Abstract
Background:
The transverse abdominis plane (TAP) block, a regional block provides effective analgesia after lower abdominal surgeries if used as part of multimodal analgesia. In this prospective, randomized double-blind study, we determined the efficacy of TAP block in patients undergoing cesarean section.
Materials and Methods:
Totally, 62 parturients undergoing cesarean section were randomized in a double-blind manner to receive either bilateral TAP block at the end of surgery with 20 ml of 0.25% bupivacaine or no TAP block, in addition to standard analgesic comprising 75 mg diclofenac 8 hourly and intravenous patient-controlled analgesia (PCA) tramadol. Each patient was assessed at 0, 4, 8, 12, 24, 36, and 48 h after surgery by an independent observer for pain at rest and on movement using numeric rating scale of 0-10, time of 1st demand for tramadol, total consumption of PCA tramadol, satisfaction with pain management and side effects. Results: Use of tramadol was reduced in patients given TAP block by 50% compared to patients given no block during 48 h after surgery (P < 0.001). Pain scores were lower both on rest and activity at each time point for 24 h in study group (P < 0.001), time of first analgesia was significantly longer, satisfaction was higher, and side effects were less in study group compared to control group.
Conclusion:
Transverse abdominis plane block was effective in providing analgesia with a substantial reduction in tramadol use during 48 h after cesarean section when used as adjunctive to standard analgesia.
Keywords: Bupivacaine, cesarean section, transverse abdominis plane block
INTRODUCTION
Pain after cesarean section is usually described as moderate to severe by most patients and failure to adequately treat may affect mother-baby bonding, care of baby, and breastfeeding.[1] It may even risk the patients for thrombo-embolism as a result of immobility due to pain.[2] The pain management should not only be adequate but also safe for the breastfeeding baby. Pain of cesarean section essentially has two components somatic (due to abdominal wall incision) and visceral (from the uterus). A substantial component of pain experienced by patients is derived from abdominal wall incision.[3]
Systemic or neuraxial opioids are the mainstay for treating postoperative pain, as they are effective against both the components. However, they are associated with a number of undesirable side effects such as nausea, vomiting, pruritus, constipation, and respiratory depression.[4,5] Nonsteroidal anti-inflammatory drug alone may be insufficient to treat postcesarean pain. Currently, multimodal analgesic technique involving abdominal nerve block with parenteral analgesics is becoming popular for these patients. Transverse abdominis plane (TAP) block is a recently introduced regional technique that blocks abdominal wall neural afferents between T6 and L1 and thus can relieve pain associated with an abdominal incision.[6,7] TAP is a neurovascular plane located between the internal oblique and transverse abdominis muscles and nerves supplying abdominal wall pass through this plane before supplying anterior abdominal wall.[8] Therefore, if the local anesthetic is deposited in this space, myocutaneous sensory blockade results.[6,7]
As postoperative pain after cesarean is predominantly due to abdominal incision we hypothesized that the TAP block if used as a part of multimodal analgesia will reduce the need of additional analgesic during 48 h after surgery (primary outcome), severity of pain and prolong the demand for first analgesic and improve patient satisfaction during postoperative period (secondary outcome).
MATERIALS AND METHODS
The study had approval of our institutional review board and was conducted according to the study protocol approved by the board. After informed written consent, 70 adult parturients belonging to American Society of Anesthesiologists physical status I and II requiring elective or nonurgent cesarean (where no fetal or maternal compromise existed) via Pfannenstiel incision were recruited in this prospective double-blind study. Patients of <50 kg or >100 kg weight, with any contraindication to spinal anesthesia or who were unable to understand numeric rating scale (NRS) or use patient-controlled analgesia (PCA) were excluded from the study.
The recruited patients were randomly assigned to one of the two groups on the basis of computer-generated random number table, concealed in opaque envelop, which was opened just before cesarean section. The patients received TAP block with 20 ml 0.25% bupivacaine (group B-study group) or received no block (group C-control group). All patients had received intravenous (IV) ranitidine (50 mg) and metoclopramide (10 mg) 20-30 min before transferring to odds ratios as institutional protocol. Each patient received spinal anesthesia with 2-2.2 ml of 0.5% heavy bupivacaine and 15 mg of fentanyl at L3-5 level in sitting position after preloading with 500 ml of Ringers lactate. Intra-operative antiemetics were not used routinely, but if needed, 4 mg of ondansetron IV was used.
At the end of surgery, all patients of study group B received TAP block using landmark technique as described by McDonnell et al.[9] With the patient in the supine position, the iliac crest was palpated from anterior to posterior until latissimus dorsi muscle insertion could be felt. Triangle of Petit was located (anteriorly bounded by external oblique and posteriorly by latissimus dorsi muscle and inferiorly by iliac crest). A 22 gauge 5 cm long blunt tip regional anesthesia needle was inserted in the triangle of Petit just above the iliac crest at right angle to the coronal plane until resistance was felt. This indicated that the needle tip pierced external oblique muscle. The needle was advanced gently in the same direction until “pop” sensation was felt, which signaled entry into facial plane between external and internal oblique muscles. Further advancement resulted in 2nd “pop” and this indicated entry into TAP. After careful negative aspiration 20 ml of 0.25% bupivacaine (group B) was slowly injected in 5 ml increments. The block was given on the other side using the same method. In the control group C, the patients did not receive the block but had their skin punctured on both sides after palpating triangle of Petit. In all the patients, abdominal wound was covered with a pressure dressing that also covered skin puncture sites and were shifted to postanesthetic care unit (PACU). The patients received standard analgesia according to obstetric department protocol consisting IV diclofenac 75 mg 8 hourly, first dose was given at the end of surgery. In addition, they also received IV tramadol through PCA (4 mg/ml) with 20 mg dose, 10 min lockout interval and 1 h limit of 50 mg.
The assessment of presence and intensity of pain (both on rest and on passive flexion of hip and knee), nausea, vomiting, and sedation was done immediately after transfer to PACU (0 h) and at 4, 8, 12, 24, 36, and 48 h after surgery. The intensity of pain was assessed on NRS (0 = no pain, and 10 = worst pain). Nausea and vomiting was assessed on a categorical scoring scale (0 = no symptoms, 1 = only nausea, 2 = nausea and/vomiting). Level of sedation was assessed as a sedation score of 0–3, where 0 = awake and alert, 1 = quietly awake, 2 = asleep but easily arousable, 3 = deep sleep, responding to painful stimulus. Patients were labeled to be sedated if score was >2. 4 mg IV ondansetron was given if patients complained of persistent nausea or vomited. The patients were also interviewed after 48 h of surgery regarding satisfaction with their pain management on scale of 0-10 (0 = very unsatisfied, 10 = highly satisfied).
In PACU, all observations were made by an independent observer who was unaware of group allocation. The primary outcome was 48 h tramadol consumption, and secondary outcome measures were pain scores at rest and movement, time of first analgesia, side effects, and satisfaction with pain management. Time of first PCA tramadol and cumulative tramadol consumption at 4, 8, 12, 24, 36, and 48 h was obtained from electronic memory of PCA device.
Sample size was determined prospectively using data from previous cesareans performed under spinal anesthesia in our institution (mean ± standard deviation 48 h consumption of tramadol 260 ± 50 mg). We considered 30% reduction in PCA tramadol in 48 h as a clinically significant end point. Based on this, power analysis indicated a minimum of 30 patients per group (α = 0.05, β = 0.2). Assuming a potential drop rate of 10%, we decided to recruit 35 patients per group. Demographic variables were analyzed using Fisher's exact test, repeated measurements by repeated measures ANOVA if normally distributed and nominal or ordinal variables by Chi-square test.
RESULTS
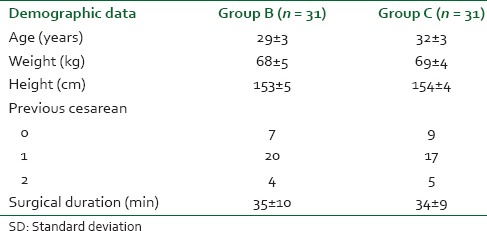
From April 2012 to August 2012, a total of 70 patients who fulfilled the criteria were randomized for this study. Four patients were removed due to inadequate spinal anesthesia, three due to analgesic protocol violation and one due to malfunctioning of PCA pump leaving 62 patients, 31 in each group. The two groups were not different regarding demographic and other data [Table 1].
Table 1.
Demographic and other data (mean ± sd)
Patient-controlled analgesia tramadol use is shown in Figure 1. Mean tramadol use within first 4 h of surgery was similar in both groups but subsequently it was significantly less 8, 12, and 24 h after surgery in group B in relation to control group. The cumulative tramadol usage during first 24 h after surgery was significantly reduced in study group B in comparison to control group C (75 ± 22 vs. 168 ± 45 mg in groups B and C, respectively, P < 0.0001). During 24-48 h, although tramadol use was less in group B compared to group C, the difference did not reach statistical significances (47 ± 15 vs. 63 ± 20 in groups B and C, respectively, P = 0.059). Overall tramadol consumption was reduced approximately by 50% in group B compared to control group in first 48 h (127 ± 24 vs. 253 ± 52 mg in groups B and C, respectively, P < 0.0001) [Table 2].
Figure 1.
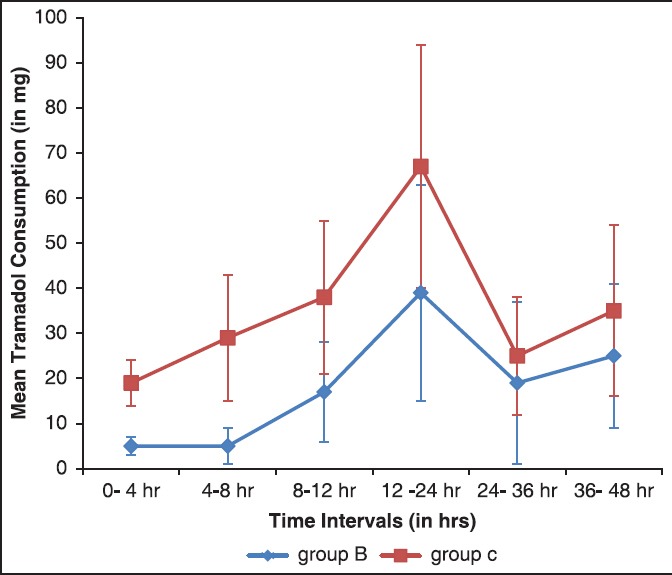
Tramadol consumption (mean ± standard deviation)
Table 2.
Time of first analgesic, 48 h consumption of tramadol and side effects
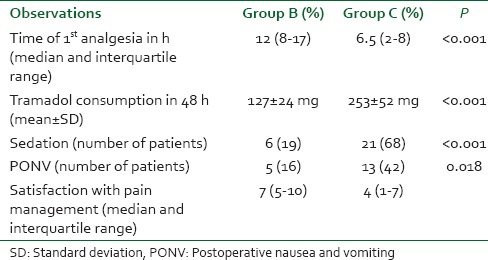
The time of the first demand for analgesia was shorter in the control group than in study group. The median time to first demand for analgesia was 6.5 h (inter-quartile range [IQR]: 2-8 h) in group C and 12 h (IQR 8-17 h) in group B (P < 0.001) [Table 2].
The NRS for pain at rest and movement is depicted in Figures 2 and 3. The scores were similar on arrival in PACU in both groups but were significantly lower at all-time points up to 24 h in group B compared to group C, both at rest and on movement (P < 0.0001). At 36 and 48 h, the scores although were lower in group B, it was not statistically significant.
Figure 2.
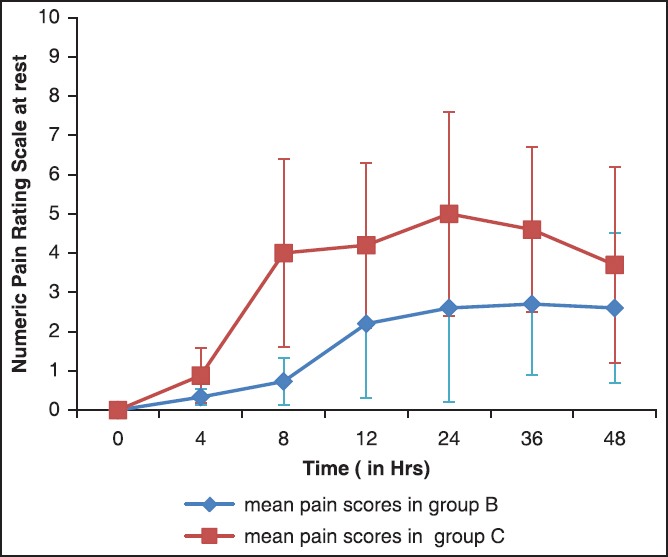
Numeric pain rating scores at rest (mean ± standard deviation)
Figure 3.
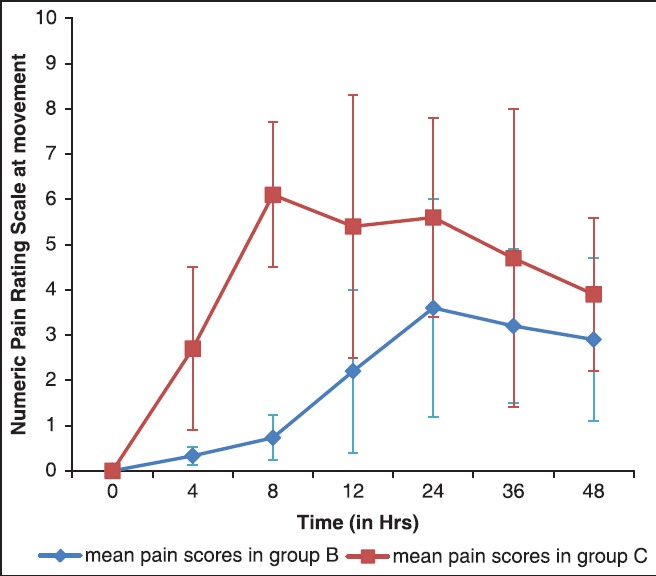
Numeric pain rating scores at movement (mean ± standard deviation)
There was no difference in number of patients who had sedation score of 1 or 2 in both the groups at 2, 4, 6, and 8 h. But more patients in the control group C were sedated (score 3) at 12 and 24 h (6 and 21 patients in groups B and C, respectively P < 0.001). Later on, no difference existed. Postoperative nausea and vomiting (PONV) was more frequently noted in the control group. Thirteen patients complained of nausea and/vomiting and required ondansetron. While five patients in study group B complained of PONV and required antiemetic medication (P = 0.018) [Table 2]. In control group, 7 patients required single dose of ondansetron while 6 required 2 doses. Satisfaction with pain relief was significantly higher in study group B. Median satisfaction scores was 7 (IQR: 5-10) in group B and 4 (IQR 1-7) in group C (P < 0.005) [Table 2].
DISCUSSION
Managing pain following cesarean section is challenging. The analgesic regimen should be effective, safe, and devoid of side effects. Over recent years, there has been growing interest in regional nerve block techniques with promising results on efficacy, as they reduce the need of supplemental analgesia[10] thereby lower the incidence of drug-related side effects.[11] TAP block is a relatively new abdominal nerve block with excellent efficacy after a variety of abdominal surgeries including cesarean section.[9,12,13,14,15]
The results of this study demonstrated that TAP block supplemented by parenteral diclofenac with PCA tramadol was effective in reducing severity of pain both at rest and on movement, delayed the demand of first postoperative analgesic and reduced the need of PCA tramadol during first 48 h after surgery in patients undergoing cesarean section under spinal anesthesia. The patients receiving TAP block also had lower PONV and were less drowsy and more satisfied with their pain management compared to those who did not receive TAP block.
Previous placebo-controlled studies have also shown clear analgesic benefit of TAP block in patients of cesarean delivery both done under spinal[4,9,14,15] or general anesthesia.[5,16] McDonnell et al. (2007) randomized 50 parturient to receive TAP block with either ropivacaine or placebo at the end of cesarean section under spinal anesthesia in addition to standard postoperative analgesia. A significant reduction in 48 h postoperative morphine consumption, pain scores, and side effects was observed in TAP group. Other authors[4,14] also reported similar benefits consistent with the study of McDonnell et al. and our study.
Similarly, Eslamian et al.[16] and Tan et al.[5] evaluated efficacy of TAP block versus no block in patients undergoing cesarean delivery under general anesthesia. Patients in TAP group had lower VAS pain scores at rest and during coughing, utilized less PCA tramadol and had a longer time to ask for first analgesia, than the patients who did not receive block.
A recent systematic review and meta-analysis[17] reviewed five randomized double-blind studies including 312 parturients receiving TAP block for management of pain after cesarean delivery. Out of five, two studies[18,19] used intrathecal morphine along with bupivacaine for spinal anesthesia while others used plain bupivacaine.[4,9,14] The conclusion was that TAP block was effective in reducing pain scores, morphine consumption, and PONV for 24 h compared to the placebo group. But in patients where morphine was used as adjuvant to subarachnoid bupivacaine, the TAP block did not provide additional analgesic benefit but at the cost of the higher incidence of side effects like pruritus.[19]
The patients receiving TAP block in our study had reduced the incidence of side effects like nausea, vomiting, or sedation. Lesser number of patients required antiemetic medication in TAP group. Sedation and nausea or vomiting could be due to higher consumption of tramadol in the control group. Significantly more number of patients in TAP block were satisfied with their pain management as they could feed and could care for their babies being pain-free. Other authors have also reported higher satisfaction in patients receiving TAP block.[4,5]
Most of the studies evaluating TAP block have included a placebo group with intervention[4,7,9,13,14] where saline was used for TAP block. However, the ethical legitimacy of using interventional placebo control regional analgesia has been questioned.[5] A Serious Harm Associated Morbidity scale was devised to categorize the potential complications of placebo-controlled interventions in the context of local anesthesia research.[20] (Grade 0 = no risk [no intervention], Grade 1 = minimal risk [skin allergy to dressing], Grade 2 = minor risk [subcutaneous hematoma, infection], Grade 3 = moderate risk [neuropraxia], and Grade 4 = major risk [blindness, pneumothorax, liver laceration]). It was found that more than half of the randomized studies subjected patients in the control group to serious risk.[20] Although TAP block is very safe and associated with lower risk of complications,[6,7,9] it cannot be labeled as no risk technique. Therefore, instead of the placebo group with the intervention, we used control group where no block was given. For blinding, skin puncture was done on both sides, but saline was injected.
There were some limitations of this study that need discussion. First, the TAP block produces sensory analgesia of the abdominal wall. Testing would have demonstrated successful block, but we avoided this for fear of loss of blinding. Second, we employed the landmark technique for performing block. Ultrasound guidance can improve the certainty and safety of the block by confirming the position of the needle. But merits of landmark technique using “double pop” method regarding safety and certainty has been proved in the literature.[6,7,9] However, ultrasound guidance for regional anesthesia has not been conclusively demonstrated to improve safety.[21] Third, although we did not encounter block-related complication in any patient, our sample size was not enough to assess the safety.
To conclude, this study observed analgesic benefit of TAP block when employed with standard postoperative analgesia after cesarean section done under spinal anesthesia. Predominant somatic pain was very well-relieved by TAP block and visceral pain at its worst did not appear to be prominent and was relieved by diclofenac. In our opinion, the TAP block has potential to become an important tool in managing postoperative pain of cesarean delivery as it is easy to perform, is safe and has definite clinical utility.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Leung AY. Postoperative pain management in obstetric anesthesia — New challenges and solutions. J Clin Anesth. 2004;16:57–65. doi: 10.1016/j.jclinane.2003.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Gadsden J, Hart S, Santos AC. Post-cesarean delivery analgesia. Anesth Analg. 2005;101:S62–9. doi: 10.1213/01.ANE.0000177100.08599.C8. [DOI] [PubMed] [Google Scholar]
- 3.Urbanczae L. Transverse abdominis plane block. Anesth Intensive Ther. 2009;35:137–41. [Google Scholar]
- 4.Belavy D, Cowlishaw PJ, Howes M, Phillips F. Ultrasound-guided transversus abdominis plane block for analgesia after Caesarean delivery. Br J Anaesth. 2009;103:726–30. doi: 10.1093/bja/aep235. [DOI] [PubMed] [Google Scholar]
- 5.Tan TT, Teoh WH, Woo DC, Ocampo CE, Shah MK, Sia AT. A randomised trial of the analgesic efficacy of ultrasound-guided transversus abdominis plane block after caesarean delivery under general anesthesia. Eur J Anaesthesiol. 2012;29:88–94. doi: 10.1097/EJA.0b013e32834f015f. [DOI] [PubMed] [Google Scholar]
- 6.Rafi AN. Abdominal field block: A new approach via the lumbar triangle. Anesthesia. 2001;56:1024–6. doi: 10.1046/j.1365-2044.2001.02279-40.x. [DOI] [PubMed] [Google Scholar]
- 7.McDonnell JG, O’Donnell B, Curley G, Heffernan A, Power C, Laffey JG. The analgesic efficacy of transversus abdominis plane block after abdominal surgery: A prospective randomized controlled trial. Anesth Analg. 2007;104:193–7. doi: 10.1213/01.ane.0000250223.49963.0f. [DOI] [PubMed] [Google Scholar]
- 8.Rozen WM, Tran TM, Ashton MW, Barrington MJ, Ivanusic JJ, Taylor GI. Refining the course of the thoracolumbar nerves: A new understanding of the innervation of the anterior abdominal wall. Clin Anat. 2008;21:325–33. doi: 10.1002/ca.20621. [DOI] [PubMed] [Google Scholar]
- 9.McDonnell JG, Curley G, Carney J, Benton A, Costello J, Maharaj CH, et al. The analgesic efficacy of transversus abdominis plane block after cesarean delivery: A randomized controlled trial. Anesth Analg. 2008;106:186–91. doi: 10.1213/01.ane.0000290294.64090.f3. [DOI] [PubMed] [Google Scholar]
- 10.Petersen PL, Mathiesen O, Torup H, Dahl JB. The transversus abdominis plane block: A valuable option for postoperative analgesia? A topical review. Acta Anaesthesiol Scand. 2010;54:529–35. doi: 10.1111/j.1399-6576.2010.02215.x. [DOI] [PubMed] [Google Scholar]
- 11.White PF. The changing role of non-opioid analgesic techniques in the management of postoperative pain. Anesth Analg. 2005;101:S5–22. doi: 10.1213/01.ANE.0000177099.28914.A7. [DOI] [PubMed] [Google Scholar]
- 12.Niraj G, Searle A, Mathews M, Misra V, Baban M, Kiani S, et al. Analgesic efficacy of ultrasound-guided transversus abdominis plane block in patients undergoing open appendicectomy. Br J Anaesth. 2009;103:601–5. doi: 10.1093/bja/aep175. [DOI] [PubMed] [Google Scholar]
- 13.El-Dawlatly AA, Turkistani A, Kettner SC, Machata AM, Delvi MB, Thallaj A, et al. Ultrasound-guided transversus abdominis plane block: Description of a new technique and comparison with conventional systemic analgesia during laparoscopic cholecystectomy. Br J Anaesth. 2009;102:763–7. doi: 10.1093/bja/aep067. [DOI] [PubMed] [Google Scholar]
- 14.Baaj JM, Alsatli RA, Majaj HA, Babay ZA, Thallaj AK. Efficacy of ultrasound-guided transversus abdominis plane (TAP) block for postcesarean section delivery analgesia – A double-blind, placebo-controlled, randomized study. Middle East J Anaesthesiol. 2010;20:821–6. [PubMed] [Google Scholar]
- 15.Sriramkes B, Sahoo N, Panigrahi S. Analgesic efficacy of ultrasound guided transverse abdominis plane block following cesarean section. Int J Perioper Ultrasound Appl Technol. 2012;1:5–8. [Google Scholar]
- 16.Eslamian L, Jalili Z, Jamal A, Marsoosi V, Movafegh A. Transversus abdominis plane block reduces postoperative pain intensity and analgesic consumption in elective cesarean delivery under general anesthesia. J Anesth. 2012;26:334–8. doi: 10.1007/s00540-012-1336-3. [DOI] [PubMed] [Google Scholar]
- 17.Abdallah FW, Halpern SH, Margarido CB. Transversus abdominis plane block for postoperative analgesia after Caesarean delivery performed under spinal anesthesia? A systematic review and meta-analysis. Br J Anaesth. 2012;109:679–87. doi: 10.1093/bja/aes279. [DOI] [PubMed] [Google Scholar]
- 18.Costello JF, Moore AR, Wieczorek PM, Macarthur AJ, Balki M, Carvalho JC. The transversus abdominis plane block, when used as part of a multimodal regimen inclusive of intrathecal morphine, does not improve analgesia after cesarean delivery. Reg Anesth Pain Med. 2009;34:586–9. doi: 10.1097/aap.0b013e3181b4c922. [DOI] [PubMed] [Google Scholar]
- 19.McMorrow RC, Ni Mhuircheartaigh RJ, Ahmed KA, Aslani A, Ng SC, Conrick-Martin I, et al. Comparison of transversus abdominis plane block vs spinal morphine for pain relief after Caesarean section. Br J Anaesth. 2011;106:706–12. doi: 10.1093/bja/aer061. [DOI] [PubMed] [Google Scholar]
- 20.McGuirk S, Fahy C, Costi D, Cyna AM. Use of invasive placebos in research on local anaesthetic interventions. Anesthesia. 2011;66:84–91. doi: 10.1111/j.1365-2044.2010.06560.x. [DOI] [PubMed] [Google Scholar]
- 21.Chin KJ, Chan V. Ultrasound-guided peripheral nerve blockade. Curr Opin Anaesthesiol. 2008;21:624–31. doi: 10.1097/ACO.0b013e32830815d1. [DOI] [PubMed] [Google Scholar]


