytes and splenocytes
Mice were injected intravenously with 200 μl of NPs and killed 30 minutes later. Leukocytes were isolated from mouse spleens and peripheral blood with Histopaque-1119 according to manufacturer's protocol (Sigma-Aldrich). Antibodies against the following molecules coupled to the indicated fluorochromes were used from BD Pharmingen (San Jose, CA, USA), eBioscience (San Diego, CA, USA) or BioLegend (San Diego, CA, USA): FITC anti-Ly-6C (HK1.4; BioLegend), PerCP anti-Ly6G (1A8; BD Pharmingen), APC anti-F4/80 (BM8; eBioscience), APC anti-TCRβ (H57-597; eBioscience), FITC anti-CD19 (1D3; BD Pharmingen). In general, 106 cells were blocked with the anti-FcR mAb 2.4G2, stained with the indicated Abs for 20 minutes at 4°C and then washed and resuspended for fluorescence-activated cell sorting (FACS) analysis. Flow cytometry was performed on the BD FACSCalibur. Data analysis was performed using BD CellQuest Pro software.
Western blot analysis of complement activation
Western blot analysis of complement activation was performed as described previously (20). C57BL/6 mice were injected intravenously with 10 μl/g of PBS, plain PFC-NP, and 2 different species of gadolinium (Gd) loaded perfluorocarbon (Gd-DTPA and Gd-DOTAP). Gd-DOTAP PFC-NP were utilized as a positive control for complement activation. Thirty minutes following administration of test doses, the mice were sacrificed and blood was collected via the inferior vena cava in EDTA-loaded tubes. Blood samples were centrifuged for 5 minutes at 4°C to obtain plasma samples. For each sample, 15 μl of plasma was diluted 1:100 in SDS running buffer and fractionated by SDS-PAGE under reducing conditions to observe complement activation in plasma. For probing of complement activation on the NP surface, blood samples were centrifuged at 960 g for 15 minutes, and the resulting pellet was rinsed 4 times in EDTA buffer, resuspended in SDS running buffer, and fractionated under reducing conditions with SDS-PAGE. In both plasma and NP surface analysis, complement activation was probed using anti-C3 (1:10,000 dilution, Valeant Pharmaceuticals International, Aliso Viejo, CA, USA) and anti-Factor H (1:1000 dilution, CompTech, Tyler, TX, USA) primary antibodies. The secondary antibody used was a horseradish peroxidase-conjugated donkey anti-goat IgG (1:10,000 dilution, Jackson ImmunoResearch Laboratories, West Grove, PA, USA). The resulting bands were visualized with a SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL, USA).
Statistics
All statistical tests were performed on R, version 3.0.1. Carotid artery occlusion time data was analyzed using Mann-Whitney-Wilcoxon rank sum test. NP accumulation trend line analysis was accomplished using a Student’s t test. The Pearson’s product-moment correlation test was utilized to determine the relationship between NP accumulation and carotid occlusion time. An ANCOVA was performed for analysis of cholesterol, NP accumulation, and carotid occlusion time data. For all statistical tests, P < 0.05 denotes statistical significance. Error bars denote sem.
Study approval
All procedures were performed with approval from the Washington University Animal Studies Committee.
RESULTS
Functional evaluation of vascular permeability after prolonged Western diet
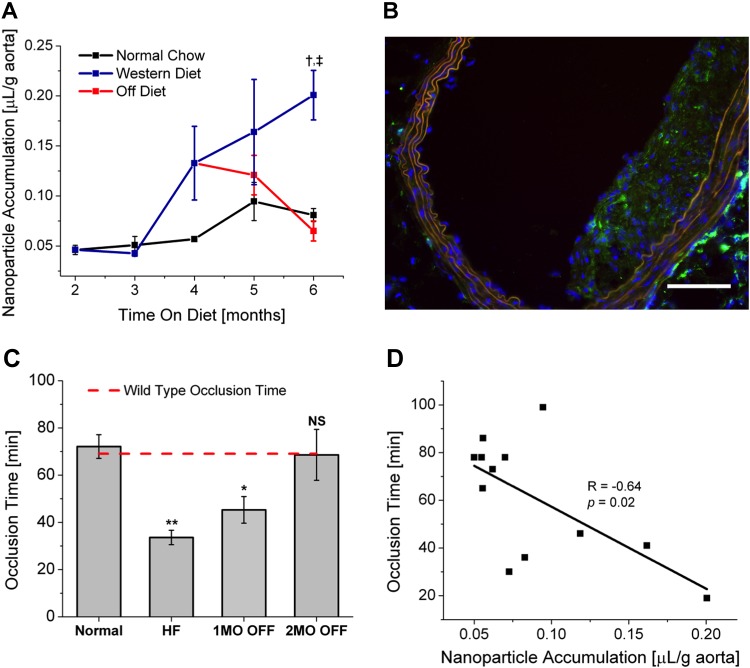
To elucidate vascular permeability in vivo in ApoE null mice subjected to selected durations on a Western diet, PFC-NP were injected intravenously and allowed to circulate for 2 hours prior to excising whole aortas for [19F]-MRS quantification of PFC-NP. A progressive monthly increase in PFC-NP accumulation was observed in the atherosclerotic aortas of mice on Western diet over and above that of mice on normal chow diets, the severity of which reflected the duration of Western diet feeding (Fig. 1A). Prior to 3 months of feeding, little accumulation of PFC-NP was observed in either Western diet fed or normal chow groups. However, beyond 3 months, increased accumulation of PFC-NP was noted in aortic plaques of fat-fed mice as compared with the normal chow group, with plaque concentrations of PFC-NP reaching 0.201 ± 0.043 μl/g aorta (n = 3) in mice fed a Western diet for 6 months compared with the age-matched group of ApoE null mice fed normal chow (0.081 ± 0.011 μl/g aorta, n = 3).
Figure 1.
A) [19F] MRS demonstrating time dependence of NP accumulation in ApoE null mouse aortas with or without a Western diet. Continuous Western diet feeding results in a significant increase in NP accumulation over age-matched normal chow controls. Return to a normal chow diet restores control levels of NP accumulation. Off-diet group at 6 months was not significantly different from age-matched normal chow controls. Trend analysis on Western diet and off-diet groups following 4 months on diet demonstrates significant difference in progression of NP accumulation (P = 0.015). †P = 0.003; ‡P = 0.0009 vs. age-matched Western diet group. B) Immunofluorescent staining confirms abundant intraplaque thrombin (green) in mouse aortic plaques. Scale bar, 100 μm. C) Return to normal chow progressively increases carotid occlusion times to control values for both normal chow ApoE null mice (leftmost bar) and wild-type mice (dashed line: based on prior published data (14). *P = 0.0056, **P = 0.004 vs. control. HF, high-fat diet; NS, not significant. D) Correlation plot of aortic NP accumulation and carotid occlusion time (R = −0.64, P = 0.02) demonstrating relationship between vascular permeability and thrombotic potential.
Tracking regression of vascular permeability to PFC-NP
To delineate the resolution of endothelial permeability after dietary cholesterol reduction, 2 additional groups of ApoE null mice were fed a Western diet for 4 months and then switched to normal chow for either 1 or 2 months before injecting PFC-NP for spectroscopic evaluation of vascular permeability. Serum cholesterol levels confirmed the effect of dietary replacement, as cholesterol levels rose to 965.45 ± 201.05 mg/dl by 5 months in mice on Western diet compared with 403 ± 39.69 mg/dl and 461.77 ± 45.15 mg/dl for mice on Western diet for 4 months and then off the Western diet for 1 or 2 months, respectively (Supplemental Fig. 1). During this period, no perceptible change in gross aortic plaque coverage was observed between experimental off-diet groups and age-matched Western diet groups (data not shown). Regression of vascular permeability was confirmed by a dramatic decrease in NP accumulation in mouse aortas by 2 months after dietary cholesterol lowering (0.065 ± 0.019 μl/g aorta, n = 4, P = 0.0009 vs. age-matched Western diet group), returning to age-matched ApoE null normal chow-fed control levels (difference not statistically significant) (Fig. 1A). Trend assessment from 4–6 months was performed by regression analysis and demonstrated a significant difference in the slope of the linear fit between the continued progression of vascular permeability in the Western diet-fed mice versus the resolution of vascular permeability in those mice removed from the diet (slope: 0.033 ± 0.019 vs. −0.026 ± 0.009 for the Western diet group and the mice removed from Western diet, respectively; P = 0.015).
Relationship between vascular permeability and vessel thrombotic potential
As increased endothelial permeability and loss of endothelial cells has been linked to future thrombotic events, we anticipated that potential differences in vessel thrombotic potential might be related to the severity of permeability. Our hypothesis was further supported by the presence of large deposits of intraplaque thrombin (Fig. 1B) that may contribute to the generation of local inflammatory and procoagulant functions. Therefore, a standard model of photochemical injury to the carotid artery was used to quantify the propensity to focal thrombosis for the different diet schedules according to the time required to attain complete vessel occlusion, which is inversely related to local procoagulant activity. Vessel injury in ApoE null mice on normal chow for 5 months resulted in an occlusion time of 72.14 ± 5.02 minutes (n = 10), which was equivalent to that previously observed for wild-type C57BL/6 (14): 69.06 ± 5.66 minutes, n = 6 (difference not statistically significant) (Fig. 1C, red dashed line). ApoE null mice fed with Western diet for 5 months exhibited dramatically shortened occlusion times of 33.62 ± 3.04 minutes (n = 6) as compared with the mice on normal chow (n = 10) for 5 months (P = 0.004). In mice fed a Western diet for 4 months followed by normal chow for either 1 or 2 subsequent months, occlusion times progressively lengthened back to control levels: 45.33 ± 5.63 minutes, n = 7 (P = 0.005 vs. control) and 68.6 ± 10.79 minutes (difference not statistically significant vs. control), respectively (Fig. 1C). By 2 months after cessation of Western diet, the occlusion time (68.6 ± 10.79 minutes, n = 5) was not significantly different as compared with wild-type C57BL/6 mice, indicating reversible diet-dependent vessel procoagulant activity. Furthermore, occlusion time measurements on ApoE−/− mice fed a Western diet for 1 month (65.73 ± 23.65, n = 3) were not significantly different compared with wild-type C57BL/6 or normal chow ApoE controls. Importantly, an inverse correlation (Fig. 1D) was observed between NP accumulation and occlusion time (P = 0.02), indicating that thrombotic potential tracks with increased vascular permeability in this model. Additionally, ANCOVA analysis demonstrated no significant effect of serum cholesterol levels on carotid occlusion time (P = 0.3849) and no significant interaction effect between serum cholesterol levels and plaque NP accumulation (P = 0.4223), ruling out an independent effect of blood cholesterol itself on occlusion times.
Mechanisms of intimal permeation of NPs
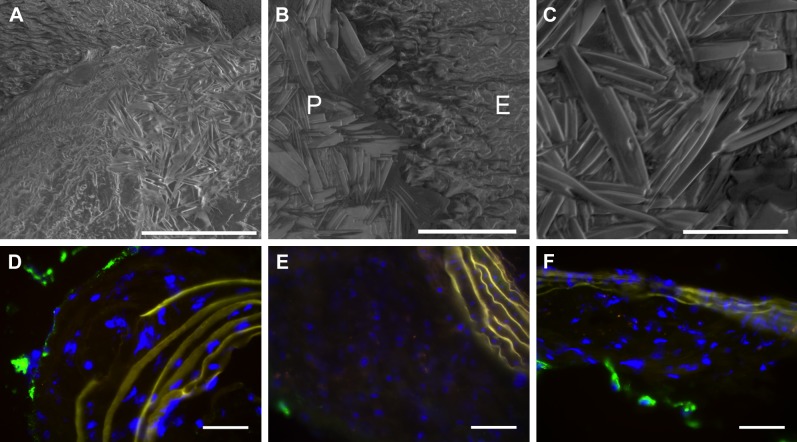
To investigate potential mechanisms of NP penetration and retention, mouse aortic tissue samples were imaged with scanning electron microscopy to examine the morphology of intimal plaques. In accord with prior reports in rabbit (12) and human (18) atherosclerosis specimens that manifest superficial deposits of protruding cholesterol crystals when properly prepared for scanning electron microscopy, we also observed analogous intimal and superficial cholesterol crystals on samples of mouse aortas presenting with plaques (Fig. 2A–C). Immunofluorescent staining of CD31 (Fig. 2D–F) revealed barely detectable intraplaque signal, consistent with prior reports of limited angiogenesis at these earlier time points in atherosclerotic mice, suggesting that passive diffusion of PFC-NP into the intima did not occur through neovascular elements (21).
Figure 2.
A) Scanning electron microscopy of cholesterol crystals densely deposited on surface of aortic plaque. Scale bar, 100 μm. (B) Scanning electron microscopy of cholesterol crystals on denuded plaque (P) but not on adjacent regular endothelium (E). Scale bar, 40 μm. C) Higher magnification scanning electron microscopy depicts morphology of cholesterol crystals. Scale bar, 20 μm. D–F) Immunofluorescent staining for CD31 (green) demonstrates little to no intraplaque angiogenesis in ApoE null fed a Western diet for (D) 4 months, (E) 5 months, and (F) 6 months. Scale bar, 50 μm.
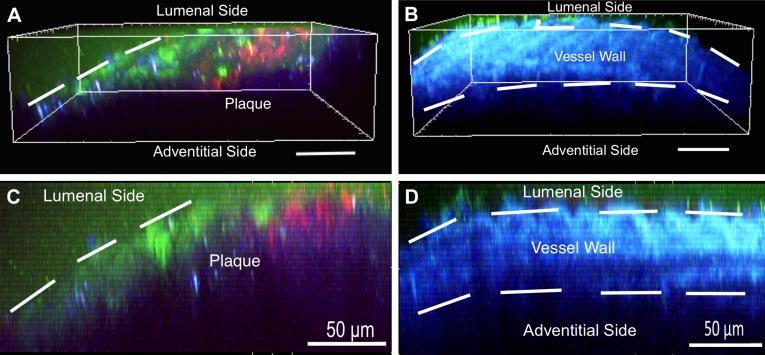
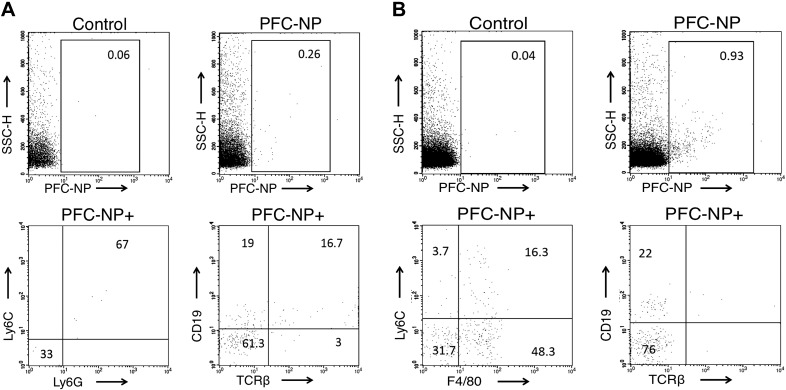
To better define the potential route of intimal penetration of these PFC-NP into plaques, we resorted to the previously validated rabbit model (12) for 2-photon assessment of permeability. Rhodamine-labeled PFC-NP were administered to rabbits fed either normal or high cholesterol chow. Aortic segments were removed from the rabbits following 2 hours of in vivo NP circulation and incubated with FITC-dextran ex vivo, demonstrating prominent accumulation of both FITC-dextran and PFC-NP beyond the lumenal surface of the plaque (Fig. 3), which is consistent with prior conclusions that active transport and blood flow are not required to achieve intimal NP penetration. Furthermore, excised atherosclerotic rabbit aortas were perfused ex vivo with PFC-NP on a custom built perfusion system for 4 hours to elucidate the contribution of passive lumenal permeation of NP without the need for active cellular transport or trafficking through vasa vasorum. Fluorine imaging of NP perfused aortas (Supplemental Fig. 2) demonstrates ample plaque-associated NPs due to passive permeation. To further rule out alternative cellular transport mechanisms, FACS analysis of isolated peripheral blood leukocytes (Fig. 4A) and splenocytes (Fig. 4B) collected 30 minutes after intravenous injection of PFC-NP demonstrated minimal active uptake of the circulating PFC-NP by cell types that might traffic to plaques: 0.26% of peripheral blood leukocytes and 0.93% of splenocytes. Western blot analysis revealed no complement activation in response to the administration of NPs, militating against immune cell trafficking of complement activated PFC-NP (Supplemental Fig. 3).
Figure 3.
A) Penetration of FITC-dextran (green) and PFC-NP (red) into a plaque is revealed with 3-dimensional 2-photon microscopy imaging of en face atherosclerotic rabbit aortic tissue. B) Two-photon microscopy of en face normal rabbit aortic tissue. Scale bar, 50 μm. Tissue autofluorescence is shown in blue. C) Side view 2-photon microscopy image of atherosclerotic rabbit tissue demonstrating penetration of FITC-dextran (green) and PFC-NP (red) into plaques. D) Side view 2-photon microscopy image of normal rabbit tissue demonstrating lack of FITC-dextran (green) and PFC-NP (red) penetration through intact tissue lumenal barriers. Scale bar, 50 μm.
Figure 4.
A) FACS analysis reveals minimal cellular active uptake of rhodamine-labeled PFC-NP by 0.26% of circulating peripheral blood leukocytes. The RhodPE+ population comprises Ly6C+Ly6G+ cells (myeloid cells) and a small number of CD19+ cells (B cells). B) FACS analysis reveals minimal active uptake of PFC-NP by 0.93% of splenocytes. The RhodPE+ population comprises F40/80+ and Ly6C+F4/80+ cells (monocytes/macrophages) and a smaller percentage of CD19+ cells (B cells). T cells (TCRb+) do not take up PFC-NP.
Imaging NP plaque permeation in vivo
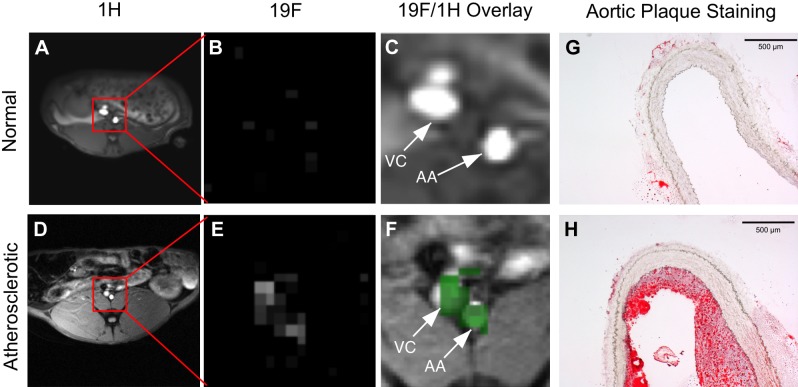
In a pilot study, [19F] MRI was performed on rabbits with or without diet-induced atherosclerotic plaques (Fig. 5). Figure 5A, D shows proton images of abdominal cross sections showing the position of the aorta in rabbits fed normal chow and Western diet, respectively; Fig. 5B, E shows the fluorine-only signatures after PFC-NP circulation in vivo for 180 minutes. Note the excellent suppression of lumenal blood [19F] signal in the normal chow rabbits due to saturation band placement (Supplemental Fig. 4), indicating that no signal arises from the circulating PFC-NP. Figure 5C, F are [19F]/[1H] overlays showing the aortic and adjacent vena cava wall [19F] signals (green) emanating from PFC-NP permeating into arterial plaques, and interestingly into adjacent inflamed venous structures that can accumulate PFC-NP when subjected to the same hyperlipidemic drive (22, 23). Figure 5C demonstrates that in a healthy rabbit, no measurable NP retention occurs in the aorta or vena cava. Corresponding Oil Red O staining of the imaged area (Fig. 5G–H) confirmed the presence of aortic plaques, or lack thereof.
Figure 5.
Cross-sectional [1H] images at 3 T of (A) normal chow rabbit and (D) cholesterol fed rabbit showing location of abdominal aorta (red box). [19F] gradient echo images of PFCE NP [19F] signal in the region of interest for (B) normal chow-fed rabbit and (E) cholesterol-fed rabbit. Saturation bands proximal and distal to imaging slice eliminate [19F] signal from blood (see Supplemental Fig. 4). [19F] signal (green) overlaid on [1H] image showing [19F] signal colocalization for the region of interest in a (C) normal chow-fed rabbit and (F) cholesterol-fed rabbit, demonstrating deposition of PFC-NP only in inflamed abdominal aorta (AA) and vena cava (VC). Representative Oil Red O stains of the imaged area showing plaque elements in the (G) normal chow rabbit aorta and the (H) cholesterol-fed rabbit aorta. Scale bars, 500 μm.
DISCUSSION
Herein we describe for the first time a quantitative in vitro and in vivo MRI/MRS approach for delineating the progression and/or regression of atherosclerotic vascular damage that bears a direct relationship to focal thrombotic risk. The use of [19F] MRS/MRI enables quantification of plaque permeability as a consequence of vascular damage induced by Western diet according to measured plaque PFC-NP content (Fig. 1A). It is notable here that we employed the entire aorta to ensure an objective definition of vascular permeability that avoided any potentially subjective bias of segmental selection. We observed a marked acceleration of endothelial permeability after 12 weeks on a Western diet in ApoE null mice, greatly exceeding that of mice maintained on normal chow. Rapid diminution of permeability to PFC-NP to baseline levels was achieved after only 2 months of dietary management, where ANCOVA analysis demonstrated no significant interaction effect (P = 0.4223) of serum cholesterol levels on endothelial permeability, suggesting that some feature of, or response to, the dietary regimen other than just serum cholesterol level may have contributed to the observed vascular damage.
Furthermore, we observed a concomitant rapid resolution of the vascular prothrombotic state in ApoE null mice after only 2 months of dietary management. A standard experimental method was used to quantify vessel thrombotic potential by inducing occlusive thrombosis in the carotid artery with Rose Bengal dye and laser injury that operates by generating caustic superoxide anions, which are known to contribute to the pathogenesis and progression of atherosclerosis (24). We observed that the functional MRI/MRS readouts for vascular permeability were correlated with the severity of prothrombotic risk (Fig. 1D) as measured by time to total occlusion of the carotid artery after photochemical injury. Furthermore, we found that these correlated measures of vascular permeability and accelerated thrombosis resolve concomitantly and rapidly to baseline values after dietary normalization. One potential caveat to note here is that the occlusion times were measured in the carotid artery territory, whereas the permeability metrics were acquired from the entire unselected aorta because experimentally it was not possible to acquire both data sets from the same vascular region in a given animal. However, because the disease process is clearly diffuse and progressive, even though some local differences in plaque severity may pertain, the correlative assessment should be informative as to the overall state of the disease process, especially in light of the rapid resolution of both metrics after cessation of the Western diet.
The use of ApoE null mice for the quantification of vascular barrier damage with PFC-NP was advantageous because immunofluorescent staining of aortic plaques for CD31 in our mouse model revealed little intraplaque neovasculature with up to 6 months of continuous Western diet (Fig. 2D–F). Prior reports of angiogenesis in mouse plaques as measured by CD31 staining indicated the presence of neovasculature only after 9 months of cholesterol feeding (21). Lack of plaque neovasculature at our measured time points, in concert with evidence of cholesterol crystals perforating the intima of diseased ApoE null mouse aortas (Fig. 2A–C), suggests that lumenal entry of PFC-NP through highly permeable endothelial barriers may be the prevailing route of NP penetration and retention, rather than through vasa vasorum or neoangiogenic routes. However, it is important to note that vasa vasorum/neoangiogenic delivery of NPs may be possible in larger subjects such as rabbits and humans. Our current data utilizing ex vivo NP perfusion of atherosclerotic rabbit aortas (Supplemental Fig. 3) coupled with prior data utilizing human endarterectomy specimens (12), suggests that lumenal entry of NPs remains a significant contributor to NP signal. Furthermore, the likelihood of predominantly lumenal entry and retention of PFC-NP in plaques accords with our prior work in atherosclerotic rabbits that showed that PFC-NP do not gain access to the intima of atherosclerotic vessels through neoangiogenic routes in early atherosclerosis (25), but may enter through eroded endothelium in later stages of plaque development (12). Additionally, investigations of alternative routes of plaque accumulation of PFC-NP through assays for complement activation (Supplemental Fig. 4) or through FACS analysis of NP uptake in circulating cells (Fig. 4) suggests that active trafficking of peripheral blood leukocytes and/or splenocytes harboring PFC-NP is unlikely in this short experimental time window (<2 hours) (26, 27). Thus, the detected [19F] signal is more probably a consequence of passive accumulation of PFC-NP entering through a highly permeable or disrupted endothelial barrier rather than influx of PFC-NP bearing cells.
With respect to potential interpretations of these data in the light of classically described endothelial dysfunction, we note that implications for endothelial dysfunction in the ApoE null model are not especially well defined in a temporal and quantitative sense. Endothelial dysfunction typically is depicted in terms of decreased endothelium-dependent vasodilation. The time course of the development of endothelial dysfunction in ApoE null mice is somewhat debatable, with various studies demonstrating differing responses to acetylcholine-induced vasodilation at selected time points after induction of hypercholesterolemia. Moreover, some studies have even reported normal responses of the aortic endothelium at 20–30 weeks of cholesterol feeding, as opposed to other studies demonstrating significant impairment at 14–15 weeks of cholesterol feeding (28). Thus, speculation as to whether enhanced PFC-NP permeation represents classic endothelial dysfunction as contrasted with more severe endothelial damage or death (e.g., erosions) cannot be supported by the present data set.
Nevertheless, it is clear that these data indicate the possibility of delineating thrombotic risk by quantification of endothelial permeability to PFC-NP that are directly associated with structural damage to the endothelial lining of vessels where plaques have formed. Other groups have previously explored noninvasive assessments of endothelial permeability in atherosclerotic ApoE null mice at very early time points after fat feeding. For example, recent work by Phinikaridou et al. utilized the clinically approved albumin-binding contrast agent gadofosveset (<6 nm diameter) for measurements of endothelial permeability as a consequence of early endothelial dysfunction with MRI. In this work, MRI of mice on a high-fat diet demonstrated accumulation of gadofosveset as early as 4 weeks after inception of a high-fat diet, which correlated well with Evans blue staining (8). These results are consistent with prior descriptions of endothelial permeability of Evans blue (5, 6) and albumin (7) through weakened and/or broken tight junctions. However, in our study at these earlier stages of atherosclerotic disease (up to 12 weeks of cholesterol feeding), the endothelium remains impermeable to the PFC-NP and there is no significant increase in vessel hypercoagulability as measured by carotid occlusion times. Together these observations suggest the appearance of more substantial structural changes in the endothelial barrier of these large vessels, when junction widths increase to ∼2 to 3 μm (9) or greater as a consequence of pathologic features such as cholesterol crystal perforation (Fig. 2A–C) and superficial plaque erosions that accord with markedly enhanced permeability over and above that which might be observed with mild cell junction widening that is associated with classic early measures of endothelial dysfunction (12). In support of this contention, it has been noted that as atherosclerotic disease progresses, mechanical (i.e., cholesterol crystals) (29) and biologic stressors contribute to endothelial apoptosis and subsequent sloughing of endothelial cells, leaving a disrupted or denuded endothelial barrier (30) exposing large (multimicron diameter) perforations in the intimal lining (12) and unfettered access of circulating blood elements to a reservoir of inflammatory cell types, lipids, cytokines, and coagulation factors that enhance prothrombotic tendencies (31). These and prior data suggest that enhanced endothelial permeability to small molecules within the first 3 months of high-fat diet feeding primarily reflects nanoscopic expansion of cell-cell junctions in intact viable but dysfunctional endothelium, whereas beyond 3 months of high-fat feeding, the endothelial permeability to PFC-NP might reflect more severe vascular damage that would be more quantitatively depictive of a focal hypercoagulable state than would endothelial dysfunction alone.
As prior pathologic studies on victims of acute coronary syndromes have established the clinical relevance of eroded or denuded plaques (32, 33), the ability to noninvasively image endothelial permeability could have clinical relevance. In view of recent clinical reports by Damani et al. of the association of plaque endothelial sloughing and acute vascular syndromes in atherosclerotic patients (10) and Makin et al., demonstrating increased CECs can be indicative of endothelial damage in patients with severe atherosclerosis (34), noninvasive evaluation of endothelial barrier disruption that corresponds directly with local thrombosis potential could be useful for detecting frank vascular damage and for depicting beneficial responses to cholesterol lowering at the vascular tissue level (35). In light of these clinical reports, we investigated a potential translatable methodology for in vivo delineation of endothelial permeability through imaging of rabbits with or without atherosclerosis in a clinical 3 T MRI scanner (Fig. 5) outfitted for [19F] detection. Our results suggest the ability to depict permeable plaques in vivo after only ∼2–3 hours of PFC-NP circulation (Fig. 5F). We note here that the voxel size required for recording a sufficient [19F] signal to enable fluorine MRI necessarily yields a comparatively low-resolution fluorine image in the rabbit (see Fig. 5B, E), as contrasted with the higher-resolution [1H] image. Nevertheless, the ability to image voxels containing PFC-NP at 3 T on a multispectral clinical scanner in rabbits suggests that this approach could be implemented on clinical scanners.
Although endothelial dysfunction as currently measured is associated with risk of eventual clinical events in large populations of selected patients (36, 37), it is not used routinely as a harbinger of thrombotic potential in individual patients. Whether the present noninvasive methods for quantification of endothelial damage and accelerated thrombosis have any value for detecting plaque rupture or vulnerable plaque, at least as these entities are now defined, remains conjectural because their evaluation in animal models is problematic.
Supplementary Material
Acknowledgments
The authors thank Todd A. Williams for MRI assistance; Mike Scott, Xiaoxia Yang, and Chandu Vemuri for animal experiment and NP formulation assistance; and Noriko Yanaba for cryostat sectioning. This work was supported in part by U.S. National Institutes of Health Grants HL073646 and HL112303 (National Heart, Lung, and Blood Institute), DK095555 (National Institute of Diabetes and Digestive and Kidney Diseases), and AR056223 (National Institute of Arthritis and Musculoskeletal and Skin Diseases); and the James R. Hornsby Family Dream Garden Investment Partnership (to S.A.W.).
Glossary
- ETL
echo train length
- FACS
fluorescence-activated cell sorting
- FOV
field of view
- MRS
magnetic resonance spectroscopy
- NP
nanoparticle
- NSA
number of signal averages
- OCT
optical cutting temperature
- PFC-NP
perfluorocarbon nanoparticles
- PFCE
perfluoro-15-crown-5-ether
- PFOB
perfluorooctylbromide
- TE
echo time
- TR
repetition time
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Stone G. W., Maehara A., Lansky A. J., de Bruyne B., Cristea E., Mintz G. S., Mehran R., McPherson J., Farhat N., Marso S. P., Parise H., Templin B., White R., Zhang Z., Serruys P. W.; PROSPECT Investigators (2011) A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 364, 226–235 [DOI] [PubMed] [Google Scholar]
- 2.Saric M., Kronzon I. (2012) Aortic atherosclerosis and embolic events. Curr. Cardiol. Rep. 14, 342–349 [DOI] [PubMed] [Google Scholar]
- 3.Croce K., Libby P. (2007) Intertwining of thrombosis and inflammation in atherosclerosis. Curr. Opin. Hematol. 14, 55–61 [DOI] [PubMed] [Google Scholar]
- 4.Demetz G, Ott I. (2012) The interface between inflammation and coagulation in cardiovascular disease. Int. J. Inflam. 2012, 860301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerrity R. G., Richardson M., Somer J. B., Bell F. P., Schwartz C. J. (1977) Endothelial cell morphology in areas of in vivo Evans blue uptake in the aorta of young pigs. II. Ultrastructure of the intima in areas of differing permeability to proteins. Am. J. Pathol. 89, 313–334 [PMC free article] [PubMed] [Google Scholar]
- 6.Caplan B. A., Gerrity R. G., Schwartz C. J. (1974) Endothelial cell morphology in focal areas of in vivo Evans blue uptake in the young pig aorta. I. Quantitative light microscopic findings. Exp. Mol. Pathol. 21, 102–117 [DOI] [PubMed] [Google Scholar]
- 7.Zimmerman M., McGeachie J. (1986) Quantitation of the relationship between aortic endothelial intercellular cleft morphology and permeability to albumin. Atherosclerosis 59, 277–282 [DOI] [PubMed] [Google Scholar]
- 8.Phinikaridou A., Andia M. E., Protti A., Indermuehle A., Shah A., Smith A., Warley A., Botnar R. M. (2012) Noninvasive magnetic resonance imaging evaluation of endothelial permeability in murine atherosclerosis using an albumin-binding contrast agent. Circulation 126, 707–719 [DOI] [PubMed] [Google Scholar]
- 9.Phinikaridou A., Andia M. E., Passacquale G., Ferro A., Botnar R. M. (2013) Noninvasive MRI monitoring of the effect of interventions on endothelial permeability in murine atherosclerosis using an albumin-binding contrast agent. J Am Heart Assoc 2, e000402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damani S., Bacconi A., Libiger O., Chourasia A. H., Serry R., Gollapudi R., Goldberg R., Rapeport K., Haaser S., Topol S., Knowlton S., Bethel K., Kuhn P., Wood M., Garragher B., Schork N. J., Jiang J., Rao C., Connelly M., Fowler V. M., Topol E. J. (2012) Characterization of circulating endothelial cells in acute myocardial infarction. Sci. Transl. Med. 4, 126ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lampka M., Grąbczewska Z., Jendryczka-Maćkiewicz E., Hołyńska-Iwan I., Sukiennik A., Kubica J., Halota W., Tyrakowski T. (2010) Circulating endothelial cells in coronary artery disease. Kardiol. Pol. 68, 1100–1105 [PubMed] [Google Scholar]
- 12.Zhang H., Zhang L., Myerson J., Bibee K., Scott M., Allen J., Sicard G., Lanza G., Wickline S. (2011) Quantifying the evolution of vascular barrier disruption in advanced atherosclerosis with semipermeant nanoparticle contrast agents. PLoS ONE 6, e26385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mousa S. A. (2010) In vivo models for the evaluation of antithrombotics and thrombolytics. Methods Mol. Biol. 663, 29–107 [DOI] [PubMed] [Google Scholar]
- 14.Myerson J., He L., Lanza G., Tollefsen D., Wickline S. (2011) Thrombin-inhibiting perfluorocarbon nanoparticles provide a novel strategy for the treatment and magnetic resonance imaging of acute thrombosis. J. Thromb. Haemost. 9, 1292–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palekar R. U., Myerson J. W., Schlesinger P. H., Sadler J. E., Pan H., Wickline S. A. (2013) Thrombin-targeted liposomes establish a sustained localized anticlotting barrier against acute thrombosis. Mol. Pharm. 10, 4168–4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keupp J., Rahmer J., Grässlin I., Mazurkewitz P. C., Schaeffter T., Lanza G. M., Wickline S. A., Caruthers S. D. (2011) Simultaneous dual-nuclei imaging for motion corrected detection and quantification of 19F imaging agents. Magn. Reson. Med. 66, 1116–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hockett F. D., Wallace K. D., Schmieder A. H., Caruthers S. D., Pham C. T., Wickline S. A., Lanza G. M. (2011) Simultaneous dual frequency 1H and 19F open coil imaging of arthritic rabbit knee at 3T. IEEE Trans. Med. Imaging 30, 22–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abela G. S., Aziz K., Vedre A., Pathak D. R., Talbott J. D., Dejong J. (2009) Effect of cholesterol crystals on plaques and intima in arteries of patients with acute coronary and cerebrovascular syndromes. Am. J. Cardiol. 103, 959–968 [DOI] [PubMed] [Google Scholar]
- 19.Zinselmeyer B. H., Dempster J., Wokosin D. L., Cannon J. J., Pless R., Parker I., Miller M. J. (2009) Chapter 16. Two-photon microscopy and multidimensional analysis of cell dynamics. Methods Enzymol. 461, 349–378 [DOI] [PubMed] [Google Scholar]
- 20.Pham C. T. N., Mitchell L. M., Huang J. L., Lubniewski C. M., Schall O. F., Killgore J. K., Pan D., Wickline S. A., Lanza G. M., Hourcade D. E. (2011) Variable antibody-dependent activation of complement by functionalized phospholipid nanoparticle surfaces. J. Biol. Chem. 286, 123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moulton K. S. K., Heller E., Konerding M. A. M., Flynn E., Palinski W., Folkman J. (1999) Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation 99, 1726–1732 [DOI] [PubMed] [Google Scholar]
- 22.Eriksson E. E., Karlof E., Lundmark K., Rotzius P., Hedin U., Xie X. (2005) Powerful inflammatory properties of large vein endothelium in vivo. Arterioscler. Thromb. Vasc. Biol. 25, 723–728 [DOI] [PubMed] [Google Scholar]
- 23.Poredos P., Jezovnik M. K. (2007) The role of inflammation in venous thromboembolism and the link between arterial and venous thrombosis. Int. Angiol. 26, 306–311 [PubMed] [Google Scholar]
- 24.Eitzman D. T., Westrick R. J., Xu Z., Tyson J., Ginsburg D. (2000) Hyperlipidemia promotes thrombosis after injury to atherosclerotic vessels in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 20, 1831–1834 [DOI] [PubMed] [Google Scholar]
- 25.Winter P. M., Morawski A. M., Caruthers S. D., Fuhrhop R. W., Zhang H., Williams T. A., Allen J. S., Lacy E. K., Robertson J. D., Lanza G. M., Wickline S. A. (2003) Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation 108, 2270–2274 [DOI] [PubMed] [Google Scholar]
- 26.Kircher M. F., Grimm J., Swirski F. K., Libby P., Gerszten R. E., Allport J. R., Weissleder R. (2008) Noninvasive in vivo imaging of monocyte trafficking to atherosclerotic lesions. Circulation 117, 388–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robbins C. S., Chudnovskiy A., Rauch P. J., Figueiredo J. L., Iwamoto Y., Gorbatov R., Etzrodt M., Weber G. F., Ueno T., van Rooijen N., Mulligan-Kehoe M. J., Libby P., Nahrendorf M., Pittet M. J., Weissleder R., Swirski F. K. (2012) Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation 125, 364–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyrelles S. S., Peotta V. A., Pereira T. M., Vasquez E. C. (2011) Endothelial dysfunction in the apolipoprotein E-deficient mouse: insights into the influence of diet, gender and aging. Lipids Health Dis. 10, 211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abela G. S. (2010) Cholesterol crystals piercing the arterial plaque and intima trigger local and systemic inflammation. J. Clin. Lipidol. 4, 156–164 [DOI] [PubMed] [Google Scholar]
- 30.Falk E. (2006) Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 47(8, Suppl), C7–C12 [DOI] [PubMed] [Google Scholar]
- 31.Mackman N. (2008) Triggers, targets and treatments for thrombosis. Nature 451, 914–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farb A., Burke A. P., Tang A. L., Liang T. Y., Mannan P., Smialek J., Virmani R. (1996) Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation 93, 1354–1363 [DOI] [PubMed] [Google Scholar]
- 33.Arbustini E., Dal Bello B., Morbini P., Gavazzi A., Specchia G., Viganò M. (2000) Immunohistochemical characterization of coronary thrombi in allograft vascular disease. Transplantation 69, 1095–1101 [DOI] [PubMed] [Google Scholar]
- 34.Makin A. J., Blann A. D., Chung N. A. Y., Silverman S. H., Lip G. Y. H. (2004) Assessment of endothelial damage in atherosclerotic vascular disease by quantification of circulating endothelial cells. Relationship with von Willebrand factor and tissue factor. Eur. Heart J. 25, 371–376 [DOI] [PubMed] [Google Scholar]
- 35.Ambrose J. A. (2008) In search of the “vulnerable plaque”: can it be localized and will focal regional therapy ever be an option for cardiac prevention? J. Am. Coll. Cardiol. 51, 1539–1542 [DOI] [PubMed] [Google Scholar]
- 36.Davignon J., Ganz P. (2004) Role of endothelial dysfunction in atherosclerosis. Circulation 109(23, Suppl 1)III27–III32 [DOI] [PubMed] [Google Scholar]
- 37.Bonetti P. O., Lerman L. O., Lerman A. (2003) Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 23, 168–175 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.