Abstract
Insulin-like growth factor 1 (IGF1) has potent trophic effects on normal or injured intestinal epithelium, but specific effects on intestinal stem cells (ISCs) are undefined. We used Sox9-enhanced green fluorescent protein (EGFP) reporter mice that permit analyses of both actively cycling ISCs (Sox9-EGFPLow) and reserve/facultative ISCs (Sox9-EGFPHigh) to study IGF1 action on ISCs in normal intestine or during crypt regeneration after high-dose radiation-induced injury. We hypothesized that IGF1 differentially regulates proliferation and gene expression in actively cycling and reserve/facultative ISCs. IGF1 was delivered for 5 days using subcutaneously implanted mini-pumps in uninjured mice or after 14 Gy abdominal radiation. ISC numbers, proliferation, and transcriptome were assessed. IGF1 increased epithelial growth in nonirradiated mice and enhanced crypt regeneration after radiation. In uninjured and regenerating intestines, IGF1 increased total numbers of Sox9-EGFPLow ISCs and percentage of these cells in M-phase. IGF1 increased percentages of Sox9-EGFPHigh ISCs in S-phase but did not expand this population. Microarray revealed that IGF1 activated distinct gene expression signatures in the 2 Sox9-EGFP ISC populations. In vitro IGF1 enhanced enteroid formation by Sox9-EGFPHigh facultative ISCs but not Sox9-EGFPLow actively cycling ISCs. Our data provide new evidence that IGF1 activates 2 ISC populations via distinct regulatory pathways to promote growth of normal intestinal epithelium and crypt regeneration after irradiation.—Van Landeghem, L., Santoro, M. A., Mah, A. T., Krebs, A. E., Dehmer, J. J., McNaughton, K. K., Helmrath, M. A., Magness, S. T., Lund, P. K. IGF1 stimulates crypt expansion via differential activation of 2 intestinal stem cell populations.
Keywords: irradiation, tissue regeneration, actively cycling ISCs, facultative/reserve ISCs
Continuous renewal of the small intestinal epithelium is driven by multipotent intestinal stem cells (ISCs) located at the base of the crypts (1–7). ISCs divide to self-renew and give rise to daughter cells, termed progenitors or transit-amplifying cells, which rapidly divide and differentiate into the 5 intestinal epithelial cell (IEC) lineages as they migrate along the crypt-villus axis. Current views suggest the existence of 2 ISC populations (4). One corresponds to crypt base columnar cells (CBCs) intercalated between Paneth cells at the base of the crypts (8). CBC-type ISCs are actively cycling and express high levels of Lgr5 (7) and other markers including Ascl2 (9), Olfm4 (10), and Smoc2 (11). CBC-ISCs were shown by lineage tracing to be multipotent for all crypt and villus cell lineages (7, 11). A second ISC population, also defined as multipotent by lineage tracing, appears to be a heterogeneous population of cells that cycle more slowly than CBCs and are marked by high levels of expression of Bmi-1 (12), Hopx (13), Lrig1 (14), or mTert (15)-reporter genes. These cells are typically located above Paneth cells, lying ∼4–6 cells up from the crypt base and correspond in location to putative reserve/facultative ISCs that were originally described as label-retaining cells (16). Available evidence suggests that a bidirectional lineage relationship exists between the 2 ISC populations, and both ISC populations have been shown to contribute to crypt regeneration after radiation (1–3, 13, 17–19).
In multiple mouse strains, radiation doses of 12–14 Gy result in ablation of small intestinal crypts followed by regeneration of crypts and ultimately villi as a result of clonal expansion of surviving ISCs (1, 2, 20). This radiation model has been used as a “gold standard” to study impact of trophic therapies on ISC-mediated crypt regeneration, which is highly relevant to protection against fatal radiation-associated enteropathy. Several growth factors including keratinocyte growth factor, transforming growth factor-β3, and insulin-like growth factor 1 (IGF1) have been shown to enhance crypt survival in early phases after high-dose radiation (21–25). However, until the development of ISC reporter mice, it was not possible to directly and specifically study the impact of trophic factors on ISCs in normal or regenerating intestinal epithelium.
IGF1 potently promotes intestinal epithelial growth or healing under a wide range of experimental conditions such as radiation-induced apoptosis (25), enteritis (23), experimentally induced colitis (26), small bowel resection (27), or total parenteral nutrition (28). IGF1 is a key mediator of the enterotrophic actions of growth hormone and glucagon-like peptide 2, which are U.S. Food and Drug Administration approved or under clinical trial as trophic therapies to promote intestinal epithelial growth and/or healing (29–32). However, whether IGF1-induced growth of intestinal epithelium reflects selective or preferential activation and expansion of ISCs is not defined, and it is not known which genes are regulated by IGF1 specifically in ISCs.
We hypothesized that IGF1 therapy for 5 days in nonirradiated mice or after crypt ablation by high-dose radiation would selectively or preferentially expand normal or regenerating ISCs. Importantly, we tested this hypothesis in Sox9-EGFP transgenic mice, which permits us to compare the impact of IGF1 on the 2 small intestinal ISC populations that are marked by different Sox9-EGFP expression levels (2, 33). Our prior work demonstrated that cells expressing low levels of Sox9-EGFP (Sox9-EGFPLow) are enriched for Lgr5 mRNA and many other mRNAs enriched in Lgr5-expressing ISCs and are multipotent for all intestinal epithelial cell lineages in vitro (2, 33). Cells expressing high levels of Sox9-EGFP (Sox9-EGFPHigh) include cells enriched for markers of the slowly cycling facultative ISCs, as well as multiple enteroendocrine cell (EEC) biomarkers (2, 33, 34). We previously demonstrated that Sox9-EGFPHigh cells are activated to proliferate and adopt a stem cell phenotype during crypt regeneration after radiation-induced injury (2). These characteristics of Sox9-EGFPHigh cells are consistent with recent reports showing that a subpopulation of secretory cells, EEC or Paneth cells, or their immediate progenitors correspond to reserve/facultative ISCs that are activated during regeneration after injury (35, 36). A third level of Sox9-EGFP expression termed “Sox9-EGFPSublow” marks progenitors (2, 33). “Sox9-EGFPNegative” cells are enriched for markers of enterocytes and other terminally differentiated IECs including goblet and Paneth cells (2). These distinct Sox9-EGFP cell types can be simultaneously identified and quantified by histology or flow cytometry and isolated by fluorescence activated cell sorting (FACS). Here we evaluated the specific impact of IGF1 on numbers, proliferation, and gene expression signatures in distinct Sox9-EGFPLow, Sox9-EGFPHigh, and other cell populations in normal or regenerating intestinal epithelium.
MATERIALS AND METHODS
Reagents
IGF1 was from Genentech (San Francisco, CA, USA). The Click-iT EdU Alexa Fluor 594 Kit, anti-chicken secondary antibody, Diaminobenzidine, High Capacity cDNA Reverse Transcription Kit, Platinum Quantitative PCR supermix, and TaqMan primers/probes were purchased from Life Technologies (Carlsbad, CA, USA). Anti-GFP antibody was from Aves Labs (Tigard, OR, USA). Anti-phospho-histone H3 (pH3) and anti-type 1 IGF receptor (IGF1R) antibodies were from Cell Signaling (Danvers, MA, USA), and the anti-rabbit-Cy3 and biotinylated anti-rabbit secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). The vectastain ABC Elite kit was purchased from Vector (Burlingame, CA, USA). The RNeasy Mini Kit was from Qiagen (Venlo, The Netherlands).
Animals
Sox9-EGFP mice expressing a bacterial artificial chromosome transgene with an ∼226.5 kb of Sox9 genomic regulatory region driving EGFP expression were established and maintained on a CD1 background at the University of North Carolina (Chapel Hill, NC, USA) as previously described (34). Animal studies were approved by the Institutional Animal Care and Use Committee of the University of North Carolina.
IGF1 treatment of nonirradiated or irradiated mice
Radiated mice were given a single dose of 14 Gy abdominal radiation. Anesthetized mice were placed in an XRad 320 irradiator (filter: 2 mm Al; 47 cm; 320 kV/s, 10 mA; 2.8 Gy/min; Precision X-Ray, North Branford, CT, USA) so that only their abdomen was exposed to radiation as previously described (2, 37). Osmotic mini-pumps (Alzet; Durect Corp., Cupertino, CA, USA) were implanted subcutaneously in anesthetized, nonirradiated controls or mice immediately following radiation and used to deliver vehicle (0.9% NaCl) or recombinant human IGF1 (10 mg/ml; 3 µg/g body weight/day). After 5 days of vehicle or IGF1 treatment, mice were killed with a lethal dose of pentobarbital (150 µg/g body weight), and small intestines were collected. This time point after radiation was chosen because previous studies demonstrated maximal ISC expansion at 5 days after 14 Gy radiation (1, 2), and we aimed to test whether IGF1 could further enhance ISC expansion during regeneration.
Histology, immunofluorescence, and immunohistochemistry
Histology and immunofluorescence were performed on frozen paraformaldehyde-fixed Swiss roll sections of entire jejunum as previously described (2). Briefly, crypt depth, villus height, and crypt and villus density were quantified on hematoxylin and eosin-stained sections. 5-ethynyl-2′-deoxyuridine (EdU) was given 90 minutes before animals were killed. EdU staining was used to localize and quantify cells in S-phase using the Click-iT EdU Alexa Fluor 594 Kit according to the manufacturer’s instructions. Cells expressing Sox9-EGFP were localized using a primary chicken polyclonal anti-GFP antibody (1:300) and a secondary anti-chicken antibody (Alexa Fluor 488; goat; 1:300). Cells in M-phase were localized using a primary rabbit polyclonal anti-pH3 antibody (Ser10; 1:800) and a secondary anti-rabbit antibody (Cy3; goat; 1:500). IGF1R was localized on formalin-fixed paraffin-embedded sections. Sections were baked at 60°C prior to heat-induced epitope retrieval. Slides were incubated in 3% H2O2, blocked, and then incubated with a rabbit polyclonal anti-IGF1R antibody (1:400) overnight at 4°C. After extensive rinses, slides were incubated with biotinylated anti-rabbit IgG (1:500; goat) and then with the avidin-biotin complex conjugated with horseradish peroxidase using the Vectastain ABC Elite kit. Peroxidase activity was detected with diaminobenzidine. Slides were examined with an AX10 Imager (Zeiss, Oberkochen, Germany) equipped with a ProgRes CF digital camera (Jenoptik, Jena, Germany).
Intestinal epithelial cell dissociation, FACS, and flow cytometry
IEC isolation, dissociation into single cells, FACS, and flow cytometry assays were performed as described previously (2). One animal from each of the 4 treatment groups (nonirradiated/vehicle, nonirradiated/IGF1, irradiated/vehicle, and irradiated/IGF1) was analyzed in each FACS or flow cytometry run. The proportion of each Sox9-EGFP cell population in each treatment group was compared with the proportion of the respective Sox9-EGFP cell population in nonirradiated vehicle-treated mice studied in that same run.
Enteroid culture on FACS-isolated cells
Enteroid culture was carried out using methods detailed previously (2). In tests of IGF1, recombinant human IGF1 was added daily at 100 and 200 ng/ml.
Quantitative real-time PCR
RNA was purified from sorted Sox9-EGFP cell populations (Sox9-EGFPNegative, Sox9-EGFPSublow, Sox9-EGFPLow, and Sox9-EGFPHigh cells) using RNeasy Mini Kit (Qiagen). RNA was reverse transcribed into cDNA using the High Capacity cDNA Reverse Transcription Kit. Quantitative real-time PCR used Platinum Quantitative PCR supermix and TaqMan primers/probes for IGF1R (Mm00802831_m1). Expression data were normalized to a standard curve generated from a pool of nonsorted/total IECs. β-Actin (Mm00607939_s1) was used as the reference gene.
Statistical analyses
Data were expressed as means ± sem. ANOVA or unpaired Student's t test compared different groups as indicated in the results or figure legends. P < 0.05 was considered statistically significant.
Gene microarray on FACS-isolated cells
Total RNA was extracted with RNeasy Mini Kit (Qiagen) from FACS-isolated Sox9-EGFPNegative, Sox9-EGFPSublow, Sox9-EGFPLow and Sox9-EGFPHigh cells, and from nonsorted total IEC obtained from jejunum of IGF1- or vehicle-treated nonirradiated mice or at 5 days after radiation (n = 3 per group). RNA integrity was verified using Agilent RNA 6000 Nano microfluidic chips and Agilent 2100 Bioanalyzer platform (Agilent, Santa Clara, CA, USA). RNA amplification and labeling were performed using Agilent Low Input Quick Amp Labeling Kit (Two-Color, Cy5 for test samples and Cy3 for the reference). Hybridization was performed using Agilent Microarray Hybridization equipment and protocol, and Agilent Mouse GE 4x44K v2 microarray. Each individual test sample was compared to a reference pool of RNA from nonsorted total IECs from nonirradiated vehicle-treated mice. Hybridized slides were scanned using Agilent Microarray Scanner and Agilent Scan Control software. Data were processed using Agilent Feature Extraction Software, version 10.7.3.1. Sample to reference ratios (Cy5/Cy3) were calculated and log2 transformed. To identify differentially expressed genes, log2 ratios were analyzed using Genespring GX v10.0 software (Agilent). Data were normalized 1) with Lowess normalization and 2) to the median of nonsorted IEC control samples from nonirradiated vehicle-treated mice. Genes regulated by IGF1 specifically/exclusively in each Sox9-EGFP cell population in uninjured intestine or at day 5 after radiation were identified in 2 steps. First, genes differentially expressed in 1 particular Sox9-EGFP cell population isolated from IGF1-treated mice versus vehicle-treated mice were identified by unpaired Student's t test with a significance threshold of 0.05. Then, using Venn diagrams, genes significantly regulated by IGF1 exclusively in one particular Sox9-EGFP cell type were identified. Ingenuity pathway analysis (IPA) was then used to identify signaling pathways and cellular functions related to the genes regulated by IGF1 in the different nonirradiated or regenerating Sox9-EGFP cell populations. Microarray data were uploaded to the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/) and are available under accession number GSE42817.
RESULTS
IGF1R mRNA is enriched in Sox9-EGFPLow ISCs
Quantitative RT-PCR on Sox9-EGFPHigh, Sox9-EGFPLow Sox9-EGFPSublow and Sox9-EGFPNegative cells showed significant enrichment of Igf1r mRNA in Sox9-EGFPLow ISCs versus all other cells. Igf1r gene expression was also higher in Sox9-EGFPHigh cells and Sox9-EGFPSublow progenitors than in Sox9-EGFPNegative cells, which primarily comprise differentiated enterocytes and other differentiated lineages (Fig. 1A). Immunohistochemistry showed that as previously described, IGF1R is expressed in lamina propria and enteric muscle cells (38, 39), and most importantly confirmed that IGF1R is strongly expressed at the base of the crypts with an intense staining observed in cells at the CBC position (Fig. 1B). High-level expression of Igf1r mRNA and protein in ISCs and progenitors thus favors selective trophic responses to IGF1.
Figure 1.
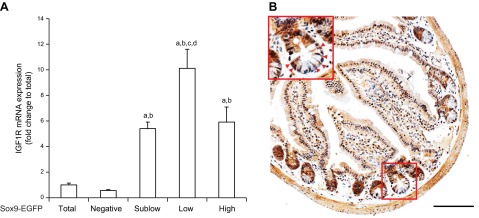
IGF1R is enriched in Sox9-EGFPLow ISCs. A) Quantitative RT-PCR for IGF1R in the 4 different FACS-isolated Sox9-EGFP cell populations. Data are expressed as means ± SEM of fold change in IGF1R gene expression relative to total (nonsorted) cells (n = 5; ANOVA; aP < 0.05 vs. total cells; bP < 0.05 vs. Sox9-EGFPNegative IECs; cP < 0.05 vs. Sox9-EGFPSublow progenitors; dP < 0.05 vs. Sox9-EGFPHigh cells). IGF1R mRNA was significantly enriched in Sox9-EGFPLow ISCs compared with other Sox9-EGFP cells, and was also expressed at higher levels in Sox9-EGFPSublow progenitors and Sox9-EGFPHigh cells vs. Sox9-EGFPNegative differentiated IECs. B) IGF1R immunoreactivity in intestinal epithelium strongly localized to the base of the crypts within the progenitor/ISC compartment. Red arrowheads show IGF1R-positive cells in the ISC zone (scale bar, 100 µm).
IGF1 stimulates mucosal growth and Sox9-EGFPLow ISC expansion in uninjured small intestine and during crypt regeneration after radiation
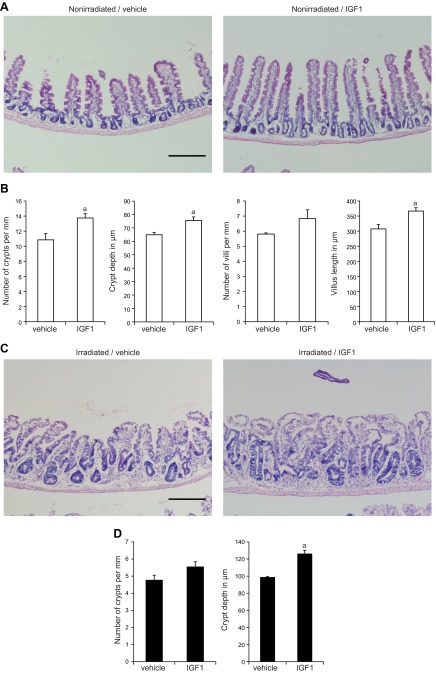
In nonirradiated and irradiated mice, IGF1 treatment profoundly impacted intestinal morphology (Fig. 2A, C). Quantitative analyses demonstrated that IGF1 induced a significant increase in crypt density, as well as modest but significant increases in crypt depth and villus height in nonirradiated intestine (Fig. 2A, B). After high-dose radiation, IGF1-treated mice showed enhanced crypt regeneration associated with increases in crypt depth compared with vehicle-treated mice (Fig. 2C, D).
Figure 2.
Five day IGF1 treatment has trophic effects in normal and regenerating intestinal epithelium. A) Hematoxylin and eosin staining in nonirradiated mice demonstrates that IGF1 induced significant changes in intestinal morphology including increased crypt density, crypt depth, and villus height (scale bar, 250 µm). B) Morphometry data were quantified on 10 zones of ∼1.5 mm spanning the entire jejunum and are expressed as means ± sem (n = 3 mice per group; n ≥ 41 villi/mouse; n ≥ 48 crypts/mouse; Student's t test; aP < 0.05 vs. vehicle-treated mice). C) Hematoxylin and eosin staining at day 5 after radiation shows hyper-regenerative crypts in IGF1-treated mice compared with vehicle-treated controls (scale bar, 250 µm). D) Quantitative morphometry was performed on 20 zones of ∼1.5 mm spanning the entire jejunum and confirmed that IGF1 induced a significant increase in crypt depth after radiation (n = 3 mice per group; n ≥ 79 crypts/mouse; Student's t test; aP < 0.05 vs. vehicle-treated mice).
IGF1 selectively stimulates expansion of normal and regenerating Sox9-EGFPLow ISCs
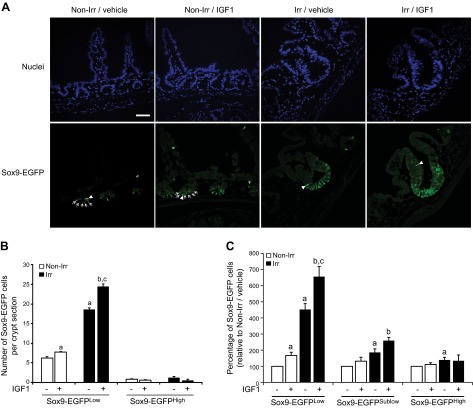
To test whether IGF1 affects actively cycling ISCs, Sox9-EGFPLow cells were quantified per crypt section, i.e., per section of crypt correctly oriented in 2-dimensional images. In nonirradiated mice, immunofluorescence demonstrated a significant 25 ± 2% increase in the number of Sox9-EGFPLow ISCs per crypt section in IGF1-treated mice versus vehicle-treated littermates (Fig. 3A, B). In vehicle-treated mice at 5 days after radiation, Sox9-EGFPLow cells were dramatically expanded compared with nonirradiated vehicle-treated mice, and importantly, IGF1 treatment led to a further 31 ± 3% increase in the number of regenerating Sox9-EGFPLow ISCs per crypt section (Fig. 3A, B). Histology did not reveal a detectable impact of IGF1 on the number of Sox9-EGFPHigh cells per crypt section in nonirradiated or radiated mice (Fig. 3A, B). An independent flow cytometry assay validated in a previous study (2) was also used to study effects of IGF1. Flow cytometry permits quantification of the proportions of the different Sox9-EGFP cell types in an entire jejunal segment rather than per crypt section and allows quantitative evaluation of Sox9-EGFPHigh cells, Sox9-EGFPLow cells, and also Sox9-EGFPSublow progenitors that are difficult to quantify using histology (2, 33). Consistent with histology data, flow cytometry demonstrated that IGF1 treatment expanded Sox9-EGFPLow ISCs in uninjured intestine and significantly enhanced the dramatic ISC expansion observed at day 5 after radiation. IGF1 did not significantly expand either Sox9-EGFPHigh cells or Sox9-EGFPSublow progenitors in nonirradiated or irradiated regenerating intestinal epithelium (Fig. 3C and Supplemental Fig. S1). Both histology and flow cytometry data therefore indicate that IGF1 preferentially stimulates the expansion of normal and regenerating Sox9-EGFPLow ISCs.
Figure 3.
IGF1 treatment preferentially expands Sox9-EGFPLow cells. A) Immunofluorescence illustrates that 5-day IGF1 therapy increased the number of Sox9-EGFPLow ISCs per crypt section in both normal (Non-Irr) and regenerating crypts (Irr) compared with vehicle (nuclei staining, DAPI, blue; Sox9-EGFP, green; scale bar, 50 µm). White arrows show Sox9-EGFPLow cells in nonirradiated crypts. White arrowheads show Sox9-EGFPHigh cells. B, C) Quantitative data are expressed as means ± sem (n = 4; Student's t test; aP < 0.05 vs. nonirradiated vehicle-treated mice; bP < 0.05 vs. nonirradiated IGF1-treated mice, cP < 0.05 vs. irradiated vehicle-treated mice). B) Quantitative analysis revealed that IGF1 significantly increased the number of Sox9-EGFPLow ISCs per crypt section in normal and regenerating crypts but did not affect the number of Sox9-EGFPHigh cells per crypt section. Thirty crypts were analyzed per animal in blinded fashion. C) Flow cytometry demonstrated that IGF1 significantly increased the proportion of Sox9-EGFPLow ISCs in both nonirradiated and irradiated regenerating crypts but did not alter percentages of Sox9-EGFPHigh and Sox9-EGFPSublow cells in either nonirradiated or irradiated intestines.
IGF1 has differential impact on S-phase and M-phase in Sox9-EGFPLow and Sox9-EGFPHigh cells during crypt regeneration
Immunostaining for the S-phase marker, EdU, or the M-phase marker, pH3, and Sox9-EGFP was performed to evaluate total numbers of EdU- or pH3-labeled cells per crypt section, and percentages of Sox9-EGFPLow or Sox9-EGFPHigh cells colabeled with EdU or pH3. Our goal was to establish whether IGF1 affected total numbers of cells per crypt, proportion of actively cycling Sox9-EGFPLow ISCs, or proportion of activatable facultative Sox9-EGFPHigh ISCs in S-phase or M-phase and whether effects differed in the basal state or at peak regeneration after radiation.
IGF1 increases proportions of Sox9-EGFPLow ISCs in M-phase at 5 days after radiation
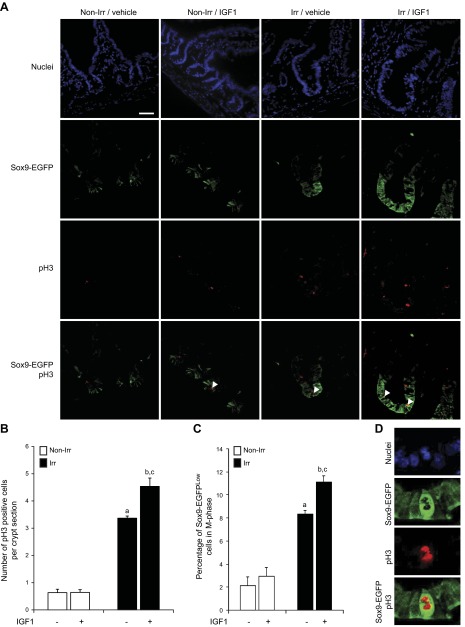
In nonirradiated mice, IGF1 did not affect total numbers of pH3-positive cells per crypt section (Fig. 4A, B) or the percentage of pH3-labeled Sox9-EGFPLow cells (Fig. 4A, C). At 5 days after radiation in vehicle-treated mice, there were significant increases in total numbers of pH3-labeled cells per crypt section (Fig. 4A, B) and the percentage of pH3-labeled Sox9-EGFPLow ISCs per crypt (Fig. 4A, C). Mice given IGF1 after radiation showed enhanced expansion of total pH3-labeled cells per crypt section (Fig. 4A, B) and increased percentage of Sox9-EGFPLow cells in M-phase (Fig. 4A, C). Colocalization of pH3 and Sox9-EGFP in uninjured intestine revealed no detectable pH3-labeled Sox9-EGFPHigh cells, and during regeneration after irradiation, only mice treated with IGF1 exhibited rare pH3-positive Sox9-EGFPHigh cells (Fig. 4D).
Figure 4.
IGF1 increases the proportion of pH3-positive Sox9-EGFPLow cells only during crypt regeneration. A) Immunofluorescence demonstrates increased number of pH3-positive cells per crypt section in IGF1-treated mice vs. vehicle-treated controls at 5 days after radiation (Irr), but no effect of IGF1 on pH3-stained cells in normal crypts (Non-Irr). White arrowheads show pH3-positive Sox9-EGFPLow cells (nuclei staining, DAPI, blue; Sox9-EGFP, green; pH3, red; scale bar, 50 µm). B, C) Quantitative data are expressed as means ± sem (n = 3; Student's t test; aP < 0.05 vs. nonirradiated vehicle-treated mice; bP < 0.05 vs. nonirradiated IGF1-treated mice, cP < 0.05 vs. irradiated vehicle-treated mice). B) Histogram shows total numbers of pH3-labeled cells per crypt section and demonstrates an increase in pH3-positive cells in regenerating crypts vs. nonirradiated controls and that IGF1 significantly enhanced this effect. Thirty crypts were studied per animal in blinded fashion. C) Histogram shows the proportion of Sox9-EGFPLow cells colabeled with pH3. IGF1 significantly increased the proportion of pH3-labeled Sox9-EGFPLow ISCs in regenerating crypts but not in nonirradiated crypts. D) Immunofluorescence illustrates that rare pH3-positive Sox9-EGFPHigh cells were only found in mice treated with IGF1 for 5 days after radiation.
IGF1 selectively increases percentages of Sox9-EGFPHigh cells in S-phase
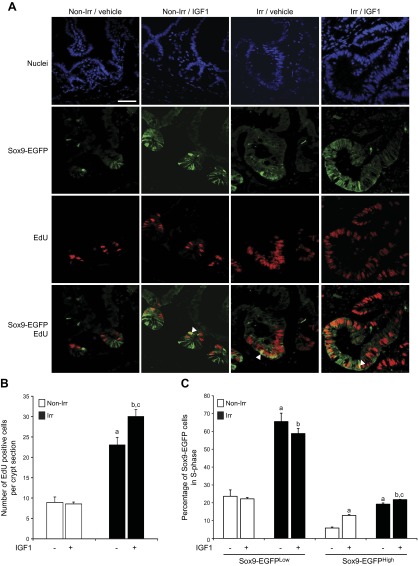
In nonirradiated mice, IGF1 did not affect total numbers of EdU-labeled cells per crypt section (Fig. 5A, B) or the percentage of EdU-labeled Sox9-EGFPLow cells per crypt (Fig. 5A, C). In contrast, IGF1 treatment resulted in a 2.2 ± 0.1-fold increase in Sox9-EGFPHigh cells colabeled with EdU (Fig. 5A, C). Consistent with prior work (2), at peak of regeneration in radiated vehicle-treated mice, there were a dramatic increase in total numbers of EdU-labeled cells per crypt section (Fig. 5A, B) and increased percentages of Sox9-EGFPLow and Sox9-EGFPHigh cells in S-phase (Fig. 5A, C) compared with nonirradiated vehicle-treated controls. IGF1 treatment significantly enhanced total numbers of EdU-positive cells per regenerating crypt (Fig. 5A, B). This did not reflect an increase in the percentage of Sox9-EGFPLow cells colabeled with EdU but rather a modest significant increase in the percentage of EdU-positive Sox9-EGFPHigh cells (Fig. 5A, C).
Figure 5.
IGF1 increases proportions of EdU-positive Sox9-EGFPHigh cells but not Sox9-EGFPLow cells. A) Immunofluorescence illustrates that IGF1 increased the numbers of EdU-positive cells per crypt section at 5 days after irradiation (Irr), but not in nonirradiated controls (Non-Irr). White arrowheads show EdU-positive Sox9-EGFPHigh cells (nuclei staining, DAPI, blue; Sox9-EGFP, green; EdU, red; scale bar, 50 µm). B, C) Quantitative data are expressed as means ± sem (n = 4; Student's t test; aP < 0.05 vs. nonirradiated vehicle-treated mice; bP < 0.05 vs. nonirradiated IGF1-treated mice, cP < 0.05 vs. irradiated vehicle-treated mice). B) Histogram demonstrates that at 5 days after radiation, there was a dramatic increase in total numbers of EdU-positive cells per crypt section compared with uninjured intestine and that IGF1 further increased the total number of EdU-labeled cells per crypt section in irradiated mice. Thirty crypts were studied per animal in blinded fashion. C) Colocalization of EdU staining and Sox9-EGFP assessed the proportion of Sox9-EGFPLow and Sox9-EGFPHigh cells in S-phase. IGF1 induced no significant change in the proportion of EdU-positive Sox9-EGFPLow ISCs in either nonirradiated or irradiated regenerating crypts. IGF1 treatment significantly increased the percentages of EdU-labeled Sox9-EGFPHigh cells per crypt section in nonirradiated and regenerating crypts.
Together, these data support a model where IGF1 promotes M-phase rather than S-phase in actively cycling Sox9-EGFPLow ISCs only after radiation. In contrast, IGF1 activates proliferation in facultative Sox9-EGFPHigh ISCs by promoting S-phase both in the basal state and during regeneration, and M-phase only during regeneration.
IGF1 therapy activates distinct molecular pathways in Sox9-EGFPLow versus Sox9-EGFPHigh cells in nonirradiated intestine and during crypt regeneration after radiation
Microarray was used to identify genes and associated regulatory pathways activated by IGF1 selectively in Sox9-EGFPLow or Sox9-EGFPHigh cells (versus all the other Sox9-EGFP cell populations) in uninjured intestine or during crypt regeneration.
IGF1 represses mRNAs linked to p53 signaling in normal Sox9-EGFPLow cells and stimulates mRNAs associated with cell cycle progression in regenerating Sox9-EGFPLow cells
In Sox9-EGFPLow cells from uninjured intestine, IGF1 induced differential expression of 1,140 genes with 876 annotated by EntrezGene (Fig. 6A and Supplemental Tables S1 and S2). IPA identified p53 signaling as a major pathway regulated by IGF1 exclusively in nonirradiated Sox9-EGFPLow cells with primarily repression of mRNAs linked to p53 signaling. These included mRNAs coding for p47ING3, which modulates p53-mediated transcription and induces apoptosis (40), JMY, a transcription cofactor that regulates the p53 response (41), DRAM, a p53 target gene that induces macroautophagy, as an effector of p53-mediated death (42), and ATM, which is critical for effective induction of p53 signaling (43). Also Efnb3, encoding Ephrin B3, was down-regulated by IGF1 in normal Sox9-EGFPLow cells. A recent study demonstrated that Ephb3 repression initiates survival and expansion of endogenous adult neural progenitor cells in a p53-dependent manner (44). Other genes modulated by IGF1 in Sox9-EGFPLow cells have been implicated in stem cell functions. Ihh (Indian Hedgehog), which regulates ISC self-renewal and differentiation (45), was up-regulated. FZD10, a Wnt receptor whose expression is up-regulated during differentiation of neuronal precursor cells (46), was repressed by IGF1. Smad4 and Bmpr2, which regulate stem cell fate in other organs (47, 48) and act together to limit proliferation and promote apoptosis and differentiation (49), were both down-regulated by IGF1.
Figure 6.
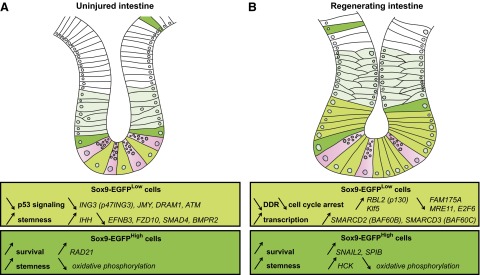
IGF1 exerts differential impact on the gene expression profiles of the 2 Sox9-EGFP ISC populations. A and B illustrate selected mRNAs significantly regulated by IGF1 selectively/exclusively in each Sox9-EGFP ISC population isolated from uninjured small intestine (A) and at day 5 after radiation (B).
Sox9-EGFPLow ISCs from regenerating crypts after radiation exhibited 651 genes significantly altered by IGF1, 516 of them being annotated with an EntrezGene ID (Fig. 6B and Supplemental Tables S1 and S2). Interestingly, only 24 genes were found to be regulated by IGF1 in both uninjured and regenerating Sox9-EGFPLow cells. This provides evidence that IGF1 has a distinct impact on gene expression signatures in normal vs. regenerating ISCs. IPA analyses of the mRNAs selectively regulated by IGF1 in Sox9-EGFPLow ISCs during crypt regeneration suggest repression of DNA damage response (DDR), exit of cell cycle arrest, and activation of transcription. Fam175A mRNA, encoding Abraxas, which is a major protein of the BRCA1 complex (50) and Mre11 mRNA, which encodes meiotic recombination 11, a protein essential to the DDR (51), were both down-regulated by IGF1 in Sox9-EGFPLow cells during crypt regeneration. E2f6, which is known to be elevated during cell cycle arrest in embryonic stem cells responding to DNA damage, was repressed, whereas Rbl2 (p130), which is predominantly found in actively proliferating embryonic stem cells (52), was up-regulated. Also mRNAs encoding chromatin remodeling-related proteins such as Smarcd2 (BAF60B) and Smarcd3 (BAF60C), which are part of complexes that regulate activation and repression of numerous genes, were up-regulated by IGF1. Evidence suggests that these chromatin remodeling complexes are also involved in cell growth (53). Interestingly BAF60C was recently shown to be required for embryonic stem cell maintenance and pluripotency (54). Other mRNAs involved in stem cell functions were altered by IGF1 in regenerating Sox9-EGFPLow cells. Among them, Klf5, which promotes intestinal epithelial cell proliferation and mucosal healing (55, 56) and accelerates mitotic entry by activating cyclin B1 and Cdc2 (57), was up-regulated. IPA analysis also revealed that IGF1 significantly impacted expression of mRNAs related to apoptosis in regenerating Sox9-EGFPLow cells. Bag5, which is known to promote cell death (58), was down-regulated and Birc3 encoding cIAP2, which delays IEC anoikis (59), was up-regulated.
IGF1 stimulates genes associated with stemness and survival in Sox9-EGFPHigh cells from uninjured and regenerating intestines
IGF1 induced differential expression of 2528 genes exclusively in Sox9-EGFPHigh cells isolated from uninjured intestine, with 2058 annotated by EntrezGene (Fig. 6A and Supplemental Tables S1 and S2). The gene signature of IGF1-treated Sox9-EGFPHigh cells from uninjured intestine predicted inhibition of cell death and activation of stemness/proliferation. The gene coding for RAD21 was up-regulated by IGF1 exclusively in Sox9-EGFPHigh cells. RAD21 regulates chromosome segregation and DDR, and has been recently shown to promote gastrointestinal radioresistance (60) and to participate in the maintenance of an embryonic stem cell phenotype (61). IGF1 treatment induced significant changes in expression of 21 genes involved in oxidative phosphorylation and 20 of them were repressed, indicating a switch of metabolism toward glycolysis, a hallmark of stem cells (62). Interestingly IGF1 treatment had strong effects on the expression of genes of the cytochrome p450 family, suggesting effects on pathways linked to metabolism of exogenous and endogenous chemicals/molecules (63) (Supplemental Tables S2).
Only 385 genes were regulated by IGF1 exclusively in Sox9-EGFPHigh cells at day 5 after radiation, when crypt regeneration is maximal (2). Among them, 284 were annotated by EntrezGene (Fig. 6B and Supplemental Tables S1 and S2). Similar to Sox9-EGFPLow ISCs, only a small number of genes (34 genes) were found to be regulated by IGF1 in both uninjured and regenerating Sox9-EGFPHigh cells, suggesting that IGF1 exerts distinct effects on normal versus regenerating Sox9-EGFPHigh cells. IPA revealed that in Sox9-EGFPHigh cells isolated after radiation, IGF1 regulated several mRNAs involved in oxidative phosphorylation, with 75% of them being repressed. In a previous study, we demonstrated that during regeneration Sox9-EGFPHigh cells are activated to hyperproliferate, and this was accompanied by repressed expression of genes linked to oxidative phosphorylation (2). Collectively, these data suggest that during crypt regeneration IGF1 enhances a switch in metabolism of Sox9-EGFPHigh cells toward preferential use of glycolysis for energy production. IGF1 also stimulated the expression of Hck, which promotes self-renewal, is silenced during differentiation in murine embryonic stem cells (64), and has been linked to IGF1 signaling in other organs (65). IGF1 increased expression of Snai2 (Snail2), which notably regulates genes involved in self-renewal and cell cycle/DNA damage control (66) and is induced by IGF1 in other systems (67). Snai2 has also been shown to promote cell survival after radiation by directly repressing the transcription of Puma (68), previously linked to IGF1 action in intestine after radiation (25). In addition, the gene Spib, coding for the ETS factor Spi-B was significantly up-regulated by IGF1 in irradiated Sox9-EGFPHigh cells and is known to promote cell survival through direct activation of the antiapoptotic gene Bcl2-a1 (69).
Together the gene microarray data provide novel evidence for distinct effects of IGF1 on gene expression profiles in Sox9-EGFPLow vs. Sox9-EGFPHigh cells, as well as in uninjured vs. regenerating ISCs.
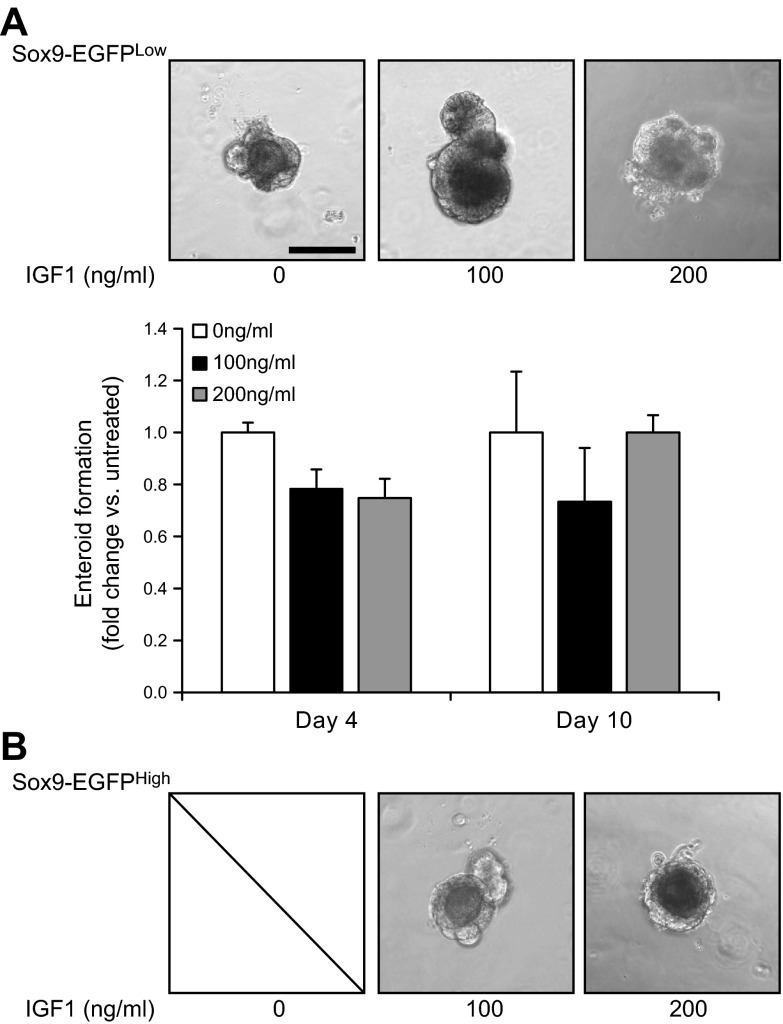
IGF1 stimulates intrinsic stem cell functions in Sox9-EGFPHigh cells but not in Sox9-EGFPLow cells in vitro
We used the ISC 3-dimensional culture system to directly test whether IGF1 affects enteroid formation abilities of Sox9-EGFPLow or Sox9-EGFPHigh cells as a measure of IGF1 impact on intrinsic functional ISC characteristics of these cells (2). Consistent with our prior report (2), only Sox9-EGFPLow cells and not Sox9-EGFPHigh cells yielded enteroids when cultured in standard conditions reported previously (70) in the absence of IGF1. Addition of exogenous IGF1 did not significantly impact numbers and sizes of enteroids grown from Sox9-EGFPLow cells in the basal state (Fig. 7A). In contrast, addition of exogenous IGF1 led to the formation of enteroids from Sox9-EGFPHigh cells as illustrated in Fig. 7B, indicating that IGF1 directly activates Sox9-EGFPHigh cells to acquire ISC ability to grow into enteroids. Of note, increased doses of IGF1 did not affect sizes of enteroids grown from Sox9-EGFPHigh cells (Fig. 7B).
Figure 7.
Exogenous IGF1 directly stimulates enteroid formation ability in Sox9-EGFPHigh cells but not in Sox9-EGFPLow cells. A) (Upper) Representative photographs of enteroids grown from Sox9-EGFPLow cells in absence or presence of IGF1 at 100 and 200 ng/ml at 10 days after plating (scale bar, 100 µm). (Lower) Quantitative data are expressed as means ± sem of fold change in numbers of enteroids formed from Sox9-EGFPLow cells grown in presence of IGF1 relative to numbers of enteroids formed from untreated Sox9-EGFPLow cells normalized to initial numbers of cells plated per well. Quantification of enteroid numbers demonstrated that IGF1 at 100 and 200 ng/ml did not significantly affect numbers of enteroids formed from Sox9-EGFPLow cells vs. untreated controls at 4 and 10 days after plating. B) Representative photographs of enteroids grown from Sox9-EGFPHigh cells in absence or presence of IGF1 at 100 and 200 ng/ml at 10 days after plating. In the absence of IGF1, Sox9-EGFPHigh cells yielded no enteroids. Addition of exogenous IGF1 resulted in the ability of Sox9-EGFPHigh cells to yield enteroids. IGF1 impact on enteroid formation from Sox9-EGFPHigh cells was similar at all doses of IGF1 tested.
DISCUSSION
A major goal of our study was to assess the effects of a short-term IGF1 treatment on ISCs during normal renewal of the intestinal epithelium and regeneration after complete crypt ablation induced by high-dose radiation. Consistent with previous studies in rats or mice (23, 29, 71), our data clearly demonstrate that 5 days of IGF1 treatment potently stimulated mucosal growth in basal conditions and enhanced crypt regeneration at the peak of the regenerative response after high-dose radiation in murine small intestine. Sox9-EGFP mice provided a useful model to study specific effects of IGF1 on 2 different ISC populations: 1) Sox9-EGFPLow cells that share many common features with Lgr5-expressing ISCs (2, 33) and 2) Sox9-EGFPHigh cells that we previously demonstrated to be enriched for mRNA biomarkers of reserve/facultative ISCs and to hyperproliferate and acquire the ISC characteristic of enteroid-forming ability during crypt regeneration after radiation (2). The present study validates previous work from our group and others (1, 2) demonstrating that in radiated vehicle-treated mice, regenerating crypts formed from expansion of hyper-proliferating Sox9-EGFPLow cells, akin to Lgr5 ISCs, as well as activation of ISC characteristics in Sox9-EGFPHigh cells. Most importantly, histology, flow cytometry, gene microarray, and in vitro culture data together provide evidence that IGF1 therapy activates both of these Sox9-EGFP ISC populations toward increased proliferation and stemness but by distinct regulatory mechanisms.
A significant finding of this work was that IGF1 therapy led to selective expansion of Sox9-EGFPLow ISCs and not other Sox9-EGFP–expressing cells including the Sox9-EGFPHigh cell population containing EECs and facultative ISCs nor Sox9-EGFPSublow progenitors in both nonirradiated intestine and during peak of crypt regeneration after irradiation. Selective effects of IGF1 on Sox9-EGFPLow ISCs were observed using both histology and flow cytometry. Preferential effects of IGF1 on Sox9-EGFPLow ISCs are consistent with our findings that IGF1R is enriched in Sox9-EGFPLow ISCs and previous work demonstrating that IGF1R is part of Lgr5-expressing ISC gene signature (11). Microarray data indicated that 5 days of IGF1 therapy did not alter Igf1r mRNA levels in Sox9-EGFPLow and Sox9-EGFPHigh cells, further reinforcing IGF1R involvement in IGF1 effects on Sox9-EGFP ISCs (data not shown). Activation of Sox9-EGFP ISCs mediated by IGF1/IGF1R signaling is also consistent with studies demonstrating that IGF1R supports stem cell proliferation, survival, and self-renewal in other organs (72, 73).
Together, gene microarray, histology, and 3-dimensional culture data support the model shown in Fig. 8 by demonstrating that 5 days of IGF1 therapy activates distinct mRNA signatures associated with different functional pathways and has differential impact on M- and S-phase in Sox9-EGFPLow ISCs vs. facultative Sox9-EGFPHigh ISCs in both the uninjured state and during regeneration after radiation. As presented in the Results section, some genes induced selectively by IGF1 in specific populations have been shown to be regulated by IGF1 in other systems, validating our data. However, we designed the current study to examine mRNAs regulated by IGF1 after 5 days of therapy with the realization that some mRNAs may not necessarily reflect direct or early IGF1 gene targets but would reflect functional pathways and processes regulated by IGF1 in the different Sox9-EGFP ISC populations in a normal setting or during regeneration. In Sox9-EGFPLow cells from uninjured intestines, IGF1 repressed expression of mRNAs linked to p53 signaling. This is consistent with previous studies demonstrating that IGF1 can attenuate p53 signaling (25, 74) and provides new evidence for specific repression of p53-dependent pathways in actively cycling ISCs. IGF1-induced repression of p53 signaling is also consistent with our histology data demonstrating that in uninjured intestine, IGF1 expanded Sox9-EGFPLow ISCs and increased crypt size, not by stimulating proliferation but rather by promoting cell survival because IGF1 did not impact EdU- or pH3-labeled Sox9-EGFPLow ISCs. Interestingly IGF1 supplementation did not induce significant changes in numbers or size of enteroids grown from Sox9-EGFPLow ISCs in vitro. This may be due to 3-dimensional culture system that contains multiple growth factors known to affect actively cycling ISCs. The ability of IGF1 to expand Sox9-EGFPLow ISCs in vivo and not enteroids from these cells in vitro may also indicate involvement of other cells such as mesenchyme in mediating in vivo actions of IGF1. In Sox9-EGFPLow ISCs isolated at times of peak regeneration, IGF1 repressed mRNAs linked to DDR and cell cycle arrest. IGF1-induced stimulation of genes related to cell cycle progression in regenerating Sox9-EGFPLow cells is consistent with our findings of increased proportions of Sox9-EGFPLow ISCs in G2/M-phase as marked by pH3 and the ability of IGF1 to expand Sox9-EGFPLow ISCs and increase size of regenerating crypts in vivo. Repression of the DDR by IGF1 may not be a desirable outcome in certain settings as IGF1-induced DDR repression in ISCs could favor survival of ISCs with potentially oncogenic mutations as it has been observed in some cancer cells after radiation (75, 76). However, in a situation of genotoxic insult caused by high-dose radiation and massive crypt loss associated with risk of radiation enteropathy, potential risks of down-regulation of DDR could be outweighed by benefits of trophic effects.
Figure 8.
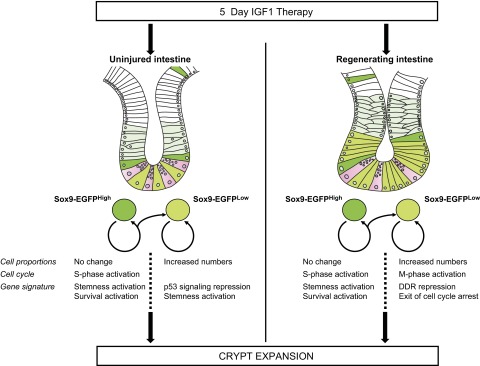
Cellular and molecular mechanisms involved in IGF1-induced crypt expansion. The schematic summarizes the cellular and molecular changes associated with crypt expansion induced by IGF1 therapy in uninjured intestine or during radiation-induced crypt regeneration.
In normal and radiated Sox9-EGFPHigh cells, our findings that IGF1 stimulated expression of mRNAs associated with stemness and survival are consistent with IGF1-induced increases in the proportion of Sox9-EGFPHigh cells in S-phase as assessed by EdU staining. In addition, only with IGF1 supplementation were Sox9-EGFPHigh cells able to generate enteroids in 3-dimensional culture, confirming and providing direct evidence that IGF1 activates facultative Sox9-EGFPHigh ISCs toward the ISC phenotype. Because we did not observe increased numbers of Sox9-EGFPHigh cells in crypts from IGF1-treated mice and we showed previously that, once activated, these cells are able to generate Sox9-EGFPLow cells (2), this supports a model where IGF1-treated Sox9-EGFPHigh cells may divide to give rise to 1 Sox9-EGFPHigh cell and 1 Sox9-EGFPLow ISC, thus participating in Sox9-EGFPLow ISC expansion and mucosal growth/crypt regeneration.
Collectively, our findings demonstrate that IGF1 activates both active and facultative Sox9-EGFP ISCs to promote crypt expansion in uninjured and regenerating intestines via distinct cellular and molecular regulatory mechanisms. To our knowledge, this study is the first reporting direct in vivo effects of a therapeutically given growth factor/hormone on 2 ISC populations. In this context, this work increases knowledge on mechanisms of IGF1 enterotrophic action and provides a model for future investigations on other growth factor/hormone impact on ISCs.
Supplementary Material
Acknowledgments
The authors thank Barry Udis, Michael Chua, Neil Kramarcy, Ashley Ezzell, Adam Gracz, Josh Robbs, and Eric Blue for technical assistance. This work was assisted by services from the University of North Carolina (UNC) Flow Cytometry core facility (Grant P30-CA06086), the Genomics and Bioinformatics core of the UNC Lineberger Comprehensive Cancer Center, the Michael Hooker Microscopy Facility, the Histology Research Core Facilities of the Center for Gastrointestinal Biology and Disease (Grant P30-DK034987), and the Department of Cell Biology and Physiology (UNC). This work was supported by grants from the North Carolina Biotechnology Center and the National Cancer Center (to L.V.L.), U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK040247-19 (to P.K.L.), and R01-DK091427 and R03-DK089126 (to S.T.M.), NIH National Institute on Aging Grant R01-AG041198-01 (to P.K.L.), and funding from the SantéDige Foundation (to L.V.L.). The authors declare no conflicts of interest.
Glossary
- CBC
crypt base columnar cell
- DDR
DNA damage response
- EdU
5-ethynyl-2′- deoxyuridine
- EEC
enteroendocrine cell
- EGFP
enhanced green fluorescent protein
- FACS
fluorescence activated cell sorting
- IEC
intestinal epithelial cell
- IGF1
insulin-like growth factor 1
- IGF1R
type 1 IGF receptor
- IPA
ingenuity pathway analysis
- ISC
intestinal stem cell
- pH3
phospho-histone H3
- Sox9
SRY (sex determining region Y)-box 9
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Hua G., Thin T. H., Feldman R., Haimovitz-Friedman A., Clevers H., Fuks Z., Kolesnick R. (2012) Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology 143, 1266–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Landeghem L., Santoro M. A., Krebs A. E., Mah A. T., Dehmer J. J., Gracz A. D., Scull B. P., McNaughton K., Magness S. T., Lund P. K. (2012) Activation of two distinct Sox9-EGFP-expressing intestinal stem cell populations during crypt regeneration after irradiation. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G1111–G1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan K. S., Chia L. A., Li X., Ootani A., Su J., Lee J. Y., Su N., Luo Y., Heilshorn S. C., Amieva M. R., Sangiorgi E., Capecchi M. R., Kuo C. J. (2012) The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl. Acad. Sci. USA 109, 466–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker N., van Oudenaarden A., Clevers H. (2012) Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell 11, 452–460 [DOI] [PubMed] [Google Scholar]
- 5.Crosnier C., Stamataki D., Lewis J. (2006) Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat. Rev. Genet. 7, 349–359 [DOI] [PubMed] [Google Scholar]
- 6.Sancho E., Batlle E., Clevers H. (2003) Live and let die in the intestinal epithelium. Curr. Opin. Cell Biol. 15, 763–770 [DOI] [PubMed] [Google Scholar]
- 7.Barker N., van Es J. H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P. J., Clevers H. (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 [DOI] [PubMed] [Google Scholar]
- 8.Cheng H., Leblond C. P. (1974) Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am. J. Anat. 141, 537–561 [DOI] [PubMed] [Google Scholar]
- 9.Van der Flier L. G., van Gijn M. E., Hatzis P., Kujala P., Haegebarth A., Stange D. E., Begthel H., van den Born M., Guryev V., Oving I., van Es J. H., Barker N., Peters P. J., van de Wetering M., Clevers H. (2009) Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell 136, 903–912 [DOI] [PubMed] [Google Scholar]
- 10.Van der Flier L. G., Haegebarth A., Stange D. E., van de Wetering M., Clevers H. (2009) OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology 137, 15–17 [DOI] [PubMed] [Google Scholar]
- 11.Muñoz J., Stange D. E., Schepers A. G., van de Wetering M., Koo B. K., Itzkovitz S., Volckmann R., Kung K. S., Koster J., Radulescu S., Myant K., Versteeg R., Sansom O. J., van Es J. H., Barker N., van Oudenaarden A., Mohammed S., Heck A. J., Clevers H. (2012) The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. EMBO J. 31, 3079–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sangiorgi E., Capecchi M. R. (2008) Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 40, 915–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeda N., Jain R., LeBoeuf M. R., Wang Q., Lu M. M., Epstein J. A. (2011) Interconversion between intestinal stem cell populations in distinct niches. Science 334, 1420–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell A. E., Wang Y., Li Y., Poulin E. J., Means A. L., Washington M. K., Higginbotham J. N., Juchheim A., Prasad N., Levy S. E., Guo Y., Shyr Y., Aronow B. J., Haigis K. M., Franklin J. L., Coffey R. J. (2012) The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 149, 146–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breault D. T., Min I. M., Carlone D. L., Farilla L. G., Ambruzs D. M., Henderson D. E., Algra S., Montgomery R. K., Wagers A. J., Hole N. (2008) Generation of mTert-GFP mice as a model to identify and study tissue progenitor cells. Proc. Natl. Acad. Sci. U S A 105, 10420–10425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potten C. S., Kovacs L., Hamilton E. (1974) Continuous labelling studies on mouse skin and intestine. Cell Tissue Kinet. 7, 271–283 [DOI] [PubMed] [Google Scholar]
- 17.Tian H., Biehs B., Warming S., Leong K. G., Rangell L., Klein O. D., de Sauvage F. J. (2011) A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478, 255–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lund P. K. (2012) Fixing the breaks in intestinal stem cells after radiation: a matter of DNA damage and death or DNA repair and regeneration. Gastroenterology 143, 1144–1147 [DOI] [PubMed] [Google Scholar]
- 19.Montgomery R. K., Carlone D. L., Richmond C. A., Farilla L., Kranendonk M. E., Henderson D. E., Baffour-Awuah N. Y., Ambruzs D. M., Fogli L. K., Algra S., Breault D. T. (2011) Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc. Natl. Acad. Sci. USA 108, 179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendry J. H., Roberts S. A., Potten C. S. (1992) The clonogen content of murine intestinal crypts: dependence on radiation dose used in its determination. Radiat. Res. 132, 115–119 [PubMed] [Google Scholar]
- 21.Booth D., Haley J. D., Bruskin A. M., Potten C. S. (2000) Transforming growth factor-B3 protects murine small intestinal crypt stem cells and animal survival after irradiation, possibly by reducing stem-cell cycling. Int. J. Cancer 86, 53–59 [DOI] [PubMed] [Google Scholar]
- 22.Farrell C. L., Bready J. V., Rex K. L., Chen J. N., DiPalma C. R., Whitcomb K. L., Yin S., Hill D. C., Wiemann B., Starnes C. O., Havill A. M., Lu Z. N., Aukerman S. L., Pierce G. F., Thomason A., Potten C. S., Ulich T. R., Lacey D. L. (1998) Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Res. 58, 933–939 [PubMed] [Google Scholar]
- 23.Howarth G. S., Fraser R., Frisby C. L., Schirmer M. B., Yeoh E. K. (1997) Effects of insulin-like growth factor-I administration on radiation enteritis in rats. Scand. J. Gastroenterol. 32, 1118–1124 [DOI] [PubMed] [Google Scholar]
- 24.Wilkins H. R., Ohneda K., Keku T. O., D’Ercole A. J., Fuller C. R., Williams K. L., Lund P. K. (2002) Reduction of spontaneous and irradiation-induced apoptosis in small intestine of IGF-I transgenic mice. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G457–G464 [DOI] [PubMed] [Google Scholar]
- 25.Qiu W., Leibowitz B., Zhang L., Yu J. (2010) Growth factors protect intestinal stem cells from radiation-induced apoptosis by suppressing PUMA through the PI3K/AKT/p53 axis. Oncogene 29, 1622–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howarth G. S., Xian C. J., Read L. C. (1998) Insulin-like growth factor-I partially attenuates colonic damage in rats with experimental colitis induced by oral dextran sulphate sodium. Scand. J. Gastroenterol. 33, 180–190 [DOI] [PubMed] [Google Scholar]
- 27.Lemmey A. B., Ballard F. J., Martin A. A., Tomas F. M., Howarth G. S., Read L. C. (1994) Treatment with IGF-I peptides improves function of the remnant gut following small bowel resection in rats. Growth Factors 10, 243–252 [DOI] [PubMed] [Google Scholar]
- 28.Gillingham M. B., Dahly E. M., Murali S. G., Ney D. M. (2003) IGF-I treatment facilitates transition from parenteral to enteral nutrition in rats with short bowel syndrome. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R363–R371 [DOI] [PubMed] [Google Scholar]
- 29.Bortvedt S. F., Lund P. K. (2012) Insulin-like growth factor 1: common mediator of multiple enterotrophic hormones and growth factors. Curr. Opin. Gastroenterol. 28, 89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMellen M. E., Wakeman D., Longshore S. W., McDuffie L. A., Warner B. W. (2010) Growth factors: possible roles for clinical management of the short bowel syndrome. Semin. Pediatr. Surg. 19, 35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garrison A. P., Dekaney C. M., von Allmen D. C., Lund P. K., Henning S. J., Helmrath M. A. (2009) Early but not late administration of glucagon-like peptide-2 following ileo-cecal resection augments putative intestinal stem cell expansion. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G643–G650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dubé P. E., Brubaker P. L. (2007) Frontiers in glucagon-like peptide-2: multiple actions, multiple mediators. Am. J. Physiol. Endocrinol. Metab. 293, E460–E465 [DOI] [PubMed] [Google Scholar]
- 33.Gracz A. D., Ramalingam S., Magness S. T. (2010) Sox9 expression marks a subset of CD24-expressing small intestine epithelial stem cells that form organoids in vitro. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G590–G600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Formeister E. J., Sionas A. L., Lorance D. K., Barkley C. L., Lee G. H., Magness S. T. (2009) Distinct SOX9 levels differentially mark stem/progenitor populations and enteroendocrine cells of the small intestine epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1108–G1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buczacki S. J., Zecchini H. I., Nicholson A. M., Russell R., Vermeulen L., Kemp R., Winton D. J. (2013) Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495, 65–69 [DOI] [PubMed] [Google Scholar]
- 36.Van Es J. H., Sato T., van de Wetering M., Lyubimova A., Nee A. N., Gregorieff A., Sasaki N., Zeinstra L., van den Born M., Korving J., Martens A. C., Barker N., van Oudenaarden A., Clevers H. (2012) Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat. Cell Biol. 14, 1099–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Landeghem L., Blue R. E., Dehmer J. J., Henning S. J., Helmrath M. A., Lund P. K. (2012) Localized intestinal radiation and liquid diet enhance survival and permit evaluation of long-term intestinal responses to high dose radiation in mice. PLoS ONE 7, e51310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ge R. T., Mo L. H., Wu R., Liu J. Q., Zhang H. P., Liu Z., Yang P. C. (2015) Insulin-like growth factor-1 endues monocytes with immune suppressive ability to inhibit inflammation in the intestine. Scientific Rep. 5, 7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuemmerle J. F. (2006) Occupation of alphavbeta3-integrin by endogenous ligands modulates IGF-I receptor activation and proliferation of human intestinal smooth muscle. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G1194–G1202 [DOI] [PubMed] [Google Scholar]
- 40.Nagashima M., Shiseki M., Pedeux R. M., Okamura S., Kitahama-Shiseki M., Miura K., Yokota J., Harris C. C. (2003) A novel PHD-finger motif protein, p47ING3, modulates p53-mediated transcription, cell cycle control, and apoptosis. Oncogene 22, 343–350 [DOI] [PubMed] [Google Scholar]
- 41.Coutts A. S., Weston L., La Thangue N. B. (2009) A transcription co-factor integrates cell adhesion and motility with the p53 response. Proc. Natl. Acad. Sci. USA 106, 19872–19877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crighton D., Wilkinson S., O’Prey J., Syed N., Smith P., Harrison P. R., Gasco M., Garrone O., Crook T., Ryan K. M. (2006) DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell 126, 121–134 [DOI] [PubMed] [Google Scholar]
- 43.Xu Y., Baltimore D. (1996) Dual roles of ATM in the cellular response to radiation and in cell growth control. Genes Dev. 10, 2401–2410 [DOI] [PubMed] [Google Scholar]
- 44.Theus M. H., Ricard J., Bethea J. R., Liebl D. J. (2010) EphB3 limits the expansion of neural progenitor cells in the subventricular zone by regulating p53 during homeostasis and following traumatic brain injury. Stem Cells 28, 1231–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kosinski C., Stange D. E., Xu C., Chan A. S., Ho C., Yuen S. T., Mifflin R. C., Powell D. W., Clevers H., Leung S. Y., Chen X. (2010) Indian hedgehog regulates intestinal stem cell fate through epithelial-mesenchymal interactions during development. Gastroenterology 139, 893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katoh M. (2002) Regulation of WNT signaling molecules by retinoic acid during neuronal differentiation in NT2 cells: threshold model of WNT action (review). Int. J. Mol. Med. 10, 683–687 [PubMed] [Google Scholar]
- 47.Pera M. F., Andrade J., Houssami S., Reubinoff B., Trounson A., Stanley E. G., Ward-van Oostwaard D., Mummery C. (2004) Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J. Cell Sci. 117, 1269–1280 [DOI] [PubMed] [Google Scholar]
- 48.Massagué J., Xi Q. (2012) TGF-β control of stem cell differentiation genes. FEBS Lett. 586, 1953–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardwick J. C., Van Den Brink G. R., Bleuming S. A., Ballester I., Van Den Brande J. M., Keller J. J., Offerhaus G. J., Van Deventer S. J., Peppelenbosch M. P. (2004) Bone morphogenetic protein 2 is expressed by, and acts upon, mature epithelial cells in the colon. Gastroenterology 126, 111–121 [DOI] [PubMed] [Google Scholar]
- 50.Wang B., Matsuoka S., Ballif B. A., Zhang D., Smogorzewska A., Gygi S. P., Elledge S. J. (2007) Abraxas and RAP80 form a BRCA1 protein complex required for the DNA damage response. Science 316, 1194–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stracker T. H., Petrini J. H. (2011) The MRE11 complex: starting from the ends. Nat. Rev. Mol. Cell Biol. 12, 90–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Becker K. A., Stein J. L., Lian J. B., van Wijnen A. J., Stein G. S. (2007) Establishment of histone gene regulation and cell cycle checkpoint control in human embryonic stem cells. J. Cell. Physiol. 210, 517–526 [DOI] [PubMed] [Google Scholar]
- 53.Muchardt C., Yaniv M. (2001) When the SWI/SNF complex remodels...the cell cycle. Oncogene 20, 3067–3075 [DOI] [PubMed] [Google Scholar]
- 54.Ho L., Ronan J. L., Wu J., Staahl B. T., Chen L., Kuo A., Lessard J., Nesvizhskii A. I., Ranish J., Crabtree G. R. (2009) An embryonic stem cell chromatin remodeling complex, esBAF, is essential for embryonic stem cell self-renewal and pluripotency. Proc. Natl. Acad. Sci. USA 106, 5181–5186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McConnell B. B., Kim S. S., Bialkowska A. B., Yu K., Sitaraman S. V., Yang V. W. (2011) Kruppel-like factor 5 protects against dextran sulfate sodium-induced colonic injury in mice by promoting epithelial repair. Gastroenterology 140, 540–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McConnell B. B., Ghaleb A. M., Nandan M. O., Yang V. W. (2007) The diverse functions of Krüppel-like factors 4 and 5 in epithelial biology and pathobiology. BioEssays 29, 549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nandan M. O., Chanchevalap S., Dalton W. B., Yang V. W. (2005) Krüppel-like factor 5 promotes mitosis by activating the cyclin B1/Cdc2 complex during oncogenic Ras-mediated transformation. FEBS Lett. 579, 4757–4762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalia S. K., Lee S., Smith P. D., Liu L., Crocker S. J., Thorarinsdottir T. E., Glover J. R., Fon E. A., Park D. S., Lozano A. M. (2004) BAG5 inhibits parkin and enhances dopaminergic neuron degeneration. Neuron 44, 931–945 [DOI] [PubMed] [Google Scholar]
- 59.Liu Z., Li H., Wu X., Yoo B. H., Yan S. R., Stadnyk A. W., Sasazuki T., Shirasawa S., LaCasse E. C., Korneluk R. G., Rosen K. V. (2006) Detachment-induced upregulation of XIAP and cIAP2 delays anoikis of intestinal epithelial cells. Oncogene 25, 7680–7690 [DOI] [PubMed] [Google Scholar]
- 60.Xu H., Balakrishnan K., Malaterre J., Beasley M., Yan Y., Essers J., Appeldoorn E., Tomaszewski J. M., Vazquez M., Verschoor S., Lavin M. F., Bertoncello I., Ramsay R. G., McKay M. J. (2010) Rad21-cohesin haploinsufficiency impedes DNA repair and enhances gastrointestinal radiosensitivity in mice. PLoS ONE 5, e12112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nitzsche A., Paszkowski-Rogacz M., Matarese F., Janssen-Megens E. M., Hubner N. C., Schulz H., de Vries I., Ding L., Huebner N., Mann M., Stunnenberg H. G., Buchholz F. (2011) RAD21 cooperates with pluripotency transcription factors in the maintenance of embryonic stem cell identity. PLoS ONE 6, e19470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rehman J. (2010) Empowering self-renewal and differentiation: the role of mitochondria in stem cells. J. Mol. Med. 88, 981–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thelen K., Dressman J. B. (2009) Cytochrome P450-mediated metabolism in the human gut wall. J. Pharm. Pharmacol. 61, 541–558 [DOI] [PubMed] [Google Scholar]
- 64.Meyn M. A. III, Schreiner S. J., Dumitrescu T. P., Nau G. J., Smithgall T. E. (2005) SRC family kinase activity is required for murine embryonic stem cell growth and differentiation. Mol. Pharmacol. 68, 1320–1330 [DOI] [PubMed] [Google Scholar]
- 65.Lakshmikuttyamma A., Pastural E., Takahashi N., Sawada K., Sheridan D. P., DeCoteau J. F., Geyer C. R. (2008) Bcr-Abl induces autocrine IGF-1 signaling. Oncogene 27, 3831–3844 [DOI] [PubMed] [Google Scholar]
- 66.Cobaleda C., Pérez-Caro M., Vicente-Dueñas C., Sánchez-García I. (2007) Function of the zinc-finger transcription factor SNAI2 in cancer and development. Annu. Rev. Genet. 41, 41–61 [DOI] [PubMed] [Google Scholar]
- 67.Lau M. T., Leung P. C. (2012) The PI3K/Akt/mTOR signaling pathway mediates insulin-like growth factor 1-induced E-cadherin down-regulation and cell proliferation in ovarian cancer cells. Cancer Lett. 326, 191–198 [DOI] [PubMed] [Google Scholar]
- 68.Wu W. S., Heinrichs S., Xu D., Garrison S. P., Zambetti G. P., Adams J. M., Look A. T. (2005) Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell 123, 641–653 [DOI] [PubMed] [Google Scholar]
- 69.Karrich J. J., Balzarolo M., Schmidlin H., Libouban M., Nagasawa M., Gentek R., Kamihira S., Maeda T., Amsen D., Wolkers M. C., Blom B. (2012) The transcription factor Spi-B regulates human plasmacytoid dendritic cell survival through direct induction of the antiapoptotic gene BCL2-A1. Blood 119, 5191–5200 [DOI] [PubMed] [Google Scholar]
- 70.Sato T., Vries R. G., Snippert H. J., van de Wetering M., Barker N., Stange D. E., van Es J. H., Abo A., Kujala P., Peters P. J., Clevers H. (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 [DOI] [PubMed] [Google Scholar]
- 71.Steeb C. B., Trahair J. F., Read L. C. (1995) Administration of insulin-like growth factor-I (IGF-I) peptides for three days stimulates proliferation of the small intestinal epithelium in rats. Gut 37, 630–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang L., Schulz T. C., Sherrer E. S., Dauphin D. S., Shin S., Nelson A. M., Ware C. B., Zhan M., Song C. Z., Chen X., Brimble S. N., McLean A., Galeano M. J., Uhl E. W., D’Amour K. A., Chesnut J. D., Rao M. S., Blau C. A., Robins A. J. (2007) Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood 110, 4111–4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bendall S. C., Stewart M. H., Menendez P., George D., Vijayaragavan K., Werbowetski-Ogilvie T., Ramos-Mejia V., Rouleau A., Yang J., Bossé M., Lajoie G., Bhatia M. (2007) IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature 448, 1015–1021 [DOI] [PubMed] [Google Scholar]
- 74.Leri A., Liu Y., Wang X., Kajstura J., Malhotra A., Meggs L. G., Anversa P. (1999) Overexpression of insulin-like growth factor-1 attenuates the myocyte renin-angiotensin system in transgenic mice. Circ. Res. 84, 752–762 [DOI] [PubMed] [Google Scholar]
- 75.Goetz E. M., Shankar B., Zou Y., Morales J. C., Luo X., Araki S., Bachoo R., Mayo L. D., Boothman D. A. (2011) ATM-dependent IGF-1 induction regulates secretory clusterin expression after DNA damage and in genetic instability. Oncogene 30, 3745–3754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Osuka S., Sampetrean O., Shimizu T., Saga I., Onishi N., Sugihara E., Okubo J., Fujita S., Takano S., Matsumura A., Saya H. (2013) IGF1 receptor signaling regulates adaptive radioprotection in glioma stem cells. Stem Cells 31, 627–640 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.