Abstract
Understanding periodontal ligament (PDL) biology and developing an effective treatment for bone and PDL damage due to periodontitis have been long-standing aims in dental medicine. Here, we first demonstrated by cell lineage tracing and mineral double-labeling approaches that murine PDL progenitor cells display a 2- and 3-fold higher mineral deposition rate than the periosteum and endosteum at the age of 4 weeks, respectively. We next proved that the pathologic changes in osteocytes (Ocys; changes from a spindle shape to round shape with a >50% reduction in the dendrite number/length, and an increase in SOST) are the key pathologic factors responsible for bone and PDL damage in periostin-null mice (a periodontitis animal model) using a newly developed 3-dimensional FITC-Imaris technique. Importantly, we proved that deleting the Sost gene (a potent inhibitor of WNT signaling) or blocking sclerostin function by using the mAb in this periodontitis model significantly restores bone and PDL defects (n = 4–5; P < 0.05). Together, identification of the key contribution of the PDL in normal alveolar bone formation, the pathologic changes of the Ocys in periodontitis bone loss, and the novel link between sclerostin and Wnt signaling in the PDL will aid future drug development in the treatment of patients with periodontitis.—Ren, Y., Han, X., Ho, S. P., Harris, S. E., Cao, Z., Economides, A. N., Qin, C., Ke, H., Liu, M., Feng, J. Q. Removal of SOST or blocking its product sclerostin rescues defects in the periodontitis mouse model.
Keywords: PDL, periostin, alveolar bone
The periodontal ligament (PDL), positioned between the bone-forming socket wall and the cementum covering the tooth root, was named in part because of the rich fibers in this unique structure. These tendon-like fibers connect the tooth to the jawbone, support the tooth in the socket, and cushion the teeth from the loads imposed on them (by tooth movement and occlusal force). Periodontitis, the most common physical disorder known to mankind, is defined as a set of complex diseases that include different forms of gingivitis and periodontitis. The causes of periodontitis are multiple and thought to be of developmental, inflammatory, traumatic, neoplastic, genetic, or metabolic origin. One major cause is pathogenic microflora in the biofilm or dental plaque, which leads to an inflammatory state and loss of bone around the tooth and PDL. As the mildest form of periodontal disease, gingivitis affects 50–90% of adults worldwide. The inflammation that extends deep into the tissues and causes the loss of supporting connective tissue and alveolar bone is known as “periodontitis” (1). The advanced form results in the impairment of connective tissue and bone and is a major cause of tooth loss in adults, occurring in 10–15% of adults in population studies (2–4). Moderate periodontitis is estimated to affect a larger number of people (5). There is also a mutual relationship between periodontitis and diabetes [i.e., diabetes increases the risk for periodontitis, and periodontitis negatively disturbs glycemic control (5–10)]. The development of an effective treatment for the bone loss caused by these diseases has been a long-standing aim in dentistry (1).
Wnt signaling plays a critical role in a variety of stem cell commitment pathways (11). The removal of Wnt activity in the periodontium results in major defects in the PDL and alveolar bone formation (12). On the other hand, enhancing Wnt activity by removing its antagonist, sclerostin (the product of the Sost gene), leads to an increase in alveolar bone volume (BV) and reduced PDL width (13). Furthermore, the sclerostin antibody (Scl-Ab) has been shown to have great efficacy in the treatment of a number of preclinical animal models and clinical trials of osteoporosis and bone fracture healing (14–18). Remarkably, this mAb can be used to treat inflammation-caused bone loss such as that in the colitis animal model (19) and periodontitis rat model (20).
Periostin, a key matrix protein required for PDL formation, is highly expressed in the PDL cells during adult life, and periostin-knockout (PKO) mice have been used for studies of periodontal diseases (21–23). In addition, it was reported that there was a significant increase in SOST expression in the PKO long bone (24). In this study, we sought to test the idea that osteocytes (Ocys), through the production of sclerostin, negatively impact the stem cell formation and differentiation of these progenitors in the periodontium by blocking Wnt signaling. By crossing Sost-knockout (KO) mice with the PKO mice, we showed that many of the periodontium and Ocy defects can be prevented in these double-knockout (DKO) mice. Using 2 different age groups of Scl-Ab PKO mice, we successfully rescued the major defects in the periodontium in these KO mice. These studies raise new hope for the future treatment of patients with periodontitis because there is currently no surgical or drug method that can be used to restore the damaged PDL structure.
MATERIALS AND METHODS
Mice, Scl-Ab treatment, adenovirus injection, and double labeling
A total of 48 PKO mice (22) and age-matched control mice with the C57BL/6 background were divided into 2 age groups: 1 and 3 months (n = 6). The mice were intraperitoneally injected with either Scl-Ab at 25 mg/kg (twice a week) or PBS for 8 weeks. The mice were euthanized at the ages of 3 and 5 months, respectively.
One-month-old Rosa26 mice (The Jackson Laboratory, Bar Harbor, ME, USA) were subjected to a local injection of Ad-CMV-Cre (5 × 106 particles; purchased from Baylor College of Medicine, Vector Development Laboratory, Houston, TX, USA) using a 0.2 mm fine needle in the lower jaw around the molars. Samples were collected at 2 hours, 5 days, and 10 days postinjection for cell lineage tracing using an X-gal staining assay as previously described (25).
DKO mice were generated by breeding PKO (21) and Sost-KO mice (26). All animal protocols were approved by the Animal Care and Use Committee at Texas A&M University Health Science Center Baylor College of Dentistry.
For measurement of the bone formation rate in the alveolar bone from the periosteum, endosteum, and PDL, double-fluorescence labeling was performed as described previously (27, 28). Briefly, calcein green (Fluka 21030, 5mg/kg; Sigma-Aldrich, St. Louis, MO, USA) was injected intraperitoneally 7 days before euthanasia, followed by alizarin red (catalog no. A3882, 20mg/kg; Sigma-Aldrich) injections 2 days before euthanasia. The distance between calcein green and alizarin red labeling is quantified to calculate bone growth rate.
Sample preparation and histochemistry
The right-lower jaws were fixed in 70% ethanol and used for radiographs, microcomputed tomography (μCT), FITC, scanning electron microscopy, and double-labeling analyses. The left-lower jaws were fixed in freshly prepared 4% paraformaldehyde in PBS (pH 7.4), decalcified, and embedded in paraffin using standard histologic procedures as previously described (29). The tissue blocks were cut into 4-μm-thick mesio-distal serial sections and mounted on glass slides. The sections were used for histologic stains using hematoxylin and eosin (H&E), tartrate-resistant acid phosphatase (TRAP), Sirius Red, and immunohistochemistry: 1:400 dentin matrix acidic phosphoprotein 1 (DMP1), donated by Dr. Chunlin Qin (Baylor College of Dentistry); 1:1000 biglycan and decorin, generously provided by Dr. Larry Fisher (National Institutes of Health, Bethesda, MD, USA); 1:1000 periostin, Innovative Research (Sarasota, FL, USA); 1:400 osterix (OSX), Abcam Incorporated (Cambridge, MA, USA); and 1:400 SOST, R&D Systems (Minneapolis, MN, USA).
The left-jaw specimens were dehydrated through a graded series of ethanol (70–100%) and embedded in methyl-methacrylate (MMA; Buehler, Lake Bluff, IL, USA) without decalcification. Twenty-micrometer sections were cut and viewed under the Leica Sp5 confocal microscope (Leica Microsystems, Buffalo Grove, IL, USA). The mean distance between the 2 fluorescent labels was determined and divided by the number of days between labels to calculate the deposition rate of bone from different origins (periosteum versus endosteum versus PDL).
Backscattered scanning electron microscopy and acid-etched scanning electron microscopy
The MMA-embedded blocks were sectioned through the center of the first mandibular molar using a water-cooled diamond-impregnated circular saw (IsoMet; Buehler). The surfaces of the sample blocks were polished using 1, 0.3, and 0.05 μm Alumina α MicroPolish II solutions (Buehler) with a soft cloth rotating wheel (27). Each sample was then cleaned in an ultrasonic bath followed by air-drying for sputter coating with carbon and scanning with a backscattered electron detector in a JEOL JSM-6300 scanning electron microscope (JEOL Limited, Tokyo, Japan). The parameters were kept constant while the backscattered scanning electron microscopy images were taken. After backscattered scanning, the sample surfaces were repolished following the same procedure described above. The surfaces were then acid etched with 37% phosphoric acid for 2–10 seconds, followed by 5% sodium hypochlorite for 20 minutes. The samples were immediately air-dried and sputter coated with gold and palladium, as described previously (30, 31), and analyzed under a scanning electron microscope.
FITC staining and Imaris analysis
Staining with FITC (32), a small molecular dye, fills in the PDL cells/fibers, as well as the Ocy cells, but does not enter the mineral matrix. Thus, the dye provides a visual representation of the organization of the PDL and Ocys under the confocal microscope. The jawbones were dehydrated through a series of ethanol solutions from 70–100% and acetone solution, followed by FITC stain (catalog no. F7250; Sigma-Aldrich) overnight, with additional dehydration and MMA embedding as described above. A cross section (300–400 μm thick) was cut with a diamond-bladed saw (Buehler), and the plastic sections were then sanded and ground to a final thickness of 30–50 μm for confocal imaging. The stacked pictures were processed through AutoQuant (MediaCybernetics, Rockville, MD, USA) for deconvolution and subsequently analyzed by Imaris (Bitplane, Zurich, Switzerland) to quantify the Ocy cell surface area, volume, and dendrite ending points as previously described (33).
Radiograph, μCT, and cementum-enamel junction-bone level area quantification
The mandibles from 4 different groups in both age sets (3 and 5 months) were dissected and analyzed by radiography (piXarray 100; Micro Photonics, Allentown, PA, USA) and by a μ-CT35 imaging system (Scanco Medical, Basserdorf, Switzerland). For the μCT analyses, we initially used a medium-resolution scan (7.0 μm slice increments) of the whole mandible to obtain an overall assessment of the tooth shape and structure. We then took a high-resolution scan (3.5 μm slice increments) of the mandible with 400 slices selected for analyses of the alveolar bone around the first molar. Of note, the tooth and PDL were excluded when contouring. The BV-to-total volume (TV) ratio was analyzed using Scanco Medical software with the data reported as the mean ± se. We used a method described by Souza et al. (34) and Kuhr et al. (35) to quantify the area under the cementum-enamel junction (CEJ), reflecting periodontal bone loss. Briefly, the lost bone area included the alveolar bone crest and CEJ in the mesial root of the first molar and the distal root of the third molar. The 3-dimensional (3D) mandible images were virtually sectioned to expose both root canals of the 3 molars in order to align the mandible perpendicularly, and then the 3D images were reconstructed for quantification. Finally, the lost bone area was contoured and calculated using ImageJ (NIH, Bethesda, MD, USA).
Micro X-ray computed tomography (Micro XCT)
The mandibles from wild-type (WT), PKO, and DKO mice at the age of 5 mo were dissected and analyzed by micro X-ray computed tomography (Micro XCT; Xradia, Pleasanton, CA, USA) at ×20 at a tungsten anode setting of 20 KVp and 4 W followed by 3D reconstruction of movies using a method previously described (36). It is of note that the total scanned bone thickness was ∼250–300 μm.
Statistical analyses
Statistical significance was determined by 1-way ANOVA followed by Bonferroni post hoc comparisons between 2 groups using SPSS 13.0 (IBM SPSS, Chicago, IL, USA). A P value <0.05 was considered statistically significant.
RESULTS
Alveolar bone formation is mainly derived from cells in the PDL
It is known that alveolar bone is formed from progenitor cells in periosteum, endosteum, and the PDL. To address the contribution of each cell resource, we compared the bone formation rate using prelabeled fluorochrome specimens (first, calcein injection, and then Alizarin Red injection 5 days later) from 1-month-old mouse jawbones. The double-labeling confocal images demonstrated a statistically significant difference among these regions with the fastest deposition rate next to the PDL, which is 2- and 3-fold faster in the PDL than in the periosteum and endosteum, respectively (Fig. 1A; P < 0.05). When the dye-labeling pattern was closely observed at the alveolar bone-PDL boundary, a weak mineralizing region was noted (orange in Fig. 1B) that represents the mineral derived from the PDL cells. FITC labeling of the PDL-bone interface further confirmed that the newly formed Ocys originated from the PDL (Fig. 1C). Using Rosa26-loxP-stop-loxP-LacZ mice, we injected Ad-CMV-Cre into only the periodontium. Over time (2 hours, 5 days, and 10 days after infections), we found the LacZ+ cells in the PDL on the bone surface and inside, following a time-dependent sequence (Fig. 1D). These results extend and confirm the finding that the stem cells in the PDL region form the major part of the alveolar bone next to the tooth (37).
Figure 1.
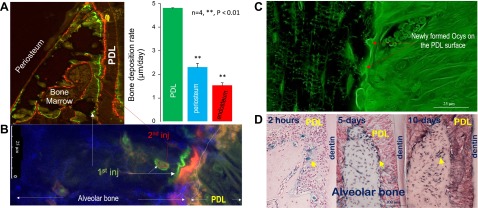
The PDL is the major resource of progenitor cells in alveolar bone formation (1-month-old murine mandible). A) The double-labeling confocal images show the widest distance between the green (first injection) and red (second injection) lines in the PDL-alveolar bone surface than in the periosteum and endosteum with a statistical difference among them (right panel). B) The enlarged fluorochrome image reveals a double-labeled layer surrounding the Ocys plus yellow-colored minerals (overlaps of green and red labels) on the PDL-alveolar bone surface, indicating the contribution of Ocys in mineralization. inj, injection. C) The FITC confocal images display a few newly formed Ocys on the alveolar bone-PDL surface, supporting the notion that the bone cells originated from the PDL. D) The X-gal-stained jawbone, in which a 1-time Ad-CMV-Cre local infection in the periosteum and PDL was performed 2 hours, 5 days, and 10 days before sample harvests, is shown. The blue cells reflect their origins from either the periosteum (left) or PDL (right) progenitors.
Removal of the Sost gene or injections of Scl-Ab at the age of 1 month averted development of the periodontal phenotype in PKO mice
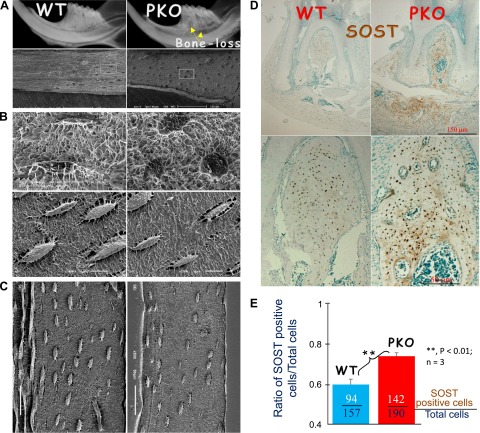
Previously, we showed that PKO mice develop a periodontitis-like phenotype due to the loss of PDL integrity (21, 22). Here, we reexamined PKO jawbone using X-ray, which indicated signs of bone loss (Fig. 2A). Acid-etched scanning electron microscopy images demonstrated an unpredicted change (from spindle to round shape) in the Ocys of the jawbone (Fig. 2B) but with no apparent changes in the PKO long bone (Fig. 2C). Moreover, there was a sharp increase in the SOST expression in the PKO jawbone (Fig. 2D). The quantitative data showed a significant difference in the ratio of SOST-positive Ocys:total Ocys between the PKO and the age-matched control (Fig. 2E). These data support the notion that the local morphologic changes in Ocy and the increased SOST expression are closely associated with the PKO bone loss.
Figure 2.
Deletion of periostin led to local changes of Ocys and a sharp increase in SOST in the PKO jawbone, but no Ocy morphology changes in the long bone. A) Representative radiographs reveal bone loss in PKO alveolar bone (right). B) Acid-etched scanning electron microscopy images display a sharp change of Ocys in jawbones from spindle to round in PKO mice. C) Acid-etched scanning electron microscopy images display no apparent change of Ocys in PKO long bone. D and E) The immunohistochemistry stain images display a dramatic increase in SOST in the PKO mandibular bone (D, right), and the quantitative data showed that the ratio of the SOST-positive Ocys:total Ocys is significantly different between the WT and PKO jawbones (a total of 1064 Ocys were counted in 3 pairs of groups) (E).
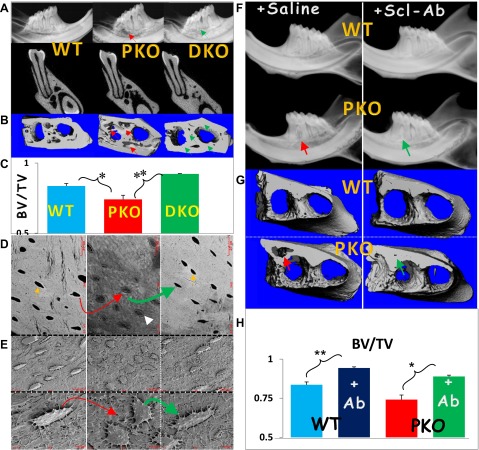
Because Sost-KO mice develop a large bone mass in both the long bone (38) and jawbone (13), we first asked if deletion of the Sost gene will change morphologies in KO jawbone. Interestingly, the murine Sost-KO jawbones developed an osteon-like structure, the basic bone function unit that occurs only in mammalians but not in rodents, by using the H&E staining, polarized light microscopy, and scanning electron microscopy techniques (Supplemental Fig. S1). We then reasoned that removing Sost from PKO mice could rescue bone loss from PKO. To test this hypothesis, we generated DKO mice by crossing PKO with Sost-KO mice and showed the full prevention of the bone phenotype using radiography (Fig. 3A) and μCT qualitatively and quantitatively (Fig. 3B, C). We also demonstrated that in the DKO mice, the following pathologic changes observed in the PKO were successfully blocked: poor bone mineral matrices were revealed using the backscattered scanning electron microscopy technique and visualized as a mixed appearance of gray (low mineral) and white (high mineral) color with a lack of white rings (indicating a high mineral content) surrounding the Ocy cell bodies (Fig. 3D). In addition, the Ocy shape changed from spindle to round as assayed with acid-etched scanning electron microscopy (Fig. 3E). Furthermore, we asked whether treatment of the PKO mice with Scl-Ab, a clinical trial drug used to treat osteoporosis (39, 40), can prevent or rescue bone loss in this periodontitis model. To achieve this goal, the animals were intraperitoneally injected with Scl-Ab starting from the age of 1 month, when they display no apparent phenotype (for prevention purposes), and lasting for 8 weeks. The radiographic images (Fig. 3F), μCT images (Fig. 3G), and quantitative data (Fig. 3H) showed full prevention of bone loss in the early-treated PKO group.
Figure 3.
Restoration of bone loss in the Sost-Periostin DKO and Scl-Ab-treated PKO mice. A) Representative radiographs reveal a restoration of bone loss in the PKO jawbone by removing the Sost gene (right; DKO). B and C) The μCT data display the restoration of bone qualitatively (right images in B) and quantitatively (lower panel in C). D) Backscattered scanning electron microscopy images confirm the rescue of bone loss in DKO mice (right). E) The acid-etched scanning electron microscopy images reveal a direct correlation between changes in Ocy morphologies and the surrounding matrices in PKO (defects; middle) and DKO (rescue; right) compared to the age-matched control (left). F) Representative radiographs reveal the restoration of bone loss in the PKO by Scl-Ab treatment. G and H) The μCT data display a restoration of BV in the PKO mice qualitatively (lower right in G) and quantitatively (lower panel in H). The yellow arrows point to white mineral rings, and the white arrow indicates a poorly formed matrix with low minerals. **P < 0.01; *P < 0.05 (n = 5).
Blocking SOST function by Scl-Ab treatment completely prevented changes in Ocy morphology in early PKO mice
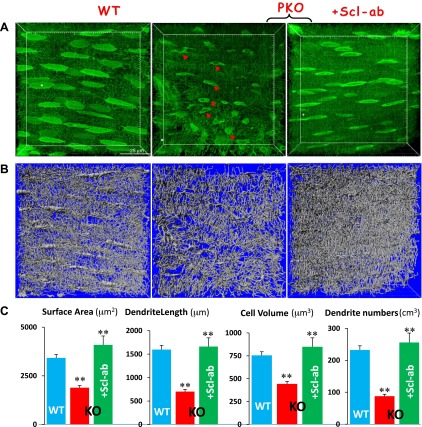
To address whether bone recovery was directly linked to the improvement of Ocy morphologies, we used a newly developed approach combining FITC images with Imaris software (33) to quantify the nondecalcified Oyc-laculo-canalicular system in an area measuring 100 × 100 × 30 μm. As shown in Fig. 4, there were significant reductions in Ocy volume and length and number of dendrites in PKO jawbones at the age of 3 months. These pathologic changes were fully restored in the Scl-Ab-treated bone, supporting the notion that the direct role of Scl-Ab on Ocy morphology is the key to recovering bone loss in this animal model.
Figure 4.
Statistically analyzing morphologic changes of Ocys in PKO jawbones with and without Scl-Ab treatment. A) Confocal FITC image shows spindle-shaped Ocys with numerous dendrites in a well-organized arrangement in the WT (left) compared to the PKO Ocys with vehicle (middle) or Scl-Ab treatment (right). B) Artificial silver images reconstructed with the Imaris software from (A) are shown. C) The statistical analysis using Imaris software reveals significant differences in Ocy surface area, dendrite length, total cell volume, and dendrite numbers among WT, PKO, and PKO Ocys treated with Scl-Ab for 8 weeks. **P < 0.01 (n = 20).
Intervention of SOST function by Scl-Ab treatment greatly improved bone loss and restored changes in Ocy morphology in late PKO mice
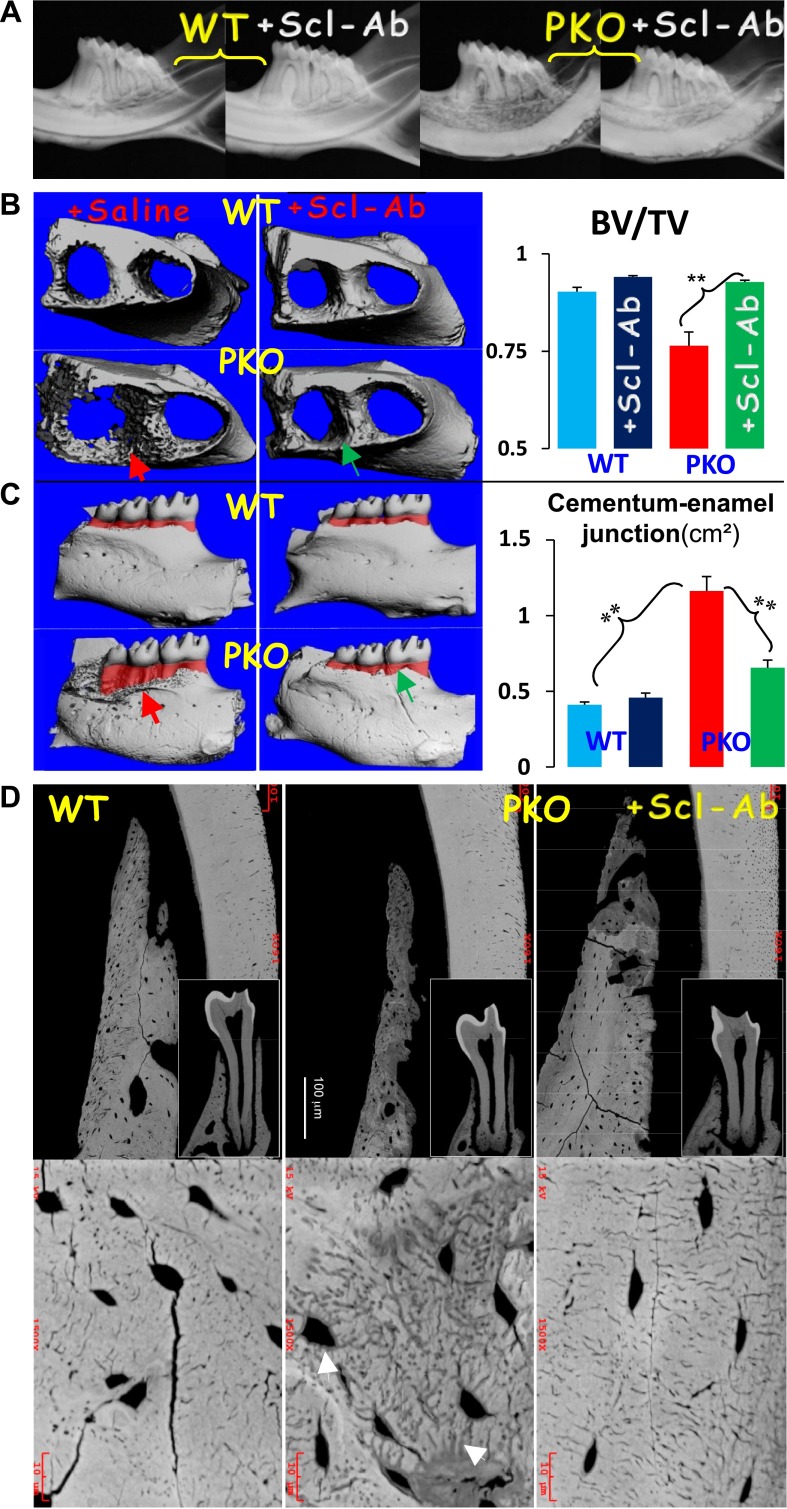
To extend the early prevention approaches using the DKO and the early treatment of Scl-Ab approaches, we examined the effectiveness of Scl-Ab on restoring bone loss in the 5-month-old PKO group (i.e., the treatment started at the age of 3 months when the periodontal phenotype is obvious in the PKO mice). Both the radiographic image (Fig. 5A) and μCT data (Fig. 5B) showed great improvement in the alveolar bone loss. We also used the distance between the alveolar bone crest and CEJ, as reflected by the bottom and the top levels of the red-highlighted area in the μCT image (Fig. 5B), to estimate the degree of alveolar bone loss. As shown in Fig. 5C, there was great improvement in the amount of bone loss in the Scl-Ab-treated group as indicated by the statistical significance. In addition, the backscattered scanning electron microscopy images (Fig. 5D) displayed a severe BV reduction throughout the alveolar bone and a great loss of minerals surrounding the Oyc-lacuna-canalicular system in the PKO alveolar bone (middle), which was restored in the Scl-Ab-treated jawbone (right). These data support a close association between the changes of Ocy morphology and the matrix mineral contents in the nontreated and treated groups in this animal model.
Figure 5.
Restoration of bone loss, defective CEJ area, and defective Ocy morphology in the 5-month-old PKO after Scl-Ab treatment for 8 weeks. A) Representative radiographic images display severe bone loss in the nontreated PKO jawbone (red arrow) and recovery of bone loss in the treated group (green arrow). B) The μCT images confirm the radiographic observation (left), and quantitative μCT data show that this change was statistically significant. C) Representative μCT images display an increase of CEJ area (pink) in the PKO mice, which was restored in the antibody treatment group (green arrow), and the quantitative analyses show that the CEJ changes in the PKO mice are significant compared to either the age-matched control or treatment group. Note that 5 animals per group were selected for a μCT scan. The images were rotated so that the crown pulp and root pulp are connected and perpendicular to the horizontal plane for estimation of the CEJ area (red color) through >100 “cutting” slides across the mandible. **P < 0.01 (n = 5). D) Representative backscattered scanning electron microscopy image reveals an expanded canalicular and lacunar system in the nontreated PKO group, which was fully reversed in the treated group. White arrow indicate lack of mineral along the canalicular systems.
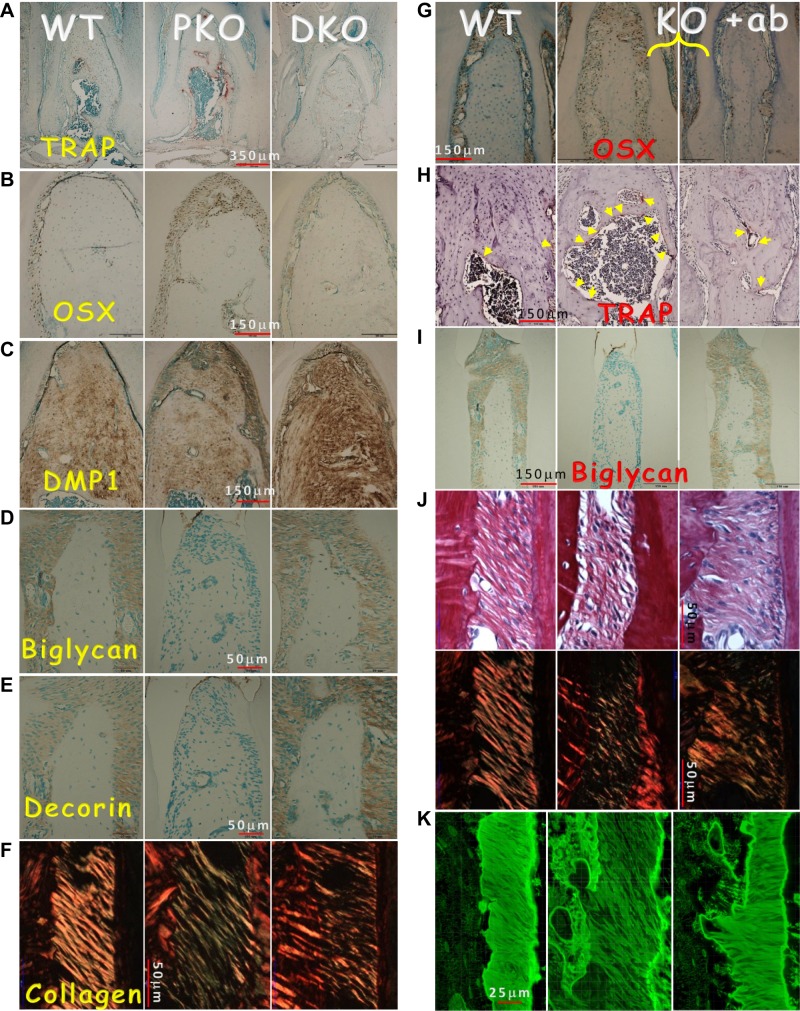
Removing the Sost gene or blocking SOST function restored the collagen and molecule critical for the PDL and alveolar bone in the PKO
To define the impact of removing the Sost gene or blocking SOST function on cellular and molecular changes in the PKO alveolar bone and PDL, we performed a series of histologic and immunohistochemical analyses. In the PKO mice, both bone resorption (as reflected by TRAP expression in Fig. 6A, middle) and formation (a compensated reaction, as revealed by levels of OSX (41); Fig. 6B, middle) were high, although the bone quality was poor, as reflected by a low level of DMP1 (Fig. 6C, middle). Remarkably, the expressions of biglycan (Fig. 6D), decorin (Fig. 6E), and collagen (indicated by polarized light; Fig. 6F, middle) were greatly reduced or largely undetectable in the PKO PDL. All these pathologic changes were reversed in the DKO mice (Fig. 6A–F, right). Furthermore, the 3D videos, which included stacked high-resolution images using Micro XCT (36), confirmed the loss of PDL integrity in the PKO mice and restoration of PDL in the DKO (Supplemental Fig. S2). Similarly, an 8-week treatment of PKO with Scl-Ab achieved the same rescue effect (Fig. 6G–K, right), supporting the notion that Scl-Ab not only restores the bone phenotype but also greatly improves the PDL phenotype in the PKO mice.
Figure 6.
Restoration of the molecular markers and collagen in the DKO mice and the PKO mice treated with Scl-Ab for 8 weeks. A) TRAP stains reveal a reduction of osteoclast number in DKO jawbones. B–E) Recovery of molecular markers in DKO mice by immunostaining, including OSX (B), DMP1 (C), biglycan (D), and decorin (E), as well as partial restoration of collagen (F) in DKO using polarized light under the microscope. G) Representative OSX immunostains in WT (left), PKO (middle), and PKO treated with Scl-Ab (right) are shown. H) TRAP stain images in 3 groups are shown. Yellow arrow indicates osteoclast (stained red by TRAP). I) Biglycan stain images in 3 groups are shown. J) Sirius stain images by regular microscopy (upper) and polarized microscopy (lower) in the 3 groups are shown. K) FITC stain images in the 3 groups are shown. The data show improvement in all these markers and PDL fibers in the Scl-Ab-treated group.
DISCUSSION
The control of chronic periodontal disease, the most common dental disease, has been the focus of intense investigation for many decades because of its destruction of the PDL and bone and tooth loss in patients. Many periodontal disease studies target changes in cytokine and inflammation processes (42). In this study, we used a different strategy and focused on the roles of the PDL in normal alveolar bone formation and the changes of the PDL and Ocys in animal studies. For example, we proved for the first time that the bone formation rate originating from PDL progenitor cells is much faster than other bone progenitor cells from the periosteum and endosteum at the age of 1 mo. Both the cell lineage tracing and FITC imaging data are in agreement with the notion that the PDL cells play a far more important role in alveolar bone formation than is commonly believed (Fig. 1).
Previously, we showed that PKO mice develop many characteristics of the human periodontitis phenotype, such as early onset of periodontal bone loss, PDL inflammation, and pocket formation (21–23). In these studies, we showed striking pathologic changes in their Ocy morphologies using multiple techniques. Because of the great increase in SOST in the PKO mice (Fig. 3), the close correlation between the optimal changes in Ocy morphologies, and the increase in alveolar BV in the Sost-KO mice (Supplemental Fig. S1), we generated DKO mice in which the Sost gene was removed in the context of the PKO condition. Full prevention of the bone phenotype development in these DKO mice confirmed our hypothesis that the changes of Ocys and production of sclerostin from these cells are linked to the periodontal defects. Furthermore, we have successfully rescued the bone phenotype in the PKO mice using monoclonal Scl-Ab. The data obtained from the prevention group (early treatment before the bone phenotype appeared) and the rescue group (the treatment initiated after the bone loss) are exciting because not only are bone loss phenotypes prevented or greatly rescued in the treatment group, but the PDL phenotype is greatly improved as well in the DKO and Scl-Ab-treated mice (Fig. 6 and Supplemental Fig. S2).
At this stage, we do not know why and how the PDL phenotype is reversed by blocking SOST function because periostin is mainly expressed in the PDL, and SOST is highly expressed in Ocys. However, based on the close association between the Sharpey’s fibers and Ocys in the alveolar bone regarding their structural connection and gene expression patterns, we speculate that there is an interaction among these structures, which is altered during periodontitis, leading to local changes in Ocy morphologies. On the other hand, the deletion of Sost or blocking SOST function may send a positive signal to the PDL progenitor cells for repairing damage in both the PDL itself and the alveolar bone through the Sharpey’s fibers. In other words, the Sharpey’s fibers not only connect the PDL and alveolar bone but also function as a signaling bridge between these 2 tissues. Because the major function of sclerostin is binding to Lrp5/6 and inhibiting Wnt signaling, increased Wnt signaling is the most likely event that alters the differentiation program in the progenitor cells in the PDL and Ocys in alveolar bone, leading to bone loss.
In summary, we demonstrated in this study that 1) the PDL functions as the most important reservoir for alveolar bone adjacent to molar roots at the age of 1 month; 2) pathologic changes in Ocys are closely linked to bone loss developed in the PKO mice; 3) deletion of Sost or blocking SOST function prevents/restores Ocy morphologies, which is directly associated with improvement of bone loss in the PKO mice; and 4) restoration of the PDL phenotype by Scl-Ab treatment in PKO mice sheds new light on future drug development, which will hopefully prove to be successful at repairing PDL damage in patients with chronic periodontitis.
Supplementary Material
Acknowledgments
The authors thank Dr. Simon J. Conway (Indiana University, Indianapolis, IN, USA) for providing the periostin knockout mice and Dr. Gabriela G. Loots (Lawrence Livermore National Laboratory, Livermore, CA, USA) for providing the Sost-knockout mice. The authors also thank Dr. Larry Fisher [U.S. National Institutes of Health (NIH), National Institute of Dental and Craniofacial Research, Bethesda, MD, USA] for providing the anti-decorin and biglycan antibodies. The authors gratefully acknowledge Ying Liu’s excellent scanning electron microscopy work and Mrs. Jeanne Santa Cruz for her English grammar editing. This study was supported in part by NIH Grants DE018486 and DE022789 (to J.Q.F.) and R01DE022032-01A1 (to S.P.H.), National Natural Science Foundation of China Grant 81371172 (to X.H.), and Amgen and Union chimique belge Pharma Research funds (to J.Q.F.). M.L. and H.K. are Amgen employees and stockholders.
Glossary
- 3D
3-dimensional
- μCT
microcomputed tomography
- BV
bone volume
- CEJ
cementum-enamel junction
- DKO
double knockout
- DMP1
dentin matrix acidic phosphoprotein 1
- H&E
hematoxylin and eosin
- KO
knockout
- Micro XCT
micro X-ray computed tomography
- MMA
methyl-methacrylate
- Ocy
osteocyte
- OSX
osterix
- PDL
periodontal ligament
- PKO
periostin knockout
- Scl-Ab
sclerostin antibody
- TRAP
tartrate-resistant acid phosphatase
- TV
total volume
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Pihlstrom B. L., Michalowicz B. S., Johnson N. W. (2005) Periodontal diseases. Lancet 366, 1809–1820 [DOI] [PubMed] [Google Scholar]
- 2.Fox C. H., Jette A. M., McGuire S. M., Feldman H. A., Douglass C. W. (1994) Periodontal disease among New England elders. J. Periodontol. 65, 676–684 [DOI] [PubMed] [Google Scholar]
- 3.Douglass C. W., Fox C. H. (1993) Cross-sectional studies in periodontal disease: current status and implications for dental practice. Adv. Dent. Res. 7, 25–31 [DOI] [PubMed] [Google Scholar]
- 4.Fox C. H. (1992) New considerations in the prevalence of periodontal disease. Curr. Opin. Dent. 2, 5–11 [PubMed] [Google Scholar]
- 5.Preshaw P. M., Alba A. L., Herrera D., Jepsen S., Konstantinidis A., Makrilakis K., Taylor R. (2012) Periodontitis and diabetes: a two-way relationship. Diabetologia 55, 21–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salvi G. E., Carollo-Bittel B., Lang N. P. (2008) Effects of diabetes mellitus on periodontal and peri-implant conditions: update on associations and risks. J. Clin. Periodontol. 35(8, Suppl)398–409 [DOI] [PubMed] [Google Scholar]
- 7.Chávarry N. G., Vettore M. V., Sansone C., Sheiham A. (2009) The relationship between diabetes mellitus and destructive periodontal disease: a meta-analysis. Oral Health Prev. Dent. 7, 107–127 [PubMed] [Google Scholar]
- 8.Khader Y. S., Dauod A. S., El-Qaderi S. S., Alkafajei A., Batayha W. Q. (2006) Periodontal status of diabetics compared with nondiabetics: a meta-analysis. J. Diabetes Complications 20, 59–68 [DOI] [PubMed] [Google Scholar]
- 9.Mealey B. L., Ocampo G. L. (2007) Diabetes mellitus and periodontal disease. Periodontol. 2000 44, 127–153 [DOI] [PubMed] [Google Scholar]
- 10.Hallmon W. W., Mealey B. L. (1992) Implications of diabetes mellitus and periodontal disease. Diabetes Educ. 18, 310–315 [DOI] [PubMed] [Google Scholar]
- 11.Clevers H., Nusse R. (2012) Wnt/β-catenin signaling and disease. Cell 149, 1192–1205 [DOI] [PubMed] [Google Scholar]
- 12.Lim W. H., Liu B., Cheng D., Williams B. O., Mah S. J., Helms J. A. (2014) Wnt signaling regulates homeostasis of the periodontal ligament. J. Periodontal Res. 49, 751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuchler U., Schwarze U. Y., Dobsak T., Heimel P., Bosshardt D. D., Kneissel M., Gruber R. (2014) Dental and periodontal phenotype in sclerostin knockout mice. Int. J. Oral Sci. 6, 70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa A. G., Bilezikian J. P., Lewiecki E. M. (2014) Update on romosozumab : a humanized monoclonal antibody to sclerostin. Expert Opin. Biol. Ther. 14, 697–707 [DOI] [PubMed] [Google Scholar]
- 15.McColm J., Hu L., Womack T., Tang C. C., Chiang A. Y. (2014) Single- and multiple-dose randomized studies of blosozumab, a monoclonal antibody against sclerostin, in healthy postmenopausal women. J. Bone Miner. Res. 29, 935–943 [DOI] [PubMed] [Google Scholar]
- 16.Jawad M. U., Fritton K. E., Ma T., Ren P. G., Goodman S. B., Ke H. Z., Babij P., Genovese M. C. (2013) Effects of sclerostin antibody on healing of a non-critical size femoral bone defect. J. Orthop. Res. 31, 155–163 [DOI] [PubMed] [Google Scholar]
- 17.Ominsky M. S., Vlasseros F., Jolette J., Smith S. Y., Stouch B., Doellgast G., Gong J., Gao Y., Cao J., Graham K., Tipton B., Cai J., Deshpande R., Zhou L., Hale M. D., Lightwood D. J., Henry A. J., Popplewell A. G., Moore A. R., Robinson M. K., Lacey D. L., Simonet W. S., Paszty C. (2010) Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J. Bone Miner. Res. 25, 948–959 [DOI] [PubMed] [Google Scholar]
- 18.Li X., Ominsky M. S., Warmington K. S., Morony S., Gong J., Cao J., Gao Y., Shalhoub V., Tipton B., Haldankar R., Chen Q., Winters A., Boone T., Geng Z., Niu Q. T., Ke H. Z., Kostenuik P. J., Simonet W. S., Lacey D. L., Paszty C. (2009) Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J. Bone Miner. Res. 24, 578–588 [DOI] [PubMed] [Google Scholar]
- 19.Eddleston A., Marenzana M., Moore A. R., Stephens P., Muzylak M., Marshall D., Robinson M. K. (2009) A short treatment with an antibody to sclerostin can inhibit bone loss in an ongoing model of colitis. J. Bone Miner. Res. 24, 1662–1671 [DOI] [PubMed] [Google Scholar]
- 20.Taut A. D., Jin Q., Chung J. H., Galindo-Moreno P., Yi E. S., Sugai J. V., Ke H. Z., Liu M., Giannobile W. V. (2013) Sclerostin antibody stimulates bone regeneration after experimental periodontitis. J. Bone Miner. Res. 28, 2347–2356 [DOI] [PubMed] [Google Scholar]
- 21.Rios H., Koushik S. V., Wang H., Wang J., Zhou H. M., Lindsley A., Rogers R., Chen Z., Maeda M., Kruzynska-Frejtag A., Feng J. Q., Conway S. J. (2005) periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol. Cell. Biol. 25, 11131–11144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rios H. F., Ma D., Xie Y., Giannobile W. V., Bonewald L. F., Conway S. J., Feng J. Q. (2008) Periostin is essential for the integrity and function of the periodontal ligament during occlusal loading in mice. J. Periodontol. 79, 1480–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rios H. F., Ye L., Dusevich V., Eick D., Bonewald L. F., Feng J. Q. (2005) DMP1 is essential for osteocyte formation and function. J. Musculoskelet. Neuronal Interact. 5, 325–327 [PubMed] [Google Scholar]
- 24.Bonnet N., Standley K. N., Bianchi E. N., Stadelmann V., Foti M., Conway S. J., Ferrari S. L. (2009) The matricellular protein periostin is required for sost inhibition and the anabolic response to mechanical loading and physical activity. J. Biol. Chem. 284, 35939–35950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng J. Q., Huang H., Lu Y., Ye L., Xie Y., Tsutsui T. W., Kunieda T., Castranio T., Scott G., Bonewald L. B., Mishina Y. (2003) The Dentin matrix protein 1 (Dmp1) is specifically expressed in mineralized, but not soft, tissues during development. J. Dent. Res. 82, 776–780 [DOI] [PubMed] [Google Scholar]
- 26.Collette N. M., Genetos D. C., Economides A. N., Xie L., Shahnazari M., Yao W., Lane N. E., Harland R. M., Loots G. G. (2012) Targeted deletion of Sost distal enhancer increases bone formation and bone mass. Proc. Natl. Acad. Sci. USA 109, 14092–14097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller S. C., Omura T. H., Smith L. J. (1985) Changes in dentin appositional rates during pregnancy and lactation in rats. J. Dent. Res. 64, 1062–1064 [DOI] [PubMed] [Google Scholar]
- 28.Lu Y., Ye L., Yu S., Zhang S., Xie Y., McKee M. D., Li Y. C., Kong J., Eick J. D., Dallas S. L., Feng J. Q. (2007) Rescue of odontogenesis in Dmp1-deficient mice by targeted re-expression of DMP1 reveals roles for DMP1 in early odontogenesis and dentin apposition in vivo. Dev. Biol. 303, 191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fen J. Q., Zhang J., Dallas S. L., Lu Y., Chen S., Tan X., Owen M., Harris S. E., MacDougall M. (2002) Dentin matrix protein 1, a target molecule for Cbfa1 in bone, is a unique bone marker gene. J. Bone Miner. Res. 17, 1822–1831 [DOI] [PubMed] [Google Scholar]
- 30.Martin D. M., Hallsworth A. S., Buckley T. (1978) A method for the study of internal spaces in hard tissue matrices by SEM, with special reference to dentine. J. Microsc. 112, 345–352 [DOI] [PubMed] [Google Scholar]
- 31.Feng J. Q., Ward L. M., Liu S., Lu Y., Xie Y., Yuan B., Yu X., Rauch F., Davis S. I., Zhang S., Rios H., Drezner M. K., Quarles L. D., Bonewald L. F., White K. E. (2006) Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 38, 1310–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciani C., Doty S. B., Fritton S. P. (2009) An effective histological staining process to visualize bone interstitial fluid space using confocal microscopy. Bone 44, 1015–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren Y., Lin S., Jing Y., Dechow P. C., Feng J. Q. (2014) A novel way to statistically analyze morphologic changes in Dmp1-null osteocytes. Connect. Tissue Res. 55(Suppl 1), 129–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Souza D. M., Prado Fde A., Prado Mde A., Rocha R. F., Carvalho Y. R. (2010) Evaluation of two morphometric methods of bone loss percentages caused by periodontitis in rats in different locations. J. Appl. Oral Sci. 18, 493–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuhr A., Popa-Wagner A., Schmoll H., Schwahn C., Kocher T. (2004) Observations on experimental marginal periodontitis in rats. J. Periodontal Res. 39, 101–106 [DOI] [PubMed] [Google Scholar]
- 36.Hurng J. M., Kurylo M. P., Marshall G. W., Webb S. M., Ryder M. I., Ho S. P. (2011) Discontinuities in the human bone-PDL-cementum complex. Biomaterials 32, 7106–7117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roguljic H., Matthews B. G., Yang W., Cvija H., Mina M., Kalajzic I. (2013) In vivo identification of periodontal progenitor cells. J. Dent. Res. 92, 709–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X., Ominsky M. S., Niu Q. T., Sun N., Daugherty B., D'Agostin D., Kurahara C., Gao Y., Cao J., Gong J., Asuncion F., Barrero M., Warmington K., Dwyer D., Stolina M., Morony S., Sarosi I., Kostenuik P. J., Lacey D. L., Simonet W. S., Ke H. Z., Paszty C. (2008) Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J. Bone Miner. Res. 23, 860–869 [DOI] [PubMed] [Google Scholar]
- 39.Padhi D., Jang G., Stouch B., Fang L., Posvar E. (2011) Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J. Bone Miner. Res. 26, 19–26 [DOI] [PubMed] [Google Scholar]
- 40.McClung M. R., Grauer A., Boonen S., Bolognese M. A., Brown J. P., Diez-Perez A., Langdahl B. L., Reginster J. Y., Zanchetta J. R., Wasserman S. M., Katz L., Maddox J., Yang Y. C., Libanati C., Bone H. G. (2014) Romosozumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 370, 412–420 [DOI] [PubMed] [Google Scholar]
- 41.Nakashima K., Zhou X., Kunkel G., Zhang Z., Deng J. M., Behringer R. R., de Crombrugghe B. (2002) The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108, 17–29 [DOI] [PubMed] [Google Scholar]
- 42.Yucel-Lindberg T., Båge T. (2013) Inflammatory mediators in the pathogenesis of periodontitis. Expert Rev. Mol. Med. 15, e7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.