Abstract
Background: The vitamin A precursor β-carotene (BC) promotes mammalian embryonic development by serving as a source of retinoids (vitamin A derivatives) to the developing tissues. In the Western world, increased consumption of dietary supplements, including vitamin A and BC, is common; however, the consequences of maternal high preformed vitamin A intake on embryonic uptake and metabolism of BC are poorly understood.
Objective: This study investigated vitamin A and BC metabolism in developing mouse tissues after a single BC administration to pregnant wild-type (WT) mice fed purified diets with different vitamin A concentrations.
Methods: WT dams fed a sufficient vitamin A (VA-S; 4.2 μg of retinol/g of diet), high vitamin A (VA-H; 33 μg of retinol/g of diet), or excess vitamin A (VA-E; 66 μg of retinol/g of diet) diet throughout gestation were intraperitoneally injected with BC or vehicle at 13.5 d postcoitum (dpc). At 14.5 dpc, retinoid and BC concentrations in maternal serum and liver, placenta, and embryo were quantified by HPLC; expressions of genes controlling retinoid and BC homeostasis were analyzed by quantitative polymerase chain reaction. Maternal lipoprotein BC concentrations were analyzed by density gradient ultracentrifugation followed by HPLC.
Results: Intact BC was undetectable only in embryos from VA-E + BC dams. Relative to the VA-S + vehicle group, placentas from VA-S + BC dams showed 39% downregulation of LDL-receptor–related protein 1 (Lrp1 ); 35% downregulation of VLDL receptor (Vldlr); 56% reduced mRNA expression of β-carotene 15,15′-oxygenase (Bco1); and 80% upregulation of β-carotene 9′,10′-oxygenase (Bco2). Placental cytochrome P450, family 26, subfamily A, polypeptide 1 (Cyp26A1) was upregulated 2-fold in the VA-E group compared with the VA-S group, regardless of maternal treatment.
Conclusions: In mice, transfer of intact BC to the embryo is attenuated by high tissue vitamin A concentrations. Maternal vitamin A intake and BC availability activate a placental transcriptional response to protect the embryo from retinoid and carotenoid excess.
Keywords: placenta, maternal-fetal β-carotene metabolism, vitamin A, embryo, retinoids
Introduction
β-Carotene (BC)4 is the most abundant dietary precursor of vitamin A, an essential nutrient that is required to sustain normal embryonic development in mammals (1). In both adult and developing tissues, generation of retinoids (vitamin A and its derivatives) from BC occurs predominantly via symmetric cleavage at the 15-15′-carbon double bond by the cytosolic enzyme β-carotene 15,15′-oxygenase (BCO1), giving rise to 2 molecules of retinaldehyde (2, 3). Retinaldehyde dehydrogenases (RALDHs) convert retinaldehyde into retinoic acid, the active form of vitamin A (4). This metabolite functions as the ligand of specific nuclear receptors (retinoic acid receptors and retinoid X receptors) that regulate the expression of hundreds of target genes, many of which are important in development (5). Alternatively, various retinaldehyde reductases, including the retinoic acid–inducible dehydrogenase reductase 3 (DHRS3) (4, 6, 7), can convert retinaldehyde to retinol, which is then esterified by lecithin:retinol acyltransferase (LRAT) (8) into retinyl ester for storage in tissues, especially liver. Hepatic retinol is recovered, bound to retinol-binding protein (RBP), and secreted into the bloodstream for delivery to other organs (9).
Asymmetric cleavage of BC by β-carotene 9′,10′-oxygenase (BCO2) also occurs, generating β-apo-10′-carotenal (3), which can be converted into one molecule of retinaldehyde by a chain-shortening mechanism (3). However, BCO2 has a broader substrate specificity (10) and seems to control a pathway through which excess carotenoids are catabolized to prevent their toxic accumulation in mitochondria, where BCO2 is localized (2, 11). Therefore, the contribution of the asymmetric cleavage pathway to the generation of retinoids from BC seems negligible, at least in adult tissues (3).
It is well established that the mammalian embryo obtains BC from the maternal circulation and metabolizes it in situ (i.e., in the developing tissues) via BCO1, to synthesize retinoids and support normal development (12, 13). However, the regulatory mechanisms involved in placental and embryonic uptake and metabolism of intact BC obtained from maternal bloodstream are largely unknown (14). Dimenstein and colleagues (15) suggested that, in humans, placental BC conversion to retinol is controlled by the nutritional status of the mother, being particularly effective in vitamin A deficiency (VAD). In agreement with this finding, we previously showed that in a mouse model of marginal VAD (16), a single maternal administration of BC at midgestation increased placental BC uptake and upregulated embryonic transcription of Bco1, likely to increase retinoic acid formation (17). Moreover, in a mouse model of severe VAD, metabolic changes in both embryos and placentas were aimed at maximizing use of BC acutely administered to the dams at midgestation (18). These results highlight the importance of BC as a source of retinoids for the developing tissues under limited availability of dietary preformed vitamin A. In contrast, the effects of maternal dietary regimens of high preformed vitamin A on uptake and metabolism of BC by the developing tissues and their potential effects on embryonic development are not known. This critical issue is especially relevant to the Western world, where almost unlimited access to food and increased consumption of dietary supplements, including vitamins such as vitamin A and BC, have become very common (19, 20).
This study aimed to investigate the changes in retinoid and carotenoid metabolism occurring in the developing tissues (i.e., placenta and embryo) at midgestation, in response to a single administration of BC to wild-type (WT) mouse dams fed purified diets containing sufficient vitamin A (VA-S), high vitamin A (VA-H), or excess vitamin A (VA-E) concentrations throughout gestation.
Methods
Mice, diet, and BC administration.
Before the onset of pregnancy, WT female mice (mixed background C57/Bl6 × 129/Sv, bred in our mouse facility) were fed a nonpurified diet [Prolab Isopro RMH3000 5p75; energy from protein, fat, and carbohydrates, 26%, 14%, and 60%, respectively; vitamin A (as retinyl palmitate), 5.4 μg of retinol/g of diet; BC, from trace to 2.6 μg/g of diet] manufactured by LabDiet (W.F. Fisher and Son, Inc.) and previously described in detail (17). At 3 mo of age, female mice were mated with WT male mice and from the time of vaginal plug detection [set as 0.5 d postcoitum (dpc), the onset of gestation] they were maintained on one of the following purified diets: VA-S diet (4.2 μg of retinol/g of diet; n = 10 dams), VA-H diet (33 μg of retinol/g of diet; n = 10 dams), and VA-E diet (66 μg of retinol/g of diet; n = 8 dams). In the purified diets, vitamin A was provided as retinyl palmitate. All of these diets were provided only during gestation (0.5–14.5 dpc) and did not contain BC or its metabolites (Research Diets, Inc.). Diets were otherwise nutritionally complete, with a composition of macro- and micronutrients similar to the nonpurified diet described previously, with the exception of the vitamin A concentration. The VA-H and VA-E diets contained 8- and 16-fold the amount of vitamin A of the sufficient diet, respectively. In summary, 3 different dietary regimens were used (VA-S, VA-H, and VA-E), and within each of them, 2 different treatments were administered (vehicle or BC intraperitoneal injection), for a total of 6 experimental groups.
Following a protocol previously established in our laboratory (12, 17, 18), BC was administered to the dams at midgestation (13.5 dpc), when the embryo is capable of regulating its own BC and retinoid metabolism. Intraperitoneal injection was chosen as a route of administration of BC to circumvent the high mouse intestinal BCO1 cleavage activity (21) and to yield detectable intact BC in the maternal bloodstream (12, 17, 18). This model allowed us to mimic the human status (varying but detectable amounts of intact BC in the maternal bloodstream) in order to study the placenta-mediated maternal-fetal transfer and metabolism of intact BC and its impact on embryogenesis. BC (Type II; Sigma Aldrich) was added in the amount of 50 μg to a 5-mL mixture of ethanol, Cremophor (Sigma), and PBS (1:11:18 ratio), under yellow light, by mixing on a vortex. The concentration of the resulting solution was determined by spectrophotometry at 450 nm. Because of poor solubility of BC, the final concentration varied from 2 to 5 g of BC/L. The mice were administered a single dose of BC of ~35 μg of BC/g body weight. This relatively high dose was chosen with the intent of overcoming the well-known high BCO1 activity of the enzyme in WT mice (21), thus, increasing our ability to detect intact BC in serum and other tissues of the mice. Similar doses of BC or its derivatives are currently being used by others (3, 22).
The chosen time of BC administration was based on an experiment in which we investigated, in WT mice, the accumulation of intact BC delivered by intraperitoneal injection into various tissues of the body over the course of 24 h (Supplemental Figure 1). Specifically, in the liver, the major site of storage and metabolism of BC, the peak of BC uptake occurred ∼8 h post–intraperitoneal injection. At 24 h postinjection, the concentration of BC declined, but it was still ~66% of the peak amount. BC concentration in the serum peaked at 4 h post–intraperitoneal injection (likely reflecting the time needed for BC to move from the peritoneum into the general circulation) with very high levels (40–60 μmol/L). Serum BC declined over time, although it still remained high at 24 h post–intraperitoneal injection (5–15 μmol/L), likely reflecting BC resecretion from the liver and its recirculation among various organs.
At 13.5 dpc, dams were given ~250 μL intraperitoneal injection of the aforementioned emulsion. Vehicle-assigned dams were injected with 250 μL of the vehicle mixture described previously (no BC). All mice were killed at 14.5 dpc by carbon dioxide asphyxiation between 0900 and 1100. Dams were continuously fed until the end of the experiment. Maternal serum and liver as well as placentas and embryos were collected, frozen, and stored at −80°C until further analyses. Overall, this study analyzed 6 experimental groups of 3–6 dams each (Table 1). Based on previous experiments and statistical power tests, the sample size was estimated to generate data with <2 CVs within a treatment group, thus making statistical analysis of the data more reliable.
TABLE 1.
Serum and hepatic retinoid concentrations 24 h after vehicle or BC administration to WT mouse dams fed diets with different vitamin A concentrations1
| Group |
P |
||||||||
| Tissue parameter | VA-S + vehicle (n = 6) | VA-S + BC (n = 4) | VA-H + vehicle (n = 6) | VA-H + BC (n = 4) | VA-E + vehicle (n = 5) | VA-E + BC (n = 3) | D | T | D × T |
| Serum, μmol/L | |||||||||
| Retinol | 0.24 ± 0.10b | 0.18 ± 0.03b | 0.45 ± 0.14a | 0.43 ± 0.12a | 0.47 ± 0.15a | 0.57 ± 010a | <0.001 | 0.88 | 0.43 |
| Retinyl esters | 0.08b (ND–0.25) | 0.04b (ND–0.07) | 0.14b (0.04–2.73) | 0.13b (0.03–0.45) | 0.57a (0.22–1.98) | 0.31a (0.13–0.36) | 0.037 | 0.51 | 0.87 |
| Liver, μmol/g | |||||||||
| Retinol | 0.09 ± 0.03c | 0.09 ± 0.01c | 0.21 ± 0.03b | 0.16 ± 0.06b | 0.27 ± 0.08a | 0.29 ± 0.07a | <0.001 | 0.51 | 0.24 |
| Retinyl esters | 8.6 ± 2.1c | 6.5 ± 0.4c | 21.3 ± 4.3b | 20.9 ± 4.0b | 48.6 ± 11.6a | 56.1 ± 13.4a | <0.001 | 0.54 | 0.34 |
Data are means ± SDs, except for retinyl esters, which are geometric means (ranges). Statistical analysis by 2-factor ANOVA and nonparametric tests (Kruskal-Wallis). Labeled means (within each row) without a common letter differ, P < 0.05. BC, β-carotene; D, diet; ND, not detected; T, treatment; VA-E, excess vitamin A; VA-H, high vitamin A; VA-S, sufficient vitamin A; WT, wild type.
Both diet and water were available to the animals ad libitum. Mice were maintained on a 12:12 light:dark cycle with the period of darkness between 1900 and 0700. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (23) and were approved by the Rutgers University Institutional Committee on Animal Care.
HPLC.
Measurements of retinol, retinyl ester, and BC concentrations in serum, lipoprotein serum fractions, and tissue (liver, placenta, and embryo) were performed by reverse-phase HPLC as previously described (12, 17, 18).
RNA extraction, cDNA synthesis, and qPCR.
RNA extraction, cDNA synthesis, and qPCR were performed as previously described (12, 17, 18, 24). Gene expression changes, determined by the ΔΔCt method, were expressed as fold of the control group (WT vehicle treated). A list of primers and amplicon sizes are reported elsewhere (12, 17, 18, 24) with the exception of those for Dhrs3: forward, 5′CAGGGCATGAGAGTCAGGTTT3′ reverse, 5′TAAGTCCCCGAGAACCTGTGA3′ amplicon size, 194 bp.
Isolation of serum lipoproteins.
Lipoprotein fractions from maternal serum were isolated by density gradient ultracentrifugation as previously described (25). Briefly, 100 μL 0.9% saline solution (d = 1.004 g/mL) was layered over 100 μL maternal serum in an airfuge ultracentrifuge tube (Beckman Coulter, Inc.). After centrifugation at 70,000 × g in a Beckman TLA 120.1 rotor (Beckman Coulter, Inc.) for 4 h, the top layer containing the VLDL + chylomicrons fraction (d < 1.006 g/mL; exactly 100 μL) was removed with a Hamilton glass syringe (Hamilton Co.) and saved. The remaining 100 μL was mixed with 100 μL potassium bromide solution (d = 1.12 g/mL) and centrifuged at the same speed as described previously for 18 h. The top 100 μL, containing the LDL + IDL fraction (d = 1.023–1.06 g/mL), and the bottom 100 μL, containing the HDL fraction (d = 1.063–1.21 g/mL), were separated using a Hamilton glass syringe. Cholesterol (Cholestrol E kit; Wako Diagnostics), and TG (Infinity Triglyceride Kit; Thermo Scientific) concentrations were measured in all serum fractions as well as total sera, according to manufacturer instructions. BC concentration of all the fractions was determined by HPLC analysis as described previously.
Statistical analyses.
Normality of the data was established by using the Shapiro-Wilk test. When data were normally distributed, statistical analysis was performed by Student’s t test, 1-factor ANOVA for BC concentrations, or by 2-factor ANOVA with diet and treatment (administration) as factors, followed by the Fisher’s least significant difference post hoc test. For data that were not normally distributed, comparisons among groups were performed using the Kruskal-Wallis test, followed by Mann-Whitney test. For Figure 2 and Table 3 only a comparison between VA-S and VA-E groups was made. When placentas or embryos were analyzed, either 1 or 2 embryos or placentas/dam were used. For those instances in which 2 of these tissue samples were used, the midpoint of the 2 values was considered as an independent observation, because they were from the same dam. Thus, these midpoints were used to calculate the final group average for statistics. Analyses were performed with SPSS statistical software (version 19; IBM SPSS Statistics,). A value of P < 0.05 was considered significant.
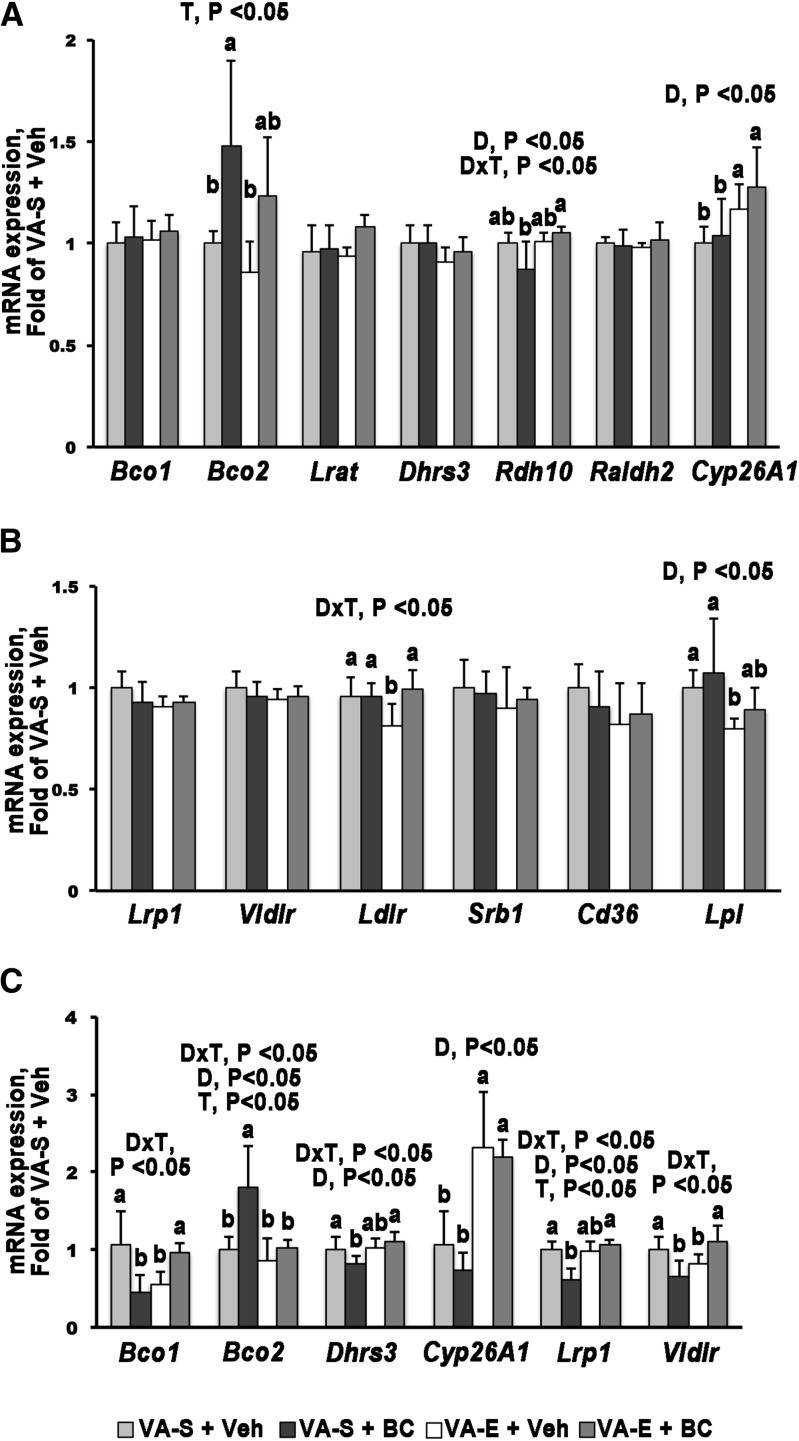
FIGURE 2.
qPCR analysis of genes involved in retinoid and carotenoid metabolism 24 h after Veh or BC administration to WT mouse dams fed VA-S or VA-E diets. Embryo (A, B) or placenta (C) from VA-S + Veh–treated dams set as a calibrator at 1. Data are means ± SDs fold change of the calibrator; n = 3–4 group. Statistical analysis by a 2-factor ANOVA, with D × T as factors. Labeled means (within each gene) without a common letter differ, P < 0.05. Figure represents data from 1 of 2 repeat analyses, which yielded similar results. BC, β-carotene; Bco1, β-carotene 15,15′-oxygenase; Bco2, β-carotene 9,10′-oxygenase; Cd36, cluster of differentiation 36; Cyp26A1, cytochrome P450, family 26, subfamily A, polypeptide 1; D, diet; Dhrs3, dehydrogenase/reductase member 3; Ldlr, LDL receptor; Lpl, lipoprotein lipase; Lrat, lecithin:retinol acyltransferase; Lrp1, LDL-receptor–related protein 1; Raldh2, retinaldehyde dehydrogenase 2; Rdh10, retinol dehydrogenase 10; Srb1, scavenger receptor B type 1; T, treatment; VA-E, excess vitamin A; VA-S, sufficient vitamin A; Veh, vehicle; Vldlr, VLDL receptor; WT, wild type.
TABLE 3.
Maternal plasma lipoprotein lipid and BC concentrations 24 h after BC administration to WT mouse dams fed diets with different vitamin A concentrations1
| VA-S + BC |
VA-E + BC |
|||||
| Lipoprotein fraction | TC, mmol/L | TG, mmol/L | BC, μmol/L | TC, mmol/L | TG, mmol/L | BC, μmol/L |
| VLDL + chylomicrons | 0.42, 0.85 | 0.37 ± 0.17 | 0.39 ± 0.16 | 0.34 ± 0.17 | 0.18 ± 0.04 | 0.40 ± 0.30 |
| LDL + IDL | 1.24 ± 0.39 | 0.26 ± 0.07 | 11.5 ± 3.37 | 1.53 ± 0.32 | 0.12 ± 0.02* | 10.4 ± 5.56 |
| HDL | 2.35 ± 0.37 | 0.20 ± 0.03 | 5.04 ± 2.01 | 1.57 ± 0.10* | 0.10 ± 0.03* | 2.32, 4.26 |
| Whole serum | 4.28 ± 0.51 | 0.48 ± 0.06 | 12.8 ± 4.20 | 3.62 ± 0.44 | 0.29, 0.41 | 12.7 ± 5.37 |
Data are means ± SDs, except when n = 2 (individual values are shown); n = 3 dams/group. Statistical analysis by Student’s t test. *Different from control (VA-S + BC group of the respective lipoprotein fraction), P < 0.05. BC, β-carotene; TC, total cholesterol; VA-E, excess vitamin A; VA-S, sufficient vitamin A; WT, wild type.
RESULTS
Retinoid and BC concentrations in maternal and developing tissues.
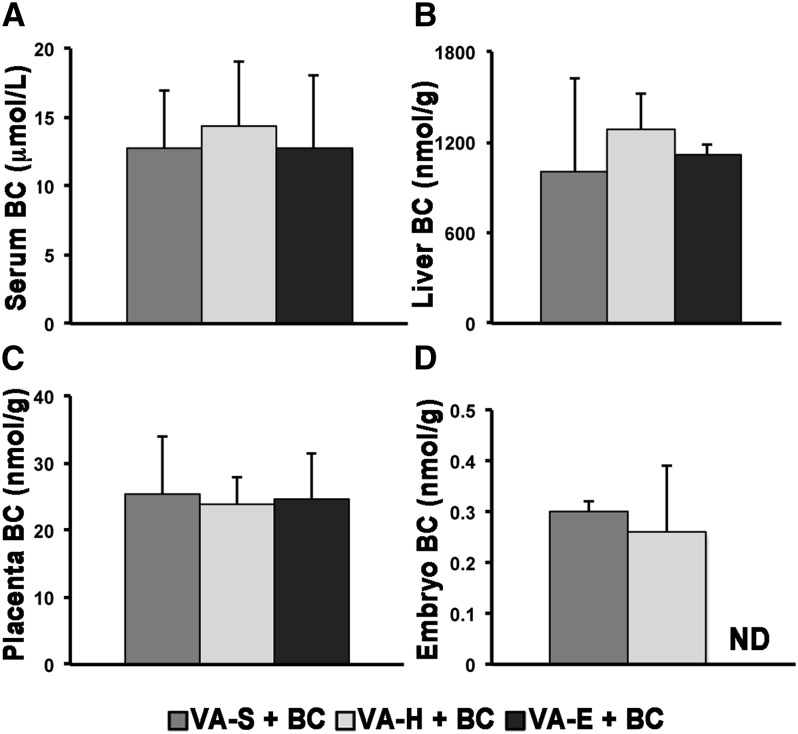
As we previously reported using the same protocol of BC administration (12, 17, 18), detectable concentrations of BC were observed in serum, liver, placentas, and embryos of the WT dams administered a single dose of BC at midgestation (Figure 1). Although maternal serum and liver as well as placental BC concentrations were similar among the groups, BC was undetectable only in embryos from dams fed the VA-E diet (Figure 1). In all vehicle-treated mice, BC was undetectable in maternal serum and liver as well as developing tissues (embryo and placenta; data not shown) (12, 17, 18, 24).
FIGURE 1.
Vitamin A concentrations in serum (A), liver (B), placenta (C), and embryo (D) 24 h after vehicle or BC administration to WT mouse dams fed diets with different vitamin A contents. Data are means ± SDs; n = 4, VA-S + BC; n = 4, VA-H + BC; n = 3, VA-E + BC. Statistical analysis by 1-factor ANOVA. BC, β-carotene; ND, not detected (serum, 0.0186 nmol/L; tissues, 0.186 nmol/g); VA-E, excess vitamin A; VA-H, high vitamin A; VA-S, sufficient vitamin A; WT, wild type.
BC administration did not affect retinoid (retinol and retinyl ester) concentrations in maternal serum and liver (Table 1) or in the placenta and embryo (Table 2) compared with the vehicle-treated dam groups. With the exception of serum, the various maternal dietary regimens resulted in different tissue retinoid concentrations in maternal liver as well as embryo and placenta (Tables 1 and 2; P < 0.05). Given that embryonic BC concentration was similar in the VA-S and VA-H groups, all of the subsequent analyses were performed by comparing only embryos and placentas from VA-S and VA-E dams.
TABLE 2.
Placental and embryonic retinoid concentrations 24 h after vehicle or BC administration to WT mouse dams fed diets with different vitamin A concentrations1
| Group |
P |
||||||||
| Tissues parameter | VA-S + vehicle (n = 6) | VA-S + BC (n = 4) | VA-H + vehicle (n = 6) | VA-H + BC (n = 4) | VA-E + vehicle (n = 5) | VA-E + BC (n = 3) | D | T | D × T |
| Placenta, nmol/g | |||||||||
| Retinol | 3.8 ± 1.1c | 3.2 ± 0.7c | 6.0 ± 3.0b | 5.7 ± 2.2b | 10.2 ± 0.7a | 9.1 ± 1.6a | <0.001 | 0.43 | 0.83 |
| Retinyl esters | 1.5 ± 0.5c | 1.2 ± 0.3c | 4.2 ± 0.9b | 5.1 ± 0.7b | 8.4 ± 0.4a | 8.2 ± 0.5a | <0.001 | 0.66 | 0.13 |
| Embryo, nmol/g | |||||||||
| Retinol | 3.3 ± 0.4c | 3.2 ± 0.5c | 4.3 ± 0.7b | 4.4 ± 0.6b | 7.5 ± 1.8a | 6.4 ± 1.1a | <0.001 | 0.33 | 0.39 |
| Retinyl esters | 6.5 ± 0.8c | 6.2 ± 0.6c | 12.9 ± 5.3b | 15.4 ± 3.5b | 26.6 ± 3.9a | 25.0 ± 1.1a | <0.001 | 0.95 | 0.63 |
Data are means ± SDs. Statistical analysis by 2-factor ANOVA. Labeled means (within each row) without a common letter differ, P < 0.05. BC, β-carotene; D, diet; T, treatment; VA-E, excess vitamin A; VA-H, high vitamin A; VA-S, sufficient vitamin A; WT, wild type.
BC concentrations in maternal serum lipoproteins.
Because carotenoids are transported in the bloodstream by lipoproteins (21, 26, 27), serum BC concentrations were determined in isolated lipoprotein fractions (VLDL + chylomicrons, LDL + IDL, and HDL) from the BC-supplemented VA-S and VA-E dams. Within each dietary regimen, the concentration of BC in the 3 isolated lipoprotein fractions was as follows: VLDL + chylomicrons < HDL < LDL + IDL (Table 3). BC concentration of each lipoprotein class was similar between the 2 dietary regimens (Table 3). Overall, the vitamin A concentration of the diet did not affect the distribution of BC within maternal lipoproteins. TG concentration in LDL + IDL and HDL from VA-E + BC dams were significantly lower compared with the corresponding fractions from VA-S + BC dams (Table 3; P < 0.05). Likewise, cholesterol concentration was lower in the HDL fraction of the dams maintained on the VA-E + BC diet compared with the VA-S + BC dams (Table 3; P < 0.05).
Expression of genes regulating retinoid and BC metabolism in the embryo.
mRNA expression of key regulators of the BC and retinoid metabolism [Bco1, Bco2, Lrat, retinol dehydrogenase 10 (Rdh10), Dhrs3, retinaldehyde dehydrogenase 2 (Raldh2), and cytochrome P450, family 26, subfamily A, polypeptide 1 (Cyp26A1)] were compared in embryos from VA-S and VA-E dams administered either vehicle or BC. Expression of Bco1, Lrat, Dhrs3, and Raldh2 were similar among the 4 groups of embryos analyzed (Figure 2A). In contrast, embryonic Bco2 was significantly upregulated upon maternal BC administration only in the VA-S group (Figure 2A; P < 0.05). Embryonic mRNA expression of Rdh10, the dehydrogenase that converts retinol into retinaldehyde (28), was slightly but significantly reduced in the VA-S + BC group when compared with the embryos from VA-E dams under the same treatment (Figure 2A; P < 0.05). Finally, mRNA expression of Cyp26A1, the enzyme that converts retinoic acid into transcriptionally inactive metabolites (29, 30), was significantly elevated in embryos from VA-E dams when compared with those of embryos from VA-S dams, within the respective treatment group (Figure 2A; P < 0.05). Overall, embryonic metabolism of BC was not enhanced by the maternal regimen of VA-E intake.
Expression of genes potentially involved in embryonic BC uptake.
We next evaluated the embryonic mRNA expression of receptors and enzymes that, by mediating cellular uptake of lipids within lipoproteins, could also facilitate the uptake of BC. LDL-receptor–related protein 1 (Lrp1), VLDL receptor (Vldlr), scavenger receptor B type 1 (Srb1), and Cd36 (FA translocase), embryonic expression were similar among the 4 groups analyzed, regardless of the maternal dietary regimen and treatment (Figure 2B). LDL receptor (Ldlr) and lipoprotein lipase (Lpl) mRNA expression were significantly attenuated in embryos from VA-E + vehicle dams compared with those from VA-S + vehicle dams (Figure 2B; P < 0.05). However, mRNA expression of these genes was similar in VA-E + BC and VA-S + BC dams (Figure 2B). Overall, these data indicate that the potential pathways of embryonic uptake of BC may be similar regardless of vitamin A diet.
Placental uptake and metabolism of BC.
Gene expression analysis, similar to that described previously for embryos, was conducted to assess retinoid and BC metabolism in the placenta. Under the VA-S regimen, a single BC administration downregulated the mRNA expression of Bco1 compared with the vehicle-treated group of the same dietary regimen (Figure 2C; P < 0.05). Bco1 transcription was significantly reduced in the placenta from VA-E + vehicle dams compared with the VA-S + vehicle group (Figure 2C; P < 0.05). However, Bco1 mRNA expression was upregulated in the placenta of VA-E + BC dams compared with VA-E + vehicle group (Figure 2C; P < 0.05), but it was not significantly different between VA-E + BC and VA-S + vehicle dams (Figure 2C). A significant upregulation in mRNA expression of Bco2 was observed only in the VA-S + BC group (Figure 2C; P < 0.05). In VA-S + BC dams, placental Dhrs3 mRNA expression was slightly, but significantly, reduced compared with those from the vehicle-treated group on the same dietary regimen (Figure 2C; P < 0.05). Cyp26A1 transcription in the placenta from VA-E dams was significantly increased compared with those from VA-S dams, regardless of the acute administration (Figure 2C; P < 0.05). BC availability did not influence the transcription of this gene in any of the 2 dietary groups (Figure 2C). Rdh10, Raldh2, and Lrat mRNA expression was similar in the placentas from the various groups (data not shown).
The mRNA expression of Lrp1 and Vldlr were significantly downregulated in placenta from VA-S + BC dams compared with the VA-S + vehicle group (Figure 2C; P < 0.05). Placental mRNA expression of Lrp1 was similar in VA-E + vehicle and VA-E + BC dams (Figure 2C). Moreover, no significant difference was observed in Lrp1 mRNA expression between VA-S + vehicle and VA-E + vehicle groups (Figure 2C). Vldlr expression was downregulated in the placenta from VA-E + vehicle dams compared with placenta from VA-S + vehicle dams and increased in the VA-E + BC group (Figure 2C; P < 0.05). Placental mRNA expression of Lpl, Ldlr, Srb, and Cd36 were not altered by either BC administration or excess of vitamin A in the diet (data not shown).
Overall, the data suggest that both high dietary vitamin A concentration and BC availability influence placental BC metabolism.
Discussion
Adequate maternal nutrition during pregnancy is essential to ensure proper development of the embryo that relies almost entirely on the maternal bloodstream as its source of nutrients, including vitamin A (13). Vitamin A is available to the developing tissues either as preformed vitamin A (mainly retinol and retinyl ester) or as provitamin A precursors, predominantly BC (13), which are taken up by the placenta and then transferred to the embryo. The metabolic capability of the placental-fetal unit to regulate its own retinoid metabolism and, hence, ensure normal embryogenesis, in response to fluctuations of the maternal vitamin A status and intake, is still not fully understood. In the current study, we evaluated how the metabolism of the developing tissues (placenta and embryo) responds to BC availability (a single administration) in the case of dams fed excessive preformed vitamin A.
Although higher than the baseline control diet and the daily dietary recommendation for vitamin A in mice (31), the concentrations of dietary vitamin A used in this study were not teratogenic (32–34), as indicated by the grossly normal morphology of all the collected embryos (data not shown). A single maternal administration of BC did not perturb retinoid concentrations at midgestation in any of the tissues analyzed, regardless of the maternal dietary regimen, confirming our previous reports with WT mice maintained on a regular unpurified diet (12, 17). However, the severely reduced concentrations of BC in embryos from dams on the VA-E diet (66 μg of retinol/g of diet) suggest that intake of excessive preformed vitamin A during pregnancy influences the ability of the embryo to acquire BC from the maternal bloodstream. Reduced embryonic BC concentrations are likely the result of a mechanism(s) aimed at protecting the developing tissues from retinoid toxicity. Intriguingly, this effect was not seen in the embryos of the dams fed the VA-H diet (33 μg of retinol/g of diet), suggesting the existence of a tissue retinoid “threshold” needed to trigger such a response.
Because it is well established that lipoprotein particles such as chylomicron remnants, VLDL, LDL, and HDL are major carriers of carotenoids in circulation (21, 26, 27), we asked whether an abnormal distribution of BC within the lipoproteins of the VA-E dams could have caused the undetectable BC in their embryos. Our results confirmed that, upon intraperitoneal administration, BC is distributed among circulating lipoproteins, as reported in humans or in other model systems (21, 26, 27). However, the lipoprotein distribution of the provitamin A carotenoid was similar between VA-S and VA-E dams, ruling out the possibility mentioned previously.
Because the main carotenoid cleavage enzyme (Bco1) as well as the genes that maintain embryonic retinoid homeostasis (Lrat, Rdh10, Dhrs3, Raldh2, and Cyp26A1) are transcriptionally regulated by retinoic acid (6, 7, 12, 17, 28, 35), changes in their mRNA expression may reflect homeostatic control of the flux of retinoids within tissues (13). Therefore, we measured, by qPCR, the embryonic mRNA concentration of the aforementioned genes to establish whether an altered carotenoid/retinoid metabolism in the embryos from the VA-E dams could have been the underlying cause for their undetectable BC. Bco2 transcription is induced by carotenoids in vivo (11). Thus, we also measured its mRNA expression as a potential control mechanism responding to changes in BC and vitamin A status. Our data show that, as already reported for the placenta (24), embryonic tissues responded to their high retinoid status (because of the elevated concentration of dietary vitamin A during pregnancy) mainly by increasing retinoic acid degradation via CYP26A1 (29, 30). These data also suggest that the transcriptional upregulation of Bco2 could aid the embryo in metabolizing the excessive dietary BC, but only in the case of embryos from VA-S dams. Therefore, it is unlikely that enhanced BC metabolism could result in undetectable BC in the embryos from VA-E dams.
Our laboratory and others have shown that elevated tissue retinoid concentrations or BC availability affect the expression levels of some receptors and enzymes that mediate cellular uptake of lipids within lipoproteins (17, 18, 24, 34–38). These observations implicate these proteins as potential regulators of tissue uptake of BC. Therefore, we examined whether maternal BC supplementation and/or excess dietary vitamin A intake would influence embryonic mRNA expression of Lrp1, Vldlr, Ldlr, Srb1, Cd36, and Lpl. The reduction of embryonic Lpl and Ldlr mRNA concentration in the VA-E group suggests that, as previously reported (24), these molecules may mediate embryonic uptake of recently ingested vitamin A and, therefore, their transcriptional downregulation would limit unnecessary acquisition of retinoids that could be developmentally detrimental. However, Ldlr and Lpl expression were restored to normal in the VA-E group upon BC administration, indicating that the ability to acquire the provitamin A carotenoid was unaffected in the embryo from VA-E dams. Therefore, it is unlikely that reduced embryonic BC uptake could result in undetectable BC in the VA-E embryos.
BC concentrations were not different in placentas from dams maintained on different vitamin A diets, suggesting that the maternal dietary regimen does not affect the total amount of BC that this organ acquires from the bloodstream. These one-time measurements do not exclude the possibility that the rate of placental accumulation and metabolism of BC could vary depending on the concentration of preformed vitamin A in the maternal diet, ultimately contributing to the differential accumulation of embryonic BC. Our data suggest that during the 24-h duration of the experiment, the placenta from VA-S dams may take up less BC, as indicated by the reduced mRNA expression of Lrp1 and Vldlr. We previously reported a similar transcriptional response of Lrp1 to BC availability in the placenta of WT dams maintained on a regular unpurified diet (17). Moreover, the downregulation of Bco1 mRNA expression in the aforementioned groups of dams could result in the suppression of unnecessary retinoic acid production by reducing retinaldehyde formation from maternal BC, which could instead be more efficiently metabolized by BCO2, as indicated by the upregulation of its gene expression. Because the contribution of BCO2 to the generation of retinoid has been proposed to be negligible (3), we speculate that an enhancement of the asymmetric cleavage pathway could further contribute to divert BC away from the generation of excessive retinoic acid. Relative to the VA-S + BC group, the placenta from VA-E dams may take up more BC (higher expression of Lrp1 and Vldlr), become more efficient at cleaving it into retinoid via Bco1 (transcriptionally enhanced on BC administration), degrade the resulting retinoic acid via Cyp26A1 (significantly upregulated in response to the increased concentration of dietary vitamin A), and likely divert retinaldehyde away from retinoic acid synthesis via Dhrs3 (slightly but significantly elevated). Overall, these data indicate that both tissue retinoid concentrations and BC availability influence placental BC metabolism. Depending on the maternal dietary regimen, different metabolic pathways seem to be activated to maintain retinoid homeostasis in the placenta and control the amount of preformed and provitamin A that is transferred to the developing embryo. Nevertheless, these differential pathways unlikely reflect the dramatic difference in embryonic BC concentrations observed between the VA-S and VA-E groups. The reason as to why maternal intake of excess preformed vitamin A during pregnancy prevents accumulation of intact BC in the embryo still remains to be fully elucidated. We speculate that dietary BC availability influences the secretion of intact BC from the placenta toward the fetal circulation in a different fashion depending on the maternal vitamin A status/intake. This hypothesis warrants further studies.
Supplementary Material
Acknowledgments
LQ designed the research; LW, VS, BC, and RR conducted the research; LW, VS, BC, and LQ analyzed the data; LW and LQ wrote the paper; LQ had the primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: BC, β-carotene; BCO1, β-carotene 15,15′-oxygenase; BCO2, β-carotene 9′,10′-oxygenase; CYP26A1, cytochrome P450, family 26, subfamily A, polypeptide 1; DHRS3, dehydrogenase/reductase member 3; dpc, days postcoitum; Ldlr, LDL receptor; Lpl, lipoprotein lipase; LRAT, lecithin:retinol acyltransferase; Lrp1, LDL-receptor–related protein 1; RALDH, retinaldehyde dehydrogenase; Raldh2, retinaldehyde dehydrogenase 2; RBP, retinol-binding protein; Rdh10, retinol dehydrogenase 10; Srb1, scavenger receptor B type 1; VAD, vitamin A deficiency; VA-E, excess vitamin A; VA-H, high vitamin A; VA-S, sufficient vitamin A; Vldlr, VLDL receptor; WT, wild type.
References
- 1.Clagett-Dame M, Knutson D. Vitamin A in reproduction and development. Nutrients 2011;3:385–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lobo GP, Amengual J, Palczewski G, Babino D, von Lintig J. Mammalian carotenoid-oxygenases: key players for carotenoid function and homeostasis. Biochim Biophys Acta 2012;1821:78–87. [DOI] [PMC free article] [PubMed]
- 3.Amengual J, Widjaja-Adhi MA, Rodriguez-Santiago S, Hessel S, Golczak M, Palczewski K, von Lintig J. Two carotenoid oxygenases contribute to mammalian provitamin A metabolism. J Biol Chem 2013;288:34081–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kedishvili NY. Enzymology of retinoic acid biosynthesis and degradation. J Lipid Res 2013;54:1744–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res 2002;43:1773–808. [DOI] [PubMed] [Google Scholar]
- 6.Billings SE, Pierzchalski K, Butler Tjaden NE, Pang XY, Trainor PA, Kane MA, Moise AR. The retinaldehyde reductase DHRS3 is essential for preventing the formation of excess retinoic acid during embryonic development. FASEB J 2013;27:4877–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams MK, Belyaeva OV, Wu L, Kedishvili NY. The retinaldehyde reductase activity of DHRS3 is reciprocally activated by retinol dehydrogenase 10 to control retinoid homeostasis. J Biol Chem 2014;289:14868–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Byrne SM, Blaner WS. Retinol and retinyl esters: biochemistry and physiology. J Lipid Res 2013;54:1731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quadro L, Hamberger L, Colantuoni V, Gottesman M, Blaner WS. Understanding the physiological role of retinol-binding protein in vitamin A metabolism using transgenic and knockout mouse models. Mol Aspects Med 2003;24:421–30. [DOI] [PubMed] [Google Scholar]
- 10.von Lintig J. Colors with functions: elucidating the biochemical and molecular basis of carotenoid metabolism. Annu Rev Nutr 2010;30:35–56. [DOI] [PubMed] [Google Scholar]
- 11.Amengual J, Lobo GP, Golczak M, Li HN, Klimova T, Hoppel CL, Wyss A, Palczewski K, von Lintig J. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J 2011;25:948–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YK, Wassef L, Chung S, Jiang H, Wyss A, Blaner WS, Quadro L. β-Carotene and its cleavage enzyme β-carotene-15,15‘-oxygenase (CMOI) affect retinoid metabolism in developing tissues. FASEB J 2011;25:1641–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiegler E, Kim YK, Wassef L, Shete V, Quadro L. Maternal-fetal transfer and metabolism of vitamin A and its precursor beta-carotene in the developing tissues. Biochim Biophys Acta 2012;1821:88–98. [DOI] [PMC free article] [PubMed]
- 14.Shete V, Quadro L. Mammalian metabolism of beta-carotene: gaps in knowledge. Nutrients 2013;5:4849–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimenstein R, Trugo NM, Donangelo CM, Trugo LC, Anastacio AS. Effect of subadequate maternal vitamin A status on placental transfer of retinol and β-carotene to the human fetus. Biol Neonate 1996;69:230–4. [DOI] [PubMed] [Google Scholar]
- 16.Kim YK, Wassef L, Hamberger L, Piantedosi R, Palczewski K, Blaner WS, Quadro L. Retinyl ester formation by lecithin:retinol acyltransferase (LRAT) is a key regulator of retinoid homeostasis in mouse embryogenesis. J Biol Chem 2008;283:5611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wassef L, Shete V, Hong A, Spiegler E, Quadro L. β-Carotene supplementation decreases placental transcription of LDL receptor-related protein 1 in wild-type mice and stimulates placental β-carotene uptake in a marginally vitamin A deficient mice. J Nutr 2012;142:1456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wassef L, Spiegler E, Quadro L. Embryonic phenotype, beta-carotene and retinoid metabolism upon maternal supplementation of beta-carotene in a mouse model of severe vitamin A deficiency. Arch Biochem Biophys 2013;539:223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulholland CA, Benford DJ. What is known about the safety of multivitamin-multimineral supplements for the generally healthy population? Theoretical basis for harm. Am J Clin Nutr 2007;85:318S–22S. [DOI] [PubMed] [Google Scholar]
- 20.Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. Am J Clin Nutr 2006;83:191–201. [DOI] [PubMed] [Google Scholar]
- 21.Erdman JW Jr, Bierer TL, Gugger ET. Absorption and transport of carotenoids. Ann N Y Acad Sci 1993;691:76–85. [DOI] [PubMed] [Google Scholar]
- 22.Lee SA, Jiang H, Trent CM, Yuen JJ, Narayanasamy S, Curley RW Jr, Harrison EH, Goldberg IJ, Maurer MS, Blaner WS. Cardiac dysfunction in beta-carotene-15,15'-dioxygenase-deficient mice is associated with altered retinoid and lipid metabolism. Am J Physiol Heart Circ Physiol 2014;307:H1675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Research Council. Guide for the care and use of laboratory animals. 7th ed. Washington (DC): National Academy Press; 1996. [Google Scholar]
- 24.Wassef L, Quadro L. Uptake of dietary retinoids at the maternal-fetal barrier: in vivo evidence for the role of lipoprotein lipase and alternative pathways. J Biol Chem 2011;286:32198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldberg IJ, Hu Y, Noh HL, Wei J, Huggins LA, Rackmill MG, Hamai H, Reid BN, Blaner WS, Huang LS. Decreased lipoprotein clearance is responsible for increased cholesterol in LDL receptor knockout mice with streptozotocin-induced diabetes. Diabetes 2008;57:1674–82. [DOI] [PubMed] [Google Scholar]
- 26.Parker RS. Absorption, metabolism, and transport of carotenoids. FASEB J 1996;10:542–51. [PubMed] [Google Scholar]
- 27.Harrison EH. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim Biophys Acta 2012;1821:70–7. [DOI] [PMC free article] [PubMed]
- 28.Farjo KM, Moiseyev G, Nikolaeva O, Sandell LL, Trainor PA, Ma JX. RDH10 is the primary enzyme responsible for the first step of embryonic vitamin A metabolism and retinoic acid synthesis. Dev Biol 2011;357:347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abu-Abed S, Dolle P, Metzger D, Beckett B, Chambon P, Petkovich M. The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev 2001;15:226–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White JA, Guo YD, Baetz K, Beckett-Jones B, Bonasoro J, Hsu KE, Dilworth FJ, Jones G, Petkovich M. Identification of the retinoic acid-inducible all-trans-retinoic acid 4-hydroxylase. J Biol Chem 1996;271:29922–7. [DOI] [PubMed] [Google Scholar]
- 31.Ross AC. Diet in vitamin A research. Methods Mol Biol 2010;652:295–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linney E, Donerly S, Mackey L, Dobbs-McAuliffe B. The negative side of retinoic acid receptors. Neurotoxicol Teratol 2011;33:631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothman KJ, Moore LL, Singer MR, Nguyen US, Mannino S, Milunsky A. Teratogenicity of high vitamin A intake. N Engl J Med 1995;333:1369–73. [DOI] [PubMed] [Google Scholar]
- 34.Collins MD, Mao GE. Teratology of retinoids. Annu Rev Pharmacol Toxicol 1999;39:399–430. [DOI] [PubMed] [Google Scholar]
- 35.Zolfaghari R, Chen Q, Ross AC. DHRS3, a retinal reductase, is differentially regulated by retinoic acid and lipopolysaccharide-induced inflammation in THP-1 cells and rat liver. Am J Physiol Gastrointest Liver Physiol 2012;303:G578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bharadwaj KG, Hiyama Y, Hu Y, Huggins LA, Ramakrishnan R, Abumrad NA, Shulman GI, Blaner WS, Goldberg IJ. Chylomicron- and VLDL-derived lipids enter the heart through different pathways: in vivo evidence for receptor- and non-receptor-mediated fatty acid uptake. J Biol Chem 2010;285:37976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blaner WS, Obunike JC, Kurlandsky SB, Al-Haideri M, Piantedosi R, Deckelbaum RJ, Goldberg IJ. Lipoprotein lipase hydrolysis of retinyl esters. J Biol Chem 1994;269:16559–65. [PubMed] [Google Scholar]
- 38.van Bennekum AM, Kako Y, Weinstock PH, Harrison EH, Deckelbaum RJ, Goldberg IJ, Blaner WS. Lipoprotein lipase expression level influences tissue clearance of chylomicron retinyl esters. J Lipid Res 1999;40:565–74. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.