Abstract
Background: Undernutrition contributes to 45% of all deaths in children <5 y of age worldwide, with a large proportion of those deaths caused by diarrhea. However, no validated tools exist for assessing undernutrition in children with diarrhea and possible dehydration.
Objective: This study assessed the validity of different measures of undernutrition in children with diarrhea.
Methods: A prospective cohort study was conducted at an urban hospital in Bangladesh. Children <60 mo of age presenting to the hospital rehydration unit with acute diarrhea were eligible for enrollment. Study staff randomly selected 1196 children for screening, of which 1025 were eligible, 850 were enrolled, and 721 had complete data for analysis. Anthropometric measurements, including weight-for-age z score (WAZ), weight-for-length z score (WLZ), midupper arm circumference (MUAC), and midupper arm circumference z score (MUACZ), were calculated pre- and posthydration in all patients. Measurements were evaluated for their ability to correctly identify undernutrition in children with varying degrees of dehydration.
Results: Of the 721 patients with full data for analysis, the median percent dehydration was 4%. Of the 4 measures evaluated, MUAC and MUACZ demonstrated 92–94% agreement pre- and posthydration compared with 69–76% for WAZ and WLZ. Although each 1% change in hydration status was found to change weight-for-age by 0.0895 z scores and weight-for-length by 0.1304 z scores, MUAC and MUACZ were not significantly affected by dehydration status. Weight-based measures misclassified 12% of children with severe underweight and 14% with severe acute malnutrition (SAM) compared with only 1–2% for MUAC and MUACZ.
Conclusions: MUAC and MUACZ were the most accurate predictors of undernutrition in children with diarrhea. WAZ and WLZ were significantly affected by dehydration status, leading to the misdiagnosis of many patients on arrival with severe underweight and SAM. This trial was registered at clinicaltrials.gov as NCT02007733.
Keywords: dehydration, diarrhea, MUAC, undernutrition, malnutrition, severe acute malnutrition, oral rehydration
Introduction
Despite the progress made in recent decades, the WHO estimates that nearly 100 million children remain underweight, with the vast majority living in low- and middle-income countries (1). Undernutrition is the leading risk factor of pediatric mortality worldwide, contributing to 45% of all deaths in children <5 y of age (1). Severe acute malnutrition (SAM)6, defined as severe wasting and/or nutritional edema, affects an estimated 18.7 million children worldwide and accounts for as many as 540,000 child deaths annually (2).
One of the key steps in addressing the burden of undernutrition in young children lies in accurately and rapidly identifying children with SAM in community and hospital settings. Historically, the WHO endorsed the diagnosis of SAM in children aged 6–60 mo presenting with at least one of the following criteria: bipedal edema, a weight-for-length (or height) or weight-for-age <3 SDs (z score < −3), or <70% of the median National Center for Health Statistics/WHO reference population (3). In 2007, the diagnostic criteria were further expanded to include a midupper arm circumference (MUAC) <115 mm (4). More recently in 2013, separate assessment criteria were established for community-based and hospital-based settings (5). In community-based settings, a child may be diagnosed with SAM if they have bilateral edema or MUAC < 115 mm, with health facilities still using weight-for-length z scores (WLZs) < −3 based on WHO growth standards (5). Using these different measurements in different settings may complicate the diagnosis of undernutrition, and more recent evidence suggests that MUAC alone may be sufficient to identify SAM in all settings (6–8).
Undernutrition and diarrhea frequently present concurrently in children. In a recent study from Malawi, almost 50% of children presenting with SAM in the outpatient setting reported having diarrhea in the previous 2 wk (9). Prior research has found that children with diarrhea have decreased weight gain, and children with undernutrition have a higher incidence of diarrhea (10, 11). Although many studies have confirmed the bidirectional relation between malnutrition and diarrheal disease, few have addressed the impact of hydration status on the assessment of malnutrition in children (12–14). Because dehydration alone will lower the weight of a child, weight-based nutritional assessments such as weight-for-length or weight-for-age in children presenting with diarrhea may be misleading (15, 16). This study aimed to assess the validity of 4 different measures of undernutrition in children <5 y of age with diarrhea by comparing performance of these measures during acute diarrheal illness and after rehydration to a stable, preillness weight.
Methods
Study design
This study used data collected as part of the Dehydration: Assessing Kids Accurately study (NCT02007733). The Dehydration: Assessing Kids Accurately study was a prospective cohort study that included a random sample of children presenting to the rehydration unit (short-stay unit) at the Dhaka Hospital of the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b).
Study setting
The study was conducted between February and June 2014. The hospital is located in Dhaka, Bangladesh, and provides treatment for ~144,000 patients yearly, over one-half of whom are children (17).
Participant selection
All children <60 mo of age presenting to the rehydration unit at the Dhaka Hospital of icddr,b with acute diarrhea were eligible for enrollment. Acute diarrhea was defined as the passage of loose or watery stools at least 3 times/d and lasting <14 d total. Research staff identified children presenting to the rehydration unit 24 h/d, 7 d/wk and randomly selected children for screening by pulling colored marbles from a blind pouch. If a child was selected, research staff screened the patient to determine if they met any of the predefined exclusion criteria. Exclusion criteria included children with <3 loose stools/d, diarrhea lasting >14 d, a clear alternative diagnosis to gastroenteritis on arrival, and children previously enrolled in the study. For patients who did not meet any of these exclusion criteria, research staff approached the patient’s parent/guardian, explained the risks and benefits of the study in the local language (Bengali), and obtained written or verbal consent.
Staff training
Research nurses received 7 d of training in all study procedures. Training consisted of 3 d of theoretical knowledge and 4 d of clinical experience practicing informed consent, weighing children, and measuring MUAC and length.
Data collection
Demographic data.
After consent was obtained, study nurses obtained basic historic and demographic data for each child from their parent or guardian including location, age, gender, days of diarrhea, stool frequency in the past 24 h, and type of diarrhea (bloody, watery, or rice water).
Nutritional measures.
Children were completely undressed and baseline weight to the nearest 100 g was obtained on arrival. Children up to 12 mo of age were measured using the Seca 383 Digital Baby Scale, with the child lying directly on the scale. Those >12 mo of age were weighed with the HealthOMeter 349KLX Digital Medical Weight Scale by subtracting the weight of the parent/guardian alone from the parent/guardian holding the undressed child. If a child received intravenous fluid before baseline weight was obtained, study staff recorded the amount of fluid received before measurements but did not alter the initial weight measurement.
Length was measured using the Seca Infantometer Measuring Board to the nearest centimeter with the child lying flat. For children >24 mo of age, 0.7 cm was subtracted from length measurements before the computation of WLZ to account for discrepancies between length and height measurements in this age group. MUAC was measured in millimeters using a standard measuring tape. Patients were treated according to standard hospital protocols. Hospital physicians determined rehydration methods independent of enrollment in the study, with a majority of patients receiving oral rehydration solution (ORS) alone and a minority receiving additional intravenous fluid at the discretion of the treating physician. Emergency care was not delayed for consent, measurements, or weights. Study patients were kept in a defined area and had study labels placed on their hospital identification card to ensure special attention was given to collecting data required for the study.
After initial enrollment, patients were weighed every 8 h to determine their posthydration stable weight, which was used as a proxy for the preillness weight to determine the percent dehydration on arrival. The stable weight was defined as the average of the highest 2 consecutive weights that differed by <2%. A repeat MUAC assessment was performed on children achieving a stable weight before discharge.
Ethics
Ethical approval for the study was obtained from the Lifespan (Rhode Island Hospital) Institutional Review Board and the icddr,b Ethical Review Committee.
Statistics
Basic demographic, historic, and clinical information were summarized using proportions for categorical variables and medians with IQR for continuous variables. The z score calculations were based on WHO Anthro growth standards for WLZ and weight-for-age z score (WAZ) (18). Malnutrition was classified as none, moderate, or severe based on MUAC cutoffs >125, 115–124, or <115 mm, respectively, and by WLZ and WAZ by cutoffs of > −2, −2 to −3, or < −3, respectively. Of note, MUAC was measured for children 6– 60 mo of age and midupper arm circumference z score (MUACZ) was calculated for children 3–60 mo of age, based on available growth standard data (5, 19).
All measurements were compared pre- and posthydration to assess for degree of agreement using Cohen’s κ. Linear regression analysis was also performed to assess the impact of dehydration on each indicator of undernutrition, controlling for both age and sex. The components of the linear regression model were assessed for linearity, additivity, independence, homoscedasticity, and normality. For each model, a value of P < 0.05 was considered significant for the regression coefficients.
For each patient enrolled, we determined the 2 highest consecutive weight measurements that differed by <2% and averaged these weights to determine a stable weight, based on standards in the literature (20). Children with dehydration will rapidly gain weight as they are rehydrated until they achieve their preillness weight, at which point they will stop gaining wait as their kidneys diurese excess fluid. We were not able to determine stable weights for children who continued gaining weight until discharge, children who lost weight during their admission, and children who had only a single weight measured. For each child who achieved a stable weight, we calculated the percent weight change with rehydration (our proxy for percent volume loss due to diarrhea) using the equation, [(stable weight − admission weight)/stable weight] ⋅ 100. Patients were classified into 3 dehydration categories based on their percent dehydration: no dehydration (<3%), some dehydration (3–9%), and severe dehydration (>9%), based on standards in the literature (21). Children who lost >3% of their body weight during their time in the rehydration unit were considered to have received inadequate rehydration despite persistent diarrhea and were excluded from analysis. ANOVA was performed to assess the impact of dehydration category on the accuracy of each of the 4 indices of malnutrition, with a value of P < 0.05 considered significant.
Finally, we estimated the percent of children misclassified as severe underweight (WAZ < −3) and SAM (MUAC < 115 mm, MUACZ < −3, or WLZ < −3) with each of the 4 measures of undernutrition. All statistical analyses were performed using STATA 12.0 (STATA Corp.).
Results
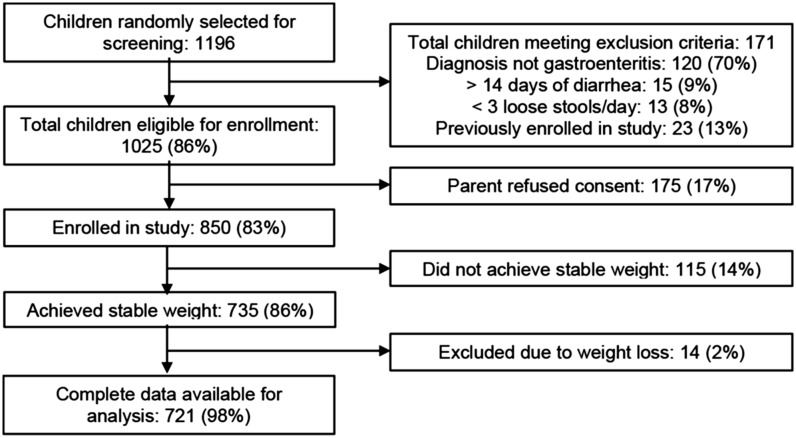
Between February and June 2014, 1196 children <5 y of age presenting to the rehydration unit of icddr,b were randomly selected for screening. Of these, 171 met exclusion criteria, 175 did not consent to participate, and 850 were enrolled. By discharge, 735 (86%) of all enrolled patients achieved a stable weight, allowing for calculation of their percent dehydration on arrival. Fourteen (2%) of these children lost weight during their stay in the rehydration unit, suggesting inadequate hydration in the setting of continued volume losses from diarrhea, and were therefore excluded from our analysis. Figure 1 provides a flow diagram for subject enrollment and Table 1 demonstrates the baseline characteristics for children included in our study analysis.
FIGURE 1.
Flow diagram of study enrollment of patients. Nutritional measurements of dehydration status were performed in 721 patients <60 mo of age before and after achieving stable weight through an independent hydration method.
TABLE 1.
Demographic data and baseline characteristics of patients enrolled in the study1
| Study sample (n = 721) | |
| Age, mo | |
| 0–6 | 44 (6) |
| 7–12 | 252 (35) |
| 13–24 | 193 (27) |
| 25–60 | 232 (32) |
| Gender | |
| Female | 308 (43) |
| Male | 413 (57) |
| Home district | |
| Urban (Dhaka) | 448 (62) |
| Rural/suburban | 273 (38) |
| Diarrhea, d | 2 [1–4] |
| Loose stools in prior 24 h, n | 15 [10–20] |
| Diarrhea type | |
| Watery | 413 (57) |
| Rice-watery | 303 (42) |
| Bloody | 4 (1) |
| Final diagnosis | |
| Gastroenteritis | 711 (99) |
| Other | 10 (1) |
Values are n (%) or median [IQR].
The mean percent weight change with rehydration was 4%. Eighty (11%) children were classified as having severe dehydration, 319 (44%) as having some dehydration, and 322 (45%) as having no dehydration. The median length of stay in the rehydration unit was 22 h (IQR: 16–38) and the median time to achieving stable weight was 14 h (IQR: 11–19). Twenty-eight percent of children received intravenous fluids before their initial admission weight. However, for these children, the median amount of fluid received before their initial weight check was 1.5 mL/kg (IQR: 1.0–2.6), which is unlikely to be clinically significant in this context.
Comparing the prehydration and posthydration malnutrition category assigned by each of the 4 indices of malnutrition in children, MUAC and MUACZ had a 92% and 94% agreement, respectively, with κ-scores of 0.78, and WAZ and WLZ had 68% and 76% agreement, respectively, with κ-scores of 0.49 and 0.62 (Table 2).
TABLE 2.
Pre- and posthydration agreement of malnutrition category by MUAC, MUACZ, WAZ, and WLZ as indices of undernutrition1
| Agreement, % | κ-Score | SE | |
| MUAC | 94 | 0.78 | 0.03 |
| MUACZ | 92 | 0.78 | 0.03 |
| WAZ | 76 | 0.62 | 0.03 |
| WLZ | 69 | 0.49 | 0.03 |
Degree of agreement between pre- and posthydration measured by Cohen’s κ. MUAC, midupper arm circumference; MUACZ, midupper arm circumference z score; WAZ, weight-for-age z score; WLZ, weight-for-length z score.
Based on a linear regression model analyzing the impact of percent dehydration on the changes in anthropometric indicators with rehydration while controlling for age and sex, each 1% increase in dehydration was associated with a change in WAZ of 0.0895 (95% CI: 0.0892, 0.0905), and a change in WLZ of 0.1304 (95% CI: 0.1297, 0.1312). There was no significant change in MUAC or MUACZ, however, with each 1% change in dehydration status (Table 3).
TABLE 3.
Linear regression model of change in MUAC, MUACZ, WAZ, and WLZ with each 1% change in dehydration status1
| β-Coefficient (95% CI) | P | |
| MUAC | 0.003 (−0.002, 0.009) | 0.20 |
| MUACZ | 0.003 (−0.002, 0.008) | 0.20 |
| WAZ | 0.090 (0.089, 0.091) | <0.001 |
| WLZ | 0.130 (0.129, 0.131) | <0.001 |
Impact of percent dehydration on measurement indices; model assessed for linearity, additivity, independence, homoscedasticity, and normality; controlled for both age and sex. MUAC, midupper arm circumference; MUAC, midupper arm circumference z score; WAZ, weight-for-age z score; WLZ, weight-for-length z score.
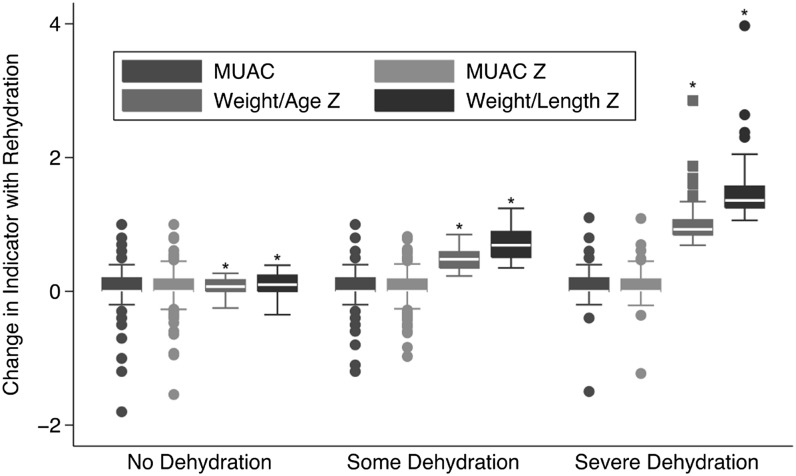
The change in each indicator with rehydration was analyzed based on baseline dehydration category (none, some, or severe). Dehydration category had no significant effect on the change in MUAC (P = 0.32) or MUACZ (P = 0.07) with rehydration. However, dehydration category had a significant effect on the change in WAZ (P < 0.001) and WLZ (P < 0.001) with rehydration. Figure 2 demonstrates the change with rehydration in each of the 4 anthropometric measures by baseline dehydration category.
FIGURE 2.
Changes in nutritional indicators between prehydration status and after rehydration to a stable weight; dehydration category assigned as no dehydration (<3% weight change with rehydration), some dehydration (3–9% weight change with rehydration), and severe dehydration (>9% weight change with rehydration); MUACZ, WLZ, and WAZ are all reported in z score units; MUAC is reported in millimeters. MUAC, midupper arm circumference; MUACZ, midupper arm circumference z score; WAZ, weight-for-age z score; WLZ, weight-for-length z score. *Signifies malnutrition indicators whose change with rehydration was signficantly different based on dehydration category.
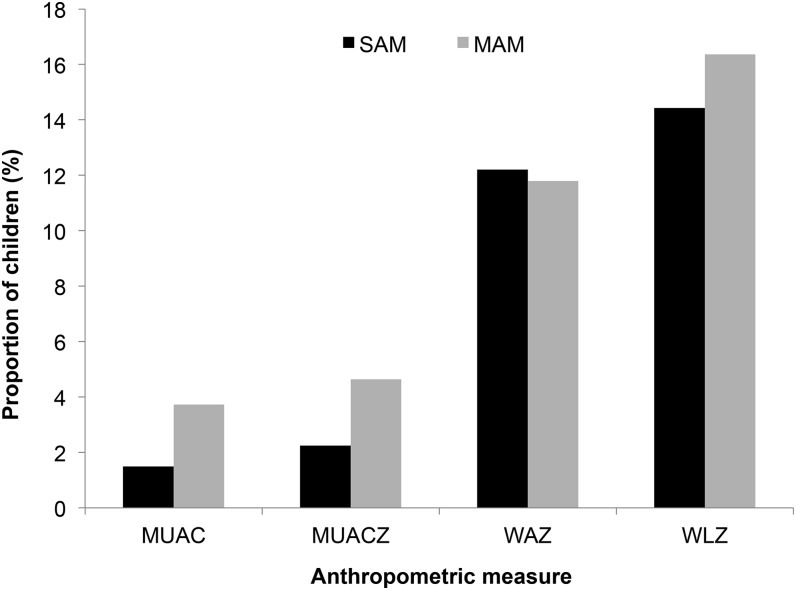
Finally, we evaluated the impact of each of the 4 measures on the diagnosis of SAM in children with complete data available. Overall, 88 (12%) children were misclassified as severe underweight on arrival using WAZ and 104 (14%) children were misclassified as SAM using WLZ. Comparatively, only 10 (1%) children were misclassified as SAM on arrival using MUAC and 16 (2%) were misclassified as SAM using MUACZ (Figure 3).
FIGURE 3.
Misclassification of malnutrition status in patients with complete data available. n = 680 for MUAC, n = 711 for MUACZ, and n = 721 for WAZ and WLZ. MAM, moderate acute malnutrition; MUAC, midupper arm circumference; MUACZ, midupper arm circumference z score; SAM, severe acute malnutrition; WAZ, weight-for-age z score; WLZ, weight-for-length z score.
Discussion
Current WHO guidelines endorse using MUAC in community-based settings and WLZ in health facilities to diagnose children with SAM. In our study, a large proportion of children with diarrhea were misclassified with SAM when using WLZ whereas MUAC and MUACZ were found to have high agreement pre- and posthydration and were far less likely to misclassify children with SAM. Furthermore, dehydration status was found to have a significant impact on the accuracy of WLZ and WAZ, but no significant impact on the accuracy of MUAC or MUACZ.
This difference may partially explain previous research, which has shown that MUAC and WLZ often identify different populations of children with malnutrition. A large study by WHO and UNICEF used both diagnostic criteria in a sample of 450,000 children aged 6–60 mo. WLZ and MUAC both identified a similar proportion of children with SAM: 3.22% and 3.27%, respectively. However, ∼60% of children who were classified as having SAM by one of these indicators were not classified as such by the other (4). These findings were confirmed by several subsequent studies (13, 16, 22, 23).
Berkley et al. (16) postulated that one of the factors causing WLZ and MUAC to identify different populations of children could be related to hydration status. To examine the influence of dehydration, Berkley et al. (16) modeled the changes in WLZ with respect to National Center for Health Statistics standards for a hypothetical severely dehydrated child with 10% loss of body weight. They found that if a child’s usual z score was 0, then a 10% body weight loss would have reduced the WLZ by ∼1 z score (16). Our study validates this theory by showing that WLZ can vary based on the severity of dehydration in the child.
Clinically, this is relevant in the diagnosis of children with severe malnutrition. Our study showed that 12–14% of children were misclassified with severe underweight or SAM using WAZ and WLZ, respectively. Previous research has found similar results of overdiagnosis of SAM using the WHO standard (15, 16). Misclassification is costly and can lead to overtreatment, poor resource use, and exacerbation of the burden of illness. Conversely, if providers are concerned that measures of undernutrition may not be accurate in children with diarrhea, they may delay treatment for undernutrition until after rehydration in some children who truly need it, with an attendant risk that they may never receive it.
MUAC has many advantages in practical use. MUAC increases by only a small amount between 1 and 4 y of age, minimizing the effect of age on the measure’s ability to predict nutrition status (22). Furthermore, MUAC measurement tapes are cheap and more readily available in resource-limited environments than scales. Finally, they have shifted the diagnosis of undernutrition to the community level, because of their ease of use and the widespread availability of MUAC measurement tapes (24).
Although there have been many studies on the diagnosis of SAM in the general population, there is a paucity of data on the diagnosis of malnutrition specifically in children with diarrhea, although these 2 conditions frequently present concurrently. To our knowledge, only one prior study has looked specifically at this population of children. Mwangome et al. (15) studied 3 measures of malnutrition in 325 children <5 y of age presenting with diarrhea to a rural hospital in Kenya. They found that every 1% change in weight with rehydration was associated with a change in MUAC of 0.40 mm, a change in MUACZ of 0.035, and a change in WLZ of 0.116 (15). Our study confirms their finding that WLZ is a poor indicator of undernutrition in children with diarrhea. However, in our study MUAC and MUACZ were not significantly affected by dehydration status.
Unlike the study by Mwangome et al. (15) our study uses the posthydration stable weight as the proxy for preillness weight to determine the severity of dehydration on arrival. Mwangome et al. (15) compared arrival weight to a second weight performed 48 h after admission, making the assumption that all children achieved a stable weight by that time and that no children were under- or overhydrated. This assumption seems particularly unlikely given that 25% of the children enrolled in their study lost weight in the first 48 h after admission, suggesting inadequate rehydration in children with persistent diarrhea. In our study, only 2% of children lost weight in the rehydration unit, and these children were excluded from our analysis to avoid biasing our results.
Our study finds MUAC and MUACZ to be the best diagnostic indicators for undernutrition in children presenting with diarrhea. Rapidly identifying vulnerable populations suffering from both diarrhea and undernutrition has important clinical implications. This high-risk population can receive targeted therapy for their severe malnutrition, such as protein-energy supplementation, while also receiving specialized ORS for their dehydration, such as ReSoMal (Nutriset, Normandy, France), developed specifically for children with severe malnutrition. Moreover, in community-based settings, it may not be possible to reassess the nutritional status of a child with diarrhea after rehydration, because they are only evaluated at a single point in time before being sent home with sachets of ORS. In these cases, MUAC or MUACZ can be used to confidently assess nutritional status, enabling prompt diagnosis and initiation of community-based nutritional supplementation, without requiring the child to return in several days for a repeat nutritional assessment after rehydration.
Limitations.
Children with bipedal edema and those with WAZ < −4 were not included in this study, because they are managed in a separate malnutrition unit at icddr,b, instead of the general rehydration unit used for all other children with diarrhea. Although this likely excluded the most severe cases of malnutrition (including those whose diagnoses were least likely to be affected by dehydration status), including them in our analysis would have presented additional complications. For instance, all children in the malnutrition unit receive supplemental nutrition, including locally made F75 and F100, so their change in weight could not be attributed solely to rehydration, making it more difficult to assess the degree of dehydration present on arrival. In addition, children with kwashiorkor may not experience the same weight gain because of loss of edema-related fluid on improvement in protein status. None of the children enrolled in our study received any high-calorie nutritional supplementary diet during their stay in the rehydration unit, as per icddr,b guidelines, so their percent weight change should accurately reflect their dehydration status on arrival.
This study was also conducted at a single urban hospital in Bangladesh, and our results may not be generalizable to more rural populations or those in other settings. However, this particular hospital has a wide catchment area, encompassing a population of >17 million people in the city of Dhaka and surrounding rural and suburban districts. For this population, icddr,b functions as a primary center for diarrhea care, with >90% of patients presenting primarily and <10% transferred from other health facilities.
Conclusions.
Our study demonstrates that MUAC and MUACZ can be used to accurately identify undernutrition in children with diarrhea, regardless of their dehydration status. Conversely, WLZ and WAZ, traditional measures of malnutrition in children still used frequently at health facilities, are significantly affected by dehydration status and misclassify a large proportion of children with SAM and severe underweight. Based on these results, clinicians and community health care workers can confidently use MUAC or MUACZ to guide nutritional supplementation for children presenting with diarrhea in resource-limited settings.
Acknowledgments
We thank Dr. Jennifer Friedman and Dr. Lindsey Locks for their contributions to this article. NHA and ACL designed the research study; PM, SN, NHA, and ACL supervised data collection; JG-B, MIH, and ACL coded and analyzed the data; PM, SN, MH, JG-B, MIH, and ACL wrote the paper. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: icddr,b, International Centre for Diarrhoeal Disease Research, Bangladesh; MUAC, midupper arm circumference; MUACZ, midupper arm circumference z score; ORS, oral rehydration solution; SAM, severe acute malnutrition; WAZ, weight-for-age z score; WLZ, weight-for-length z score.
References
- 1.WHO. World health statistics 2014. Geneva (Switzerland): WHO; 2014.
- 2.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, Onis M, Ezzati M, Grantham-McGregor M, Katz J, Martorell R, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013;382:427–51. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Management of severe malnutrition: a manual for physicians and other senior health workers. Geneva (Switzerland): WHO; 1999. [Google Scholar]
- 4.UNICEF, WHO. WHO child growth standards and the identification of severe acute malnutrition in infants and children. Geneva (Switzerland): WHO; 2009. [PubMed] [Google Scholar]
- 5.WHO. Updates on the management of severe acute malnutrition in infants and children. Geneva (Switzerland): WHO; 2013. [PubMed] [Google Scholar]
- 6.Goossens S, Bekele Y, Yun O, Harcizi G, Ouannes M, Shepherd S. Mid-upper arm circumference based nutrition programming: evidence for a new approach in regions with high burden of acute malnutrition. PLoS ONE 2012;7:e49320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali E, Zachariah R, Shams Z, Vernaeve L, Alders P, Salio F, Manzi M, Allaouna M, Draquez B, Delchevalerie P, et al. Is mid-upper arm circumference alone sufficient for deciding admission to a nutritional programme for childhood severe acute malnutrition in Bangladesh? Trans R Soc Trop Med Hyg 2013;107:319–23. [DOI] [PubMed] [Google Scholar]
- 8.Briend A, Marie B, Fontaine O, Garenne M. Mid-upper arm circumference and weight-for-height to identify high-risk malnourished under-five children. Matern Child Nutr 2012;8:130–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trehan I, Goldbach HS, LaGrone LN, Meuli GJ, Wang RJ, Maleta KM, Manary MJ. Antibiotics as part of the management of SAM. N Engl J Med 2013;368:425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wierzba TF, El-Yazeed RA, Savarino SJ, Mourad AS, Rao M, Baddour M, El-Deen AN, Naficy AB, Clemens JD. The interrelationship of malnutrition and diarrhea in a periurban area outside Alexandria, Egypt. J Pediatr Gastroenterol Nutr 2001;32:189–96. [DOI] [PubMed] [Google Scholar]
- 11.Martorell R, Habicht JP, Yarbrough C, Lechtig A, Klein RE, Western KA. Acute morbidity and physical growth in rural Guatemalan children. Am J Dis Child 1975;129:1296–301. [DOI] [PubMed] [Google Scholar]
- 12.Nel ED. Diarrhoea and malnutrition. South Afr J Clin Nutr 2010;23:S15–8. [Google Scholar]
- 13.Olimen P, Onyaye E. Nutritional assessment of children aged twelve to fifty-nine months with diarrhoeausing mid-upper arm circumference (MUAC). BMJ 2013;4:1315–26. [Google Scholar]
- 14.Talbert A, Thuo N, Karisa J, Chesaro C, Ohuma E, Ignas J, Berkley JA, Toromo C, Atkinson S, Maitland K. Diarrhoea complicating severe acute malnutrition in Kenyan children: a prospective descriptive study of risk factors and outcome. PLoS ONE 2012;7:e38321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mwangome MK, Fegan G, Prentice AM, Berkley JA. Are diagnostic criteria for acute malnutrition affected by hydration status in hospitalized children? A repeated measures study. Nutr J 2011;10:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berkley J, Mwangi I, Griffths K, Ahmed I, Mithwani S, English M, Newton C, Maitland K. Assessment of severe malnutrition among hospitalized children in rural Kenya: comparison of weight for height and mid upper arm circumference. JAMA 2005;294:591–7. [DOI] [PubMed] [Google Scholar]
- 17.Bardhan PK. Annual statistics of Dhaka Hospital. Dhaka (Bangladesh): International Center for Diarrheal Disease Research; 2012. [Google Scholar]
- 18.WHO [Internet]. WHO Anthro and macros. Version 3.2.2, January 2011. Geneva (Switzerland): WHO [cited 2014 Nov 10]. Available from: http://www.who.int/childgrowth/software/en/.
- 19.WHO. Child growth standards: Methods & development—head-circumference-for-age, arm circumference-for-age, triceps skinfold-for-age and subscapular skinfold-for-age. Geneva (Switzerland): WHO; 2007. [Google Scholar]
- 20.Gorelick MH, Shaw KN, Murphy KO. Validity and reliability of clinical signs in the diagnosis of dehydration in children. Pediatrics 1997;99:E6. [DOI] [PubMed] [Google Scholar]
- 21.King CK, Glass R, Bresee JS, Duggan C; Centers for Disease Control and Prevention. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep 2003;52:1–6. [PubMed] [Google Scholar]
- 22.McDowell I, King FS. Interpretation of arm circumference as an indicator of nutritional status. Arch Dis Child 1982;57:292–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myatt M, Khara T, Collins S. A review of methods to detect cases of severely malnourished children in the community for their admission into community-based therapeutic care programs. Food Nutr Bull 2006;27(3, Suppl)S7–23. [DOI] [PubMed] [Google Scholar]
- 24.WHO, World Food Programme, UN System Standing Committee on Nutrition, UNICEF. Community-based management of severe acute malnutrition. Geneva (Switzerland): WHO; 2007. [Google Scholar]