Abstract
Background:
Postoperative acute kidney injury (AKI) is common in patients with chronic liver disease. We prospectively evaluated effectiveness of the N-acetylcysteine (NAC) in preserving postoperative renal functions in cirrhotic patients undergoing major abdominal surgeries.
Materials and Methods:
A total of 60 cirrhotic patients child A to B were randomized into two groups of 30 each. NAC groupwas received intravenous infusion of NAC (1200 mg/12h starting immediately before surgery and continued for 72h h postoperative) and controls group received a similar volume of glucose 5% solution as a a placebo. Systemic hemodynamics, hepatic and renal functions, serum cystatin C and cystatin C glomerular filtration rate (GFR) (GFR) were compared between both groups.
Results:
Serum level of cystatin C was raised significantly above the basal value at postoperative day 1 and day 3 associated with significantly decreased in cystatin C GFR below the basal value in the control group (P = 0.001). 6 (20%) (PP = 0.03) in control group developed AKI based on cystatin C GFR criteria (GFR <55 ml/min/1.73m2). Mean values of alanine aminotransferase and aspartate aminotransferase were increased significantly above the basal values in both groups, but the increases were significantly lower in NAC group (P = 0.00). Chest infection was significantly lower associated with shorter hospital stay in the NAC group than the control group.
Conclusion:
Intravenous administration of NAC NAC in cirrhotic patients undergoing major abdominal surgeries reduces the incidence of cystatin C GFR-based AKI, postoperative renal and liver functions were well-preserved and improved outcome.
Keywords: Acute Kidney injury, cirrhotic patients, N-acetylcysteine
INTRODUCTION
Renal dysfunction is one of the most common complications in cirrhosis with high morbidity and mortality.[1] Hepatorenal disorders commonly encountered in subjects with cirrhosis are either a direct consequence of the underlying cirrhosis affecting the kidneys (hepatorenal syndrome [HRS])[2] or secondary to etiologies other than cirrhosis per se. In patients with cirrhosis, either excessive or insufficient production of nitric oxide (NO) results in reduced renal blood flow that result in a substantial reduction in glomerular filtration rate (GFR).[3] The functional renal abnormalities associated with advanced liver disease and cirrhosis increase the susceptibility of the kidneys to the development of acute renal failure(ARF) (ARF) following insults such as hypovolemic shock, major surgical procedures, and use of nephrotoxic drugs, infection. Depletion of glutathione(GSH) (GSH) is believed to be the cause of both ARF and acute liver failure acute liver failure (ALF).[4] Mindikoglu and Weir and Weir[5] proposes a new classification of renal dysfunction in cirrhosis. Serum creatinine is not an accurate marker of kidney function in cirrhosis. The synthesis of creatine that is a precursor of creatinine is impaired in cirrhosis.[6] Protein-restricted diet and increased tubular secretion further lower creatinine levels and reduce the accuracy of 24 h-h creatinine clearance to estimate GFR.[7] Although measuring GFR is a gold standard method to assess kidney function, it is laborious, expensive, time-consuming, and not practical in clinical practice.[7] The second alternative to creatinine in estimating kidney function in cirrhosis is serum biomarker cystatin C.[8] Institution of prophylactic measures in these high-risk patients in Perioperative periods is the best opportunity to prevent acute kidney injury (AKI), and many pharmacologic strategies have been proposed but with conflicting results.[9] Recently, antioxidant N-acetylcysteine (NAC), an agent that buffers a variety of oxygen-derived free radicals and improves renal vasodilatation through an endothelium-dependent mechanism, has been shown to confer protection against contrast-induced renal dysfunction[10] and preliminary data suggest that NAC ameliorates renal ischemia-reperfusion injury(IRI) (IRI).[11] We designed this study to evaluate the effect of the NAC in postoperative renal function in cirrhotic patients undergoing major abdominal surgeries assessed by cystatin C and cystatin C GFR.
MATERIALS AND METHODS
The study protocol was approved by the Institutional Ethics Committee. After obtaining written informed consent from the patients, 60 cirrhotic patients child A to B scheduled for open major abdominal surgery (intestinal resection, liver resection, Whipple, hepato-biliary procedures) were enrolled in this prospective double-blinded randomized controlled study. Those with known allergy to NAC, and those having received NAC for the the contrast-induced nephropathy prevention in the previous 7 days or contrast agents in the previous 72 h were excluded.
Study protocol
Pharmacy department supplied the infusions to the anaesthesia department prior to the planned surgery. Both the anaesthesia provider and the accessors were blind to the content of the infusion. Sealed opaque envelopes were only opened by the pharmacist to allocate the patient to his group. The 1st group (NAC group) received an intravenous bolus of 1200 mg of NAC (Hidonac 5 g & 200 mg/ml) (Zambon S.P.A.,) dilute in 5% glucose or physiological solution via an infusion pump (Fresenius Kabi, Germany) over 55 h given immediately after induction of anesthesia, followed by additional boluses administered at 12-h intervals post-operative for 72 h in the intensive care unit intensive care unit ((ICU)) (total dose of NAC = 7200 mg). This protocol similar to that used by Sisillo et al et al.[12] The 2nd group (controls) received similar volume of glucose 5% bolus and infusion as placebo. General anesthesia was induced with fentanyl 2 μg/kg, propofol 2 mg/kg, and rocuronium 0.9 mg/kg. After intubation of the trachea, the lungs were ventilated to maintain normocapnia (end-expiratory partial pressure of carbon dioxide level (32-38 mm Hg) using a constant fresh gas flow of 1 L/min. Maintenance of anesthesia were performed with sevoflurane, fentanyl, and rocuronium keeping entropy reading (GE healthcare, Helsinki, Finland) between 40 and and 60. Normothermia was achieved with a forced-air warming device (Bair Hugger, Arizant, UK). Standard monitoring for both groups included electrocardiogram, invasive arterial blood pressure via left radial artery catheter after performing Modified Allen's test, central venous pressure (7 Fr, 20 cm; arrow international reading) was placed through the right internal jugular vein by ultrasound-guided method (SonoSite, NanoMax ultrasound system, USA), pulse oximetry, nasopharyngeal core temperature, inspiratory and expiratory gas concentrations. After surgery, patients were transferred to the ICU, with adequate pain control. The hemodynamic management consisted of maintaining the mean arterial pressure (MAP) (MAP) at ≥65 mm Hg with adequate vascular filling with crystalloids and colloids (Voluven hydroxyethyl starch 130/0.4 6%), and dopamine or norepinephrine infusion were used, if necessary. If urinary output was <1 ml/kg/h, incremental doses of 20 mg of furosemide, followed by continuous infusion of (0.02-0.04) mg/kg/h. Baseline liver-specific variables included etiology of chronic liver disease, Child-Pughscore.[13] All data were collected by a resident blinded to the study design and group allocation. To ascertain comparable preconditions between the groups with respect to preoperative comorbidity and type of surgery, all patients underwent physiological and operative severity score for the enumeration of mortality and morbidity (POSSUM) (POSSUM) scoringusing an online software calculator (http://www.vasgbi.com/riskpossum.htm).[14] Physiological variables recorded included heart rate(HR) (HR), blood pressure, calculated mean arterial blood pressure, Daily collection of serum and plasma samples was undertaken to provide aliquots for routine biochemistry analysis; additional aliquots were immediately stored at (−80°C°C) for subsequent analysis of serum cystatin C. Cystatin C concentrations were measured in the main biochemistry laboratory using a latex-enhanced immune turbid metric assay[15] (Roche/Hitachi cobas C systems human cystatin C agglutinates with latex particles coated with anti-cystatin C antibodies The aggregate is determined turbid metrically at 546 nm). The cystatin C-based equation of Hoek et al.[16] allowed the calculation of cystatin C GFR from the cystatin C concentrations. Perioperative adverse or side effects such as such as arrhythmias, nausea, vomiting, flushing, hypotension, cough, and urticaria were all recorded. Serum creatinine mg/dl levels, blood urea nitrogen (BUN), cystatin C, cystatin C GFR, aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, and serum lactate all measured at the following time-: T0 — Baseline before surgery, T1 — Post-operative day- 1, T2 — Post-operative day 3.
Estimation ofglomerular filteration rate glomerular filtration rate
The cystatin C-based equation of Hoek et al.[16] allowed the calculation of cystatin C GFR from the cystatin C concentrations.
GFR/1.73 m2 = −4.32 + 80.35/cyst C.
Sample size and power of the study
In the present study, α was set to 0.05 (priori), and maximum b accepted = 20% with a minimum power of the study of 80%. Primary outcome of this randomized controlled trialwas cystatin C GFR between experimental group (1200 mg NAC NAC) (73 ± 17) and control group (60 ± 22) with a mean difference of (13),[17] one-tailed analysis will be adopted. Calculation of sample size was done using (IBM Statistical Package for Social Science SPSS sample power) software and was also confirmed using Lenth Java applets for power and sample size.
Normally distributed data were analyzed using t-test, and categorical data were analyzed using the Chi-square test. Continuous data are presented as mean and standard deviation, whereas categorical data are presented as number of patients and percentage. Data were analyzed using IBM SPSS statistics 20.0 software. P < 0.05 was considered statistically significant.
RESULTS
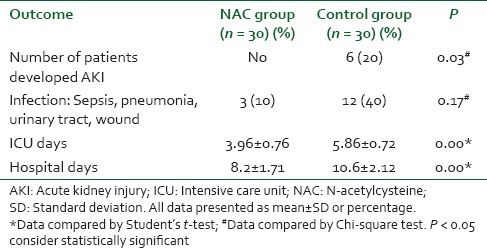
There were no significant differences between the groups with regard to demographic data, clinical, and perioperative characteristics [Table 1]. There were no significant differences with regard to baseline indexes of renal function, child classification, co-morbid risk factors, physiological or operative POSSUM score, surgery type or duration, blood loss or postoperative hematology, of the two groups were remarkably similar overall. Hence Hence, it is, therefore therefore, very unlikely that patient characteristic variables might influence the results of the study significantly. There were were no significant differences between both groups regarding the physiological POSSUM score consequently it is very unlikely that preoperative Factors made a significant contribution to the difference in the the outcome. Furthermore Furthermore, the operative POSSUM scores were virtually identical indicating an even overall distribution of operative difficulty [Table 1]. There were no significant differences in the volume of crystalloids or colloids transfused to both groups [Table 2] with a mean value of 2470 (1680) ml and 570 (330) ml in the NAC group while mean values were 2530 (1760) ml and 590 (290) ml in the control group, respectively (P > 0.05). There was no statistically significant different between the two groups as regards serum creatinine and BUN BUN all over the time of study (P > 0.05) [Table 3]. There were no statistically significant different in preoperative values of serum cystatin C in both groups a mean value of 1.032 (0.169) versus (1.045 [0.166] mg/L, P = 0.68) associated with significant increase in serum cystatin C at postoperative day 1 and postoperative day 3 a mean value of (1.151 [0.184] and and 1.204 [0.201] 1.003 [0.111] and and 1.027 [0.142] mg/L, P = 0.00) in the control group and NAC group, respectively [Table 4 and Figure 1]. Estimation of GFR calculated according to Hoek et al. equation used the values of cystatin C showed that There were no statistically significant different in preoperative values of cystatin C GFR in both groups a mean value of (76.33 [11.329] 75 [11.199] ml/min/1.73 m2, PP = 0.52) that associated with significant decreased in cystatin C GFR at postoperative day 1 and postoperative day 3 a mean value of (68.53 [9.886] and 65.33 [10.162] vs vs. 76.26 [6.414] and 75.13 [7.696] ml/min/1.73 m2, PP = 0.00) in the control group and NAC group, respectively [Table 4 and Figure 2]. Of the 30 patients enrolled, 6 (20%) in the control group developed AKI as urine output <0.5 ml/kg/h for more than 12 h and based on results of cystatin C GFR criteria (GFR <55 ml/min/1.73 m2) they admitted to the ICU ICU. AKI resolved with medical intervention in five patients suggestive of reversible prerenal etiology. One patient developed AKI, due to either acute tubular necrosis or HRS that required the initiation of renal replacement therapy. Our results showed that the increase in AST and ALT from baseline values were significantly lower postoperative day 1 and postoperative day 3 in NAC group than in control group a mean values (67.53 [17.45] and and 73.20 [16.43] vs. 84.60 [15.14] and and 95.60 [17.27] IU/L, P = 0.00) and (74 [10.18] and 78.26 [17.27] vs. 94.73 [20.39] and 104.40 [23.35] IU/L, P = 0.00 <0.001), respectively [Table 3]. There were no significant differences between both groups regarding serum bilirubin (mg/dl), there was statistically significant prolonged in prothrombin time at postoperative day 1 and postoperative day 3 in both group with no statistically significant different between both groups (P > 0.05) [Table 3]. There was no statistical difference in systolic arterial pressure, MAP, diastolic arterial pressure, HR, and SpO2 values at the preoperative, postoperative day 1 and day 3 [Table 2]. Complications in the form of chest infection and postoperative intolerance to oral feeding were significantly lower in the NAC group than control group; this was accompanied with a significant reduction in the hospital stay, (P < 0.05) [Table 5].
Table 1.
Demographic data and preoperative criteria in both groups
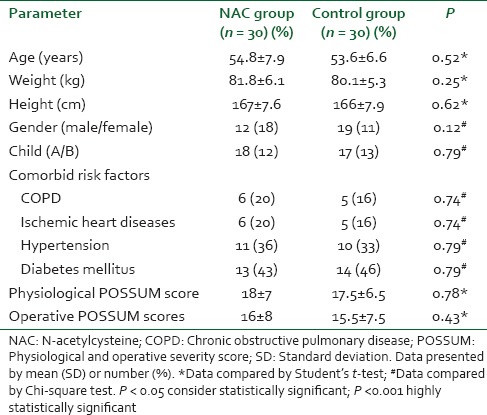
Table 2.
Operative data, hemodynamic and volume of replacement
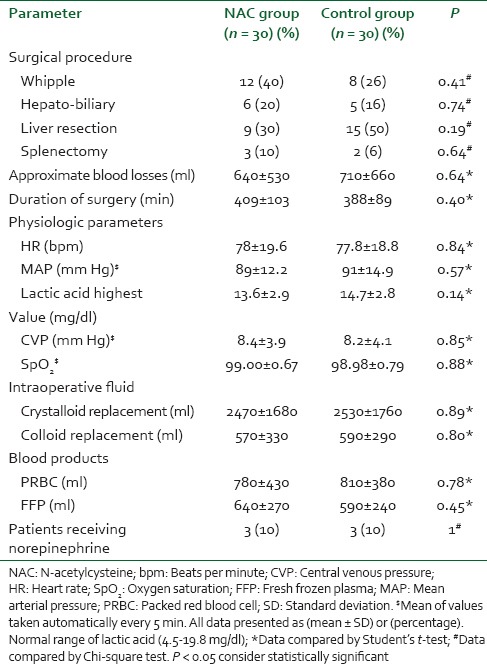
Table 3.
Kidney function and liver function tests in both groups
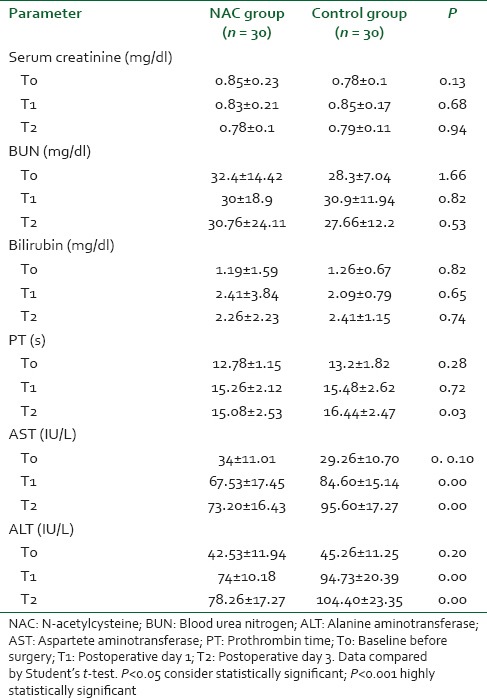
Table 4.
Cystatin C and cystatin C GFR in both groups
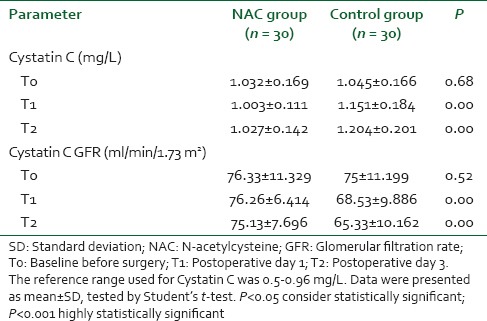
Figure 1.
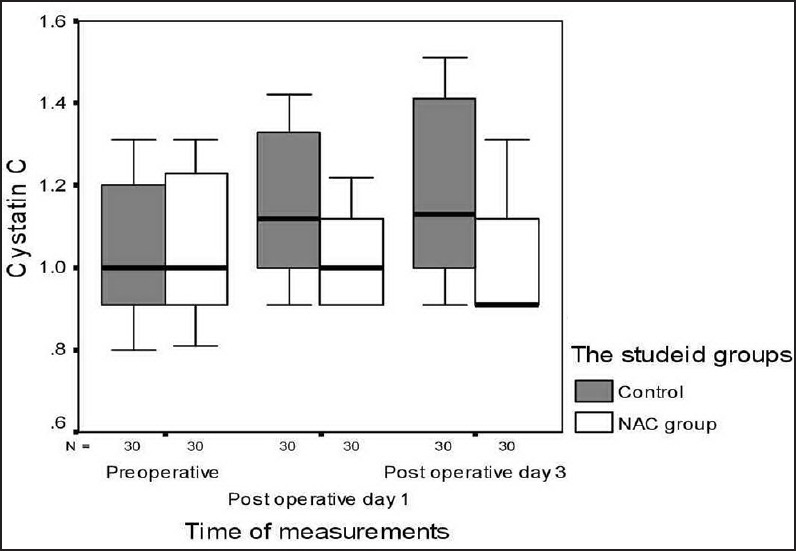
Box and whiskers graph of cystatin C (mg/L) in the two studied groups. N-acetylcysteine group
Figure 2.
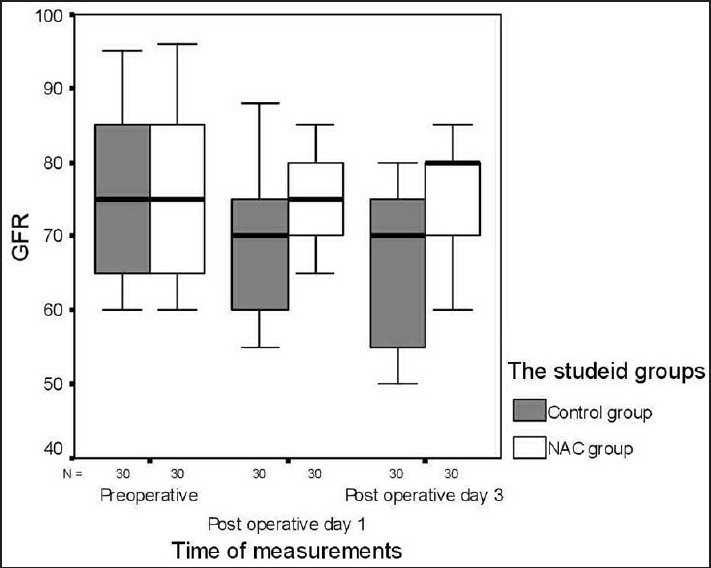
Box and whiskers graph of cystatin C glomerular filtration rate (ml/min/1.73 m2) in the two studied groups. N-acetylcysteine group
Table 5.
Postoperative complications in both groups
As all included patients completed the study (with no withdrawal or exclusion) so there is no need for the flow chart of drop out (the number of patients that drop out of a study should give concern if the number is very high. Conventionally, a 20% drop out rate is regarded as acceptable, but this may vary). (Ref. SIGN: Methodology Checklist 2: Controlled trial)
DISCUSSION
Acute kidney injuryis observed in approximately 20% of patients admitted to hospital with decompensated chronic liver disease and ascites common at postoperative period it is associated with high mortality, morbidity and a more complicated hospital course. It is either ischemic or toxic in origin and results from a reduction in GFR GFR and formation of oxygen free radicals (GFR).[18] Therefore, prevention of AKI and development of new pharmacological approaches are needed in cirrhotic patients with preexisting renal insufficiency. NAC is a modified form of L-cysteine, an amino acid that is a precursor to reduced GSH GSH. It is known to be a potent antioxidant that scavenges oxygen-free radicals thus preventing direct oxidative tissue damage in the body. It also has vasodilatory properties derived from enhanced NO NO availability.[19] NAC has direct vasodilating effects in the kidneys, it also causes vasodilatation by increasing cyclic guanosine monophosphate levels, inhibits platelet aggregation, acts as a sulfhydryl donor to regenerate endothelial-derived relaxing factor and reduces interleukin (IL8)- and tumor necrosis factor-alpha production. NAC carries out an anti-inflammatory action by suppressing cytokine expression/release and inhibiting adhesion molecule expression.[20] Our results demonstrate that NAC preserved postoperative renal function in cirrhotic patients undergoing major abdominal surgeries assessed by cystatin C concentration and cystatin C GFR. Tepel et al. was the first to report that NAC reduced the incidence of contrast-induced nephrotoxicity (CIN).[10] The promising results of this study led to widespread use of NAC to prevent CIN, particularly following intra-arterial administration of CM. More recent studies have used higher doses and found that it is more efficacious in preventing CIN.[21,22] These observations call our attention to the unique aspects of NAC pharmacology, including its low bioavailability, extensive first pass metabolism,[23] and possible dose-dependent antioxidant characteristics.[24] The use of NAC to prevent ARF in patients undergoing cardiac surgery has been recently investigated with conflicting results.[12,25] Supported our results but in rate Abdelrahman et al.[26] Disagree with our results Nigwekar and Kandula[27] and Alioglu et al.,[28] small dose of NAC used in their studies can explain this results. Cystatin C is a low molecular weight protein produced at a constant rate by all nucleated cells. It is freely filtered by the glomerulus, not secreted but reabsorbed and metabolized in the tubules. Cystatin C is not dependent on an intact hepatic function and subjects with cirrhosis do not have increased tubular secretion of cystatin C. Cystatin C levels are not affected or are less affected than creatinine by muscle mass, gender, race, protein-restricted diet, and other factors. All of these features make cystatin C an attractive endogenous GFR marker in cirrhosis.[8] Declining kidney function has been associated with increased biomarker concentrations.[29] Several investigators investigated the accuracy of cystatin C in estimating kidney function in cirrhosis.[30] Serum cystatin c is a better biomarker for predicting the development of AKI in liver cirrhosis.[17,31] Data suggest a significant improvement of GFR estimation in cirrhotic patients by means of cystatin C-based Hoek FJ et al. equations.[16] NAC can increase intracellular reduced GSH levels in erythrocytes andliver and lung cells and replenish GSH stores following experimental depletion.[32] Results in significant preservation of membrane fluidity andthe activities of catalase, mitochondrial superoxide dismutase and the different forms of GSH peroxidase in biliary obstructed rats. These effects of NAC suggest it may be a useful agent to preserve liver function.[33] Liver-specific Transaminase is commonly used to determine the extent procurement, preservation, and reperfusion injuries to the graft in liver transplantation. Serum concentration of ALT and AST are good markers of hepatocyte loss. Our results showed that liver functions were well-preserved with administration of NAC as indicated by the Increase in ALT and AST was significantly lower POD1 and POD3 in NAC group than control group Supported our results Beyaz et al. who found that NAC preserved hepatic function during isoflurane anesthesia in laparoscopic surgery patients.[34] Todd Stravitz et al. showed that NAC may improve transplant-free survival in patients with nonacetaminophen ALF by ameliorating the production of IL-17.[35] Lee et al. found that intravenous NAC improves transplant-free survival in patients with early stage nonacetaminophen-related ALF.[36] Use of NAC in liver transplant IRI showed that IRI is not totally preventable, but its negative effects may be reduced.[37,38] NAC improves our respiratory health through a very unique characteristic it possesses. NAC breaks disulfide bonds in mucus, essentially liquefying it. This allows it to be coughed up and frees up the congestion of the lungs that's can explain our results as there was statistically significant decreased in number of patients complicated by chest infection. NAC has been demonstrated to maintain the endothelium-dependent vasodilatation in the pulmonary circulation to prevent, or attenuate, the deterioration of lung mechanics and gas exchange in endotoxemic sheep to improve oxygenation and reduce ventilator days after acute lung injury in humans.[39] A possible limitation of our study is the definition of AKI. Although this definition is widely used[9,22] and clearly related to clinical outcome in our study, it remains, at least in part, arbitrary. Additional studies should investigate the relevance of renal effects of NAC using a consensus definition of AKI, and of its extrarenal effects (particularly on hepatic and respiratory function), in cirrhotic patients.
CONCLUSION
Intravenous administration of NAC in cirrhotic patients undergoing major abdominal surgeries reducess the incidence of cystatin C-based AKI, postoperative renal and liver functions were well-preserved and improved outcome.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ginès P. Pharmacological management of hepatorenal syndrome: Lessons from non-responders. J Hepatol. 2011;55:268–9. doi: 10.1016/j.jhep.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–8. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lluch P, Mauricio MD, Vila JM, Segarra G, Medina P, Del Olmo JA, et al. Accumulation of symmetric dimethylarginine in hepatorenal syndrome. Exp Biol Med (Maywood) 2006;231:70–5. doi: 10.1177/153537020623100108. [DOI] [PubMed] [Google Scholar]
- 4.Moore K. Renal failure in acute liver failure. Eur J Gastroenterol Hepatol. 1999;11:967–75. doi: 10.1097/00042737-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Mindikoglu AL, Weir MR. Current concepts in the diagnosis and classification of renal dysfunction in cirrhosis. Am J Nephrol. 2013;38:345–54. doi: 10.1159/000355540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherman DS, Fish DN, Teitelbaum I. Assessing renal function in cirrhotic patients: Problems and pitfalls. Am J Kidney Dis. 2003;41:269–78. doi: 10.1053/ajkd.2003.50035. [DOI] [PubMed] [Google Scholar]
- 7.Mindikoglu AL, Regev A, Seliger SL, Magder LS. Gender disparity in liver transplant waiting-list mortality: The importance of kidney function. Liver Transpl. 2010;16:1147–57. doi: 10.1002/lt.22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mindikoglu AL, Dowling TC, Weir MR, Seliger SL, Christenson RH, Magder LS. Performance of chronic kidney disease epidemiology collaboration creatinine-cystatin C equation for estimating kidney function in cirrhosis. Hepatology. 2014;59:1532–42. doi: 10.1002/hep.26556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bove T, Landoni G, Calabrò MG, Aletti G, Marino G, Cerchierini E, et al. Renoprotective action of fenoldopam in high-risk patients undergoing cardiac surgery: A prospective, double-blind, randomized clinical trial. Circulation. 2005;111:3230–5. doi: 10.1161/CIRCULATIONAHA.104.509141. [DOI] [PubMed] [Google Scholar]
- 10.Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180–4. doi: 10.1056/NEJM200007203430304. [DOI] [PubMed] [Google Scholar]
- 11.Conesa EL, Valero F, Nadal JC, Fenoy FJ, López B, Arregui B, et al. N-acetyl-L-cysteine improves renal medullary hypoperfusion in acute renal failure. Am J Physiol Regul Integr Comp Physiol. 2001;281:R730–7. doi: 10.1152/ajpregu.2001.281.3.R730. [DOI] [PubMed] [Google Scholar]
- 12.Sisillo E, Ceriani R, Bortone F, Juliano G, Salvi L, Veglia F, et al. N-acetylcysteine for prevention of acute renal failure in patients with chronic renal insufficiency undergoing cardiac surgery: A prospective, randomized, clinical trial. Crit Care Med. 2008;36:81–6. doi: 10.1097/01.CCM.0000295305.22281.1D. [DOI] [PubMed] [Google Scholar]
- 13.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–9. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 14.Copeland GP. The POSSUM system of surgical audit. Arch Surg. 2002;137:15–9. doi: 10.1001/archsurg.137.1.15. [DOI] [PubMed] [Google Scholar]
- 15.Lewis AV, James TJ, McGuire JB, Taylor RP. Improved immunoturbidimetric assay for cystatin C. Ann Clin Biochem. 2001;38(Pt 2):111–4. doi: 10.1258/0004563011900399. [DOI] [PubMed] [Google Scholar]
- 16.Hoek FJ, Kemperman FA, Krediet RT. A comparison between cystatin C, plasma creatinine and the Cockcroft and Gault formula for the estimation of glomerular filtration rate. Nephrol Dial Transplant. 2003;18:2024–31. doi: 10.1093/ndt/gfg349. [DOI] [PubMed] [Google Scholar]
- 17.Slack AJ, McPhail MJ, Ostermann M, Bruce M, Sherwood R, Musto R, et al. Predicting the development of acute kidney injury in liver cirrhosis – An analysis of glomerular filtration rate, proteinuria and kidney injury biomarkers. Aliment Pharmacol Ther. 2013;37:989–97. doi: 10.1111/apt.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Betrosian AP, Agarwal B, Douzinas EE. Acute renal dysfunction in liver diseases. World J Gastroenterol. 2007;13:5552–9. doi: 10.3748/wjg.v13.i42.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Efrati S, Dishy V, Averbukh M, Blatt A, Krakover R, Weisgarten J, et al. The effect of N-acetylcysteine on renal function, nitric oxide, and oxidative stress after angiography. Kidney Int. 2003;64:2182–7. doi: 10.1046/j.1523-1755.2003.00322.x. [DOI] [PubMed] [Google Scholar]
- 20.Spapen H, Zhang H, Demanet C, Vleminckx W, Vincent JL, Huyghens L. Does N-acetyl-L-cysteine influence cytokine response during early human septic shock? Chest. 1998;113:1616–24. doi: 10.1378/chest.113.6.1616. [DOI] [PubMed] [Google Scholar]
- 21.Briguori C, Colombo A, Violante A, Balestrieri P, Manganelli F, Paolo Elia P, et al. Standard vs double dose of N-acetylcysteine to prevent contrast agent associated nephrotoxicity. Eur Heart J. 2004;25:206–11. doi: 10.1016/j.ehj.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Marenzi G, Assanelli E, Marana I, Lauri G, Campodonico J, Grazi M, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354:2773–82. doi: 10.1056/NEJMoa054209. [DOI] [PubMed] [Google Scholar]
- 23.Sjödin K, Nilsson E, Hallberg A, Tunek A. Metabolism of N-acetyl-L-cysteine. Some structural requirements for the deacetylation and consequences for the oral bioavailability. Biochem Pharmacol. 1989;38:3981–5. doi: 10.1016/0006-2952(89)90677-1. [DOI] [PubMed] [Google Scholar]
- 24.Cotgreave I, Moldéus P, Schuppe I. The metabolism of N-acetylcysteine by human endothelial cells. Biochem Pharmacol. 1991;42:13–6. doi: 10.1016/0006-2952(91)90674-t. [DOI] [PubMed] [Google Scholar]
- 25.El-Hamamsy I, Stevens LM, Carrier M, Pellerin M, Bouchard D, Demers P, et al. Effect of intravenous N-acetylcysteine on outcomes after coronary artery bypass surgery: A randomized, double-blind, placebo-controlled clinical trial. J Thorac Cardiovasc Surg. 2007;133:7–12. doi: 10.1016/j.jtcvs.2006.05.070. [DOI] [PubMed] [Google Scholar]
- 26.Abdelrahman AM, Al Salam S, AlMahruqi AS, Al husseni IS, Mansour MA, Ali BH. N-acetylcysteine improves renal hemodynamics in rats with cisplatin-induced nephrotoxicity. J Appl Toxicol. 2010;30:15–21. doi: 10.1002/jat.1465. [DOI] [PubMed] [Google Scholar]
- 27.Nigwekar SU, Kandula P. N-acetylcysteine in cardiovascular-surgery-associated renal failure: A meta-analysis. Ann Thorac Surg. 2009;87:139–47. doi: 10.1016/j.athoracsur.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 28.Alioglu E, Saygi S, Turk U, Kirilmaz B, Tuzun N, Duman C, et al. N-acetylcysteine in preventing contrast-induced nephropathy assessed by cystatin C. Cardiovasc Ther. 2013;31:168–73. doi: 10.1111/j.1755-5922.2011.00309.x. [DOI] [PubMed] [Google Scholar]
- 29.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A, NGAL Meta-analysis Investigator Group Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–24. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 30.Ahn HS, Kim YS, Kim SG, Kim HK, Min SK, Jeong SW, et al. Cystatin C is a good predictor of hepatorenal syndrome and survival in patients with cirrhosis who have normal serum creatinine levels. Hepatogastroenterology. 2012;59:1168–73. doi: 10.5754/hge11616. [DOI] [PubMed] [Google Scholar]
- 31.Orlando R, Mussap M, Plebani M, Piccoli P, De Martin S, Floreani M, et al. Diagnostic value of plasma cystatin C as a glomerular filtration marker in decompensated liver cirrhosis. Clin Chem. 2002;48(6 Pt 1):850–8. [PubMed] [Google Scholar]
- 32.Nakata K, Kawase M, Ogino S, Kinoshita C, Murata H, Sakaue T, et al. Effects of age on levels of cysteine, glutathione and related enzyme activities in livers of mice and rats and an attempt to replenish hepatic glutathione level of mouse with cysteine derivatives. Mech Ageing Dev. 1996;90:195–207. doi: 10.1016/0047-6374(96)01771-x. [DOI] [PubMed] [Google Scholar]
- 33.Pastor A, Collado PS, Almar M, González-Gallego J. Antioxidant enzyme status in biliary obstructed rats: Effects of N-acetylcysteine. J Hepatol. 1997;27:363–70. doi: 10.1016/s0168-8278(97)80183-3. [DOI] [PubMed] [Google Scholar]
- 34.Beyaz SG, Yelken B, Kanbak G. The effects of N-acetylcysteine on hepatic function during isoflurane anesthesia for laparoscopic surgery patients. Indian J Anaesth. 2011;55:567–72. doi: 10.4103/0019-5049.90610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stravitz RT, Sanyal AJ, Reisch J, Bajaj JS, Mirshahi F, Cheng J, et al. Effects of N-acetylcysteine on cytokines in non-acetaminophen acute liver failure: Potential mechanism of improvement in transplant-free survival. Liver Int. 2013;33:1324–31. doi: 10.1111/liv.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856–64e1. doi: 10.1053/j.gastro.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson J. A Knack for NAC? USE of N-Acetyl Cystatin in Liver Transplant Ischemia Reperfusion Injury. Research. J Nelson, 2011. 2004 Jan;52:2. [Google Scholar]
- 38.Bromley PN, Cottam SJ, Hilmi I, Tan KC, Heaton N, Ginsburg R, et al. Effects of intraoperative N-acetylcysteine in orthotopic liver transplantation. Br J Anaesth. 1995;75:352–4. doi: 10.1093/bja/75.3.352. [DOI] [PubMed] [Google Scholar]
- 39.Angdin M, Settergren G, Starkopf J, Zilmer M, Zilmer K, Vaage J. Protective effect of antioxidants on pulmonary endothelial function after cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2003;17:314–20. doi: 10.1016/s1053-0770(03)00053-3. [DOI] [PubMed] [Google Scholar]


