Abstract
Recent studies have shown diagnostic and prognostic values of circulating tumor cells (CTCs) and disseminated tumor cells (DTCs) in various cancers, including ovarian cancer. We aimed to evaluate the association of CTCs and/or DTCs with the clinical outcomes of ovarian cancer. Clinical studies of CTCs/DTCs of ovarian cancer were included for systematic review and meta-analysis. A total of 236 studies were screened but only 16 qualified studies with 1623 subjects were included. Odds ratio (OR) showed CTCs/DTCs were not significantly associated with serous carcinoma (OR = 0.71 [0.49, 1.05]), lymph node metastasis (OR 1.14 [0.67, 1.93]), and residual disease (OR 1.45 [0.90, 2.34]); but significantly associated with advanced tumor staging (OR = 1.90 [1.02, 3.56]). The overall pooled hazard ratio (HR) of CTCs/DTCs on OS and PFS/DFS was 1.94 [1.56– 2.40] and 1.99 [1.59–2.50], respectively. Subgroup analyses revealed that CTCs were significantly associated OS (HR 1.97 [1.50-2.58]) and PFS/DFS (HR 2.52 [1.83-3.48]), while DTCs was significantly associated OS (HR 1.89 [1.33, 2.68]) and PFS/DFS (HR 1.60 [1.17, 2.19]). Meta-analysis showed strong relationship of CTCs/DTCs with advanced staging, treatment response and poor prognosis in patients with ovarian cancer.
Electronic supplementary material
The online version of this article (doi:10.1186/s13048-015-0168-9) contains supplementary material, which is available to authorized users.
Keywords: Ovarian cancer, Circulating tumor cells, Disseminated tumor cells, Prognosis
Introduction
Ovarian cancer is the most frequent cause of death amongst gynecological cancers worldwide. Majority of cases diagnosed in late stage of the disease and resulted in poor survival [1]. The five-year survival rate of patients with ovarian cancer is only around 30 % in Stage III or IV [2]. The reasons of delayed diagnosis are partly due to lack of sensitive signs and symptoms and effective screening methods [3]. Although survival has been improved with the use of cyto-reduction surgery along with platinum- and/or taxane-based chemotherapy, nearly 80 % eventually relapse within 5 years [4]. Therefore, methods that help detection of ovarian cancer in early stage and monitoring of tumor progression have great potential to improve survival of the patients.
It was considered that ovarian cancer spreads primarily through direct dissemination in the abdominal cavity. While the presence of disseminated tumor cells (DTCs) in bone marrow of patients with ovarian cancer have been reported [5, 6]. However, bone marrow sampling is rather an invasive procedure, which is not widely accepted in the clincial management. In recent years, focus has been shifted to the detection of circulating tumor cells (CTCs) in peripheral blood. CTCs are tumor cells release from the primary tumor and then circulate through the bloodstream, resulting in spreading to different organs and subsequent outgrowth of the tumor cells in new microenvironment. These CTCs thereby have the potential to contribute to the development of local and systematic relapses [7]. Either DTCs or CTCs have potential to predict prognosis and to monitor treatment efficacy in cancer patients. Presence of CTCs has been reported in several solid tumors, including breast [8], colorectal [9], lung [10], kidney [11], esophageal [12], liver [13], prostate [14], and pancreatic cancers [15, 16]. Studies of CTCs/DTCs in ovarian cancer patients had been investigated, most of them demonstrated that CTC or DTC is associated with poor clinical outcome [17–22]. However, other studies failed to show the positive correlation [5, 23, 24] and even demonstrated negative association in terms of progression free survival/disease free survival (PFS/DFS) and overall survival (OS) [25, 26]. The prognosis value of CTCs/DTCs in ovarian cancer remains controversial. A recent systematic review of CTCs and DTCs in ovarian cancer concluded the association of CTCs and DTCs with adverse clincopathological characteristics and poor clinical outcomes [27], but no appropriate statistics and detailed analysis were provided.
The objective of this study was to conduct a meta-analysis of published clinical studies of CTCs/DTCs in ovarian cancer and to investigate the association of CTCs/DTCs with clinical outcomes.
Methods
An independent systematic review of the literature across PubMed and EMBASE database was conducted on April 27, 2015. The search strategy included keywords such as “ovarian cancer”, “ovarian carcinoma”, “circulating tumor cell (s)”, “disseminated tumor cell (s)”, and “prognos*”. Only studies published in peer reviewed journals were included, data from letters and conference abstracts were not included. The study selection process is shown in Fig. 1 and search strategies and results are provided in Additional file 1.
Fig. 1.
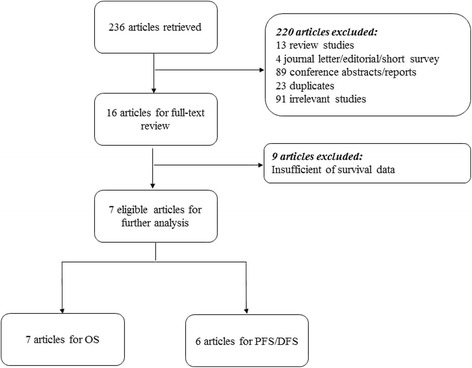
Flow diagram of study inclusion decisions
We recorded the following information from each eligible study, including author’s name, publication year, number of participants, sampling time, methods and results of CTCs/DTCs detection, and OS and/or PFS/DFS. We also collected clinopathological parameters, including histology, lymph node metastasis, cancer stage, residual diseases and treatment response. Histological types of the ovarian cancer were classified mainly as serous carcinoma or non-serous carcinoma. Lymph node metastasis was confirmed by pathological examination in the lymph nodes collected during cyto-reduction surgery. According to FIGO staging of ovarian cancer, Stage I and Stage II were combined and Stage III and Stage IV were combined. Complete resection was referred to no residual disease for no residual tumor, minimal residual disease for residual tumor in 1 cm or less, and gross residual disease for residual tumor in greater than 1 cm [28, 29]. Treatment response was classified as platinum-sensitive as defined when the patients with platinum-free interval of ≥ 6 months or platinum-resistant disease as defined when the patients relapse ≥ 6 months from the end of first line platinum-base therapy [30].
Inclusion criteria included: 1) clinical studies measured CTCs/DTCs, regardless of randomized or case-controlled studies; prospective or retrospective studies; and detection methods; 2) study outcomes provided clinical and pathological information; and 3) studies provided information of survival outcomes such as OS and PFS/DFS. Studies were excluded based on: 1) laboratory studies without clinical outcomes; 2) review, editorials, and commentary articles; 3) no survival data or insufficient data to be extracted. Two reviewers independently screened titles and abstracts of the studies for inclusion and then retrieved the full text for details data extraction.
Meta-analyses were conducted according to the PRISMA and MOOSE Checklist and the quality of the included studies was assessed with the Newcastle-Ottawa Scale (NOS) for cohort studies [31]. (Additional file 2 and Additional file 3). We examined the association between CTCs/DTCs and clinopathological outcomes. Odds ratio (OR) was used as the measure of index to describe the association. Survival analysis for natural logarithm of HR (lnHR) and standard error (SE) were calculated. If these statistical variables were not explicitly provided in studies, lnHR, SE and p values will be calculated from the available numerical data and Kaplan-Meier survival curves according to Tierney et al. [32]. Fixed or random-effects models will be employed to calculate the pooled hazards ratio (HR) with 95 % confidence intervals (CIs) for survival. Heterogeneity among studies was conducted by Cochran-Mantel-Haenszel test with the Cochran’s Q test and P values. When P value less than 0.05, a random-effects model was used. Otherwise, a fixed-effects model was presented. Subgroup analyses of sample types and detection method were performed. Publication bias was assessed using funnel plot, then further examined by Begg and Egger’s test [33]. All statistical analyses were conducted using the software R/metafor version 2.14.0. P values less than 0.05 were considered statistically significant.
Results
236 records were identified from the literature search. The selection processes are summarized in Fig. 1. After screening of titles and abstracts, 220 studies were excluded. 91 were irrelevant, 13 were review articles, 4 were letter/editorial/survey articles, 89 were conference abstracts/reports, and 23 were duplicated publications. Finally, 16 studies met the inclusion criteria for data extraction. We recorded the following information of each eligible study: author’s names, year of publication, number of patients analyzed, sampling timing, CTCs and/or DTCs studied, detection method of CTCs/DTCs, markers used for the detections, definition of positive CTCs/DTCs and survival data, and results of the studies. Details of the included studies are summarized in Table 1.
Table 1.
Main characteristics of studies
| Author | No. of Patients | Sampling time | CTCs or DTCs | Detection methods | Markers | Definition of positive | Outcome measures | Results |
|---|---|---|---|---|---|---|---|---|
| [17] | 80 | Pre-therapy | CTC | RT-PCR | MAGE-As | ≥1 tumor-associated transcript over expressed | OS & PFS/DFS | Positive for PFS/DFS and OS |
| [18] | 129 | Pre-therapy | CTC | CAM-initiated CTC enrichment or identification | EpCAM, CD45 clone 5B1 and CD66b | iCTCs ≥ 5 | OS & PFS/DFS | Positive for PFS/DFS and OS |
| [19] | 143 | Pre-therapy | CTC | RT-PCR | EpCAM, MUC1,or MUC16 | ≥1 tumor-associated transcript over expressed | OS & PFS/DFS | Positive for PFS/DFS and OS |
| [35] | 216 | Pre-therapy and post-therapy | CTC | RT-PCR | PPIC, GPX8, CDH3, TUSC3, COL3A1, LAMB1, MAM, ESRP2, AGR2, BAIAP2L1, TFF1, EpCAM | ≥1 tumor-associated transcript over expressed | OS & PFS/DFS | Negative pre-therapy but positive for post-therapy |
| [34] | 216 | Pre-therapy | CTC | CellSearch (IHC) | EpCAM | ≥2 cell stained | OS & PFS/DFS | Positive for PFS/DFS; negative for OS |
| [26] | 122 | Pre-therapy and/or post-therapy | CTC | RT-PCR | EpCAM, MUC-1, HER2 A45-B/B3 (BM) | ≥1 tumor-associated transcript over expresse ≥1 CK cell positive (BM) | OS & PFS/DFS | CTC: positive for OS but negative for PFS/DFS; DTC: negative for PFS/DFS and OS |
| [20] | 90 | Pre-therapy | DTC | IHC | A45-B/B3 | ≥1 cell positive | OS & PFS/DFS | Positive for PFS/DFS and OS |
| [37] | 112 | Pre-therapy | DTC | IHC | A45-B/B3 | ≥1 CK cell positive | OS & PFS/DFS | Positive for PFS/DFS but negative for OS |
| [21] | 62 | Pre-therapy and post-therapy | DTC | IHC (Epimet® kit) | A45-B/B3 | ≥1 CK cell positive | OS & PFS/DFS | Positive for PFS/DFS and OS |
| [22] | 69 | Pre-therapy | DTC | IHC | A45-B/B3 | ≥1 CK cell positive | PFS/DFS | Positive for PFS/DFS |
| [24] | 90 | Pre-therapy | CTC/DTC | IHC | MOC-31 | Presence of ≥2 rosettes (≥5 beads bound to a cell) | OS & PFS/DFS | Negative for PFS/DFS and OS |
| [5] | 59 | Pre-therapy | CTC | IHC | CK8 and 18 TFS-2, CK7, CK20 EGFR | ≥1 cell positive | OS & PFS/DFS | Negative for PFS/DFS and OS |
| [25] | 66 | Pre-therapy | CTC | Cell invasion assay | EpCAM | ≥1 cell positive | OS & PFS/DFS | Positive for PFS/DFS; Negative for OS |
| [23] | 43 | Pre-therapy and intra-therapy | CTC | CellSearch (IHC) | EpCAM | ≥1 cell positive | OS & PFS/DFS | Negative for PFS/DFS and OS |
| [6] | 57 | Pre-therapy and intra-therapy | DTC | IHC (Epimet® kit) | A45-B/B3 EpCAM | ≥1 cell positive | PFS/DFS | Positive for PFS/DFS |
| [38] | 69 | Pre-therapy | DTC | IHC | A45-B/B3 | ≥1 CK cell positive | PFS/DFS | Negative for PFS/DFS |
CTCs Circulating tumor cells; DTCs Disseminated tumor cells; RT-PCR Reverse transcription-polymerase chain reaction; IHC Immunocytochemistry; MAGE-A Melanoma-associated antigens A; PPIC Peptidylprolyl isomerase C (cyclophilin C); GPX8 Glutathione peroxidase 8; CDH3 Cadherin-3; TUSC3 Tumor suppressor candidate 3; COL3A1 Collagen, Type III, alpha 1; LAMB1 Laminin subunit beta-1; MAM Mammaglobin A; ESRP2 Epithelial splicing regulatory protein 2; AGR2 Anterior gradient protein 2 homolog; BAIAP2L1 Brain-specific angiogenesis inhibitor 1-associated protein 2-like protein 1; TFF1 Trefoil factor 1; EpCAM Epithelial cell adhesion molecule; MUC-1 Mucin 1; MUC-16 Mucin 16; HER2 Human growth factor receptor 2; A45-B/B3 Pan-cytokeratin antibody (CK 8, 18, 19); MOC-31 Epithelial glycoprotein 2 mouse monoclonal antibody; EGFR Epithelial growth factor receptor; iCTC Invasive circulating tumor cells; CK Cytokeratin; CAM Cell adhesion matrix; BM Bone marrow; OS Overall survival; PFS/DFS Progression-free survival/disease-free survival
In total, there were 1623 patients, and the sample size of each study was ranged from 43 to 216. Most studies were published between 2002 and 2014, 4 studies from US, 11 studies from Europe and 1 study from Asia. There were 6 studies including 459 patients recorded the prognostic values of DTCs detected in bone marrow and 10 studies including 1164 patients recorded the prognostic values of CTCs detected in peripheral blood. Seven out of 16 studies had positive results of CTC/DTC effects on survival. Four out of 16 had negative results, remaining 5 studies had controversial conclusions.
Associations of CTCs/DTCs with clinicopathological parameters were analyzed (Table 2). Six studies [5, 17, 25, 34–36] with defined pathological diagnosis of serous carcinoma or non-serous carcinoma were included to study the relationship between CTCs/DTCs and histological types of the ovarian cancer. The estimated pooled OR was 0.72 (95 % CI: 0.48–1.06; Z = −1.71; P = 0.088 fixed-effect), demonstrating that CTCs were not associated with the tumour histology. The heterogeneity among studies was not significant (Q = 5.24, p = 0.387). Three studies [17, 35, 37] assessing metastasis in lymph node or not were included to study the relationship between CTCs/DTCs and lymph node metastasis. Of the results showed that CTCs/DTCs were not significantly associated with lymph node metastasis in ovarian cancer patients (pooled OR = 1.14; 95 % CI: 0.67–1.93; Z = 0.481; P = 0.630 fixed-effect). The heterogeneity among studies was not significant (Q = 3.82, p = 0.148). In six studies [5, 17, 25, 35–37], there was significant association between CTC and advanced tumor stage (Stage III-IV, pooled OR = 1.90; 95 % CI: 1.02–3.56; Z = 2.02; P = 0.044 fixed-effect), indicating that CTCs/DTCs were significantly increased with the risk of disease progression in ovarian cancer. The heterogeneity among studies was not significant (Q = 10.84, p = 0.055). Three studies [17, 25, 35], were included to study the relationship between CTCs/DTCs and debulking surgery, CTCs were not significantly associated with the optimal or suboptimal surgery in ovarian cancer patients (pooled OR = 1.45; 95 % CI: 0.90–2.34; Z = 1.53; P = 0.126 fixed-effect). However, one study [35] showed that DTCs significant association with residual diseases (OR = 2.31, CI: 1.19-4.50). The heterogeneity among studies was not significant (Q = 3.71, p = 0.157). Two studies [34, 35] assessing platinum sensitive or resistant were included to study the relationship between CTCs and treatment response, the result showed that CTCs were significantly associated with treatment response in ovarian cancer patients (pooled OR = 0.55; 95 % CI: 0.34–0.90; Z = −2.37; P = 0.017 fixed-effect). The heterogeneity among studies was not significant (Q = 0.930, p = 1.0000).
Table 2.
Association of CTCs/DTCs and clinicopathological datasets
| No. of Studies (sample size) | OR (95 % CI) | Model | OR, p value | P-H | Begg’s test, p value | |
|---|---|---|---|---|---|---|
| Serous carcinoma vs. Non-serous carcinoma | 6 (789) | 0.71 [0.49,1.05] | FE | 0.0878 | 0.3876 | 0.719 |
| FIGO stage III-IV vs. FIGO stage I-II | 6 (687) | 1.90 [1.02, 3.56] | FE | 0.0438 | 0.0546 | 0.1361 |
| Lymph node metastasis vs. No lymph node metastasis | 3 (404) | 1.14 [0.67, 1.93] | FE | 0.6304 | 0.1484 | 1.0000 |
| Suboptimal debulking vs. optimal debulking | 2 (141) | 0.78 [0.32, 1.88] | FE | 0.5751 | 0.3232 | 1.0000 |
| Platinum sensitive vs. Platinum resistant | 2 (508) | 0.55 [0.34, 0.90] | FE | 0.0178 | 0.9298 | 1.0000 |
FE Fixed-Effects; P-H P value –Heterogeneity
OS was analyzed in 7 studies [17–19, 22, 34, 35, 37] including 965 patients in total. Since the heterogeneity across the studies was larger than 0.05 (Q = 3.3, P = 0.770), the estimated pooled HR for studies was calculated using a fixed effect model. The pooled HR showed that CTCs/DTCs were significantly associated with OS (HR = 1.94; 95 % CI: 1.56– 2.40; Z = 6.02; P < 0.0001 fixed effects), indicating CTCs/DTCs significantly increased the risk of overall mortality in ovarian cancer (Fig. 2).
Fig. 2.
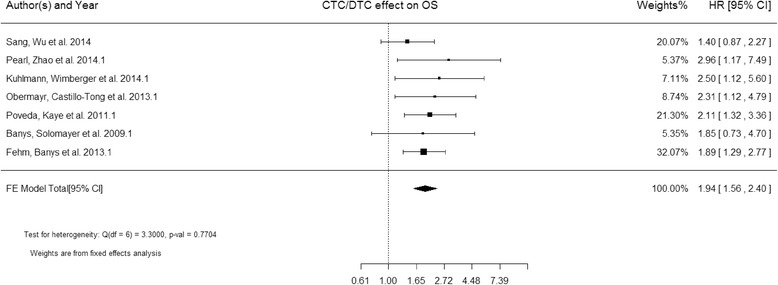
Forest plot of HRs for OS from 7 studies (965 patients)
PFS/DFS were analyzed in 6 studies [18, 19, 22, 34, 35, 37] including 885 patients in total. Because the heterogeneity across the studies was also larger than 0.05 (Q = 9.11, P = 0.105), the estimated pooled HR for studies was calculated using a fixed effect model. The estimated pooled HR showed that CTCs/DTCs was also significantly associated with PFS/DFS (HR = 1.99; 95 % CI: 1.59–2.50; Z = 6.01; P < 0.0001 fixed effects), indicating CTCs/DTCs significantly increased with the risk of low survival in ovarian cancers (Fig. 3).
Fig. 3.
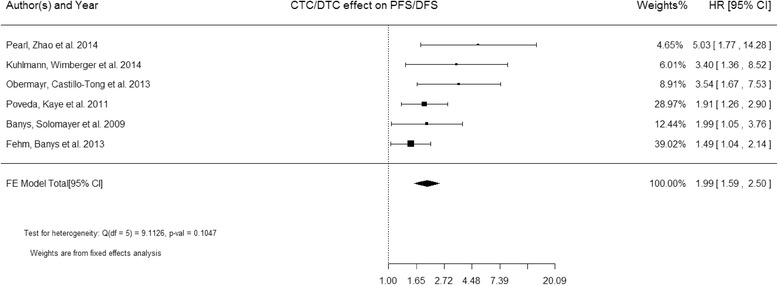
Forest plot of HRs for PFS/DFS from 6 studies (885 patients)
Results for subgroup analysis is summarized in Table 3. CTCs and DTCs could be detected in peripheral blood (PB) and bone marrow (BM), respectively, we divided the studies into either CTCs or DTCs subgroups to investigate the influence of sampling types on the survival of patients with ovarian cancer. CTCs from PB were detected and correlated with PFS/DFS and OS in 4 studies [18, 19, 34, 35] and 5 studies [17–19, 34, 35], respectively. The results showed that CTCs were significantly associated with both PFS/DFS (HR =2.52 [1.83, 3.48], z = 5.614 %, p < 0.0001) and OS (HR = 1.97 [1.50, 2.58], z = 4.878, p < 0.0001). DTCs from BM were detected and correlated with PFS/DFS and OS in 3 studies [22, 34, 37]. The results showed that DTCs were also significantly associated with PFS/DFS (n = 2, HR = 1.60 [1.17, 2.19], z = 2.926, p < 0.0034) and OS (n = 2, HR = 1.89 [1.33, 2.68], z = 2.358, p < 0.0004). These suggested both CTCs and DTCs could be useful for evaluating prognostic value of ovarian cancer.
Table 3.
Subgroup analyses of CTCs/DTCs
| Subgroup analysis | PFS/DFS | OS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies (sample size) | HR (95 % CI) | Model | P value | P-H | Begg’s test, p value | No. of Studies (sample size) | HR (95 % CI) | Model | P value | P-H | Begg’s test, p value | |
| Total (CTCs and DTCs) | 6 (885) | 1.99 [1.59, 2.50] | FE | <.0001 | 0.1047 | 0.0167 | 7 (965) | 1.94 [1.56 2.40] | FE | <.0001 | 0.7704 | 0.2389 |
| Sampling from BM (DTCs) | 2 (181) | 1.60 [1.17, 2.19] | FE | 0.0034 | 0.4417 | 1.000 | 2 (181) | 1.89 [1.33, 2.68] | FE | 0.0004 | 0.9687 | 1.0000 |
| Sampling from PB (CTCs) | 4 (704) | 2.52 [1.83, 3.48] | FE | <.0001 | 0.2064 | 0.3333 | 5 (784) | 1.97 [1.50, 2.58] | FE | <.0001 | 0.5147 | 0.0833 |
| Total (IHC and RT-PCR) | 5 (756) | 1.91 [1.51, 2.40] | FE | <.0001 | 0.2037 | 0.0833 | 6 (818) | 1.89 [1.52, 2.36] | FE | <.0001 | 0.7839 | 0.7194 |
| Detected DTCs by IHC | 3 (397) | 1.70 [1.33, 2.19] | FE | <.0001 | 0.5934 | 1.0000 | 3 (397) | 1.96 [1.48, 2.60] | FE | 0.0004 | 0.9314 | 1.0000 |
| Detected CTCs by RT-PCR | 2 (359) | 3.45 [1.95, 6.24] | FE | <.0001 | 0.9452 | 1.0000 | 3 (439) | 1.78 [1.24, 2.54] | FE | 0.0017 | 0.3463 | 0.3333 |
FE Fixed-Effects; P-H P-Heterogeneity
Four studies detected CTC by RT-PCR methods [17, 19, 26, 35]. Eleven studies detected by CellSearch system or other IHC methods [5, 6, 20–25, 34, 37, 38]. We divided the studies into molecular-based and immunological-based detection methods for sub-group analysis. For molecular-based subgroup, HR and 95 % CI for OS was 1.78 [1.24, 2.54] (z = 3.1369, p < 0.0001) among three studies [35, 19, 17] whereas HR and 95 % CI for PFS/DFS was 3.49 [1.95, 6.24] (z = 4.2007, p < 0.0001) among two studies [35, 19]. For immunological-based subgroup, HR and 95 % CI for OS and PFS/DFS was 1.96 [1.48, 2.60] (z = 3.5380, p < 0.0001) and 1.70 [1.33, 2.19] (z = 4.1628, p < 0.0001) amongst three studies [37, 34, 22], respectively. The results indicated that both RT-PCR and IHC detection methods were able to detect CTCs/DTCs in predicting patient’s survival.
Begg’s funnel plot was used to identify individual studies in relation to their respective standard deviation, which revealed no evidence of asymmetry (p = 0.2389) in an overall analysis of the studies of OS (Fig. 4a). There was no evidence of asymmetry (p = 0.0167) in an overall analysis of the studies of PFS/DFS (Fig. 4b). Using Egger’s test, there was no significant publication bias for overall analysis of the studies of OS (p = 0.3405), but significant bias was found for overall analysis of the studies of PFS/DFS (p = 0.0051). There was no publication bias for all subsequent subgroup analyses.
Fig. 4.
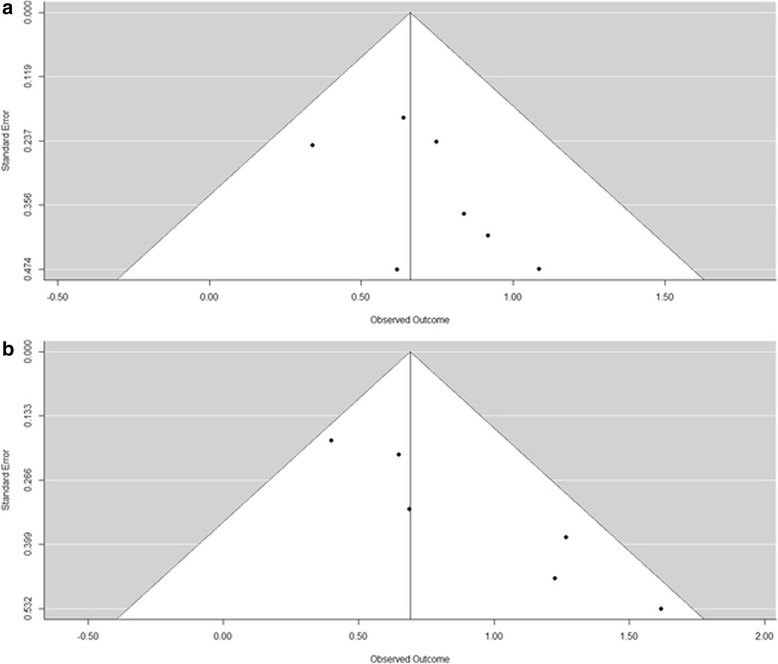
Begg’s funnel plots of publication bias summary. a Overall survival (OS); b Progression-free Disease-free survival (PFS/DFS)
Discussion
In our systematic review and meta-analysis, the results showed strong association of CTCs/DTCs not only with advanced staging and poor prognosis in patients with ovarian cancer, but also with treatment response. Although there was publication bias in Pearl et al. 2014 and Kuhlmann et al. 2014 studies for PFS/DFS group analysis, CTCs/DTCs were still significantly associated with PFS/DFS after bias removed. The combined HRs of CTCs/DTCs for OS and PFS/DFS was nearly 2.0, suggesting that the detected CTCs/DTCs had a strong predictive values for OS and PFS/DFS. For CTCs alone, the pooled HRs of CTCs for PFS/DFS were more than 2.0, indicating that CTCs could be used as a prognostic marker for ovarian cancer.
Association of CTCs/DTCs with clinopathological characteristics revealed that CTCs/DTCs significantly associated with advanced tumor stage and treatment response. No significant association were observed with histological subtypes, debulking surgery and lymph nodes metastasis. Experimental studies had demonstrated detection of CTCs were significantly correlated with the advanced stage [39, 40]. It may be one of the reasons why these patients have a high incidence of tumor recurrence after surgical resection. Residual disease after cyto-reduction surgery was associated with poor prognosis of ovarian cancer [41]. Moreover, in terms of the response to chemotherapy, CTCs/DTCs were significantly associated with treatment response. It suggested that CTCs/DTCs could be used as an early predictive marker of tumor response in ovarian cancer patients undergo chemotherapy. In our meta-analysis, detection of CTC was not significantly associated with the evidence of optimal or suboptimal surgery, only one study [35] reached a conclusion of positive (OR 2.31 [1.19-4.50]) with residual diseases by detecting DTC. This confusing result could be only resolved when more studies were conducted to confirm clinical values of the CTCs in ovarian cancer. On the other hand, ovarian cancer grows and recurs mainly in direct dissemination in the abdominal cavity [42]. Lymph nodes metastasis occur only when cancer cells invade lymphatic vessels while CTCs/DTCs occur only when cancer cells invade blood vessels. Although both lymph nodes metastasis and CTCs/DTCs were associated with poor prognosis in ovarian cancer patients [43], CTCs/DTCs were not significantly associated with lymph nodes metastasis, indicating the cancer cells may spread differently.
In subgroup analysis, comparing the HR of CTCs with the HR of DTCs for survival, CTCs for PFS/DFS was larger than DTCs for PFS/DFS (HR 2.50 vs 1.60) while CTCs for OS was similar to DTCs for OS (HR 1.97 vs 1.89). This indicated that CTCs could be more sensitive than DTC in evaluating tumor progression. In addition, detection of CTC seems more practical than DTC in terms of monitoring of progression of disease. Although bone marrow is the major site of metastasis [44], CTC could be systematically evaluated in peripheral blood stream. For CTCs/DTCs detection methods, IHC and RT-PCR were two main methods. Compared with IHC, although both methods were significantly associated with poor prognosis, RT-PCR seems to be more sensitive than IHC (HR 3.49 vs 1.70) [45]. This suggested that RT-PCR could be a promising methods in identifying CTC/DTC in patients with ovarian cancer. However, significant challenges include the frequency of both false positive and false negative results and the difficulty in quantitating relative levels of expression. Although genomic analyses of cell-free DNA fragments in peripheral blood have been reported [46, 47] and recently extended to the whole-genome scale [48], in situ and morphological analyses by fluorescent in situ hybridization (FISH) and IHC will be not possible. Apart from nucleic acid-based detection of CTC, detection and isolation of CTC by virtue of their physical properties distinguished from normal blood cells, including cell size, cell density, membrane charge and migratory properties, has advantageous to analyze CTCs with intact functional cancer cells circulating in peripheral blood [49]. It shows promising in CTC detection but requires further validation [50, 51].
CTCs/DTCs were associated with a poor survival outcome in our meta-analysis. However, there were limitations in this meta-analysis. Firstly, the number of patients in each study were relatively small. The results should be confirmed by larger prospectively clinical study. Secondly, the methodology varied in different studies lead to heterogeneity in experimental design, detection methods and defining the presence of CTCs/DTCs. However, there is still no gold standard in the definition of positive results in detection of CTCs/DTCs. And validation studies are still lacking. An international agreement of the definition of ‘positive’ CTCs in future trial is necessary. Thirdly, logHR and SE results were extracted from either multivariate analysis or univariate analysis studies. To ensure data integrity, we combined these univariate analysis and multivariate analysis together.
Conclusion
In conclusion, available evidence supports that CTCs/DTCs were significantly associated with advanced tumor stage, residual diseases, and treatment response, but not with histological types and lymph node metastasis in patients with ovarian cancer. Moreover, CTCs also were significantly associated with a poorer survival. CTCs/DTCs could be a reliable non-invasive prognostic marker for ovarian cancer. Clinical management based on CTCs/DTCs could be useful for determining which patients would potentially benefit from adjuvant therapy.
Acknowledgements
This work was supported by the research grant from National Natural Science Foundation of China (Grant No: 81301827).
Abbreviations
- CTCs
Circulating tumor cells
- DTCs
Disseminated tumor cells
- RT-PCR
Reverse transcription-polymerase chain reaction
- IHC
Immunocytochemistry
- MAGE-A
Melanoma-associated antigens A
- PPIC
Peptidylprolyl isomerase C (cyclophilin C)
- GPX8
Glutathione peroxidase 8
- CDH3
Cadherin-3
- TUSC3
Tumor suppressor candidate 3
- COL3A1
Collagen, type III, alpha 1
- LAMB1
Laminin subunit beta-1
- MAM
Mammaglobin A
- ESRP2
Epithelial splicing regulatory protein 2
- AGR2
Anterior gradient protein 2 homolog
- BAIAP2L1
Brain-specific angiogenesis inhibitor 1-associated protein 2-like protein 1
- TFF1
Trefoil factor 1
- EpCAM
Epithelial cell adhesion molecule
- MUC-1
Mucin 1
- MUC-16
Mucin 16
- HER2
Human growth factor receptor 2
- A45-B/B3
Pan-cytokeratin antibody (CK 8, 18, 19)
- MOC-31
Epithelial glycoprotein 2 mouse monoclonal antibody
- EGFR
Epithelial growth factor receptor
- iCTC
Invasive circulating tumor cells
- CK
Cytokeratin
- CAM
Cell adhesion matrix
- FISH
Fluorescent in situ hybridization
- BM
Bone marrow
- HR
Hazards ratio
- OR
Odds ratio
- lnHR
Natural logarithm of HR
- SE
Standard error
- CIs
Confidence intervals
- OS
Overall survival
- PFS/DFS
Progression-free survival/disease-free survival
Additional files
Search strategies and results of Embase.
Meta-analysis of Observational Studies in Epidemiology (MOOSE) Checklist.
Quality assessment of included cohort studies with the Newcastle-Ottawa Scale (NOS).
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LC, JK and CCW conceived and designed the study. LC collected and analyzed the data. LC drafted the manuscript in collaboration with JK and CCW. All authors read and approved the final manuscript.
References
- 1.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53(1):5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin LA, Huang B, Miller RW, Tucker T, Goodrich ST, Podzielinski I, et al. Ten-year relative survival for epithelial ovarian cancer. Obstet Gynecol. 2012;120(3):612–618. doi: 10.1097/AOG.0b013e318264f794. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs IJ, Menon U. Progress and challenges in screening for early detection of ovarian cancer. Mol Cell Proteomics. 2004;3(4):355–366. doi: 10.1074/mcp.R400006-MCP200. [DOI] [PubMed] [Google Scholar]
- 4.Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer. 2009;9(3):167–181. doi: 10.1038/nrc2583. [DOI] [PubMed] [Google Scholar]
- 5.Judson PL, Geller MA, Bliss RL, Boente MP, Downs LS, Jr, Argenta PA, et al. Preoperative detection of peripherally circulating cancer cells and its prognostic significance in ovarian cancer. Gynecol Oncol. 2003;91(2):389–394. doi: 10.1016/j.ygyno.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Wimberger P, Heubner M, Otterbach F, Fehm T, Kimmig R, Kasimir-Bauer S. Influence of platinum-based chemotherapy on disseminated tumor cells in blood and bone marrow of patients with ovarian cancer. Gynecol Oncol. 2007;107(2):331–338. doi: 10.1016/j.ygyno.2007.07.073. [DOI] [PubMed] [Google Scholar]
- 7.da Lu Y, Chen XL, Ding J. Individualized cancer chemotherapy integrating drug sensitivity tests, pathological profile analysis and computational coordination - an effective strategy to improve clinical treatment. Med Hypotheses. 2006;66(1):45–51. doi: 10.1016/j.mehy.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim SH, Becker TM, Chua W, Caixeiro NJ, Ng WL, Kienzle N, et al. Circulating tumour cells and circulating free nucleic acid as prognostic and predictive biomarkers in colorectal cancer. Cancer Lett. 2014;346(1):24–33. doi: 10.1016/j.canlet.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 10.Turner AM, McGowan L, Millen A, Rajesh P, Webster C, Langman G, et al. Circulating DBP level and prognosis in operated lung cancer: an exploration of pathophysiology. Eur Respir J. 2013;41(2):410–416. doi: 10.1183/09031936.00002912. [DOI] [PubMed] [Google Scholar]
- 11.Kolostova K, Cegan M, Bobek V. Circulating tumour cells in patients with urothelial tumours: enrichment and in vitro culture. Can Urol Assoc J. 2014;8(9–10):E715–E720. doi: 10.5489/cuaj.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Driemel C, Kremling H, Schumacher S, Will D, Wolters J, Lindenlauf N, et al. Context-dependent adaption of EpCAM expression in early systemic esophageal cancer. Oncogene. 2014;33(41):4904–4915. doi: 10.1038/onc.2013.441. [DOI] [PubMed] [Google Scholar]
- 13.Morris KL, Tugwood JD, Khoja L, Lancashire M, Sloane R, Burt D, et al. Circulating biomarkers in hepatocellular carcinoma. Cancer Chemother Pharmacol. 2014;74(2):323–332. doi: 10.1007/s00280-014-2508-7. [DOI] [PubMed] [Google Scholar]
- 14.Medina-Villaamil V, Martinez-Breijo S, Portela-Pereira P, Quindos-Varela M, Santamarina-Cainzos I, Anton-Aparicio LM, et al. Circulating MicroRNAs in blood of patients with prostate cancer. Actas Urol Esp. 2014;38(10):633–639. doi: 10.1016/j.acuro.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486(7403):395–399. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tjensvoll K, Nordgard O, Smaaland R. Circulating tumor cells in pancreatic cancer patients: methods of detection and clinical implications. Int J Cancer. 2014;134(1):1–8. doi: 10.1002/ijc.28134. [DOI] [PubMed] [Google Scholar]
- 17.Sang M, Wu X, Fan X, Zhou X, Zhou N. Multiple MAGE-A genes as surveillance marker for the detection of circulating tumor cells in patients with ovarian cancer. Biomarkers. 2014;19(1):34–42. doi: 10.3109/1354750X.2013.865275. [DOI] [PubMed] [Google Scholar]
- 18.Pearl ML, Zhao Q, Yang J, Dong H, Tulley S, Zhang Q, et al. Prognostic analysis of invasive circulating tumor cells (iCTCs) in epithelial ovarian cancer. Gynecol Oncol. 2014;134(3):581–590. doi: 10.1016/j.ygyno.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhlmann JD, Wimberger P, Bankfalvi A, Keller T, Scholer S, Aktas B, et al. ERCC1-positive circulating tumor cells in the blood of ovarian cancer patients as a predictive biomarker for platinum resistance. Clin Chem. 2014;60(10):1282–1289. doi: 10.1373/clinchem.2014.224808. [DOI] [PubMed] [Google Scholar]
- 20.Schindlbeck C, Hantschmann P, Zerzer M, Jahns B, Rjosk D, Janni W, et al. Prognostic impact of KI67, p53, human epithelial growth factor receptor 2, topoisomerase IIalpha, epidermal growth factor receptor, and nm23 expression of ovarian carcinomas and disseminated tumor cells in the bone marrow. Int J Gynecol Cancer. 2007;17(5):1047–1055. doi: 10.1111/j.1525-1438.2007.00920.x. [DOI] [PubMed] [Google Scholar]
- 21.Wimberger P, Roth C, Pantel K, Kasimir-Bauer S, Kimmig R, Schwarzenbach H. Impact of platinum-based chemotherapy on circulating nucleic acid levels, protease activities in blood and disseminated tumor cells in bone marrow of ovarian cancer patients. Int J Cancer. 2011;128(11):2572–2580. doi: 10.1002/ijc.25602. [DOI] [PubMed] [Google Scholar]
- 22.Fehm T, Banys M, Rack B, Janni W, Marth C, Blassl C, et al. Pooled analysis of the prognostic relevance of disseminated tumor cells in the bone marrow of patients with ovarian cancer. Int J Gynecol Cancer. 2013;23(5):839–845. doi: 10.1097/IGC.0b013e3182907109. [DOI] [PubMed] [Google Scholar]
- 23.Behbakht K, Sill MW, Darcy KM, Rubin SC, Mannel RS, Waggoner S, et al. Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: a gynecologic oncology group study. Gynecol Oncol. 2011;123(1):19–26. doi: 10.1016/j.ygyno.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marth C, Kisic J, Kaern J, Trope C, Fodstad O. Circulating tumor cells in the peripheral blood and bone marrow of patients with ovarian carcinoma do not predict prognosis. Cancer. 2002;94(3):707–712. doi: 10.1002/cncr.10250. [DOI] [PubMed] [Google Scholar]
- 25.Fan T, Zhao Q, Chen JJ, Chen WT, Pearl ML. Clinical significance of circulating tumor cells detected by an invasion assay in peripheral blood of patients with ovarian cancer. Gynecol Oncol. 2009;112(1):185–191. doi: 10.1016/j.ygyno.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aktas B, Kasimir-Bauer S, Heubner M, Kimmig R, Wimberger P. Molecular profiling and prognostic relevance of circulating tumor cells in the blood of ovarian cancer patients at primary diagnosis and after platinum-based chemotherapy. Int J Gynecol Cancer. 2011;21(5):822–830. doi: 10.1097/IGC.0b013e318216cb91. [DOI] [PubMed] [Google Scholar]
- 27.Romero-Laorden N, Olmos D, Fehm T, Garcia-Donas J, Diaz-Padilla I. Circulating and disseminated tumor cells in ovarian cancer: a systematic review. Gynecol Oncol. 2014;133(3):632–639. doi: 10.1016/j.ygyno.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Polterauer S, Vergote I, Concin N, Braicu I, Chekerov R, Mahner S, et al. Prognostic value of residual tumor size in patients with epithelial ovarian cancer FIGO stages IIA-IV: analysis of the OVCAD data. Int J Gynecol Cancer. 2012;22(3):380–385. doi: 10.1097/IGC.0b013e31823de6ae. [DOI] [PubMed] [Google Scholar]
- 29.Zapardiel I, Morrow CP. New terminology for cytoreduction in advanced ovarian cancer. Lancet Oncol. 2011;12(3):214. doi: 10.1016/S1470-2045(10)70292-8. [DOI] [PubMed] [Google Scholar]
- 30.Ledermann JA, Kristeleit RS. Optimal treatment for relapsing ovarian cancer. Ann Oncol. 2010;21(Suppl 7):vii218–vii222. doi: 10.1093/annonc/mdq377. [DOI] [PubMed] [Google Scholar]
- 31.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 32.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Begg CB, Berlin JA. Publication bias and dissemination of clinical research. J Natl Cancer Inst. 1989;81(2):107–115. doi: 10.1093/jnci/81.2.107. [DOI] [PubMed] [Google Scholar]
- 34.Poveda A, Kaye SB, McCormack R, Wang S, Parekh T, Ricci D, et al. Circulating tumor cells predict progression free survival and overall survival in patients with relapsed/recurrent advanced ovarian cancer. Gynecol Oncol. 2011;122(3):567–572. doi: 10.1016/j.ygyno.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 35.Obermayr E, Castillo-Tong DC, Pils D, Speiser P, Braicu I, Van Gorp T, et al. Molecular characterization of circulating tumor cells in patients with ovarian cancer improves their prognostic significance – a study of the OVCAD consortium. Gynecol Oncol. 2013;128(1):15–21. doi: 10.1016/j.ygyno.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 36.Liu JF, Kindelberger D, Doyle C, Lowe A, Barry WT, Matulonis UA. Predictive value of circulating tumor cells (CTCs) in newly-diagnosed and recurrent ovarian cancer patients. Gynecol Oncol. 2013;131(2):352–356. doi: 10.1016/j.ygyno.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Banys M, Solomayer EF, Becker S, Krawczyk N, Gardanis K, Staebler A, et al. Disseminated tumor cells in bone marrow may affect prognosis of patients with gynecologic malignancies. Int J Gynecol Cancer. 2009;19(5):948–952. doi: 10.1111/IGC.0b013e3181a23c4c. [DOI] [PubMed] [Google Scholar]
- 38.Fehm T, Becker S, Bachmann C, Beck V, Gebauer G, Banys M, et al. Detection of disseminated tumor cells in patients with gynecological cancers. Gynecol Oncol. 2006;103(3):942–947. doi: 10.1016/j.ygyno.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 39.Magnowski P, Bochynski H, Nowak-Markwitz E, Zabel M, Spaczynski M. Circulating tumor cells (CTCs)--clinical significance in patients with ovarian cancer. Ginekol Pol. 2012;83(4):291–294. [PubMed] [Google Scholar]
- 40.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matias-Guiu X, Davidson B. Prognostic biomarkers in endometrial and ovarian carcinoma. Virchows Arch. 2014;464(3):315–331. doi: 10.1007/s00428-013-1509-y. [DOI] [PubMed] [Google Scholar]
- 42.Pereira A, Perez-Medina T, Magrina JF, Magtibay PM, Rodriguez-Tapia A, Perez-Milan F, et al. The impact of pelvic retroperitoneal invasion and distant nodal metastases in epithelial ovarian cancer. Surg Oncol. 2014;23(1):40–44. doi: 10.1016/j.suronc.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Fehm T, Banys M, Rack B, Jager B, Hartkopf A, Taran FA, et al. Presence of disseminated tumor cells in bone marrow correlates with tumor stage and nodal involvement in cervical cancer patients. Int J Cancer. 2014;134(4):925–931. doi: 10.1002/ijc.28417. [DOI] [PubMed] [Google Scholar]
- 44.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Ring AE, Zabaglo L, Ormerod MG, Smith IE, Dowsett M. Detection of circulating epithelial cells in the blood of patients with breast cancer: comparison of three techniques. Br J Cancer. 2005;92(5):906–912. doi: 10.1038/sj.bjc.6602418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forshew T, Murtaza M, Parkinson C, Gale D, Tsui DW, Kaper F, et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci Transl Med. 2012;4(136):136ra68. doi: 10.1126/scitranslmed.3003726. [DOI] [PubMed] [Google Scholar]
- 47.Ninomiya S, Kawano M, Abe T, Ishikawa T, Takahashi M, Tamura M, et al. Potential small guide RNAs for tRNase ZL from human plasma, peripheral blood mononuclear cells, and cultured cell lines. PLoS One. 2015;10(3):e0118631. doi: 10.1371/journal.pone.0118631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss L, Hufnagl C, Greil R. Circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;369(1):93. doi: 10.1056/NEJMc1306040. [DOI] [PubMed] [Google Scholar]
- 49.Alix-Panabieres C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14(9):623–631. doi: 10.1038/nrc3820. [DOI] [PubMed] [Google Scholar]
- 50.Ali A, Furusato B, Ts’o PO, Lum ZP, Elsamanoudi S, Mohamed A, et al. Assessment of circulating tumor cells (CTCs) in prostate cancer patients with low-volume tumors. Pathol Int. 2010;60(10):667–672. doi: 10.1111/j.1440-1827.2010.02584.x. [DOI] [PubMed] [Google Scholar]
- 51.Wu S, Liu Z, Liu S, Lin L, Yang W, Xu J. Enrichment and enumeration of circulating tumor cells by efficient depletion of leukocyte fractions. Clin Chem Lab Med. 2015;53(2):337. doi: 10.1515/cclm-2015-5000. [DOI] [PubMed] [Google Scholar]


