Abstract
Background: The purpose of food fortification is to affect those at the lower end of the distribution curve for nutrient status while avoiding unintended consequences for those at the high end of the distribution. Vitamin D presents challenges in this regard.
Objectives: We used scenarios to model changes in concentrations of serum 25-hydroxyvitamin D [25(OH)D] based on increases made because of assumptions about fortification. We then examined the outcomes for balance between improving serum 25(OH)D status for those at risk of inadequacy while avoiding high concentrations for those not at risk.
Methods: Data from NHANES 2001–2006 served as baseline serum 25(OH)D concentrations and were used to model shifts in serum 25(OH)D distribution after application of 3 fortification scenarios, including conceptual scenarios and an experiential predictive scenario we developed with the use of statistical modeling of changes in NHANES serum folate concentrations between prefortification and postfortification time periods.
Results: All scenarios suggested the possibility of increasing serum 25(OH)D above 125 nmol/L among the proportion of the population at the high end of baseline serum 25(OH)D distribution. The scenario based on serum folate change struck a middle ground between the 2 conceptual scenarios. It predicted a prevalence of 11% <40 nmol/L serum 25(OH)D compared with 17% currently (study baseline), and 8% prevalence of serum 25(OH)D >125 nmol/L compared with <1% currently (study baseline). It also confirmed that fortification affects those at the low end of the status distribution curve differently from those at the high end.
Conclusions: Nutrient inadequacy of the type demonstrated by vitamin D—in which the risk is not universal—requires a thorough exploration of the unintended consequences of the overall shift in the distribution of serum 25(OH)D if efforts are made to use fortification to increase the status of persons at risk of deficiency. Fortification is at best a blunt instrument that must be implemented with caution. Moreover, fortification must be preceded by more research to elucidate the dose-response relation between intake and changes in serum 25(OH)D.
Keywords: fortification, vitamin D, serum 25-hydroxoyvitamin D, folate, modeling scenarios
Introduction
The 2010 Dietary Guidelines for Americans identified vitamin D as a nutrient of public health concern for underconsumption (1). The current focus on vitamin D has been associated with increased supplement use (2), and calls for intervention strategies to increase concentrations of serum 25-hydroxyvitamin D [25(OH)D] (3). In turn, there has been interest in a comprehensive, nationally implemented effort to fortify the US food supply with vitamin D.
Because food fortification is a balancing act between reaching those in need of improved status while avoiding unintended consequences for those whose status is adequate, it is important to examine the consequences of adding vitamin D to the food supply before steps are taken to implement such interventions. The need for such information is particularly relevant to vitamin D because inadequacy, although of concern, is not universal. The continued focus on vitamin D, even in the face of conflicting data about its health consequences, warrants attention at this time. There are varying estimates of vitamin D inadequacy depending upon the cutoffs selected for serum concentrations, but a probability approach based on NHANES data and the Institute of Medicine (IOM) recommendations for serum 25(OH)D concentrations estimated that 17–20% of the US adults may be at risk of inadequacy (4). These estimates suggest that an initial task when considering the addition of vitamin D to the food supply is to understand how it will affect all members of the population, not just those at risk. Moreover, this question is consistent with the FDA Fortification Policy (5) that specifies the need to anticipate unintended consequences and ensure safety while also confirming the public health need and technical feasibility. Others have stipulated the importance of simulating the potential approach before finalizing fortification decisions and ensuring postfortification monitoring (6).
The focus of this study was the potential shifts in the distribution of serum 25(OH)D concentrations based on scenarios that simulated fortification, in order to examine differences in response among groups at the lower end of the distribution of serum 25(OH)D in contrast to those at the higher end of the distribution. Previous research related to fortification had underscored the importance of considering the fortification-driven change across the entire distribution of the measure of interest, rather than only changes in the mean (6, 7); the unintended consequences and safety issues of a fortification intervention are likely to be seen at the tails of the distribution (8). Because the nature of potential vitamin D fortification strategies is unknown, 2 of the scenarios used a fortification approach that would either cause a consistent increase in the measure of interest for all members of the population or reflect a proportional increase in the measure, most likely because of a selective response akin to heeding dietary advice to increase intake. We also took advantage of the available serum folate data from NHANES for both prefortification and postfortification time periods to develop a predictive equation for fortification, which served as a third scenario. When taken as a whole, these scenarios reflected a reasonable range of potential fortification approaches. Our work, however, is at best a first step in exploring the myriad of questions that would need to be answered before considering a fortification initiative, ranging from public health need to technical feasibility. Given the limited nature of the data available to model fortification effects on serum 25(OH)D, as well as the unknowns related to changes in serum 25(OH)D concentrations in response to intake as well as the role of sun exposure, our outcomes best serve as a basis for further consideration and research rather than as definitive conclusions.
Methods
Survey design and participants.
The NHANES is a nationally representative, cross-sectional survey of the noninstitutionalized US population (9). The National Center for Health Statistics within the CDC administers the NHANES and obtains written informed consent from all participants or proxies. The survey uses a complex, stratified, multistage probability cluster sampling design. Beginning in 1999, it became a continuous survey formulated in 2 y cycles. Survey personnel initially interview participants in their homes, during which interviewers collect information on demographic characteristics, dietary supplement use, and some health-related issues. One to two weeks after the household interview, participants undergo a standardized physical examination and blood collection in a mobile examination center.
To evaluate the 3 fortification scenarios, we used NHANES data for serum 25(OH)D from the 2001–2006 cycles, the data available at the time of our analyses. The overall unweighted NHANES response rates for the interview samples of the 3 continuous 2 y surveys were 84% (2001–2002), 79% (2003–2004), and 80% (2005–2006) (10). We included persons ≥1 y of age because fortification is a broad approach that targets the entire population and cannot be directed at different age, ethnic, or lifestage groups. The NHANES 2001–2006 sample reflected a total of 30,070 examined individuals; our analytic sample was 24,411 after excluding those with missing survey weights and missing serum 25(OH)D concentrations. To derive a fortification scenario based on the folate fortification experience, we used serum folate concentrations from NHANES to reflect a prefortification time period (1988–1994; n = 39,695 examined) and a postfortification time period (1999–2004; n = 38,684 examined). Federal regulations requiring the addition of folate to enriched cereal grain products became effective in 1998 (11). We limited our folate postfortification analyses to the 1999–2004 survey cycles. These cycles reflected the available postfortification data closest in time to implementation of folate fortification, thus minimizing the likelihood that important factors other than fortification were altering serum folate concentrations while maintaining an analytic sample size similar to that available for serum 25(OH)D. Our folate analytic sample was limited to persons ≥4 y of age because data on serum folate were not available for persons <4 y of age for the prefortification survey time. After excluding those with missing survey weights and missing serum folate concentrations, the analytic sample for folate was 23,703 for NHANES 1988–1994 and 23,200 for NHANES 1999–2004.
Serum concentrations: Serum 25(OH)D and serum folate.
NHANES 2001–2006 serum 25(OH)D concentrations were determined at the CDC with the use of a radioimmunoassay kit (DiaSorin) (12). The CDC adjusted the 2003–2004 and 2005–2006 serum 25(OH)D data to correct for assay drifts (12). We reported serum 25(OH)D in nmol/L (a nmol/L value is ∼2.5 times the amount in μg/L).
Serum folate, similar to serum 25(OH)D, is an indicator of the availability of the circulating nutrient to body tissues (13). The CDC used the Bio-Rad Quantaphase I radioassay from 1988 to 1991 and the Quantaphase II from 1991 to 2006 (14). The CDC applied corrections to the publicly released NHANES folate data to account for method differences between the Quantaphase I and II (14, 15). Because Bio-Rad assays underestimate serum folate concentrations, we also adjusted the results to make them comparable to the microbiological growth assay, which is considered to be more accurate (16). Serum folate concentrations are reported in nmol/L (1.0 μg/L = 2.266 nmol/L).
Descriptive analyses.
We used SAS version 9.3 to create initial datasets. As described in an earlier publication (4), serum concentrations should be corrected for within-person variability to ensure the best estimate of a serum concentration because a distribution based on a single measurement for each individual will inflate variability across the distribution (17, 18). We corrected for the within-person variability with the use of a subset of NHANES data that reflected replicate serum samples from a subset of NHAHES participants as reported in more detail elsewhere (4). The software PC-Side, version 1.01, was used for this correction for both serum 25(OH)D and folate concentrations, and to generate descriptive statistics. In addition, the software program R (2008; Foundation for Statistical Computing) was used to derive scenario calculations and create figures.
Initial analyses were carried out to determine the observed (i.e., baseline) distribution of serum 25(OH)D, the risk of inadequacy, and the proportion experiencing excess among the NHANES 2001–2006 sample of persons ≥1 y of age and for IOM age and pregnancy groups. Serum 25(OH)D, which reflects exposure from both diet and sunlight, is considered to be a more appropriate measurement of vitamin D status than estimates of vitamin D intake (7). Preliminary analyses found no statistical relation between time of year of blood draw and serum 25(OH)D concentrations in NHANES, likely because of NHANES sampling protocol, which generally samples in northern states in the summer and southern states in the winter (9). Nonetheless, we included a season indicator as a covariate in estimating serum 25(OH)D concentrations to ensure that the factor of seasonality was minimized, and thus sun exposure was held constant across the data. Estimates of the risk of vitamin D inadequacy or excess were based on IOM reference values—that is, values established to serve as reference measurements for nutrient status—for serum 25(OH)D (7). Specifically, a serum concentration of 40 nmol/L was used as the mean reference value, whereas 125 nmol/L was stipulated at the upper level of intake. Prevalences of inadequacy were calculated with the use of a statistical probability approach with data corrected for within-person variability (4).
Application of scenarios.
We applied 3 scenarios, including 2 conceptual scenarios and 1 experiential scenario derived from US folate prefortification and postfortification measurements. The conceptual scenarios were first informally described by Beaton (Supplemental Text) as part of preliminary work to establish principles for application of reference values in assessing and planning diets (19, 20). The ability to use intake-based dietary assessment principles with serum 25(OH)D values was demonstrated in earlier work (4), allowing us to incorporate these scenarios into our research. The first scenario, termed Additive, increases the measure of interest by a fixed amount for all persons in the population (Table 1). The second, termed the Proportional Scenario, causes each person in the population to experience the same percent increase in the measure. As a theoretical construct, the Additive Scenario is predicated on a consistent increase, similar to a situation in which all consume a dietary supplement of constant dosage. The Proportional Scenario rested on the assumption that the percent change required to shift the low-intake percentile group to a more desirable status would also be the percent change experienced by higher percentile groups. The Proportional Scenario could occur when all followed dietary advice such as “consume more foods fortified with vitamin D.” We applied these 2 scenarios in order to increase serum 25(OH)D concentrations to 40 nmol/L at lower percentiles of the baseline serum 25(OH)D distribution (i.e., 2.5th, 5th, 10th, and 15th) while noting the concurrent changes of serum 25(OH)D within the higher percentiles. The IOM specified 40 nmol/L as the mean reference value for the US population (7). Further, baseline serum 25(OH)D concentrations were specified to the nearest 0.5 nmol/L, causing the observed distribution of concentrations to contain numerous peaks and valleys. For this reason in the case of the Additive Scenario, we added random noise with mean zero and very small variance to the serum measurements to smooth the empirical distribution and ease the process of normalization, consistent with the accepted statistical procedure to improve properties of data without changing summary statistics (21). Because of the inability to quantitatively factor in a change in response as a function of baseline status, the scenario was based on a linear response.
TABLE 1.
Additive Scenario and Proportional Scenario to achieve serum 25(OH)D concentrations of 40 nmol/L among lower percentiles at baseline: Example of calculation with the use of the 10th percentile from NHANES 2001–20061
| Scenario | Approach | Calculation |
| Additive | Quantity of serum 25(OH)D needed to increase 10th percentile to 40 nmol/L is systematically added to serum 25(OH)D for all individuals | • 10th percentile serum 25(OH)D is 34 nmol/L |
| • 6 nmol/L needed to achieve 40 nmol/L | ||
| • Serum 25(OH)D of all individuals is increased by 6 nmol/L | ||
| Proportional | Proportional increase needed for 10th percentile to achieve 40 nmol/L is systematically used to increase serum 25(OH)D for all individuals | • 10th percentile serum 25(OH)D is 34 nmol/L |
| • 10th percentile must increase 1.2 times to achieve 40 nmol/L | ||
| • Serum 25(OH)D of all individuals is increased 1.2 times |
Includes persons ≥1 y of age; n = 24,411. Methods for calculations are the same when percentiles are targeted to achieve 50 nmol/L. 25(OH)D, 25-hydroxyvitamin D.
Additionally, for illustrative purposes, we examined the 2 conceptual scenarios on the basis of achieving 50 nmol/L serum 25(OH)D among the lower percentiles. Misunderstandings about the application of reference values for dietary planning and assessment of population groups may lead some to conclude that the desirable goal is to ensure that the entire population achieves the 97.5th percentile reference value, which in the case of vitamin D is 50 nmol/L serum 25(OH)D. This is contrary to IOM guidelines for the use of reference values (19, 20) that demonstrate the validity of reliance on the mean reference value in this situation, but nonetheless it is at times used inappropriately as a basis for research.
We operationalized the third scenario, termed Folate Change Scenario, by modeling the population-based changes in serum folate prefortification vs. postfortification. This scenario used NHANES serum folate data from 1988–1994 (prefortification) and 1999–2004 (postfortification) for persons ≥4 y of age with adjustments as described by Pfeiffer et al. (14).
Before analysis, the ability to relate serum folate changes to changes for serum 25(OH)D was explored. No other fortification intervention with nationally representative data on prefortification and postfortification was available. Although it is expected that the nature of the 2 vitamins may influence dose and response, the correspondence in pattern and magnitude of the dose-response relation between intake and serum concentrations for folate and vitamin D appeared to be sufficiently similar. Both are known to show a flattened response at a higher intake (7, 22). For folate, noncurvilinearity occurs at a folate intake >400 μg/d (22). For vitamin D, noncurvilinearity occurs at 10–15 μg/d (7, 22). The extent to which the metabolism of vitamin D—such as conversion to its 24,25-hydroxyvitamin D form—may play a role in causing this nonlinearity is uncertain. A recent meta-analysis concluded that a doubling of folate intake to 400 μg/d resulted in an increase in serum folate of 71% (22). For vitamin D, the quantitative aspects of the dose-response curve is less clear (7). A vitamin D meta-analysis found that with each additional 2.5 μg/d intake of vitamin D3, when total dose was <20 μg, serum 25(OH)D increased by 1–2 nmol/L with considerable variation depending upon baseline and administration of the vitamin D form (23). This suggested a lesser quantitative response for vitamin D than for serum folate. It may be that serum folate curves are narrower with a higher peak than those that characterize serum 25(OH)D, which appear to be broad and shallow. Depending upon the relation between folate and vitamin D, it may be more difficult to increase the status of those at the low end of the distribution curve for 25(OH)D without causing a greater percentage of excessively high concentrations than was experienced for serum folate. With these caveats, serum folate data were used as the basis of the prediction equation.
Furthermore, use of serum folate data required the assumption that all changes in serum folate between prefortification (1988–1994) and postfortification (1999–2004) were due to increases in folate intake and not to nonfortification related changes, such as changes in dietary patterns. This 5 y time interval was dictated by the availability of NHANES surveys. Whereas steady-state biomarker responses to change in folate intake would likely occur in less time than 5 y (24), the possibility that other factors unrelated to the fortification program could have affected postfortification serum folate concentrations cannot be ruled out. Further, we assumed that vitamin D fortification would involve cereal grains, akin to the intervention for folate. Cereal grains are presently widely used by manufacturers to deliver vitamin D in the diet and this delivery vehicle in general has been demonstrated to be effective for fortification because cereal grains are consumed by virtually all persons on a daily basis (25)
The development of the predictive equation for the Folate Change Scenario was initiated by plotting plotted serum folate percentiles for NHANES 1988–1994 against the ratio of the prefortification and postfortification serum folate percentiles for NHANES 1999–2004, and then a curve was fitted to these data. These calculations are described in detail in the Supplemental Methods (including Supplemental Figures 1–4). The fitted equation was yhat = c + a/(x − b), with estimates of parameters a, b, and c as ahat = 28.28 ± 0.37, bhat = −2.28 ± 0.06, and chat = −1.14 ± 0.02. The variable x denotes serum folate percentiles for NHANES 1988–1994; yhat is the predicted ratio of serum folate percentiles for NHANES 1999–2004. This equation was applied to the baseline serum 25(OH)D concentrations to derive a predicted change in the distribution of serum 25(OH)D with the assumption that sun exposure was a constant across the data set.
Results
Results are presented primarily in the context of comparing the predicted serum 25(OH)D distribution shifts for each scenario relative to increasing serum 25(OH)D for persons at the lower end of the distribution at baseline while noting concurrent changes for serum 25(OH)D among persons at the high end of the distribution. We assumed that comparison of distribution shifts would be independent of the analytic methodology, because the NHANES used the same assay for the time period of this study and the concentrations were adjusted for assay drift (12) as described earlier. Although there are important debates about the accuracy and reliability of the available serum 25(OH)D assays (26), the NHANES data are the currently available national estimates of serum 25(OH)D concentrations for the US population. The available data from NHANES indicated that the mean serum 25(OH)D for persons ≥1 y of age was 59 ± 0.13 nmol/L (Table 2). Different age groups ≥14 y of age and pregnant women within NHANES 2001–2006 showed similar prevalence estimates (17–20%) for inadequacy. Relatively low prevalences were observed for children 1–8 y of age (2–3%), whereas children 9–13 y of age had prevalences between these 2 groupings (9%). Percentages of the population with serum 25(OH)D concentrations >125 nmol/L were low (<1%) across all age groups and for pregnant women (2%).
TABLE 2.
NHANES 2001–2006 serum 25(OH)D concentrations1
| Age and pregnancy group | n | Serum 25(OH)D, nmol/L | Risk of inadequacy,2 % | 25(OH)D >125 nmol/L |
| Total ≥1 y | 24,411 | 59 ± 0.13 | 17 ± 0.34 | <1 |
| 1–3 y | 1165 | 71 ± 0.48 | 2 ± 0.006 | <1 |
| 4–8 y | 1959 | 70 ± 0.31 | 3 ± 0.005 | <1 |
| 9–13 y | 2989 | 62 ± 0.28 | 9 ± 0.008 | <1 |
| 14–18 y | 3915 | 60 ± 0.33 | 17 ± 0.009 | <1 |
| 19–30 y | 3644 | 59 ± 0.38 | 20 ± 0.009 | <1 |
| 31–50 y | 4606 | 59 ± 0.31 | 19 ± 0.008 | <1 |
| 51–70 y | 3719 | 58 ± 0.32 | 19 ± 0.009 | <1 |
| ≥71 y | 2414 | 58 ± 0.39 | 19 ± 0.01 | <1 |
| Pregnant | 928 | 66 ± 0.38 | 17 ± 0.02 | 23 |
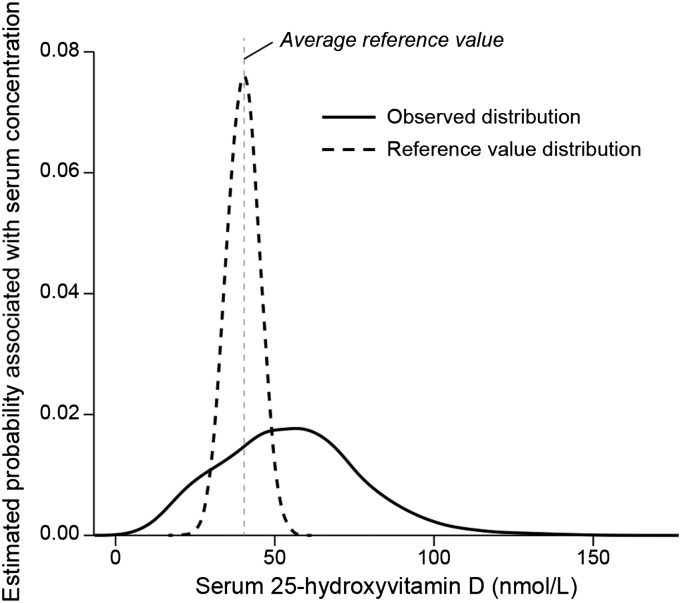
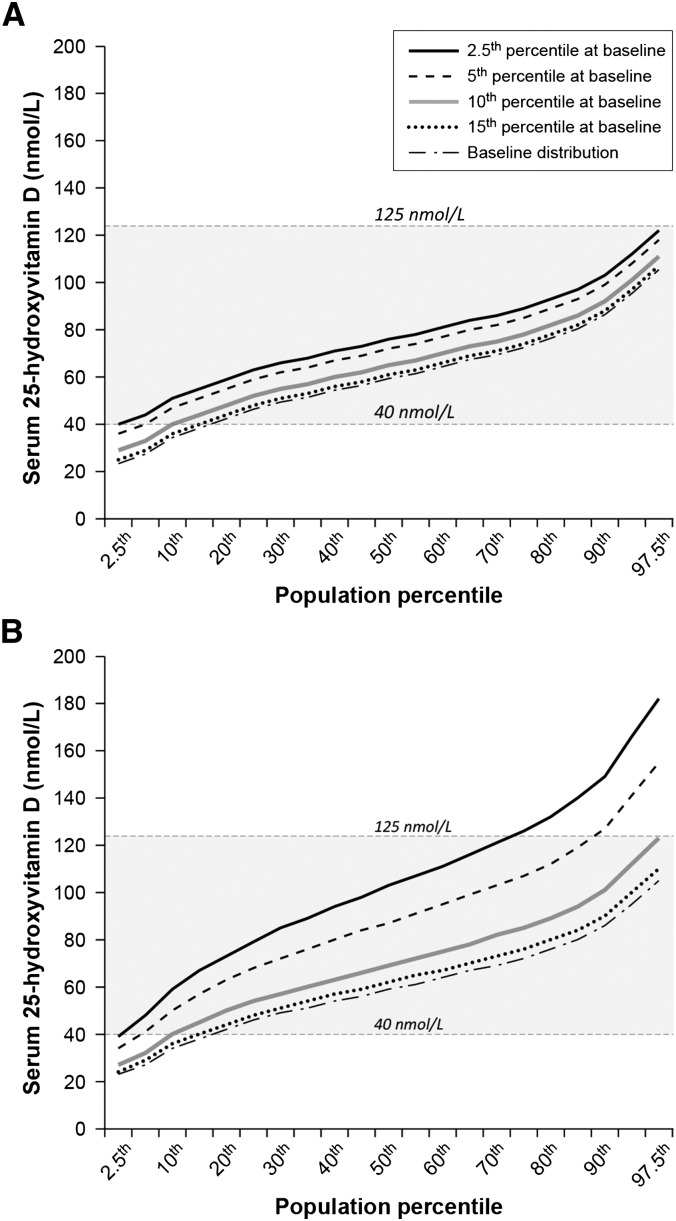
The baseline serum 25(OH)D distribution for persons ≥1 y of age showed a wide distribution range skewed to the right (Figure 1). The IOM reference value distribution is assumed to be normally distributed with a narrow range (7). Persons between the 15th and 20th percentile in this population already had a serum 25(OH)D ≥40 nmol/L. Thus, we set the target for the serum 25(OH)D increase when applying the 2 conceptual scenarios as persons below the 20th percentile at baseline serum 25(OH)D. The outcomes when the 2.5th, 5th, 10th, and 15th percentiles were subjected to each of the 2 conceptual scenarios and, in turn, achieved a serum 25(OH)D of 40 nmol/L resulted in different patterns of fortification impacts with larger changes in the Proportional Scenario than did the Additive Scenario (Figure 2). In the Additive Scenario, all projected shifts generally remained below 125 nmol/L, although targeting the 2.5th percentile to achieve a serum 25(OH)D of 40 nmol/L resulted in persons at the high end of the serum 25(OH)D distribution coming close to the 125 nmol/L concentration. Clearly, the greatest reduction in persons with a serum 25(OH)D <40nmol/L would occur when the target was the 2.5th percentile.
FIGURE 1.
Observed (baseline) distribution of serum 25-hydroxvitamin D concentrations from NHANES 2001–2006, persons ≥1 y of age, n = 24,411; mean ± SE: 50 ± 0.13 nmol/L. The Institute of Medicine reference value distribution for serum 25(OH)D is also shown (7).
FIGURE 2.
Serum 25-hydroxyvitamin D distribution shifts to achieve 40 nmol/L with the use of the Additive Scenario (A) and Proportional Scenario (B). Shifts are shown across population percentiles for each of 4 percentile groups at low-end at baseline. Data are from NHANES 2001–2006, persons ≥1 y of age, n = 24,411, corrected for within-person variability. The shaded area indicates the range between the Institute of Medicine mean reference value of 40 nmol/L and the upper level reference value of 125 nmol/L (7).
The distribution shifts to achieve 40 nmol/L at the low end of the distribution based on the Proportional Scenario were notably elevated at the high end of the distribution, to the extent that persons at the high end of the 5th as well as the 2.5th percentile shift readily surpassed the 125–150 nmol/L range (Figure 2). The 10th percentile shift was elevated compared with the Additive Scenario, but it did not exceed 125 nmol/L. For the Proportional Scenario, the 95% CI for the 97.5th percentile within the 10th percentile shift was 121–125 nmol/L.
The effect of targeting the lower percentile groups to achieve, inappropriately, a serum 25(OH)D of 50 nmol/L (i.e., the IOM 97.5th reference value), rather than the 40 nmol/L value (i.e., the IOM mean reference value) associated with statistical probability (4, 19), resulted in predicted serum 25(OH)D concentrations that were notably increased for those with baseline measurements at the high end of the distribution serum 25(OH)D (Table 3). For the 75th percentile at baseline, both the 2.5th and the 5th percentile scenarios exceeded the 125 nmol/L upper limit. At the 97.5th percentile, all serum concentrations surpassed the 125–150 nmol/L range.
TABLE 3.
Increased serum 25(OH)D concentrations for higher baseline percentile groups when serum distribution is inappropriately shifted to achieve 50 nmol/L vs. correctly shifted to achieve 40 nmol/L for 4 lower percentile groups at baseline1
| Predicted serum 25(OH)D, nmol/L |
||||
| 75th percentile at baseline |
97.5th percentile at baseline |
|||
| Scenario | To achieve 40 nmol/L | To achieve 50 nmol/L | To achieve 40 nmol/L | To achieve 50 nmol/L |
| Additive | ||||
| 2.5th percentile shifted | 89 ± 0.23 | 99 ± 0.23 | 122 ± 0.80 | 132 ± 0.80 |
| 5th percentile shifted | 85 ± 0.23 | 95 ± 0.23 | 118 ± 0.80 | 128 ± 0.80 |
| 10th percentile shifted | 78 ± 0.23 | 88 ± 0.23 | 111 ± 0.80 | 121 ± 0.80 |
| 15th percentile shifted | 74 ± 0.23 | 84 ± 0.23 | 107 ± 0.80 | 117 ± 0.80 |
| Proportional | ||||
| 2.5th percentile shifted | 126 ± 0.40 | 157 ± 0.49 | 182 ± 1.40 | 228 ± 1.75 |
| 5th percentile shifted | 107 ± 0.34 | 134 ± 0.42 | 155 ± 1.19 | 194 ± 1.49 |
| 10th percentile shifted | 85 ± 0.27 | 106 ± 0.33 | 123 ± 0.95 | 154 ± 1.19 |
| 15th percentile shifted | 76 ± 0.24 | 95 ± 0.30 | 110 ± 0.85 | 138 ± 1.06 |
Concentrations are means ± SEs. Predicted increases are derived from the Additive Scenario and Proportional Scenario. NHANES 2001–2006 (persons ≥1 y of age; n = 24,411). 25(OH)D, 25-hydroxyvitamin D.
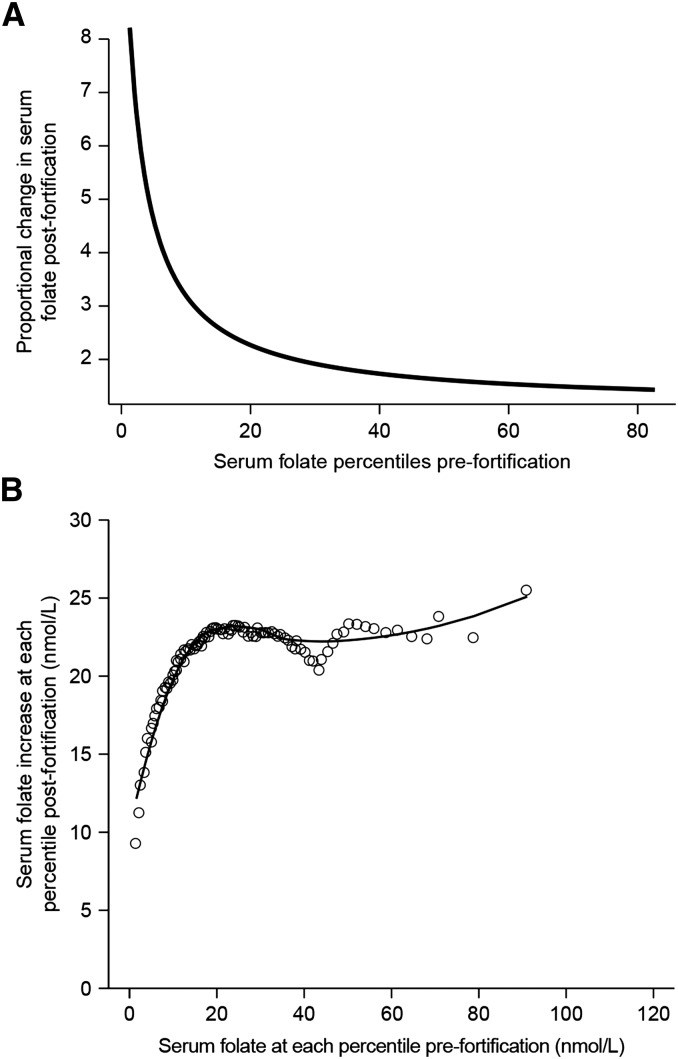
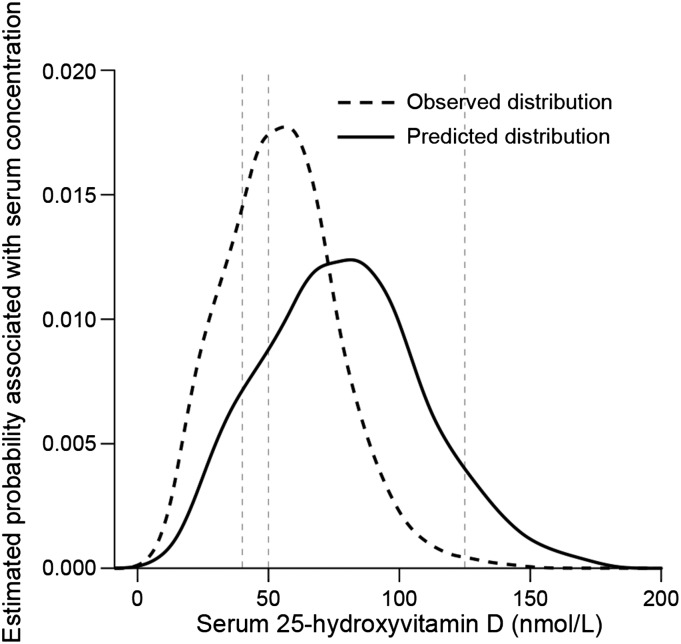
The population-based changes in the serum folate distribution between prefortification and postfortification that served as the basis for the Folate Change Scenario demonstrated that the proportional change in serum concentrations was greater for the measurements at the low end of the serum folate distribution than for values above ∼20 nmol/L (Figure 3A). However, the quantitative increase in serum folate concentrations was much less in the low end than in the high end (Figure 3B). That is, the low end of concentrations changed proportionally 5–6 times more than the high end, but experienced only a 9–10 nmol/L increase in serum folate compared with the high-end increase of as much as 25–30 nmol/L. Application of the folate-derived predictive equation to the baseline serum 25(OH)D distribution resulted in a distribution shifted to the right and higher concentrations compared with baseline distribution (Figure 4). Approximately 11% of the population remained below the IOM mean reference serum concentration of 40nmol/L, and 8% surpassed 125 nmol/L.
FIGURE 3.
Proportional change (A) and quantitative change (B) in serum folate postfortification for persons ≥4 y of age from NHANES 1999–2004 (postfortification; n = 38,684) and NHANES 1988–1994 (prefortification; n = 39,695), both corrected for within-person variability. Proportional change is plotted against population percentiles prefortification; proportional increases in serum folate ranged from ∼2–8 times baseline concentrations. Quantitative change is plotted as the nmol/L increase from prefortification to postfortification for each percentile; quantitative increases in serum folate ranged from ∼9–25 nmol/L. Fitted predictive equation yhat = c + a/(x − b); estimates of parameters a, b, and c are ahat = 28.28 ± 0.37, bhat = −2.28 ± 0.06, and chat = −1.14 ± 0.02, where x = serum folate percentiles for NHANES 1988–1994.
FIGURE 4.
Observed (baseline) vs. predicted serum 25-hydroxyvitamin D distribution based on the Folate Change Scenario. Baseline data from NHANES 2001–2006, persons ≥1 y of age, n = 24,411. Vertical lines from left to right reflect, respectively, the IOM mean reference value (40 nmol/L), the IOM 97.5th reference value (50 nmol/L), and the IOM upper level reference value (125 nmol/L) for serum 25-hydroxyvitmin D (7). Serum concentrations decline to 0 because of statistical smoothing of data. The prevalence of serum 25-hydroxyvitamin D <40 nmol/L was 17% for the baseline distribution and 11% for the predicted distribution. The prevalence of serum 25-hydroxyvitamin D concentrations >125 nmol/L was <1% for the baseline distribution and 8% for the predicted distribution. IOM, Institute of Medicine.
Discussion
Unless the potential consequences of food fortification are explored and studied in advance of implementing a wide-ranging fortification initiative on a national basis, the increased intake associated with fortification runs the risk of being an unplanned experiment. At the same time, the blunt-instrument nature of fortification makes its application a balancing act. The challenges associated with fortification are increased when the inadequacy is not universal, because there may be unintended consequences for those who are not targets of the intervention. For this reason, understanding and clarifying the potential shift in the total distribution of the measure of interest is one of the key components of the exploration needed before the development of fortification strategies.
In the case of vitamin D, the distribution of interest is serum 25(OH)D, which is the preferred measure of status, and it would be monitored in tracking a fortification program. Thus, the goal of increased vitamin D intake is not increased intake per se, but rather increased serum concentrations among those at risk. Achieving this goal via diet is complicated by the need to factor in sun exposure, which currently is very poorly characterized. Moreover, the serum response to increased intake appears to vary depending upon baseline status as well as dose. Further, although fortification would be uniform across the population, the ability to achieve desirable serum concentrations may vary by characteristics of population subgroups such as genetically related differences in free serum 25(OH)D. Additionally, inadequacy is not universal and shows notable heterogeneity across age groups, adding further complications. Our work was intended to raise questions, and we could not provide quantitatively based outcomes given the limited data and need for more research related to vitamin D dose response. Rather, the outcomes illustrated patterns, some of which signal the need for caution in considering vitamin D fortification of the food supply.
The Additive Scenario, which reflected a uniform increase consistent with all persons consuming a constant dosage, appeared likely to result in increases in lower percentiles without causing higher percentiles to exceed the upper level (Figure 2, Table 3). Its practicality as an intervention is, of course, limited. The Proportional Scenario, which assumed that consumers at ends of the distribution would experience increases that were proportionally equivalent, demonstrated that absolute amounts of increase in the measure of interest would be larger at high baseline percentiles than at low baseline percentiles. Application of this scenario resulted in greater difficulty in achieving improvements at the low percentiles because of the tendency for higher percentiles to exceed the upper concentration of 125 nmol/L (Figure 2, Table 3). In all cases, the paucity of data to describe the nature of the dose-response relation between vitamin D intake and increases in serum concentrations required that we assume the relation to be linear. Although likely a valid assumption when vitamin D intake is low to moderate, linearity is less certain when intake reaches higher amounts (7). Were the dose-response relation to be established as seriously nonlinear, it would suggest that both scenarios would have fewer persons approaching serum concentrations near 125 nmol/L than we report, and the response to fortification could be somewhat muted for those at the high end of the distribution.
The Folate Change Scenario was empirically derived. It allowed us to incorporate for the first time to our knowledge the longitudinal outcomes from a nationally regulated folate fortification. Specifically, it allowed the development of a predictive equation for dietary fortification based on a biomarker of status and to be used in predicting a fortification effect on another biomarker of status having a nominally similar dose-response relation through the use of an established food delivery system. Our work showed this scenario reflected a middle ground between the 2 polar conceptual scenarios. The folate multiplicative factor was high for the lower percentiles, but then approached a plateau at ∼30th percentile of the distribution curve (Figure 3) in contrast to reflecting a constant across the distribution as suggested by the Proportional Scenario. Moreover, the differences between prefortification and postfortification serum folate concentrations increased as the percentiles increased (Figure 4), and therefore did not meet the assumption of a constant additive value as suggested by the Additive Scenario.
We further highlighted the possibility that inappropriate use of reference values in exploring scenarios for fortification could lead to erroneous conclusions in future work focused on fortification. Specifically, misunderstandings about the application of reference values for dietary planning and assessment of population groups has led some to conclude that the desirable goal is to ensure that the entire population achieves the 97.5th percentile reference value, i.e., 50 nmol/L serum 25(OH)D. Application of the 97.5th percentile reference value would lead to unacceptably high serum concentrations for some of the population (Table 3).
Our study was strengthened by use of a large, nationally representative sample. Further, the NHANES data reduced confounding of serum 25(OH)D concentrations from sun exposure across the data because survey protocol causes the mobile examination units to “follow the sun,” with the result that most blood draws take place in the summer in northern states and in the winter in southern states. Although development of a predictive equation for shifts in biomarker distributions based on folate status biomarker distributions is a unique aspect of our research and sets the stage for more sophisticated modeling of fortification, the approach will require further validation and included some uncertainties related to the correspondence between the response for serum folate and serum 25(OH)D, or for any other nutrient that may be modeled for fortification based on the serum folate response.
Although uncertainties, especially those related to the equivalence of folate and vitamin D response, require a cautious application of the predictive equation, the folate change scenario supported evidence that a dietary fortification program affects consumers at the high end of the distribution to a greater extent than those at the low end (6). In the case of vitamin D, this is likely to occur even if sun exposure is not assumed to be constant. Our outcomes shed light on potential diet-related shifts in serum 25(OH)D distributions, and are intended to inform the complicated steps of modeling approaches to identify vitamin D intake and food vehicles to achieve desirable serum 25(OH)D concentrations. Not only is more information needed before we can mesh intake recommendations with contributions from sun exposure, but the ability to reliably describe dietary sources of vitamin D and identify fortification routes is currently limited. These activities are necessary should government regulations be pursued for vitamin D food fortification. Our work sets the stage for further studies to examine the effect of fortification for the persons at the lower and higher ends of the serum 25(OH)D distribution. The outcomes signaled that attempting to increase those at the lower end of the distribution could potentially put those at the higher end of the distribution at risk of excessive serum concentrations, based on the IOM reference values. There were 2 questions that could not be answered in this work: 1) whether the curvilinear nature of the dose-response relation would slow the trajectory of serum increases for persons at the higher end and thereby allow the higher amounts of fortification that would be needed to affect persons at the lower end of the distribution; and 2) whether persons at risk of vitamin D inadequacy could be sufficiently characterized and reached by means other than a broad fortification program, or alternatively whether modest fortification overall could be safely accomplished and then accompanied by enhanced efforts to reach those at highest risk.
Supplementary Material
Acknowledgments
We thank Christine Pfeiffer and Vicki Burt from the CDC for their assistance; Kevin Dodd from the National Cancer Institute for his helpful initial analyses; Elizabeth Yetley for her valuable input on the research design and manuscript development; and Joyce Merkel, who provided technical assistance in preparing the manuscript. CLT and ALC designed the research; ALC analyzed the data and developed the predictive models; RLB provided data support; CLT wrote and had primary responsibility for the final manuscript; and RLB and ALC contributed to the manuscript. All authors read and approved the final manuscript.
References
- 1.US Department of Agriculture and US Department of Health and Human Services. Dietary guidelines for Americans, 2010. 7th ed Washington (DC): US Government Printing Office; 2010. [Google Scholar]
- 2.Nutrition Business Journal. Supplement business report. New York, NY; 2013. Table 4.2.6.
- 3.Murphy SP, Barr SI. Steps forward in assessing populations. Am J Clin Nutr 2013;97:1–2. [DOI] [PubMed] [Google Scholar]
- 4.Taylor CL, Carriquiry AL, Bailey RL, Sempos CT, Yetley EA. Appropriateness of the probability approach with a nutrient status biomarker to assess population inadequacy: a study using vitamin D. Am J Clin Nutr 2013;97:72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services, Food and Drug Administration. Code of federal regulations. Fortification policy. 21CFR10420, 2013.
- 6.Crane NT, Wilson DB, Cook DA, Lewis CJ, Yetley EA, Rader JI. Evaluating food fortification options: general principles revisited with folic acid. Am J Public Health 1995;85:660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington (DC): National Academies Press; 2011. [PubMed] [Google Scholar]
- 8.Yetley EA, Rader JI. Modeling the level of fortification and post-fortification assessments: US experience. Nutr Rev 2004;62:S50–9; discussion S60–1. [DOI] [PubMed]
- 9.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey. Atlanta (GA): Centers for Disease Control and Prevention; [cited 2014 May 14]. Available from: http://www.cdc.gov/nchs/nhanes.htm. [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). NHANES response rates and population totals. Atlanta (GA): Centers for Disease Control and Prevention; [cited 2014 May 14]. Available from: http://www.cdc.gov/nchs/nhanes/response_rates_CPS.htm. [Google Scholar]
- 11.Lewis CJ, Crane NT, Wilson DB, Yetley EA. Estimated folate intakes: data updated to reflect food fortification, increased bioavailability, and dietary supplement use. Am J Clin Nutr 1999;70:198–207. [DOI] [PubMed] [Google Scholar]
- 12.National Center for Health Statistics. Revised analytic note for NHANES 2000–2006 and NHANES III (1988–1994) 25-hydroxyvitamin D analysis. Atlanta (GA): Centers for Disease Control and Prevention; 2010. [cited 2014 May 14]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes3/VitaminD_analyticnote.pdf. [Google Scholar]
- 13.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate OBV and Choline. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): National Academies Press; 1998. [PubMed] [Google Scholar]
- 14.Pfeiffer CM, Hughes JP, Lacher DA, Bailey RL, Berry RJ, Zhang M, Yetley EA, Rader JI, Sempos CT, Johnson CL. Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortification using assay-adjusted data from the NHANES 1988–2010. J Nutr 2012;142:886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yetley EA, Johnson CL. Folate and vitamin B-12 biomarkers in NHANES: history of their measurement and use. Am J Clin Nutr 2011;94:322S–31S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yetley EA, Coates PM, Johnson CL. Overview of a roundtable on NHANES monitoring of biomarkers of folate and vitamin B-12 status: measurement procedure issues. Am J Clin Nutr 2011;94:297S–302S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nusser SM, Carriquiry AL, Dodd KW, Fuller WA. A semiparametric transformation approach to estimating usual daily intake distributions. J Am Stat Assoc 1996;91:1440–9.
- 18.Guenther PM, Kott PS, Carriquiry AL. Development of an approach for estimating usual nutrient intake distributions at the population level. J Nutr 1997;127:1106–12. [DOI] [PubMed] [Google Scholar]
- 19.National Research Council. Dietary reference intakes: applications in dietary assessment. Washington (DC): The National Academies Press; 2000. [PubMed] [Google Scholar]
- 20.National Research Council. Dietary reference intakes: applications in dietary planning. Washington (DC): The National Academies Press; 2003. [PubMed] [Google Scholar]
- 21.Box GEP, Hunter WG, Hunter JS. Statistics for experimenters. New York: John Wiley and Sons, Inc.; 1978. [Google Scholar]
- 22.Duffy ME, Hoey L, Hughes CF, Strain JJ, Rankin A, Souverein OW, Dullemeijer C, Collings R, Hooper L, McNulty H. Biomarker responses to folic acid intervention in healthy adults: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2014;99:96–106. [DOI] [PubMed] [Google Scholar]
- 23.Cranney A, Horsley T, O'Donnell S, Weiler H, Puil L, Ooi D, Atkinson S, Ward L, Moher D, Hanley D, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep) 2007;158:1–235. [PMC free article] [PubMed] [Google Scholar]
- 24.Tighe P, Ward M, McNulty H, Finnegan O, Dunne A, Strain J, Molloy AM, Duffy M, Pentieva K, Scott JM. A dose-finding trial of the effect of long-term folic acid intervention: implications for food fortification policy. Am J Clin Nutr 2011;93:11–8. [DOI] [PubMed] [Google Scholar]
- 25.National Research Council. Proposed fortification policy for cereal-grain products. Washington (DC): National Academy Press; 1974. [Google Scholar]
- 26.Carter GD, Phinney KW. Assessing vitamin D status: time for a rethink? Clin Chem 2014;60:809–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.