Doxorubicin and the IGF-1R antibody cixitumumab is an overall well-tolerated combination in patients with soft tissue sarcoma. Subsequent studies are needed to determine the incremental efficacy benefit of cixitumumab to standard doxorubicin in this patient population. Incremental toxicities of the combination include hyperglycemia along with a potential for an increased incidence of cardiac toxicity.
Keywords: soft tissue sarcoma, cixutumumab, doxorubicin, IGF-1R, cardiotoxicity, TITE-CRM
Abstract
Background
Insulin-like growth factor receptor (IGF-1R) has been studied as an oncologic target in soft tissue sarcoma (STS), but its role in sarcoma biology is unclear. Anti-IGF-1R antibody cixutumumab demonstrated acceptable toxicity but limited activity as a single agent in STS. We carried out a dose-escalation study of cixutumumab with doxorubicin to evaluate safety and dosing of the combination.
Patients and methods
Eligible patients with advanced STS were treated with cixutumumab intravenously on days 1/8/15 at one of three dose levels (A: 1 mg/kg, B: 3 mg/kg, C: 6 mg/kg) with doxorubicin at 75 mg/m2 as a 48 h infusion on day 1 of a 21 day cycle. After six cycles of the combination, patients could receive cixutumumab alone. The Time-to-Event Continual Reassessment Method was used to estimate the probability of dose-limiting toxicity (DLT) and to assign patients to the dose with an estimated probability of DLT≤20%.
Results
Between September 2008 and January 2012, 30 patients with advanced STS received a median of six cycles of therapy (range <1–22). Two DLTs were observed, grade 3 mucositis (dose level B) and grade 4 hyperglycemia (dose level C). Grade 2 and 3 reduced left ventricular ejection fraction was seen in three and two patients, respectively. Five partial responses were observed, and estimated progression-free survival was 5.3 months (95% confidence interval 3.0–6.3) in 26 response-assessable patients. Immunohistochemical staining of 11 available tumor samples for IGF-1R and phospho-IGF-1R was not significantly different among responders and non-responders, and serum analysis of select single-nucleotide polymorphisms did not predict for cardiotoxicity.
Conclusion
The maximum tolerated dose was doxorubicin 75 mg/m2 on day 1 and cixitumumab 6 mg/kg on days 1/8/15 of a 21 day cycle. Cardiac toxicity was observed and should be monitored in subsequent studies, which should be considered in STS only if a predictive biomarker of benefit to anti-IGF-1R therapy is identified.
Trial registration
ClinicalTrials.gov:NCT00789633.
introduction
Soft tissue sarcomas (STS) represent a therapeutic challenge based on their rarity, heterogeneity, and their overall limited responsiveness to chemotherapy. Doxorubicin and ifosfamide are the only cytotoxic single agents with objective response rates over 10% [1]. Single-agent doxorubicin is often used as a first-line agent for patients with metastatic disease with palliative intent, given its relative tolerability and ease of administration, and it often serves as the backbone for new agents in clinical trials.
The insulin-like growth factor receptor (IGF-1R) pathway has been implicated in many malignancies and is a logical target for anti-STS therapy [2–4]. When phosphorylated, IGF-1R signaling can result in downstream activation of a number of potential oncogenes within the PI3K-Akt-mTOR as well as RAF-MAPK pathways. IGF-1R is overexpressed in many sarcoma histologies, and inhibition of IGF-IR has resulted in vitro in tumor apoptosis and growth inhibition and in vivo in tumor formation in mouse models of Ewing's sarcoma treated with IGF-1R anti-sense mRNA [2, 5–8]. Cixutumumab (IMC-A12) is a fully human IgG1/λ monoclonal antibody directed at the type I IGF-1R (ImClone Systems, Inc.). In a single-agent phase II study in STS, cixutumumab was well tolerated, but demonstrated minimal activity with the exception of the adipocytic cohort [9]. Pre-clinical studies revealed IGF-1R activation as a possible mechanism of doxorubicin resistance in STS [10] and that anti-IGF-IR therapy showed synergistic cytotoxicity with doxorubicin in osteosarcoma in vitro [10, 11]. Given pre-clinical rationale to study anti-IGF1R therapy with doxorubicin in STS, we carried out a phase I study of doxorubicin and cixutumumab limited to patients with STS.
methods
patients
Eligible patients were 16 years and older with histologically confirmed, measurable, advanced STS excluding pediatric rhabdomyosarcoma, GIST, alveolar soft part sarcoma, and clear cell sarcoma. Other key eligibility criteria included: ECOG performance status ≤ 2, number of prior chemotherapies ≤ 1, adequate organ, fasting glucose <120 mg/dl, and left ventricular ejection fraction (LVEF) ≥50%.
The study was conducted in accordance with US Food and Drug Administration, Good Clinical Practice, the Declaration of Helsinki, and applicable local health authority requirements. The institutional review board of participating institutions approved the study protocol, and all patients provided written informed consent. The study is registered at http://www.clinicaltrials.gov as NCT00720174.
study design and treatment plan
This multicenter, open-label phase I study was sponsored by the Cancer Therapeutics Evaluation Program (CTEP) and carried out at participating centers of the University of Chicago Phase II Consortium. Patients were treated with doxorubicin and cixutumumab for up to six cycles. Patients could continue on therapy with cixutumumab alone thereafter in the absence of progression.
The primary objective was to collect safety data on the combination of doxorubicin and cixutumumab. Secondary objectives included assessment of confirmed response rate as assessed by Response Evaluation Criteria in Solid Tumors (RECISTv1), 3- and 6-month progression-free survival (PFS) rates, median PFS and overall survival (OS), and changes in left ventricular (LV) ejection fraction.
Three dose levels were tested. Cixutumumab was dosed at 1, 3, or 6 mg/kg weekly on dose levels A/B/C, respectively. Doxorubicin was dosed at 75 mg/m2/IV continuous infusion over 48 h on day 1 of a 21 day cycle.
safety evaluations
Evaluations were repeated after each 3 week cycle except for blood counts that were carried out weekly and MUGA scans and tumor imaging, which was carried out every two cycles. After 24 weeks, assessments were carried out less frequently.
dose-limiting toxicities
Current versions of NCI CTCAE (3.0 and subsequently 4.0) were used to assess toxicity. Reporting of toxicities was carried out according to version 4.0, with the exception of cardiotoxicity as stopping rules were based on version 3.0. Dose-limiting toxicities (DLTs) were defined as the following events occurring within the first two cycles felt to be attributable to study treatment: (i) any drug-related death, (ii) any grade 3/4 treatment-related non-hematologic toxicity (excepting grade 3 hyperglycemia not require dose change), (iii) cardiac LV dysfunction resulting in discontinuation in treatment, (iv) grade 4 neutropenia >7 days, (v) grade 3 febrile neutropenia, (vi) grade 4 thrombocytopenia, (vii) any other grade 4 hematologic toxicity, and (viii) any toxicity leading to ≥1 dose interruption or requiring dose reduction.
statistical considerations
The Time-to-Event Continual Reassessment Method (TITE-CRM) was used to estimate the probability of DLT at each dose level and to assign patients to the dose with the estimated probability of DLT≤20%. This model was chosen to allow for continuous enrollment after initial dose escalation and to allow the maximum number of patients to be treated at the maximum tolerated dose. The sample size for estimation of the dose-toxicity function was 30 patients. The relationship between dose and toxicity was summarized by a single-parameter logistic model. The posterior distribution of toxicity, which displays the probability that a future patient will experience toxicity at a given dose on the basis of the current data, was calculated with 95% credible intervals for each dose level. The dose closest to but not exceeding the target rate of toxicity (20%) was estimated as the MTD.
LVEF was calculated from MUGA scans. The estimated mean value and range were reported for each measurement point and differences from baseline after two, four, and six cycles. The longitudinal course was modeled using a random effects, repeated measures model, using an unstructured correlation matrix for measurements within a patient over time.
All statistical tests were estimated using SAS software, version 9.2 (SAS Institute, Cary, NC).
correlative studies
immunohistochemistry evaluation
Immunohistochemistry (IHC) staining was carried out on the DAKO Autostainer (DAKO, Carpinteria, CA). Serial sections of de-paraffinized sections were labeled with either IGF-1r (clone JBW902, 1:1000, Millipore, Billerica, MA, Cat. No 05-656) or pY1158/1162/1163-IGF-1r (1:500, Millipore, Cat. No 07-841), overnight at 4C. Microwave epitope retrieval in 10 mM Tris/HCl, pH9 containing 1 mM EDTA was used before staining. Slides were scored with methodology of Harvey et al. [12].
DNA extraction and SNP analysis
Genomic DNA was extracted from blood using DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA). DNA was subjected to polymerase chain reaction (PCR) using Platinum PCR supermix (Invitrogen, Carlsbad, CA) and 200 nM primers. After an initial denaturation and Taq DNA polymerase activation, templates were amplified for 40 cycles, followed by a 10 min extension. PCR products were visualized on 2% agarose gels and purified using a Wizard SV PCR clean-up kit (Promega, Madison, WI). Amplicons were sequenced directly in both directions using an ABI 377 DNA sequencer (ABI, Foster City, CA). Chromatograms were downloaded to CodonCode Aligner software (v4.2.7, Dedham, MA) and compared with reference sequence downloaded from NCBI.
results
patient characteristics
Thirty patients enrolled from September 2008 to January 2012 at six participating centers. Patient characteristics are depicted in Table 1. Twenty-nine patients were evaluable for safety (received at least one dose of treatment), and 27 patients were evaluable for efficacy (received at least one cycle). There was one death during cycle 1 of treatment due to complications of port infection, not associated with neutropenia that was felt to be unrelated to treatment. Reasons patients came off study included: toxicity (5), progression (21), withdrawal (3), and death (1).
Table 1.
Patient demographics
| Characteristic | No. of patients (%) | Characteristic | No. of patients (%) |
|---|---|---|---|
| Age | Median: 64 | Prior chemotherapy | |
| Range: 19–80 | Yes | 7 (23%) | |
| No | 23 (77%) | ||
| Sex | Histologic subtypes | ||
| Male | 17 (56.7%) | Leiomyosarcoma | 12 (40%) |
| Female | 13 (43.3%) | High grade undifferentiated | |
| Pleomorphic sarcoma | 8 (27%) | ||
| Race | Liposarcoma | 8 (27%) | |
| White | 26 (86.7%) | Dedifferentiated | 4 (13%) |
| Black | 3 (10%) | Myxoid/round cell | 1 (3%) |
| Asian | 1 (3.3%) | Not otherwise specified | 3 (9%) |
| Malignant peripheral nerve sheath tumor | 1 (3%) | ||
| Angiosarcoma | 1 (3%) |
toxicity
Two patients experienced DLTs. The first experienced grade 3 mucositis at dose level B during the first cycle of therapy, and the second experienced grade 4 hyperglycemia at dose level C during the second cycle of therapy. The final study estimates for the probability of DLT are listed in Table 2. For all dose levels tested, the estimated mean probability of DLT is below our target for acceptability, 20%. Toxicities by dose level are presented in Table 3 and serious adverse effects are shown in Supplementary Table S1, available at Annals of Oncology online.
Table 2.
Outcomes and estimates of the probability of dose-limiting toxicity at each study dose level
| Dose level | Cixutumumab weekly dose (mg/kg) | Prior estimate for DLT (%) | Patients treated | Patients with DLT (s) | Posterior estimate for DLT (%) | 95% Bayesian credible interval (%) |
|---|---|---|---|---|---|---|
| A | 1 | 10 | 4 | 0 | 4.5 | 1.0–14.1 |
| B | 3 | 13 | 13 | 1 | 6.2 | 1.6–17.8 |
| C | 6 | 18 | 12 | 1 | 9.4 | 2.7–23.6 |
Table 3.
Adverse events by dose level
| Toxicity categorya | Dose level A (n = 4) |
Dose level B (n = 13) |
Dose level C (n = 12) |
Total (n = 29) |
||||
|---|---|---|---|---|---|---|---|---|
| All grades, n (%) | Grade 3+, n (%) | All grades, n (%) | Grade 3+, n (%) | All grades, n (%) | Grade 3+, n (%) | All grades, n (%) | Grade 3+, n (%) | |
| Blood and lymphatic system disorders | 3 (75) | 2 (50) | 11 (85) | 4 (31) | 4 (31) | 0 | 18 (60) | 6 (20) |
| Cardiac disorders | 0 | 0 | 5 (39) | 2 (15) | 2 (15) | 0 | 5 (17) | 2 (7) |
| Ear and labyrinth disorders | 1 (25) | 0 | 0 | 0 | 0 | 0 | 2 (7) | 0 |
| Eye disorders | 1 (25) | 0 | 5 (39) | 0 | 0 | 0 | 10 (33) | 0 |
| Fatigue | 4 (100) | 1 (25) | 12 (92) | 1 (8) | 1 (8) | 0 | 17 (59) | 3 (10) |
| Gastrointestinal disorders | 3 (75) | 1 (25) | 12 (92) | 2 (15) | 2 (15) | 1 (8) | 22 (73) | 4 (13) |
| Hepatobillary disorders | 0 | 0 | 1 (8) | 1 (8) | 1 (8) | 0 | 1 (3) | 1 (3) |
| Infections and infestations | 2 (50) | 1 (25) | 7 (54) | 2 (15) | 2 (15) | 2 (17) | 12 (40) | 5 (17) |
| Procedural complications | 0 | 0 | 1 (8) | 0 | 0 | 0 | 1 (3) | 0 |
| Investigations | 2 (50) | 1 (25) | 11 (85) | 5 (39) | 5 (36) | 1 (8) | 18 (60) | 7 (23) |
| Metabolism and nutrition disorders | 2 (50) | 1 (25) | 7 (54) | 2 (15) | 2 (15) | 1 (8) | 12 (40) | 4 (13) |
| Musculoskeletal and connective tissue disorders | 3 (75) | 0 | 7 (54) | 1 (8) | 1 (8) | 0 | 13 (43) | 1 (3) |
| Neoplasms benign, malignant, and unspecified | 0 | 0 | 1 (8) | 0 | 0 | 0 | 1 (3) | 0 |
| Nervous system disorders | 0 | 0 | 6 (46) | 0 | 0 | 0 | 8 (27) | 0 |
| Other | 1 (25) | 1 (25) | 0 | 1 (8) | 0 | 0 | 2 (7) | 0 |
| Psychiatric disorders | 1 (25) | 0 | 1 (8) | 0 | 0 | 0 | 2 (7) | 0 |
| Renal and urinary disorders | 2 (50) | 0 | 4 (31) | 1 (8) | 1 (8) | 0 | 7 (23) | 1 (3) |
| Respiratory, thoracic and mediastinal disorders | 2 (50) | 0 | 8 (61) | 0 | 0 | 0 | 14 (47) | 0 |
| Skin and subcutaneous tissue disorders | 2 (50) | 0 | 9 (69) | 0 | 0 | 0 | 16 (53) | 0 |
| Vascular disorders | 2 (50) | 2 (50) | 3 (23) | 1 (8) | 1 (8) | 0 | 5 (17) | 3 (3) |
aCommon toxicity criteria for adverse events version 4.03.
hyperglycemia
Based on toxicity profile of cixutumumab alone, hyperglycemia was of special concern and occurred in seven patients (grade 2, 3, and 4 events in 4, 2, and 1 patients, respectively). Events resolved with standard therapy including oral hypoglycemic medications and/or insulin. Four (13.3%) patients required a dose reduction in cixutumumab for hyperglycemia. All events resolved to ≤grade 1 on study discontinuation without the need for ongoing anti-diabetic medications.
cardiac toxicity
Five patients experienced significant LVEF decrease (grade 2 or greater, LVEF <50% per CTCAE v3) over the course of study (supplementary Figure S1, available at Annals of Oncology online). Toxicity of at least grade 2 was apparent after 2, 4, and 6 cycles of therapy in 1, 3, and 1, patients, respectively. Grade 3 events were not apparent until after six cycles of therapy. Therapy was held for LVEF<50% or for decrease in LVEF >15% relative to baseline. Two patients with a decline in ejection fraction below the institutional upper limits of normal were treated with standard heart failure therapy, and both discontinued therapy because of lack of cardiac improvement with medical management.
Model estimates of LVEF change closely mirror observed mean value at each time point (supplementary Table S2, available at Annals of Oncology online). Change in LVEF was significantly different after four cycles when compared with two cycles; suggesting a 4% decrease on average. The trend continued at six cycles, although the rate of decrease appears largest between two and four cycles of therapy.
efficacy
The median overall number of cycles was 6 (range <1–22). The first 6 cycles consisted of combination doxorubicin plus cixutumumab therapy. Thirteen patients received cixutumumab alone following combination, for a median of 4 cycles of single-agent therapy (range 1–16). The median follow-up time for the entire study was 12 months.
Among patients evaluable for response (n = 26), the response rate was 19% (5 PRs, 0 CR) and stable disease rate was 58%. Responses were seen the following histologies: myxoid liposarcoma (dose level A), undifferentiated pleomorphic sarcoma (UPS) and angiosarcoma (dose level B), and in UPS and leiomyosarcoma (dose level C). Patients who received over six cycles had the following histologies: (leiomyosarcoma—7, liposarcoma—3, UPS—3). The median PFS was 5.3 months [95% confidence interval (CI) 3.0–6.3] with 3- and 6-month PFS rate of 69.2% (95% CI 47.8–83.3) and 34.3 (95% CI 16.1–53.3), respectively. A plot of the Kaplan–Meier estimates of PFS is provided in Figure 1.
Figure 1.
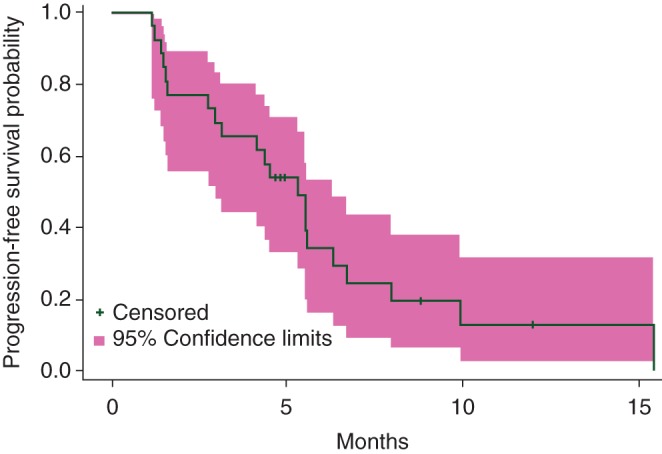
Progression-free survival for assessable patients (n = 27).
Three patients had a seemingly exceptional response to study treatment. A myxoid liposarcoma patient had over 70% shrinkage to large volume target lesions and a leiomyosarcoma patient had stable disease on treatment for 22 cycles. Figure 2 depicts images from a patient with a uterine leiomyosarcoma metastatic to lung with significant shrinkage after combination therapy (Figure 2B) and cystic changes with continued cixitumumab alone (Figure 2C). After nine cycles of cixitumumab therapy, the patient underwent metastatectomy, which revealed leiomyosarcoma with cystic degeneration.
Figure 2.
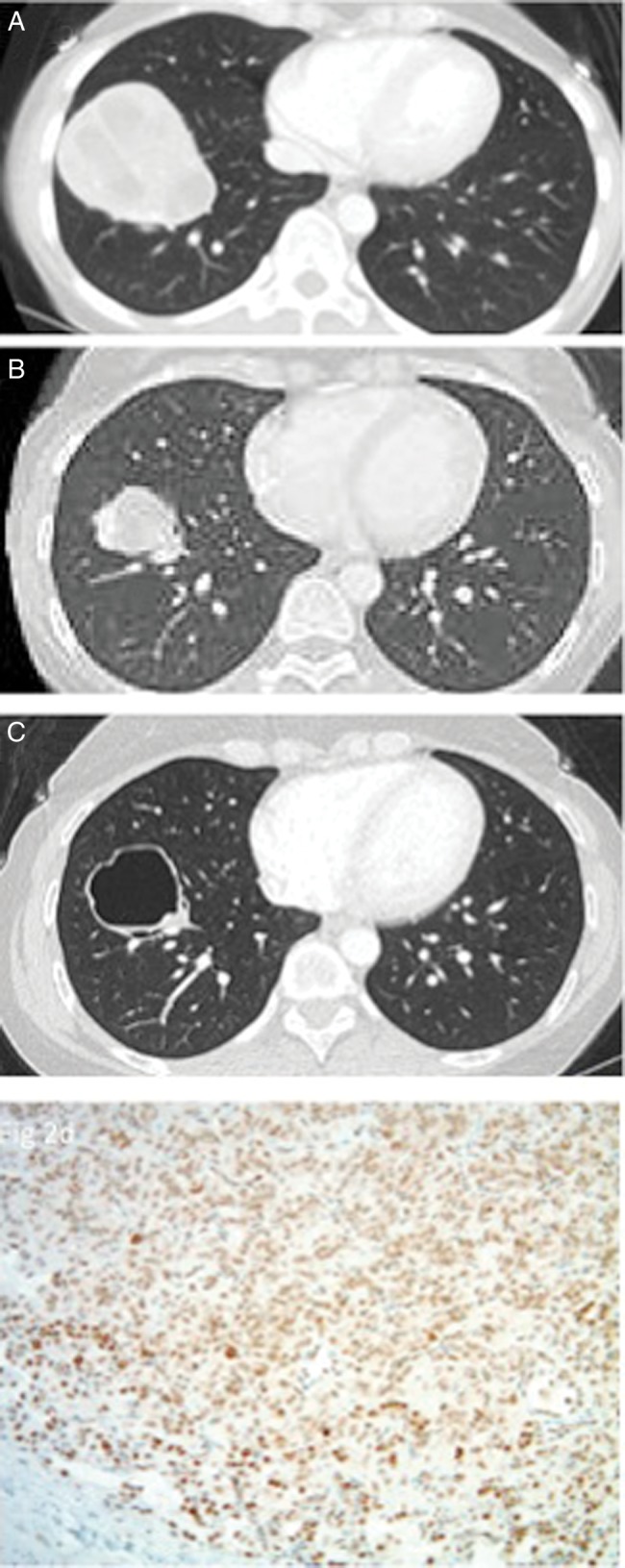
Partial response and cystic change in uterine leiomyosarcoma patient. Lung metastasis at baseline (A) demonstrated shrinkage after six cycles of combination chemotherapy and two cycles of single-agent cixitumumab (B). Lesion underwent cystic degeneration after an additional seven cycles of single-agent cixitumumab therapy (C). High expression of IGF-1R by immunohistochemistry in resected tumor specimen (D).
correlative assessments
immunohistochemistry
Eleven tissue samples were available for analysis of IGF-IR and phospho-IGF-IR (pIGF-IR). Ten of 11 samples showed overexpression of IGF-IR and all demonstrated overexpression of pIGF-IR. No clear correlation was detected between expression of IGF-IR and tumor response or PFS.
SNP analysis
Twenty serum samples were available for evaluation of SNPs in candidate genes previously described as either conferring protection or increased risk from anthracycline-related cardiac toxicity [13, 14]. For protective variant SNPs in three candidate genes (FMO2, SLC10A2, and SLC28A3), only one patient demonstrated homozygosity with a single protective variant. For risk variant SNPs (CBR1, CBR3, HNMT, UGT1A6, ABCB4, ABCC1), homozygosity was seen in several patients with cardiotoxicity as well as in patients without (supplementary Table S3, available at Annals of Oncology online). Overall within the limits of this small analysis, there were no significant SNP findings in relation to cardiac toxicity or protection.
discussion
Although doxorubicin remains a first-line treatment for metastatic STS, it is a relatively unsatisfactory standard. Many attempts to improve on this standard with combination strategies have been made, but have only offered incremental improvements in response rate and increased toxicity with no proven overall survival benefit [15–18].
Most recently, doxorubicin ± ifosfamide (10 g/m2/cycle) yielded significant differences in response rates of 14% versus 26% and the median PFS of 4.6 versus 7.4 months, but non-significant differences in overall survival of 12.8 versus 14.3 months in single agent versus combination therapy, respectively [18]. In our study, the response rate was 19% with a median PFS of 5.3 months across dose levels. While some dramatic responses and prolonged PFS were seen, a randomized study is necessary to understand whether the addition of cixutumumab represented an improvement over doxorubicin alone.
Overall, studies of anti-IGF-1R therapy in sarcoma have yielded disappointing results. Cixitumumab in multiple subtypes of sarcoma showed only the potential of benefit in one histologic subtype, liposarcoma. Ewing's sarcoma has been evaluated in multiple settings with anti-IGF-1R therapy and has consistently shown activity in ∼10% of patients treated [19, 20]. While the benefit seen in some of these patients is impressive, without a biomarker predictive of benefit, development of anti-IGF-1R therapy in Ewing's has come to a halt.
In this study, we evaluated IGF-1R and pIGF-1R expression, which has been described potentially as predictive factors. Only a minority of tissue samples was available for this study, and the majority demonstrated increased expression. Despite this, there was limited response to anti-IGFR therapy plus doxorubicin, suggesting that overexpression of these kinases as detected by IHC were not predictive of response.
IGF-1 signaling plays a critical role in cardiac contractility and metabolism and appears to have a protective effect on the heart [21]. While the mechanism of doxorubicin-associated cardiac toxicity has not been fully elucidated, much data exist that heart failure is uncommon with doses of doxorubicin <300 mg/m2 [22]. Although damage likely occurs shortly after drug exposure, cardiac compensatory mechanisms usually prevent the presentation until years later. Given the frequency and early onset of the changes on MUGA scans, the cardiac toxicity seen in this study was suggestive as potentially increased with the use of cixitumumab when compared with doxorubicin alone.
In this study, the maximum tolerated dose was doxorubicin 75 mg/m2 on days 1–2 and cixitumumab 6 mg/kg IV on days 1, 8, and 15 of a 21 day cycle. This regimen was relatively well tolerated, and studies to better establish the efficacy of this combination would best be carried out in a randomized setting, particularly if a predictive biomarker of benefit to anti-IGF-1R-1 therapy is identified, with close attention to the potential for cardiotoxicity.
funding
This work was supported by the Cancer Therapeutics Evaluation Program of the National Cancer Institute at the National Institutes of Health (N01-CM-62001).
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
Special appreciation to the patients and families who participated, University of Michigan Clinical Trials Office, and University of Chicago Phase II Consortium.
references
- 1.Ryan CW, Desai J. The past, present, and future of cytotoxic chemotherapy and pathway-directed targeted agents for soft tissue sarcoma. Am Soc Clin Oncol Educ Book 2013; 2013: 386–393. [DOI] [PubMed] [Google Scholar]
- 2.Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer 2003; 107: 873–877. [DOI] [PubMed] [Google Scholar]
- 3.Bohula EA, Playford MP, Macaulay VM. Targeting the type 1 insulin-like growth factor receptor as anti-cancer treatment. Anticancer Drugs 2003; 14: 669–682. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Yee D. The therapeutic potential of agents targeting the type I insulin-like growth factor receptor. Expert Opin Investig Drugs 2004; 13: 1569–1577. [DOI] [PubMed] [Google Scholar]
- 5.Beech D, Pollock RE, Tsan R et al. . Epidermal growth factor receptor and insulin-like growth factor-I receptor expression and function in human soft-tissue sarcoma cells. Int J Oncol 1998; 12: 329–336. [DOI] [PubMed] [Google Scholar]
- 6.Mitsiades CS, Mitsiades NS, McMullan CJ et al. . Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell 2004; 5: 221–230. [DOI] [PubMed] [Google Scholar]
- 7.Roholl PJ, Skottner A, Prinsen I et al. . Expression of insulin-like growth factor 1 in sarcomas. Histopathology 1990; 16: 455–460. [DOI] [PubMed] [Google Scholar]
- 8.Scotlandi K, Maini C, Manara MC et al. . Effectiveness of insulin-like growth factor I receptor antisense strategy against Ewing's sarcoma cells. Cancer Gene Ther 2002; 9: 296–307. [DOI] [PubMed] [Google Scholar]
- 9.Schoffski P, Adkins D, Blay JY et al. . An open-label, phase 2 study evaluating the efficacy and safety of the anti-IGF-1R antibody cixutumumab in patients with previously treated advanced or metastatic soft-tissue sarcoma or Ewing family of tumours. Eur J Cancer 2013; 49: 3219–3228. [DOI] [PubMed] [Google Scholar]
- 10.Beech DJ, Perer E, Helms J et al. . Insulin-like growth factor-I receptor activation blocks doxorubicin cytotoxicity in sarcoma cells. Oncol Rep 2003; 10: 181–184. [PubMed] [Google Scholar]
- 11.Luk F, Yu Y, Walsh WR, Yang JL. IGF1R-targeted therapy and its enhancement of doxorubicin chemosensitivity in human osteosarcoma cell lines. Cancer Invest 2011; 29: 521–532. [DOI] [PubMed] [Google Scholar]
- 12.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 1999; 17: 1474–1481. [DOI] [PubMed] [Google Scholar]
- 13.Visscher H, Ross CJ, Rassekh SR et al. . Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol 2012; 30: 1422–1428. [DOI] [PubMed] [Google Scholar]
- 14.Wojnowski L, Kulle B, Schirmer M et al. . NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation 2005; 112: 3754–3762. [DOI] [PubMed] [Google Scholar]
- 15.Borden EC, Amato DA, Rosenbaum C et al. . Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J Clin Oncol 1987; 5: 840–850. [DOI] [PubMed] [Google Scholar]
- 16.Demetri GD, Elias AD. Results of single-agent and combination chemotherapy for advanced soft tissue sarcomas. Implications for decision making in the clinic. Hematol Oncol Clin North Am 1995; 9: 765–785. [PubMed] [Google Scholar]
- 17.Lorigan P, Verweij J, Papai Z et al. . Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 2007; 25: 3144–3150. [DOI] [PubMed] [Google Scholar]
- 18.Judson I, Verweij J, Gelderblom H et al. . Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol 2014; 15: 415–423. [DOI] [PubMed] [Google Scholar]
- 19.Pappo AS, Patel SR, Crowley J et al. . R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research through Collaboration study. J Clin Oncol 2011; 29: 4541–4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tap WD, Demetri G, Barnette P et al. . Phase II study of ganitumab, a fully human anti-type-1 insulin-like growth factor receptor antibody, in patients with metastatic Ewing family tumors or desmoplastic small round cell tumors. J Clin Oncol 2012; 30: 1849–1856. [DOI] [PubMed] [Google Scholar]
- 21.Troncoso R, Ibarra C, Vicencio JM et al. . New insights into IGF-1 signaling in the heart. Trends Endocrinol Metab 2014; 25: 128–137. [DOI] [PubMed] [Google Scholar]
- 22.Ades F, Zardavas D, Pinto AC et al. . Cardiotoxicity of systemic agents used in breast cancer. Breast 2014; 23: 317–328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


