Abstract
Background:
Postoperative recurarization remains a risk following the use of the conventional neuromuscular blocking agents. In addition, none of the commonly used reversal agents, such as neostigmine or edrophonium are capable of reliably reversing profound blockade. The present comparative and randomized study investigated the use of sugammadex for reversing profound neuromuscular blockade (NMB) in pediatric neurosurgical patients undergone posterior fossa tumor excision.
Patients and Methods:
Forty pediatric patients undergoing elective craniotomy for posterior fossa tumor excision were randomly divided into either of neostigmine or sugammadex group in which muscle relaxant was reversed at the end of anesthesia either with neostigmine 0.04 mg/kg added to atropine 0.02 mg/kg or sugammadex 4 mg/kg alone, respectively. The primary endpoint was the time from the administration of sugammadex or neostigmine to recovery of the train of four (TOF) ratio to 90% after rocuronium-induced neuromuscular block. Unpaired t-test was used to compare continuous variables between groups. Meanwhile, repeated ANOVA was used to detect intragroup differences.
Results:
Patients in sugammadex group attained a TOF ratio 90% in statistically shorter time (1.4 ± 1.2 min) than those in neostigmine group (25.16 ± 6.49 min) for reversal of the rocuronium. Mean arterial pressure and heart rate were significantly higher in neostigmine group at 2, 5 and 10 min after administration of the reversal agents and returned nonsignificantly different after that. With no recurarization in any patient throughout the study period.
Conclusion:
Sugammadex rapidly and effectively reverses rocuronium-induced NMB in pediatric patients undergoing neurosurgery when administered at reappearance of T2 of TOF at dose 4 mg/kg.
Keywords: Neostigmine, reversal of neuromuscular blockade, sugammadex
INTRODUCTION
Neuromuscular blockade (NMB) is routinely used worldwide as part of a modern concept of balanced anesthesia during surgery to facilitate endotracheal intubation and to keep patient immobilized throughout the surgery. In spite of its few adverse effects (mainly allergic reactions) during anesthesia, Postoperative recurarization always remain a risk following the use of neuromuscular blocking agents even for those with an intermediate duration of action.[1] In addition, none of the commonly used reversal agents, such as neostigmine or edrophonium, are capable of reliably reversing profound blockade.[2] Side-effects may impede the use of higher doses of anticholinesterase agents. Consequently, a reversal agent with greater safety and efficacy that would lead to complete recovery and be able to reverse profound blockade will be of great advantage. The achievement of early recovery is targeted in neurosurgical cases in order to achieve early neurological evaluation and hence to hasten the diagnosis and treatment of a life-threatening complication.
Sugammadex (Bridionw, MSD, Oss, The Netherlands), a modified γ-cyclodextrin, is a selective relaxant-binding agent which rapidly and completely reverses the effects of the neuromuscular blocking agent rocuronium[3,4] by encapsulating the muscle relaxant and rendering it inactive. It has been approved by the European Union since 2008 for the reversal of moderate and deep NMB induced by rocuronium.
The present comparative and randomized study investigated the use of sugammadex for reversing profound NMB in pediatric neurosurgical patients undergone posterior fossa tumor excision.
PATIENTS AND METHODS
Following Institutional Review Board approval and written informed consent, 40 pediatric patients were randomly enrolled in this study in the Children Cancer Hospital-Egypt (CCHE). The selected patients were American Society of Anesthesiologists physical status I-III, aged 7-18 years, and undergoing scheduled, elective craniotomy for posterior fossa tumor excision. The criteria of exclusion were known allergies to any used anesthetic drug, mid-line shift, Glasgow Coma Scale <15, neuromuscular disorder; a history of malignant hyperthermia; significant renal dysfunction; if they were hemodynamically or neurologically unstable and/or refusal to sign the consent. In addition, patients who had been exposed to general anesthesia in the preceding 7 days before study were excluded. The tumor characteristics were assessed from the relevant preoperative magnetic resonance imaging.
Using a computer-generated randomization list, the patients were randomly allocated into one of two groups (20 patients each): Neostigmine group, and sugammadex group in which muscle relaxation was reversed at the end of surgery using neostigmine 0.04 mg/kg combined with atropine 0.02 mg/kg, or sugammadex 4 mg/kg solely, respectively.
All patients were sedated with midazolam (0.05 mg/kg IV) in the preoperative holding area, and then transferred to the operating theatre where they were preoxygenated with 100% oxygen for 3 min during which the noninvasive monitoring incorporated into the anesthesia work station (Zeus®, Draeger, Luebeck, Germany) were applied. These monitors include pulse oximetry (SpO2), electrocardiography, noninvasive blood pressure, axillary temperature probe and peripheral nerve stimulator. Apart from the reversal of the muscle relaxant administered at recovery from anesthesia at the end of surgery, anesthesia was standardized to all patients. Induction of anesthesia was achieved intravenously using thiopental (6 mg/kg), fentanyl (2 μg/kg), and rocuronium bromide (0.6 mg/kg) to facilitate endotracheal intubation after the count of twitches of the train of four (TOF) stimulation reached 0-1 and was displayed digitally. Then, all patients were mechanically ventilated using fresh gas flow at 2 L/min with a mixture of air-oxygen (50% oxygen) using the anesthesia workstation (Zeus®, Dräger, Luebeck, Germany). Tidal volume was set at 8 ml/kg and respiratory frequency was adjusted in order to maintain an end-tidal CO2 (EtCO2) between 30 and 35 mmHg. EtCO2, inspired and expired tidal volumes, airway pressure as well as oxygen and agent concentrations were continuously monitored. All data were recorded using electronic anesthesia record (Cerner Millennium software, USA).
Arterial catheter was inserted into the radial artery and connected to a transducer (Hemomed, Siemens, Germany) to monitor the invasive blood pressure throughout the anesthesia as well as postoperatively for the first 2 h. Muscle relaxation was monitored throughout the anesthesia by peripheral nerve stimulator (INFINITY TRIDENT, Dragger Medical Systems, Inc., USA) applied to the adductor pollicis using TOF mode targeting to keep the count of twitches of the TOF stimulation 0-1.
A local anesthetic of 1% lidocaine with epinephrine 1:200,000 was infiltrated at the sites of fixation of Mayfield head holder into the patient's head and on the scalp over the surgical field. Maintenance of anesthesia was obtained using intravenous infusion of fentanyl at rate of 0.5 μg/kg/h, and rocuronium bromide at rate of 0.4 mg/kg/h together with sevoflurane starting at 1 minimum alveolar concentration (MAC) and then subsequently adjusted to maintain mean arterial blood pressure (MAP) and heart rate (HR) within a range of 20% of preanesthesia level. MAC of sevoflurane was recorded by the anesthetic machine in real-time for MAC-hour calculation which is the average MAC length of exposure.
Persistent relative hypertension or tachycardia episodes lasting >1 min (defined as MAP or HR rises >20% of baseline value) not responding to the maximal allowed anesthetic concentration were treated with labetalol 0.5 mg/kg intravenous bolus.
Episodes of hypotension (MAP <20% of the baseline value) not responding to intra-operative fluid replacement could be managed with vasopressor (ephedrine 0.2 mg/kg IV). Bradycardia (HR <20% of the baseline value) and lasting >1 min were treated with atropine sulfate 0.02 mg/kg IV.
Arterial blood samples for gas analysis were collected every hour during the anesthesia as well as postoperatively for the first 2 h to monitor oxygen and carbon dioxide tensions as well as blood sodium and potassium levels.
Intra-operative normothermia was actively maintained with a forced air warming system (Bair Hugger) and continuous monitoring of body temperature using axillary temperature probe.
During securing the bone flap, the rocuronium and fentanyl infusion were discontinued. At the end of surgery, after skin closure and detachment of Mayfield head holder from the patient, the inhalational anesthetics were discontinued. When the TOF count reached 2, the residual muscle relaxation was antagonized with neostigmine 0.04 mg/kg IV and atropine 0.02 mg/kg IV for patients in neostigmine group or with sugammadex 4 mg/kg alone for patients in sugammadex group. Vital signs (blood pressure and HR) were measured at screening, before administration of rocuronium, immediately before and then at 2, 5, 10 and 15 min after administration of the used neuromuscular blocking agent as well as 24 h postoperatively.
Patients were extubated when respiratory function was adequate (as indicated by adequate respiratory rate according to the age; tidal volume >4 ml/kg; SpO2 continued maintained above 95% on FiO2 <60%), hemodynamics were stable and upper airway reflexes were fully recovered.
Patients then were transferred into the Neurosurgical Intensive Care Unit (NSICU) where they received paracetamol (Perfalgan) 10 mg/kg IV as well as supplemental oxygen at a flow rate of 6-8 L/min (fraction of inspired oxygen 40%) throughout the period of observation.
Upon arrival to NSICU, pulse oximetry and respiratory rate were measured for at least 60 min to assess the adequacy of spontaneous ventilation.
Any other adverse effects elicited during the procedure or in the NSICU were recorded along with its time of occurrence, severity, and duration.
Three blood samples (3 ml each) were collected from each patient for safety assessment to measure hematocrit, blood count, liver and kidney enzymes just before administration of muscle relaxant at induction of anesthesia, at stopping of rocuronium and at 24 h postoperative.
The primary study endpoint was the time from the administration of sugammadex or neostigmine to recovery of the TOF ratio to 90% after rocuronium-induced neuromuscular block.
Intraoperative HR and MAP during administration of reversal agents were considered as secondary endpoints as well any incidence of adverse events in the first 24 h after surgery.
Statistical analysis
Previous studies on efficacy of sugammadex for the reversal of the block induced by rocuronium were based on recovery of TOFR. Sugammadex has been shown to effectively reverse profound neuromuscular block within 2-3 min[5,6,7] time started from administration of sugammadex until TOF ratio reaches 90%. Hence, 19 patients in each group were required to detect at least 5 min difference or more in this variable between groups and to be able to reject the null hypothesis, which the population means of each group are equal with probability (power) 0.85. The Type I error probability associated with this test of this null hypothesis (α) is 0.05. We decided to take 20 patients in each group.
Data were analyzed using the Statistical Package for Social Sciences for windows (SPSS 13.0.1; SPSS Inc.; Chicago, IL, USA).
Quantitative variables were expressed as means ± standard deviation categorical variables were expressed as the number (%).
Unpaired t-test was used to compare continuous variables between groups. Meanwhile, repeated ANOVA was used to detect intragroup differences. Categorical variables were compared using the Chi-square test or Fisher's exact test as appropriate. P < 0.05 was considered as statistically significant.
RESULTS
This prospective, comparative, randomized clinical study was conducted in CCHE between August 2013 and July 2014. Data were normally distributed. Baseline demographic data, including age, sex, weight and tumor characteristics were comparable in both groups and summarized in Table 1.
Table 1.
Patient's demographic data
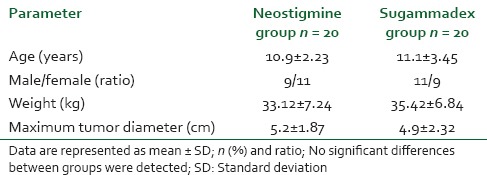
There were no significant differences between groups regarding surgery or anesthesia times. Furthermore, the total amount of fentanyl and MAC hour sevoflurane used intra-operatively as well as the temperature at extubation were comparable in both groups [Table 2].
Table 2.
Intraoperative characteristics
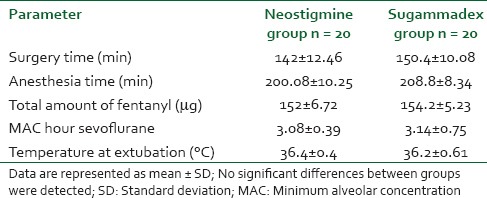
Although, the mean time from the first administration of rocuronium until the reappearance of T2 was comparable in both groups 166 ± 6.46 and 144 ± 10.23 min in neostigmine and sugammadex groups respectively, the patients in sugammadex group attained a TOF ratio of 90% in statistically shorter time (1.4 ± 1.2 min) than those in neostigmine group (25.16 ± 6.49 min) for reversal of the rocuronium [Table 3].
Table 3.
Mean time from administration of roucuronium till T2
Mean arterial blood pressure and HRs showed no significant difference between both groups at preinduction and throughout the maintenance of anesthesia, with the exception of a statistically higher MAP and mean HR at 2, 5, and 10, min after administration of neostigmine and atropine in the neostigmine group than the preinduction levels as well as than that in the sugammadex group and returned nonsignificantly different at 15 min and at 24 h postoperatively. However, the mean MAP and HRs did not exceed 20% beyond the baseline throughout the previous time periods. The MAP and HR levels were stable and showed no changes than the preinduction levels in the sugammadex group [Figures 1 and 2].
Figure 1.
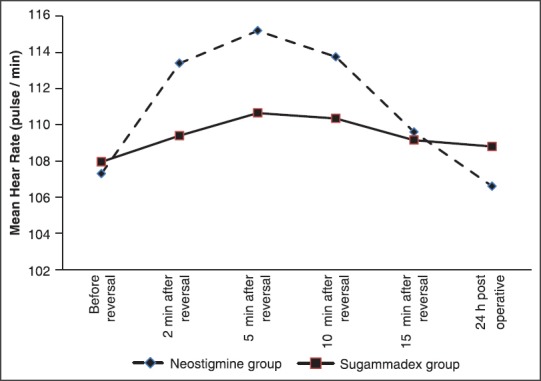
Mean heart rate during reversal of neuromuscular blockade
Figure 2.
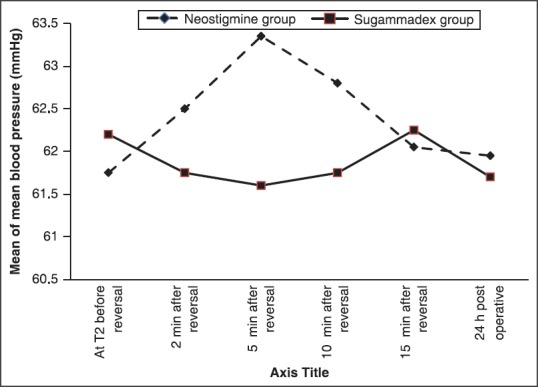
Mean of mean blood pressure during reversal of neuromuscular blockade
There was no evidence of recurarization in any patient during the period of neuromuscular monitoring or during their stay in the NSICU.
Apart from two patients (10%) in the sugammadex group and 4 (20%) in the neostigmine group developed postoperative vomiting, no serious adverse events were documented in any study group. No patient was withdrawn from the study because of any major adverse event.
No significant difference could be detected at any study time compared to the baseline in the same group or even between both groups regarding the hematological or biochemical variables.
DISCUSSION
Sugammadex, a novel γ-cyclodextrin and selective relaxant binding agents, was specifically developed to reverse the neuromuscular block elicited by rocuronium by its encapsulation.
Our study showed that sugammadex at a dose of 4 mg/kg was associated with a prompt and complete reversal from profound rocuronium-induced neuromuscular block in neurosurgical pediatric patients recovering from general anesthesia when compared to the conventional cholinesterase-inhibiting drugs, neostigmine. The use of sugammadex also averted the need for the anticholinergic drugs atropine.
It is well established that neostigmine provides slow recovery when administered for reversal of profound neuromuscular block.[2]
The sugammadex at dose of 4 mg/kg reversed the neuromuscular block and the TOF reached 90% in a mean of 1.4 min which was 18 folds faster than that achieved by the neostigmine at the dose of 40 μg/kg combined with atropine 0.02 mg/kg.
These results come in agreement with the results observed by Sorgenfrei et al.,[8] who demonstrated that sugammadex allowed return of the TOF ratio to 90% within 5 min when administered to reverse a moderately profound rocuronium-induced neuromuscular block.
This prompt and smooth reversal of the neuromuscular block appears to have important clinical implications during prolonged surgeries particularly the neurosurgery that require maintenance of deep NMB throughout the procedure followed by rapid recovery to allow early neurological evaluation of the patient after neurosurgery and hence to hasten the diagnosis and treatment of a life-threatening complication.
In the current study, we selected the dose 4 mg/kg for sugammadex according to phase II dose-ranging studies[6,8,9] and the dose of neostigmine and atropine were administered based on the standard recommended doses.
The results in our study support the findings of previous studies that concluded that sugammadex provides fast and safe recovery from rocuronium-induced neuromuscular block.[8,10,11]
In a randomized study by Groudine et al., the mean times for the TOF ratio to reach 90% after 4 mg/kg sugammadex for reversal of rocuronium at 0.6 or 1.2 mg/kg were 3.3 and 1.9 min, respectively which come in agreement with our findings.[6]
Furthermore, Sacan et al. found that sugammadex at 4 mg/kg reversed rocuronium-induced NMB (by reaching TOF ration to 90%) by 10 folds faster than that achieved by neostigmine combined with glycopyrrolate.[12]
It is worthy to mention that the reversal of muscle relaxation in our study was sustained without any evidence of recurarization in any patient in both groups during the period of neuromuscular monitoring or during their stay in the postanesthetic neurosurgical care unit.
The mean recovery times were similar to that previously found with Shields et al.[9] when they administered Org 25,969 at recovery of T2 following at least 2 h of neuromuscular block by rocuronium 0.6 mg/kg as an initial dose followed by increments to maintain a deep block. Thus, the effective dose of sugammadex seems to be independent of whether the rocuronium was administered in several maintenance doses or continuous infusion.
The patients in sugammadex group remained stable regarding the MAP and HR during the entire observation time during and after reversal in contrast to those patients in the neostigmine group who demonstrated significant rise in MAP and HR values in the first 10 min after administration of the reversal drugs when administered at the end of the steady state anesthesia in our study and this augment the results of Sacan et al.[12]
In the first 10 min after reversal with neostigmine and atropine but not after sugammadex there were significant higher HR and arterial pressure than the preinduction level although, this did not exceed the 20% beyond the baseline.
As sugammadex binds the rocuronium molecules in a 1:1 ratio[13] without having an effect on the plasma cholinesterase or on any muscarinic receptor in the human body,[8,14] no muscarinic effects results from its use. Such effects are responsible for the side-effects observed with the use of anticholinesterase agents combined with anticholinergic drug (atropine). Atropine may produce the undesirable tachycardia and/or arrhythmias.[15,16] The absence of muscarinic and cardiovascular effects at the time of reversal will be of great advantage especially in patients with cardiovascular and respiratory disease.
Sugammadex was well tolerated and confirmed in previous clinical studies. Peeters et al. concluded in a randomized double-blind crossover and placebo-controlled study that sugammadex is highly safe drugs with few adverse effects. In the phase I clinical study, a high intravenous dose of 96 mg/kg when administered into human subjects, showed taste disturbance as characteristic adverse reaction.[17] Otomo et al. added that overdose in itself is not hazard and the risk of underdose is not likely to warrant a deliberate reduction in dose.[18]
CONCLUSION
Sugammadex, a selective relaxant binding agent, rapidly and effectively reverses rocuronium-induced NMB in pediatric patients undergoing neurosurgery when administered at reappearance of T2 of TOF at dose 4 mg/kg.
Additional studies on pediatrics, particularly in infants <2 years will be required to determine fully the efficacy and safety of sugammadex in this category of patients, particularly when more profound levels of NMB are required.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hayes AH, Mirakhur RK, Breslin DS, Reid JE, McCourt KC. Postoperative residual block after intermediate-acting neuromuscular blocking drugs. Anaesthesia. 2001;56:312–8. doi: 10.1046/j.1365-2044.2001.01921.x. [DOI] [PubMed] [Google Scholar]
- 2.van den Broek L, Proost JH, Wierda JM. Early and late reversibility of rocuronium bromide. Eur J Anesthesiol Suppl. 1994;9:128–32. [PubMed] [Google Scholar]
- 3.Blobner M, Eriksson LI, Scholz J, Motsch J, Della Rocca G, Prins ME. Reversal of rocuronium-induced neuromuscular blockade with sugammadex compared with neostigmine during sevoflurane anaesthesia: Results of a randomised, controlled trial. Eur J Anesthesiol. 2010;27:874–81. doi: 10.1097/EJA.0b013e32833d56b7. [DOI] [PubMed] [Google Scholar]
- 4.Lemmens HJ, El-Orbany MI, Berry J, Morte JB, Jr, Martin G. Reversal of profound vecuronium-induced neuromuscular block under sevoflurane anesthesia: Sugammadex versus neostigmine. BMC Anesthesiol. 2010;10:15. doi: 10.1186/1471-2253-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duvaldestin P, Kuizenga K, Saldien V, Claudius C, Servin F, Klein J, et al. A randomized, dose-response study of sugammadex given for the reversal of deep rocuronium- or vecuronium-induced neuromuscular blockade under sevoflurane anesthesia. Anesth Analg. 2010;110:74–82. doi: 10.1213/ANE.0b013e3181c3be3c. [DOI] [PubMed] [Google Scholar]
- 6.Groudine SB, Soto R, Lien C, Drover D, Roberts K. A randomized, dose-finding, phase II study of the selective relaxant binding drug, Sugammadex, capable of safely reversing profound rocuronium-induced neuromuscular block. Anesth Analg. 2007;104:555–62. doi: 10.1213/01.ane.0000260135.46070.c3. [DOI] [PubMed] [Google Scholar]
- 7.Jones RK, Caldwell JE, Brull SJ, Soto RG. Reversal of profound rocuronium-induced blockade with sugammadex: A randomized comparison with neostigmine. Anesthesiology. 2008;109:816–24. doi: 10.1097/ALN.0b013e31818a3fee. [DOI] [PubMed] [Google Scholar]
- 8.Sorgenfrei IF, Norrild K, Larsen PB, Stensballe J, Ostergaard D, Prins ME, et al. Reversal of rocuronium-induced neuromuscular block by the selective relaxant binding agent sugammadex: A dose-finding and safety study. Anesthesiology. 2006;104:667–74. doi: 10.1097/00000542-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Shields M, Giovannelli M, Mirakhur RK, Moppett I, Adams J, Hermens Y. Org 25969 (sugammadex), a selective relaxant binding agent for antagonism of prolonged rocuronium-induced neuromuscular block. Br J Anaesth. 2006;96:36–43. doi: 10.1093/bja/aei314. [DOI] [PubMed] [Google Scholar]
- 10.de Boer HD, van Egmond J, van de Pol F, Bom A, Booij LH. Chemical encapsulation of rocuronium by synthetic cyclodextrin derivatives: Reversal of neuromuscular block in anaesthetized Rhesus monkeys. Br J Anaesth. 2006;96:201–6. doi: 10.1093/bja/aei306. [DOI] [PubMed] [Google Scholar]
- 11.Vanacker BF, Vermeyen KM, Struys MR, Rietbergen H, Vandermeersch E, Saldien V, et al. Reversal by Org 25969 is not affected by sevoflurane compared with propofol (abstract) Eur J Anesthesiol. 2005;22 Suppl 34:A453. [Google Scholar]
- 12.Sacan O, White PF, Tufanogullari B, Klein K. Sugammadex reversal of rocuronium-induced neuromuscular blockade: A comparison with neostigmine-glycopyrrolate and edrophonium-atropine. Anesth Analg. 2007;104:569–74. doi: 10.1213/01.ane.0000248224.42707.48. [DOI] [PubMed] [Google Scholar]
- 13.Bom A, Bradley M, Cameron K, Clark JK, Van Egmond J, Feilden H, et al. A novel concept of reversing neuromuscular block: Chemical encapsulation of rocuronium bromide by a cyclodextrin-based synthetic host. Angew Chem Int Ed Engl. 2002;41:266–70. doi: 10.1002/1521-3773(20020118)41:2<265::aid-anie265>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 14.Naguib M. Sugammadex: Another milestone in clinical neuromuscular pharmacology. Anesth Analg. 2007;104:575–81. doi: 10.1213/01.ane.0000244594.63318.fc. [DOI] [PubMed] [Google Scholar]
- 15.Fielder DL, Nelson DC, Andersen TW, Gravenstein JS. Cardiovascular effects of atropine and neostigmine in man. Anesthesiology. 1969;30:637–41. doi: 10.1097/00000542-196906000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Mirakhur RK, Dundee JW, Jones CJ, Coppel DL, Clarke RS. Reversal of neuromuscular blockade: Dose determination studies with atropine and glycopyrrolate given before or in a mixture with neostigmine. Anesth Analg. 1981;60:557–62. [PubMed] [Google Scholar]
- 17.Peeters PA, van den Heuvel MW, van Heumen E, Passier PC, Smeets JM, van Iersel T, et al. Safety, tolerability and pharmacokinetics of sugammadex using single high doses (up to 96 mg/kg) in healthy adult subjects: A randomized, double-blind, crossover, placebo-controlled, single-centre study. Clin Drug Investig. 2010;30:867–74. doi: 10.1007/BF03256915. [DOI] [PubMed] [Google Scholar]
- 18.Otomo S, Iwasaki H, Takahoko K, Onodera Y, Sasakawa T, Kunisawa T, et al. Prediction of optimal reversal dose of sugammadex after rocuronium administration in adult surgical patients. Anesthesiol Res Pract 2014. 2014 doi: 10.1155/2014/848051. 848051. [DOI] [PMC free article] [PubMed] [Google Scholar]


