Abstract
Biologists and chemists of the world have been attracted towards marine natural products for the last five decades. Approximately 16,000 marine natural products have been isolated from marine organisms which have been reported in approximately 6,800 publications, proving marine microorganisms to be a invaluable source for the production of novel antibiotic, anti tumor, and anti inflammatory agents. The marine fungi particularly those associated with marine alga, sponge, invertebrates, and sediments appear to be a rich source for secondary metabolites, possessing Antibiotic, antiviral, antifungal and antiyeast activities. Besides, a few growth stimulant properties which may be useful in studies on wound healing, carcinogenic properties, and in the study of cancers are reported. Recent investigations on marine filamentous fungi looking for biologically active secondary metabolites indicate the tremendous potential of them as a source of new medicines. The present study reviews about some important bioactive metabolites reported from marine fungal strains which are anti bacterial, anti tumour and anti inflammatory in action. It highlights the chemistry and biological activity of the major bioactive alkaloids, polyketides, terpenoids, isoprenoid and non-isoprenoid compounds, quinones, isolated from marine fungi.
Keywords: Marine fungi, bioactive metabolites, anti tumor, alkaloids, isoprenoids, polyketides
Background
Oceans have provided a complacent base for biological activities on earth. Many biological compounds with varying degrees of action, such as anti-tumor, anti-cancer, antiproliferative, cytotoxic as well as antibiotic properties have been isolated from marine sources. The marine environment is an unexplored source for isolation of new microbes (bacteria, fungi, actinomycetes, cyanobacteria and diatoms) that are potent producers of bioactive secondary metabolites. The bacteria and fungi from sea are reported to produce substances which affect central nervous system (CNS), respiratory system (RS), neuromuscular system (NMS), autonomic nervous system (ANS), cardiovascular system (CVS) and gastrointestinal system (GI). Marine secondary metabolites can easily impede other micro organisms [1]. Among marine microorganisms, particularly fungi have gained an important role as a source of biologically active secondary metabolites [2]. Marine fungi have proved to be a rich source of new biological natural products. Because of their characteristic properties with reference to temperature, nutrients, competition and salinity, they have developed specific secondary metabolic pathways compared with terrestrial fungi [3]. Marine filamentous fungi have proved to possess tremendous potential as a source of new medicines even at low concentrations of their secondary metabolites, as some recent studies prove too [4]. Approximately 56 species of Facultative marine fungi had been described until 1999 [5]. Between 2000 and 2005, approximately 100 marine fungal metabolites were described and between 2006 and 2010, a total of 690 natural products were reported as being isolated from fungi in marine habitats [6]. Major anti – bacterial compounds isolated from marine fungi have been listed as under the table tiled „Major Antibacterial compounds from marine derived fungi׳ [7] Table 1 (see supplementary material).
Main Bioactivity Groups of the Novel Compounds
According to a study by Hu et al., (2015) [8], out of 4196 bioactive compounds isolated from marine organisms, 2225 had anticancer activity (56% of the total bioactive compounds). This was followed by antibacterial activity of 521 compounds (13%). The other five groups were represented 14%, including antifungus, pest resistance and anti-virus. The remaining 16% of the bioactive compounds could not be classified into the above bioactive groups and were termed the “other” activity group.
Bioactive metabolites from marine derived fungi
Marine fungal strains are potent producers of polyketide derived alkaloids, terpenes, peptides and mixed biosynthesis compounds which represent chemical groups of secondary metabolites produced by fungi. Miriam et al. (2012) [9] isolated marine fungal strains, they yielded several bioactive secondary metabolites among which are (E)-4-methoxy-5-(3-methoxybut- 1-enyl)-6- methyl-2H-pyran-2-one, a new metabolite was isolated from the Penicillium paxilli (strain MaG) K. Norliquexanthone, also known as 1,3,6- trihydroxy-8-methyl- 9H-xanthen-9-one, was isolated from the fungus P. raistrikii obtained from the sponge Axinella corrugate. Two new indole alkaloids, (2-3, 3-dimethylprop-1-ene)-costaclavine and (2-3, 3- dimethylprop-1- ene)-epicosta-clavine, together with the known compounds costaclavine, fumgaclavine A and C, were isolated from the marine fungus Aspergillus fumigatus. An essential antibacterial compound, xanthocillin X(3) (Figure 1), and 14 other known compounds comprising three steroids, two ceramides, six aromatic compounds and three alkaloids were isolated from Penicillium commune SD-118, a fungus obtained from a deep-sea sediment sample [10].
Figure 1.
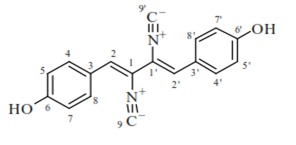
Xanthocillin
Chrodrimanin B, along with five new phenolic bisabolane type sesquiterpenoids were isolated from fermentation broth of the marine fungus Aspergillus sp. isolated from South China Sea by Mei Yan (2011) [11]. Antibacterial and cytotoxic activities were reported from marine isolates of the fungal strain Myceliophthora lutea Costantin by Smetanina et al. (2011) [12]. Antimicrobial activity of marine fungi isolate was evaluated against six human pathogenic bacteria, they are Enterobacter aerogenes, Escherichia coli, Proteus mirabilis, Bacillus subtilis, Staphylococcus aureus and Klebsiella pneumoniae, with different solvents like n-butanol, chloroform, water and acetone. Anticancer activity of the selective fungi Aspergillus protuberus SP1 extracts were tested and they showed activity against Hep 2 cells using MTT assay [13]. Alkaloids with anticancer activity were isolated from Penicillium citrinum, Fusarium sp., Apiospora montagnei and polyketide asco-sali pyrrolidinone-A40 isolated from the Ascochyta Sali-corniae having potential antimalarial activity [14]. The fungal genus Aspergillus has been reported to produce a considerable number of cytotoxic compounds as well as other bioactive compounds (Figure 2).
Figure 2.
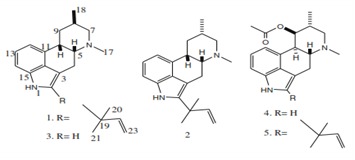
Metabolites from Aspergillus fumigatus
Anti tumour compounds from marine derived fungi
In the year 2005, the pharmacology of Verrucarin A, a chemical compound isolated from the culture broth of the Palauan marine fungus Myrothecium roridum was described [15]. Verrucarin A significantly inhibited interleukin-8 production from human promyelocytic leukemia cells, by a mechanism that involved inhibition of the activation of the mitogen activated kinases c-JUN and p38. A new chromone derivative, (2-hydroxymethyl)-8-methoxy-3-methyl-4H-chromen-4-one chromanone A44 was isolated from a marine fungal isolate of Penicillium sp. which acts as an active tumour anti-initiating via modulation of carcinogen metabolizing enzymes and protection from DNA damage [2]. Penicillium sp.( strain DG M3) 6׳C, isolated from the ascidian Didemnum granulatum, was reported to yield 13-desoxy-phomenone [4]. Penicillium paxilli strain (Ma G) K, obtained from the sponge M. angulosa gave an extract which was cytotoxic against MDA-MB435 human mammalian cancer cells (HCT8 human colon), CNS 295 central nervous system cancer cells and HL60 leukemia cells [4]. The marine fungus Trichoderma virens, originated from ascidian Didemnum molle has been used for the isolation of Trichodermamides A and B. In vitro cytotoxicity against human colon carcinoma has been reported from Trichodermamide B (Figure 3) [16].
Figure 3.
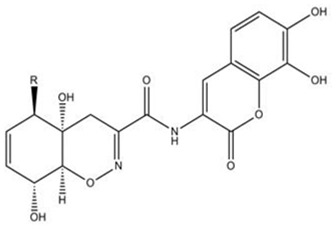
Trichodermamide A R=OH, Trichodermamide BR=Cl
Leptosins belong to a series of epipolysulfanyldioxopiperazine class of chemical compounds produced mainly by various strains of Leptosphaeria sp. that have been originally isolated from the marine alga Sargassum tortile. These secondary metabolites have similar structures (indole derivatives) and exhibit in vivo cytotoxic activity, to broad spectrum of cell lines. The anticancer potential of leptosins M, M1 and N were examined using the murine P-388 lymphocytic leukemia cell line and a disease-oriented panel of 39 human cancer cell lines (HCC panel) (Figure 4) [17].
Figure 4.
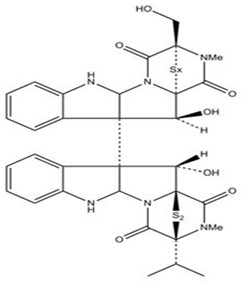
Leptosin A x=4, Leptosin B x=3, Leptosin C x=2
Zygosporamide is a cyclic depsipeptide isolated from the seawater-based fermentation broth of a marine-derived fungus Zygosporium masonii, this compound was tested for cytotoxicity against 60 cancer cell lines in NCI (Figure 5).
Figure 5.
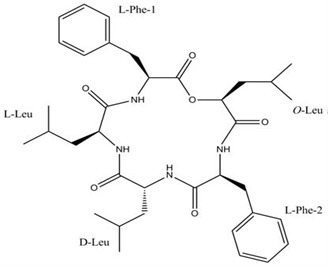
Zygosporamide
Cephalimysin A belongs to a family of natural products containing a unique 1-oxa-7-azaspiro non-2-ene-4,6- dione core structure, isolated from a strain of Aspergillus fumigatus OPUST106B- 5 originally separated from the marine fish Mugil cephalus. Cephalimysin A exhibited significant cytotoxic activity against the murine P-388 leukemia cell line and the human HL- 60 leukemia cell line (Figure 6) [18].
Figure 6.
Cephalimycin
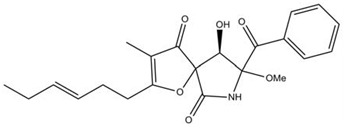
The diterpene conidiogenone C was isolated from Penicillium sp. Several more members of conidiogenone were isolated from the culture extracts of Penicillium chrysogenum QEN-24S, an endophytic fungus derived from an unidentified marine red algal species of the genus Laurencia. This compound has shown potent cytotoxic effect to HL-60 [19]. Fusaranthraquinone, fusarnaphthoquinones A-C and fusarone were obtained from Fusarium spp. (sea fan Annella sp., Koh Hin Ran Pet, Suratthani Province, Thailand) along with austrocortirubin, a metabolite of Australian Cortinarius toadstools (C. basirubescens and C. persplendidus) [20] and a known Fusarium metabolite, the naphtoquinone anhydrofusarubin (Figure 7 a,b). Austrocortirubin was selectively toxic to MCF-7 cells (breast) while anhydrofusarubin displayed potent and selective cytotoxicity. Anhydrofusarubin also showed cytotoxic effects on human cancer cell lines HCT-8 (colon), MDA-MB-435 (melanoma) and SF-295 (brain).
Figure 7.
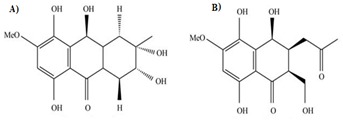
Fusaranthraquinone; (b) Fusarnapthoquinone A
Cryptosphaerolide is an ester-substituted sesquiterpenoid related to the eremophilane class, isolated from Cryptosphaeria sp. (unidentified ascidian, Bahamas). It is an inhibitor of the protein Mcl-1 (a cancer drug target implicated in apoptosis) in the Mcl-1/Bak fluorescence resonance energy transfer assay (FRET). Cryptosphaerolide is also significantly cytotoxic against HCT-116 cells (Figure 8) ([21, 22]).
Figure 8.
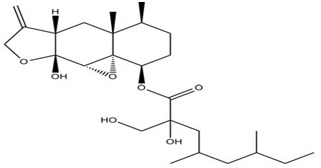
Cryptosphearolide
Paeciloxocins A and B, (2-(1-hydroxy-3-methylbutyl) and 2-(1- acetoxy-3-methylbutyl)-11-hydroxy-9-methyl- 1-methoxy- 5H,7H-dibenzo[b, g]-1,5-dioxocin-5-ones), are two new aromatic metabolites isolated from the mangrove fungus Paecilomyces sp. collected from the Taiwan Strait. Both paeciloxocin A and paeciloxocin B exhibited cytotoxicity against the HepG2 cell line (liver) (Figure 9) [23].
Figure 9.
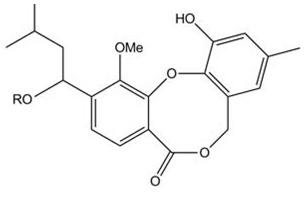
Paeciloxocin A R=H, Paeciloxocin B R=Ac
The monomeric C-methylated hexaketide (2E,4E)-1- (2,6- dihydroxy-3,5-dimethyl-phenyl)hexa-2,4-dien-1-one, a marine secondary metabolite with strong activity to HeLa (cervical cancer) and SW-620 cells has been reported from Penicillium sp.. The anthraquinone SZ-685C was isolated from the mangrove endophytic fungus Halorosellinia sp. (South China Sea). It suppressed the proliferation of six cancer cell lines derived from human breast cancer, prostate cancer, glioma and hepatoma [24].
Recent research
In a recent study by Henrik Harms (2015) [25], isolation, identification and biological evaluation of secondary metabolites from the marine-derived fungus Dichotomomyces cejpii proved that it serves as powerful producer of compounds exhibiting activities toward cannabinoid and type II nuclear receptors and possesses Aβ-42 lowering activity potentially useful to treat Alzheimer dementia. In a research by Didha Andidni Putria (2015) [26], soft coral Sinularia sp. as source of marine fungal symbionts from Panjang island of the North Java Sea was collected. A total of 15 fungal symbionts that were capable of growth inhibition of pathogenic fungi were successfully isolated and screened using overlay method against pathogenic fungi Candida albicans and Aspergillus. Phytochemical tests showed that phenolic, triterpenoid and flavanoid compounds were detected within fungal extract. Molecular identification based on 18S rRNA gene showed that the active fungi was closely related to Aspergillus unguis. Arumugam et al., 2015 [27] successfully isolated a piezotolerant fungus Nigrospora sp. NIOT from deep sea environment and cultured it under submerged fermentation. The secondary metabolites produced from this organism showed potent antimicrobial and anticancer activities with immediate application to cosmetics and pharmaceutical industries. According to a study by El- Hady et al., 2015 [28], the fungus Aspergillus unguis RSPG_204 associated to the marine Sponge (Agelas sp., Red Sea , Egypt) was investigated. The supernatant and mycelial extracts [culture static (clt.st.)] had the highest free radical scavenging activity against superoxide anion radical. The fungus Aspergillus unguis RSPG_204 2ry metabolites showed significant acetyl cholinesterase and high α - glucosidase inhibition, beside its high antioxidant activities. For the first time it was evidenced that these 2ry metabolites in further in-vivo studies could play an important role as acetyl cholinesterase and α -glucosidase inhibitors, besides their antioxidant activities. In a study by Virupakshaiah (2014) [29], seven secondary metabolites obtained from marine derived fungi were taken that were experimentally proven to be cytotoxic against various human/cancer cell lines. Using Pharmmapper - a web server for drug target identification, the potential anti tumor targets of these compounds were found and their cancer relevance was determined. Seven new alkaloids including tryptoquivaline K (1) and fumiquinazolines K–P (2–7), bearing a rare 1-aminocyclopropane-1-carboxylic acid residue, together with six known compounds, were isolated from the fungus Aspergillus sp. obtained from the Mediterranean sponge Tethya aurantium which exhibited pronounced cytotoxicity against the mouse lymphoma cell line, but only moderate activity was observed against human ovarian cancer and human Philadelphia chromosome-positive chronic myelogenous leukemia cell lines [30].
Conclusion
Marine environments present an invaluable source of new natural products that may hold important leads for future drug discovery and development [31]. Natural products of marinederived fungi are expected to inspire medicinal chemists in their search for better antitumor agents than existing ones, bearing in mind that simplification and computer-aided design may be particularly useful tools. Study on the bioactivity of marine natural products should be emphasized to develop new natural compounds from marine organisms. The novel structures and metabolism (biosynthesis) pathways fascinate chemists and the biological activities of these compounds are interesting to the drug development community [32].
Supplementary material
Footnotes
Citation:Hasan et al, Bioinformation 11(4): 176-181 (2015)
References
- 1.Jeffrey JC, et al. PLoS ONE. 2011;6:9. [Google Scholar]
- 2.Amira MGE, et al. Environ Toxicol Pharmacol. 2009;28:317. doi: 10.1016/j.etap.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Liberra K, Lindequist U. Pharmazie. 1995;50:583. [PubMed] [Google Scholar]
- 4.Swathi J, et al. Mintage J Pharmaceut Med Sci. 2013;2:45. [Google Scholar]
- 5.Aline MVM, et al. Quim Nova. 2008;31:1099. [Google Scholar]
- 6.Katia D, et al. Trends Analy Chem. 2012 [Google Scholar]
- 7.Punyasloke B, et al. J Ind Microbiol Biotechnol. 2006;33:325. [Google Scholar]
- 8.Yiwen Hu, et al. Mar Drugs. 2015;13:202. doi: 10.3390/md13010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miriam H, et al. Brazilian J Pharmacognosy. 2012 [Google Scholar]
- 10.Dahai Z, et al. J Nat Med. 2012;66:222. [Google Scholar]
- 11.Mei-Yan W, et al. Chem Nat Compound. 2011;47:4. [Google Scholar]
- 12.Smetanina OF, et al. Chem Nat Compounds. 2011;47:3. [Google Scholar]
- 13.Mathan S, et al. Chin J Nat Med. 2011;94:286. [Google Scholar]
- 14.Joel Q, et al. Adv Marine Genomics. 2010 [Google Scholar]
- 15.Oda T, et al. Marine Drugs. 2005;3:64. [Google Scholar]
- 16.Garo E, et al. J Nat Prod. 2003;66:423. doi: 10.1021/np0204390. [DOI] [PubMed] [Google Scholar]
- 17.Yanagihara M, et al. Cancer Sci. 2005;96:816. doi: 10.1111/j.1349-7006.2005.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y, et al. Eur J Org Chem. 2013;5:894. [Google Scholar]
- 19.Gao SS, et al. Chem Biodiversity. 2011;8:1748. doi: 10.1002/cbdv.201000378. [DOI] [PubMed] [Google Scholar]
- 20.Beattie KD, et al. Phytochemistry. 2010;71:948. doi: 10.1016/j.phytochem.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Oh H, et al. J Nat Prod. 2010;73:998. doi: 10.1021/np1000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blunt JW, et al. Nat Prod Rep. 2012;29:144. doi: 10.1039/c2np00090c. [DOI] [PubMed] [Google Scholar]
- 23.Wen L, et al. Russ Chem Bull. 2010;59:1656. [Google Scholar]
- 24.Liu S, et al. Chem Nat Comp. 2010;46:116. [Google Scholar]
- 25.Harms Henrik, et al. Dissertation. 2015 [Google Scholar]
- 26.Didha AP, et al. Procedia Environmental Sciences. 2015;23:351. doi: 10.1016/j.proenv.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arumugam GK, et al. J of Appl Microbiology. 2015;118:99. doi: 10.1111/jam.12693. [DOI] [PubMed] [Google Scholar]
- 28.Abd FK, et al. Int J Pharm Sci Rev Res. 2015;30:272. [Google Scholar]
- 29.Virupakshaiah DBM, et al. Journal of Advanced Bioinformatics Applications and Research. 2014;5:78. [Google Scholar]
- 30.Zhou Yaming, et al. European Journal of Organic Chemistry. 2013 [Google Scholar]
- 31.Montaser R, Luesch H. Future Med Chem. 2011;3:1475. doi: 10.4155/fmc.11.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molinski TF, et al. Nat Rev Drug Discov. 2009;8:69. doi: 10.1038/nrd2487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


