Abstract
Background:
Recent reports of increased intracranial pressure (ICP) due to steep Trendelenburg (ST) position causing neurological deterioration, decreased regional cerebral oxygen saturation and postoperative visual loss after robotic urological and gynecological surgeries led us to consider a simple technique of ICP monitoring. Ours is one of the first instances reported of quantitative noninvasive measurement of increase in ICP with ST position by serial measurement of binocular optic nerve sheath diameter (ONSD) in patients undergoing robot assisted urological and gynecological oncosurgery. We tested whether ONSD values rose to above the upper limits of normal and for what length of time they remained elevated.
Materials and Methods:
Prospective, randomized, interventional, parallel group, active control study conducted on 252 American Society of Anesthesiologists I and II patients. ONSD was measured using 7.5 MHz linear ultrasound probe in supine and Trendelenburg positions.
Statistics:
Student's t-test to compare the inter-group mean ONSD and the repetitive t-test for intra-group analysis.
Result:
Comparison of the mean ONSD values of both groups yielded a 2-tailed significance P <0.01 at all compared time points intra- and post-operatively. In Group-O (open surgery; supine position), the baseline mean bilateral ONSD was 4.36 mm, which did not show any statistically significant change throughout open surgery and postoperative period. On de-docking the robot, 6.2 mm was the mean ONSD value in Group-R (robotic group) while 4.3 mm was the corresponding value in control Group-O.
Conclusion:
ONSD evaluation is a simple, quick, safe, readily available, reliable, cost effective, noninvasive, potential standard of care for screening and monitoring of patients undergoing robotic surgery in ST position.
Keywords: Optic nerve sheath diameter, raised intracranial pressure, robotic surgery, Trendelenburg position
INTRODUCTION
Raised intracranial pressure (ICP) may cause secondary brain ischemia that adds insult to pathologies like traumatic brain injury, stroke and intracranial hemorrhages. It can cause complications like visual impairment, reversible or permanent neurological problems, seizures, stroke and even death.[1,2,3,4] We frequently noticed conjunctival chemosis and edema, postoperative delirium, cognitive dysfunction and delayed awakening in our patients undergoing robotic surgery in steep Trendelenburg (ST) position and attributed these to the raised ICP. Neurological deterioration and visual loss have been reported post-robot assisted laparoscopic prostatectomy (RALP). However, in routine clinical management of patients, ICP monitoring is often excluded since the standard monitoring methods (ventriculostomy, dural bolt) are rather invasive. Contraindications like coagulopathy, high costs, additional patient risk, dedicated neurosurgeon and prolonged insertion time are also deterrents. Hence, the demand for more practical, alternative noninvasive methods for raised ICP detection. These include cranial computed tomography (time-consuming), ophthalmoscopic papilledema measurement, transcranial Doppler ultrasound and sonographic optic nerve sheath diameter (ONSD) measurement.
The evaluation of the ONSD is a simple, quick, safe, readily available, reliable and noninvasive procedure,[1,2,3,4,5,6] which is a potential standard of care in the assessment and monitoring of patients undergoing robotic surgery in ST position. Neurosurgical patients develop raised ICP intraoperatively due to prolonged hours of steep head low position.[7]
A dilated optic nerve sheath is indicative of transmission of increased ICT to the perineural subarachnoid space. This is especially noteworthy because in vivo sonographic quantification of ONSD along with being noninvasive gives an impressive resolution of below 0.5 mm. Ours is the first instance reported of utilizing ONSD for ICP monitoring in patients undergoing robotic surgery in ST position. Our null hypothesis was that steep head low position has no effect on the ONSD. Our trial (registered with the Clinical Trial Registry of India [CTRI] as NIMORT Trial) has the following aims and objectives:
Quantitative measurement of the increase in ICP with ST position by serial measurement of binocular ONSD in patients undergoing robotic surgery keeping patients undergoing the same surgeries in the supine position as a control.
Study of the rate of return of ONSD toward normal with resumption of the supine position.
Our null hypothesis was that ST position has no effect on the ONSD while the alternate hypothesis was that ONSD changes with steep head low position.
MATERIALS AND METHODS
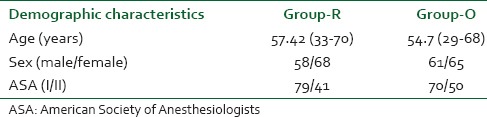
Ours was a prospective, single blind, parallel group, active-controlled, single-centric, randomized, interventional study conducted at Rajiv Gandhi Cancer Institute and Research Center in the major OT and postoperative ward. The study is registered retrospectively with the CTRI (Registration No: CTRI/2014/08/004809 and the date of registration being 1/08/14). At the time of recruitment, ours being the first study comparing ONSD in supine and head low positions we did not have a reference study for sample size estimation. Our pilot study with 10 patients in each group gave significant results. To enhance the power (1-β) of the study keeping the α or type-1 error as 5%, we selected a sample size of 126 cases in each group. After requisite approval of the institutional ethics committee review board and written informed consent from all subjects, 252 American Society of Anesthesiologists I and II patients of either sex, aged between 25 and 70 years [Table 1] were included in the study. Robot assisted radical prostatectomy (RALP-54 patients) robot assisted radical hysterectomy (RRH-68 patients) and robot assisted radical cystoprostatectomy (4 patients) with ileal conduit/neobladder were the urogynecological surgeries performed using the da Vinci robotic operating system (Intuitive Surgical Sunnyvale, CA, USA). One hundred and twenty-six patients undergoing robotic surgery in steep (45°) Trendelenburg position were included in Group-R while 126 patients who underwent open urogynecological surgeries in the supine position formed the control Group-O. Out of 270 patients enrolled, 18 patients were excluded from the study as per following exclusion criteria — history of neurological disease, transient ischemic attack, carotid disease, raised ICP, cerebral edema, glaucoma, ocular surgery, difficult mechanical ventilation/decreased vital capacity, lower limb ischemia and docking interval <2 h or more than 3 h.
Table 1.
Demographic Data in Robotic and Open Surgery Groups
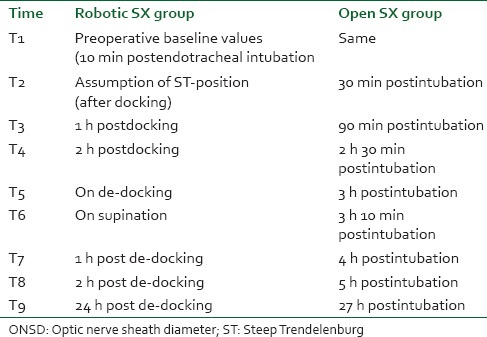
Optic nerve sheath diameter (both eyes), ETCO2, heart rate and mean arterial pressure (MAP) were measured at following intervals of time in both the groups [Table 2].
Table 2.
Time points for serial ONSD measurement
We utilized a standardized anesthetic technique utilizing air-oxygen (40%), end tidal sevoflurane between 1% and 1.5% and a BIS-guided propofol infusion. Goal MAP was within 20% of baseline while the goal ETCO2 range was fixed at 30-35 mmHg. Fluid was restricted to 1000 ml of ringers lactate and ephedrine boluses (3 mg) were utilized as rescue drug to maintain the MAP goal. Core temperature was kept above 35.5°C using fluid warmer and patient warming blankets.
At 10 min postinduction, a preoperative baseline value was obtained in both the groups. ONSD reading on assumption of Trendelenburg position, hourly ONSD charting thereafter, a reading each at de-docking and supination and then at one, three and 24 h post supination was recorded for Group-R. In Group-O, ONSD was recorded at comparable points of time. Simple computer generated randomization was done, and the method of concealment was sequentially numbered, sealed opaque envelopes.
After application of coupling gel over the closed upper eyelid, the ultrasound probe was positioned over it [Figure 1]. ONSD was measured 3 mm posterior to the posterior scleral margin using a 7.5 MHz linear ultrasound probe (Micromaxx Ultrasound System; SonoSite Inc., Bothell, WA, USA). A hypoechoic linear zone stemming away from the hypoechoic eyeball posteriorly represents the optic nerve [Figure 2]. As per the departmental protocol, three separate readings were taken for each optic nerve sheath utilizing the digital cursor and standardized adjustment of SonoSite software.(Micromaxx, Bothell, USA) The anesthetist recording the measurements was not blinded to the preliminary clinical diagnosis or the stage of surgery (time elapsed in ST position). Later the saved ultrasound images were independently reviewed by the chief investigator who calculated the mean ONSD. He was blinded to the clinical details including the timing of the ultrasonography (USG) scan whether pre-, intra- or post-operative. The trial was participant and outcome assessor blinded. Primary outcome measure was binocular ONSD at 3 h/de-docking (T5). Secondary outcome measures were binocular ONSD at remaining points of time mentioned above.
Figure 1.
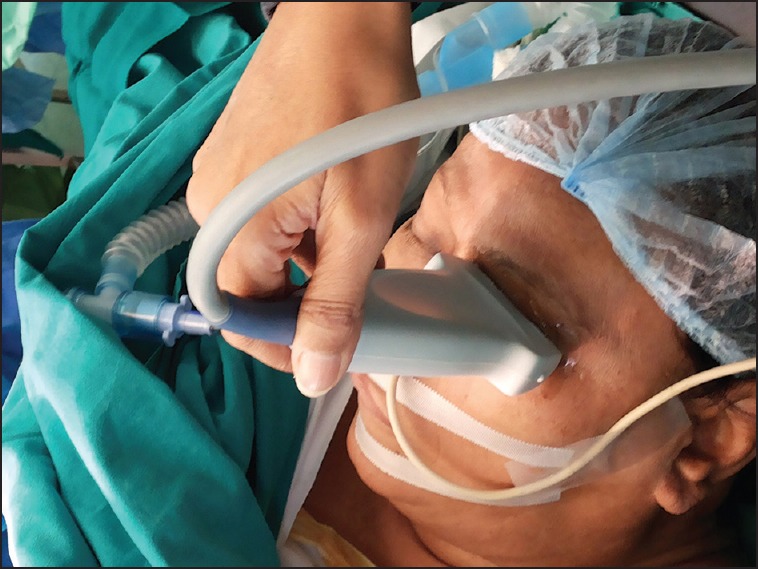
Recording of optic nerve sheath diameter using 7.5 MHz ultrasound probe
Figure 2.
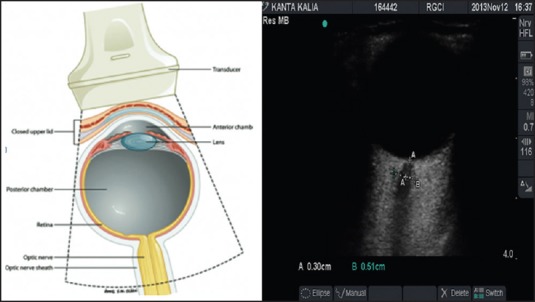
Sonoanatomy of the human eye
Statistical analysis
Paired t-test for intra-group comparison of mean ONSD at various time points with baseline ONSD values (10 min postendotracheal intubation) and Student's t-test for inter-group comparison of mean ONSD values at corresponding time points were utilized using the SPSS-22 software(IBM-International Business Machines Corporation, Armonk, New York, United States)
Patients were also examined for occurrence of postoperative delayed awakening, emergence delirium and postoperative nausea-vomiting.
RESULTS
Participant flow is depicted by the CONSORT flow diagram [Figure 3]. The first patient was enrolled in April 2013, and the trial ended in May 2014 after the requisite number of cases were successfully completed.
Figure 3.
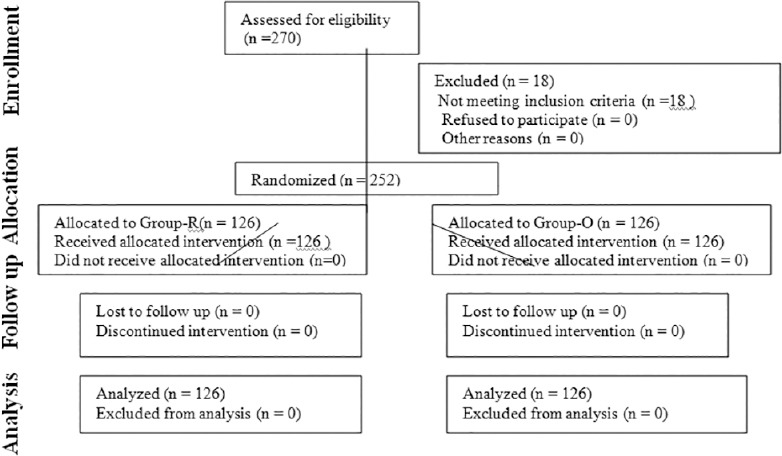
CONSORT flow diagram
The primary outcome measure was ONSD at the time of de-docking the robot in Group-R (robotic surgery in ST position) and at the corresponding time interval (3 h postendotracheal intubation in Group-O (traditional open surgery in supine position for the same disease). Mean bilateral ONSD at all other time points were the secondary outcome measures. The mean bilateral ONSD in Group-R (n = 126) was 4.3 mm, 10 min posttracheal intubation. This increased to 5.1 mm on docking, 5.7 mm at the end of the first hour and 5.9 mm at the end of the second hour of pneumoperitoneum. At the time of de-docking, the robot (between 2 and 3 h of ST position) 6.2 mm (6.126 mm and 6.345 mm being the upper and lower limits of 95% confidence interval) was the mean ONSD value (primary outcome). Immediately after supination, ONSD decreased to 5.8 mm. At the end of 1st h post de-docking, ONSD was 5.5 mm and at 2 h it was 5.2 mm. 24 h later 4.7 mm was the mean ONSD [Table 3 and Figure 4].
Table 3.
Variation in ONSD over time
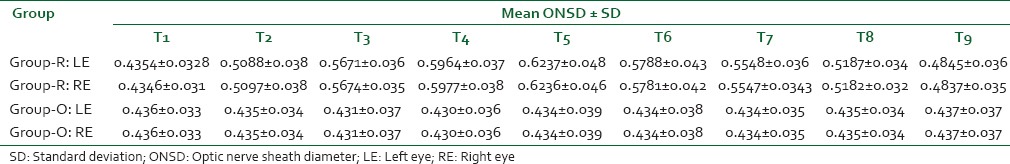
Figure 4.
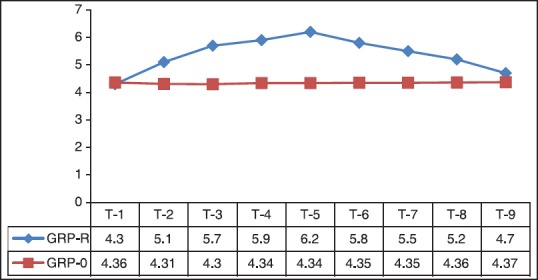
Graphical representation of variation in optic nerve sheath diameter over time
The mean bilateral ONSD in Group-O (n = 126) was 4.36 mm, 10 min posttracheal intubation. At 30 min, 1 h 30 min, 2 h 30 min, 3 h and 3 h 10 min, ONSD was 4.35, 4.31 mm, 4.30 mm, 4.34 mm and 4.34 mm (4.276-4.433 mm 95% confidence interval: primary outcome). In the postoperative period ONSD values were 4.34 mm, 4.35 mm and 4.37 mm at 4 h, 5 h and 27 h postendotracheal intubation.
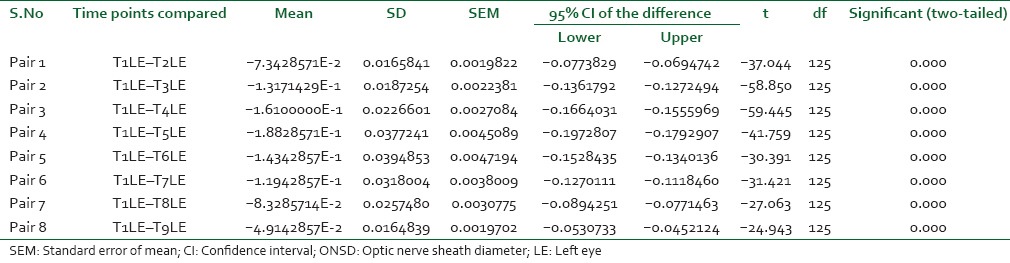
The ETCO2 was strictly maintained between 30 and 35 mmHg throughout surgery by adjusting mechanical ventilatory parameters. The paired t-test was utilized to compare the baseline value with intra-group mean ONSD values at various points of time during surgery and postoperative period in both the groups. In Group-R, the P <0.001 in all the pairs, which is statistically highly significant [Table 4]. In Group-O, statistically insignificant P values were obtained. The Student's t-test was utilized for inter-group analysis. At 10 min posttracheal intubation, the P value was statistically insignificant while at all other time points it was statistically highly significant (P < 0.001).
Table 4a.
Comparison of ONSD in Robotic Surgery Group at Various Time Points RE
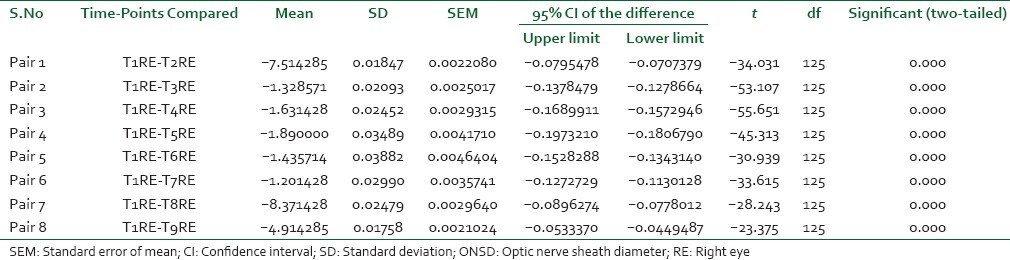
The average baseline MAP was 102 mmHg. At T2, T3 and T4, the MAP values were 100 mmHg, 91 mmHg and 88 mmHg respectively. Immediately on assumption of the supine position MAP dropped to 71 mmHg. It was 78 and 88 mmHg respectively at 1 and 2 h post de-docking. Twenty-four hours postsurgery it was 97 mmHg.
Eleven patients in the robotic surgery group showed delayed awakening despite use of short-acting anesthetics while only 3 patients in the open surgery showed delayed recovery. Postoperative emergence delirium was observed in 27 patients in the robotic surgery group and 15 patients in the open surgery group. Postoperative nausea-vomiting was observed in 42 patients in the robotic surgery group and 26 patients in the open surgery group.
DISCUSSION
Steep inclination of 35-45° for a prolonged period can lead to raised cerebral venous pressure, upper airway and brain edema,[8,9] increase in ICP and cerebral blood flow. To preserve cerebrovascular homeostasis, normocarbia should be maintained. The Molloy study showed that even under anesthesia, cerebrovascular and ophthalmic circulatory autoregulation do not prevent complications such as increased intraocular pressure.[10] As per Theelen et al., raised ICP leads to a rise in episcleral venous pressure, which in turn leads to an elevation in intraocular pressure.[11]
B-scan (planar) ultrasound provides longitudinal cross-section images of the optic nerve and its sheath. The method has been successfully validated in several relatively large studies that included patients with severe head trauma[1,2] hydrocephalus,[12] intracranial hemorrhage,[1] stroke, acute application of subarachnoid pressure,[13] liver failure[14] and climbers with acute mountain sickness. ONSD can be used for identification of patients with intracranial hypertension that requires treatment. Ours is one of the first instances reported of quantitative noninvasive measurement of the increase in ICP with steep (45°) Trendelenburg position by serial measurement of binocular ONSD in patients undergoing robotic surgery. Using an ROC curve, Kimberly et al.[6] systematically confirmed the commonly used threshold of ONSD >5 mm to detect ICP >20 mmHg. Their study directly correlates ventriculostomy measurements of ICP with US ONSD measurements.
The optic nerve, ontogenetically a part of the central nervous system, is surrounded by cerebrospinal fluid (CSF) and duramater [Appendix: CONSORT Checklist]. Owing to a connection with the intracranial subarachnoid space, CSF pressure variations influence the ONSD. Histological studies revealed a segment of the optic nerve in which maximal diameter fluctuations could be expected (bulging duramater region approximately 3 mm behind the papilla). After widening of the subarachnoid space with gelatine in cadavers, the mean diameter increased by 60% at 3 mm behind optic nerve head, but only by 35% at 10 mm distance. Independent measurements by two examiners correlated highly, which indicates excellent reproducibility of the USG measurements.[12,15,16,17] The optimal experimental scanning position was at right angle to the optic nerve (longitudinal section). Under clinical conditions, however, only axial sections can be obtained using anterior probe positions with transbulbar sound directions. ONSD changes almost concurrently with CSF pressure variations and have a good reproducibility.[1] Upper limit of normal for ONSD is 4.5-5.2 mm in patients over 1 year of age[1,2,4,5,6,12,13,14] and 4 mm in children <1 year old as per the various studies conducted so far. A limitation of our trial could be that the absolute values for ONSD in the Indian subcontinental population may vary from those in other races across the globe. The trends in ONSD values can be generalized to the entire globe though. Our study demonstrates that ONSD (surrogate for ICP) rises steadily with pneumoperitoneum and hours in ST position during robotic surgery to dangerous levels that are well above the upper limit for normal. The values fail to return to baseline even 24 h after surgery. This may result in devastating complications that are preventable if detected early. The incidence of delayed awakening (8.7% in Group-R and 2.4% in Group-O), emergence delirium (21.4% in Group-R and 11.9% in Group-O) and postoperative nausea-vomiting (33.3% in Group-R and 20.6% in Group-O) were significantly higher in the robotic surgery group as per our study. Two cases of robotic radical cystectomy with ileal conduit urinary diversion surgeries having neurological deterioration (stupor, incoherent talking, rapid shallow respiratory pattern, vomiting, hypertension and bradycardia) postextubation probably due to iatrogenic positional cerebral edema have been reported by Pandey et al.[18] Postoperative visual loss due to posterior ischemic optic neuropathy after RALP[19,20] has occurred. Lee et al. observed a significant decline in regional cerebral oxygen saturation as a result of decreased cerebral perfusion pressure consequent to raised ICP during ST position in 24 female patients undergoing robot assisted gynecological surgery.[21] ONSD measurement can aid in screening and excluding patients with raised ICP and glaucoma for robotic surgery in ST position. A possible drawback of the study could be that the end tidal CO2 was maintained at the same levels in the supine and Trendelenburg positions. This probably means a greater minute ventilation in the head low group. This might be a variable via mean intrathoracic pressure on the ONSD results. But that mentioned, serial ONSD measurement in our view, is a potential standard of care for intraoperative monitoring and postoperative vigilance.
Table 4b.
Intra-group comparison of ONSD in LE
CONCLUSION
To conclude, ST position results in increasing venous congestion within and outside the cranium leading to cerebral edema and raised ICP. US-ONSD should be used routinely as a screening test for raised ICP and glaucoma in patients scheduled for robotic surgery in ST position, and such patients declared unfit for robotic surgery and offered open surgery as an option. Serial ONSD monitoring intra- and post-operatively can also serve as a guide to decide whether the raised ICP merits any intervention (intravenous [IV] fluid restriction, IV dexamethasone, 20%mannitol, furosemide, reverse trendelenburg, break in surgery). Adverse neurological morbidity after robotic surgery could be due to raised ICP if the ONSD value is persistently more than 5 mm.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Moretti R, Pizzi B, Cassini F, Vivaldi N. Reliability of optic nerve ultrasound for the evaluation of patients with spontaneous intracranial hemorrhage. Neurocrit Care. 2009;11:406–10. doi: 10.1007/s12028-009-9250-8. [DOI] [PubMed] [Google Scholar]
- 2.Rajajee V, Vanaman M, Fletcher JJ, Jacobs TL. Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care. 2011;15:506–15. doi: 10.1007/s12028-011-9606-8. [DOI] [PubMed] [Google Scholar]
- 3.Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: A systematic review and meta-analysis. Intensive Care Med. 2011;37:1059–68. doi: 10.1007/s00134-011-2224-2. [DOI] [PubMed] [Google Scholar]
- 4.Soldatos T, Chatzimichail K, Papathanasiou M, Gouliamos A. Optic nerve sonography: A new window for the non-invasive evaluation of intracranial pressure in brain injury. Emerg Med J. 2009;26:630–4. doi: 10.1136/emj.2008.058453. [DOI] [PubMed] [Google Scholar]
- 5.Moretti R, Pizzi B. Ultrasonography of the optic nerve in neurocritically ill patients. Acta Anaesthesiol Scand. 2011;55:644–52. doi: 10.1111/j.1399-6576.2011.02432.x. [DOI] [PubMed] [Google Scholar]
- 6.Kimberly HH, Shah S, Marill K, Noble V. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. 2008;15:201–4. doi: 10.1111/j.1553-2712.2007.00031.x. [DOI] [PubMed] [Google Scholar]
- 7.Mavrocordatos P, Bissonette B, Ravussin P. Effects of neck position and head elevation on intracranial pressure in anaesthetized neurosurgical patients. J Neurosurg Anesthesiol. 2000;12:10–4. doi: 10.1097/00008506-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Goswami S, Nishanian E, Mets B. Anesthesia for robotic surgery. In: Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Young WL, editors. Miller's Anesthesia. 7th ed. Philadelphia, PA: Elsevier; 2010. pp. 1103, 2389–95. [Google Scholar]
- 9.Awad H, Santilli S, Ohr M, Roth A, Yan W, Fernandez S, et al. The effects of steep trendelenburg positioning on intraocular pressure during robotic radical prostatectomy. Anesth Analg. 2009;109:473–8. doi: 10.1213/ane.0b013e3181a9098f. [DOI] [PubMed] [Google Scholar]
- 10.Molloy BL. Implications for postoperative visual loss: Steep trendelenburg position and effects on intraocular pressure. AANA J. 2011;79:115–21. [PubMed] [Google Scholar]
- 11.Theelen T, Meulendijks CF, Geurts DE, van Leeuwen A, Voet NB, Deutman AF. Impact factors on intraocular pressure measurements in healthy subjects. Br J Ophthalmol. 2004;88:1510–1. doi: 10.1136/bjo.2004.049924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman WD, Hollman AS, Dutton GN, Carachi R. Measurement of optic nerve sheath diameter by ultrasound: A means of detecting acute raised intracranial pressure in hydrocephalus. Br J Ophthalmol. 2002;86:1109–13. doi: 10.1136/bjo.86.10.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen HC, Lagrèze W, Krueger O, Helmke K. Dependence of the optic nerve sheath diameter on acutely applied subarachnoidal pressure — An experimental ultrasound study. Acta Ophthalmol. 2011;89:e528–32. doi: 10.1111/j.1755-3768.2011.02159.x. [DOI] [PubMed] [Google Scholar]
- 14.Krishnamoorthy V, Beckmann K, Mueller M, Sharma D, Vavilala MS. Perioperative estimation of the intracranial pressure using the optic nerve sheath diameter during liver transplantation. Liver Transpl. 2013;19:246–9. doi: 10.1002/lt.23591. [DOI] [PubMed] [Google Scholar]
- 15.Helmke K, Hansen HC. Fundamentals of transorbital sonographic evaluation of optic nerve sheath expansion under intracranial hypertension. I. Experimental study. Pediatr Radiol. 1996;26:701–5. doi: 10.1007/BF01383383. [DOI] [PubMed] [Google Scholar]
- 16.Helmke K, Hansen HC. Fundamentals of transorbital sonographic evaluation of optic nerve sheath expansion under intracranial hypertension II. Patient study. Pediatr Radiol. 1996;26:706–10. doi: 10.1007/BF01383384. [DOI] [PubMed] [Google Scholar]
- 17.Hansen HC, Helmke K. The subarachnoid space surrounding the optic nerves. An ultrasound study of the optic nerve sheath. Surg Radiol Anat. 1996;18:323–8. doi: 10.1007/BF01627611. [DOI] [PubMed] [Google Scholar]
- 18.Pandey R, Garg R, Darlong V, Punj J, Chandralekha, Kumar A. Unpredicted neurological complications after robotic laparoscopic radical cystectomy and ileal conduit formation in steep trendelenburg position: Two case reports. Acta Anaesthesiol Belg. 2010;61:163–6. [PubMed] [Google Scholar]
- 19.Gainsburg DM. Anesthetic concerns for robotic-assisted laparoscopic radical prostatectomy. Minerva Anestesiol. 2012;78:596–604. [PubMed] [Google Scholar]
- 20.Weber ED, Colyer MH, Lesser RL, Subramanian PS. Posterior ischemic optic neuropathy after minimally invasive prostatectomy. J Neuroophthalmol. 2007;27:285–7. doi: 10.1097/WNO.0b013e31815b9f67. [DOI] [PubMed] [Google Scholar]
- 21.Lee JR, Lee PB, Do SH, Jeon YT, Lee JM, Hwang JY, et al. The effect of gynaecological laparoscopic surgery on cerebral oxygenation. J Int Med Res. 2006;34:531–6. doi: 10.1177/147323000603400511. [DOI] [PubMed] [Google Scholar]


