Abstract
Setting: Multidrug-resistant tuberculosis (MDR-TB, defined as resistance to isoniazid and rifampicin) is poorly detected in Nepal; one reason may be poor functioning of culture and drug susceptibility testing (CDST) services for retreatment tuberculosis (TB) patients.
Objectives: To determine, among retreatment TB patients in mid-west Nepal, 1) the number of patients registered for treatment between July 2011 and July 2012; 2) the number submitting sputum specimens for CDST to the Central Reference Laboratory (CRL), Kathmandu, along with the results; and 3) the length of time for submission and receipt of specimens.
Design: Retrospective cohort study involving the review of treatment and laboratory registers from the Nepalgunj TB Referral Centre and the CRL.
Results: Of 431 retreatment patients, 66 (15%) submitted sputum samples, of which 63 reached the CRL. Of these, 39 (62%) were culture-positive; 13 (33%) patients had MDR-TB. The CDST results of 19 patients were received back at the TB Referral Centre. The median turnaround time from sending specimens to receipt of results at the TB Referral Centre was 119 days.
Conclusion: Less than 10% of retreatment TB patients in mid-West Nepal had CDST results recorded, leading to the underdiagnosis of MDR-TB in the region. Urgent solutions are needed to rectify this problem.
Keywords: retreatment tuberculosis, multidrug-resistant tuberculosis, Nepal, culture and drug susceptibility testing, central reference laboratory, operational research
Abstract
Contexte : La tuberculose multi-résistante (TB-MDR, définie comme une résistance à l'isoniazide et à la rifampicine) est mal détectée au Népal; une des raisons pourrait être le mauvais fonctionnement des services de culture et de tests de sensibilité aux médicaments (CDST) pour les tuberculeux en retraitement.
Objectifs : Déterminer parmi les patients tuberculeux du Centre Ouest du Népal 1) le nombre de patients enregistrés pour un traitement de juillet 2011 à juillet 2012; 2) le nombre de patients ayant eu un examen de crachats pour CSDT au laboratoire central de référence (CRL) à Katmandou ainsi que les résultats; et 3) le délai de fourniture et de réception des échantillons.
Schéma : Etude rétrospective de cohorte par revue des registres de traitement et de laboratoire du centre de référence de la TB Nepalgunj et du CRL.
Résultats : Sur 431 patients en retraitement, 66 ont fourni des échantillons de crachats dont 63 (15% du total) sont parvenus au CRL. Parmi eux, 39 (62%) étaient positifs; 13 patients (33%) avaient une TB-MDR. Les résultats du CDST sont parvenus au centre de référence de la TB pour 19 patients. Le délai médian entre l'envoi des échantillons et la réception des résultats au centre de référence de la TB était de 119 jours.
Conclusion : Dans le Centre Ouest du Népal, moins de 10% des patients ont eu des résultats d'examens de CSDT, ce qui induisait un sous-diagnostic de la TB-MDR dans cette région. Il est urgent de trouver des solutions à ce problème.
Abstract
Marco de referencia: La detección de la tuberculosis multidrogorresistente (TB-MDR), que se define como la presencia de resistencia a isoniazida y rifampicina, es precaria en Nepal. Una de las causas podría ser una deficiencia en el funcionamiento de los servicios de cultivo y pruebas de sensibilidad a los medicamentos (CDST), en el caso del retratamiento de los pacientes tuberculosos.
Objetivos: Determinar los siguientes aspectos de los pacientes registrados en retratamiento en el occidente medio de Nepal: 1) el número de pacientes registrados en tratamiento entre julio del 2011 y julio del 2012; 2) el número de pacientes cuyas muestras de esputo se remitieron al Laboratorio Central de Referencia (CRL) en Katmandú para CDST y los resultados obtenidos; y 3) el tiempo transcurrido entre la obtención de la muestra y la recepción de los resultados.
Métodos: Fue este un estudio retrospectivo de cohortes en el cual se examinaron los registros de tratamiento y de laboratorio del Centro de Referencia de Tuberculosis de Nepalgunj y del CRL.
Resultados: De los 431 pacientes en retratamiento, en 66 se recogieron muestras de esputo y de estas muestras, 63 llegaron al CRL (15%). De esos, 39 obtuvieron un cultivo positivo (62%) y 13 pacientes exhibieron TB-MDR (33%). En el Centro de Referencia de TB, se recibieron los resultados del cultivo y las pruebas de sensibilidad de 19 pacientes. La mediana del tiempo necesario a la obtención del resultado desde el envío de las muestras fue 119 días.
Conclusión: En menos del 10% de los pacientes en retratamiento se practicaron el CDST a los medicamentos antituberculosos en el occidente medio de Nepal, lo cual origina una deficiencia en el diagnóstico de la TB-MDR en la región. Se precisan con urgencia intervenciones que solucionen esta carencia.
Nepal is a landlocked country with five geographically diverse regions divided into 75 districts. The country has had a tuberculosis (TB) control programme for many years and rigorously follows the World Health Organization (WHO) DOTS strategy. In 2011, the country notified 35 434 TB cases to the WHO, of which 2882 (8%) were retreatment cases.1 In the same year, Nepal reported 213 cases of multi-drug-resistant TB (MDR-TB, i.e., TB resistant to at least isoniazid [INH] and rifampicin [RMP]); however, it was estimated that there were 1100 MDR-TB cases, giving an MDR-TB case detection rate of 21% for 2011.1
Patients with MDR-TB are most likely to have failed first-line treatment, relapsed after completing treatment, or returned after default.1 According to Nepal National TB Programme (NTP) recommendations,2 all such patients are supposed to submit sputum specimens for culture and drug susceptibility testing (CDST), so that DST patterns can be identified and MDR-TB detected. One of the reasons for the poor detection of MDR-TB in the country may be the failure to perform and obtain CDST results in this group of patients at risk. The logistics of getting sputum samples to a central reference laboratory (CRL), as is the case in Nepal, is not easy, and this has proved difficult in other countries.3–6 We therefore carried out a retrospective study to determine how the CDST process worked among retreatment patients in the mid-west region of Nepal, the results of CDST when specimens did successfully reach the CRL, and the time taken for all procedures to be performed.
The aim of the present study was to assess CDST management for retreatment TB patients (relapses, failures or returns after default) in the mid-west region of Nepal who were registered between mid-July 2011 and mid-July 2012 (1 year). Specific objectives were to determine 1) the number and proportion of all registered TB patients who failed, relapsed or who returned after default during the 1-year period, 2) the number and proportion of patients who submitted sputum specimens for CDST to the CRL in Kathmandu, 3) the DST and resistance patterns determined at the CRL, and 4) the time required for the process of submission of specimens and receipt of CDST results.
METHODS
Study design
This was a retrospective cohort study of retreatment TB patients and CDST performance and results.
Setting
General setting
Nepal is a mountainous country situated in Asia between India and China. The population of Nepal is 26 620 809 (population in the mid-west region: 3546682).7 In the mid-west region, there are 15 districts with marked geographical diversity in which there are small urban cities and rural villages. The majority of the population is of low socio-economic status and has a low education level. Transport facilities, educational institutions and hospitals are mainly located in cities and district headquarters. However, health coverage is provided for urban and rural areas, the latter particularly through primary health care facilities.
Nepal National Tuberculosis Control Programme
Nepal's NTP has officers at the central level, in Kathmandu, and at the regional and district levels, with diagnostic and treatment services integrated into general health care. TB case finding is passive, and diagnosis is established through sputum smear microscopy and chest radiography for pulmonary TB and other systemic investigations for extra-pulmonary disease. All diagnosed patients are recorded with unique registration numbers, given standardised treatment and monitored for treatment outcomes per national and international recommendations.2,8
All TB microscopy services and treatment are free. If patients remain smear-positive at 5 months or at the end of treatment they are classified as failures. If patients default (are lost to follow-up) for ≥2 months and return to treatment still smear-positive, they are classified as return after default (RAD). If patients complete treatment and subsequently develop smear-positive TB, they are classified as relapse TB. In all these patients categorised as ‘retreatment TB’, it is recommended by the Nepal NTP that sputum be collected and submitted to the GENETUP (German-Nepal TB Project) CRL located in the capital, Kathmandu, for CDST.9 In practice, priority is given to those patients who have failed treatment. Patients are then started on a standardised retreatment regimen, except for those with MDR-TB, who are treated with second-line anti-tuberculosis drugs per Nepal NTP guidelines.2
The current 8-month retreatment regimen in Nepal is 2SHRZE+1HRZE+5HRE.1* The current regimen for MDR-TB comprises an intensive phase of 8–12 months with kanamycin, pyrazinamide (Z, PZA), levofloxacin (LFX), ethionamide (ETH) and cycloserine (CS), followed by a continuation phase of 12–16 months with PZA, LFX, ETH and CS.9
In mid-west Nepal, retreatment patients are referred to the International Nepal Fellowship Nepalgunj TB Referral Centre (INF-NTRC), a regional referral centre; two sputum specimens are collected from each patient into special containers, which are sent by air and courier to the CRL in Kathmandu. Culture is performed on Löwenstein-Jensen solid medium. Positive culture isolates are identified for Mycobacterium tuberculosis and subjected to DST for four of the first-line anti-tuberculosis drugs using the indirect proportion method, as recommended by the WHO, at the following critical drug concentrations: SM, 4 μg/ml; INH, 0.2 μg/ml; RMP, 40 μg/ml; and EMB 2 μg/ml.10,11 The CDST results are sent back to the INF-NTRC. Pending their CDST results, patients are referred back to their DOTS centre and started on a standardised retreatment regimen. If the results show MDR-TB, the patient is changed to the MDR-TB treatment regimen. The most recent drug resistance surveillance survey in Nepal found an MDR-TB rate of 2.6% among new TB cases and 17.6% among retreatment TB cases.12
Patient population
All retreatment patients diagnosed and registered as relapse, failure and RAD in the mid-west region of Nepal in the National TB Report and retreatment patients who were registered at INF-NTRC for CDST from 16 July 2011 to 15 July 2012 were included in the study.
Data variables, sources of data and data collection
Data variables and sources of data included 1) the number of all registered TB patients and the number of those with retreatment TB, stratified by type (relapse, failure and RAD) in mid-west Nepal (data source: 2012 Nepal National TB Report); 2) the number and proportion of retreatment patients who submitted sputum specimens for CDST, with name, age, sex, TB/suspect number, sputum specimen and date of submission to the INF-NTRC (data source: INF-NTRC register); 3) the number and proportion of retreatment patients whose sputum specimens were received by the CRL, with name, age, sex, TB/suspect number, date of receipt, sputum culture and DST result, and whether CDST results were sent back to the INF-NTRC, with date of dispatch. The source of data was the CRL register, which was examined for matching names from 16 July 2011 to 31 December 2012. A search was made for matching names for a minimum of 6 months after registration; if names from the INF-NTRC could not be found, the sputum specimen was declared not to have arrived at the CRL. A search was also made for names in the CRL register from mid-west Nepal and recorded even if the names did not appear in the INF-NTRC register; and 4) the number and proportion of retreatment patients whose sputum specimen results were returned to the INF-NTRC, along with the date of arrival of the results (data source: INF-NTRC register). All data were collected into a structured proforma between March and September 2013.
Analysis and statistics
Data were double-entered into EpiData (version 3.1, EpiData Association, Odense, Denmark), and validated and analysed using EpiData Analysis. Descriptive analyses were performed. Comparisons were made between males and females and between different categories of retreatment with respect to the different stages of the CDST pathway using the χ2 test and odds ratios (ORs) with 95% confidence intervals (CIs). Levels of significance were set at 5%.
Ethics approval
Permission for the study was sought from INF-NTRC, the Nepal Anti Tuberculosis Association, GENETUP CRL (Kathmandu), and the Nepal National Tuberculosis Centre, Bhaktapur, Nepal. Ethics approval was obtained from the INF Ethics Committee, the Nepal Health Research Council in Kathmandu, Nepal, and the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France.
RESULTS
Of the 5279 registered TB patients, 431 (8%) were retreatment TB cases. Details of type and category of TB by sex are shown in Table 1. For all types of patients, males exceeded females, but there were significantly more males with retreatment smear-positive pulmonary TB (PTB) than with new smear-positive PTB (OR 1.5, 95%CI 1.2–1.9, P < 0.01).
TABLE 1.
All TB patients registered in mid-west Nepal over 12 months, with number and proportion of males, 16 July 2011–15 July 2012
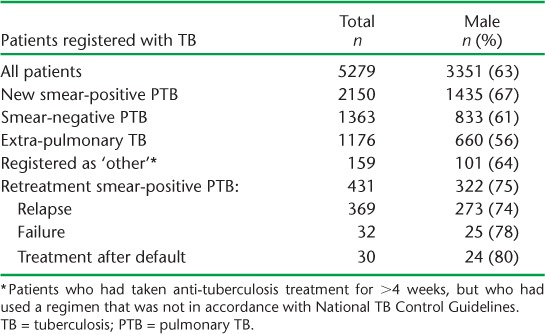
The pathway of retreatment patients in mid-west Nepal registered at the INF-NTRC, from having sputum specimens sent to and received at the CRL with CDST results subsequently obtained and then sent back to the INF-NTRC, is shown in the Figure and Table 2. Patients were lost to follow-up at each step. Only 164 (38%) patients were registered at the INF-NTRC, of whom 66 (40%) submitted sputum specimens that were sent to the CRL. Altogether, 63 sputum specimens (from 15% of retreatment patients) arrived at the CRL, with the majority coming from the INF-NTRC; four patients self-presented to the CRL. Of these, 39 sputum specimens (from 9% of all retreatment patients) showed a positive M. tuberculosis culture with associated DST. Nineteen results (15 positive cultures and 4 negative cultures) from the CRL were received back at the INF-NTRC, representing 4% of all retreatment patients. Performance along the pathway was better for females than males, except for the last component of CDST results returning to the INF-NTRC, in which the performance for females and males was similar.
FIGURE.

Patients with retreatment TB, their registration at the INF-NTRC and the flow of sputum specimens and culture and DST results to and from the CRL, mid-west Nepal. PTB = pulmonary TB; INF-NTRC = International Nepal Fellowship Nepalgunj TB Referral Centre; CRL = Central Reference Laboratory; RMP = rifampicin; INH = isoniazid; DST = drug susceptibility testing; TB = tuberculosis.
TABLE 2.
Patients with retreatment TB, their registration at the INF-NTRC and the flow of sputum specimens and culture and DST results to and from the CRL by sex, mid-west Nepal
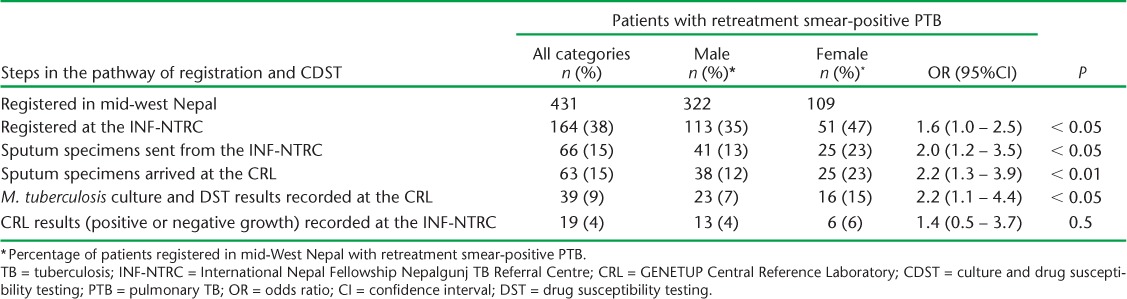
Relapse, failure and treatment after default patients were only recorded at the INF-NTRC register as ‘retreatment Category II’; specific categories of retreatment were not recorded. However, patient category was obtained from the sputum culture form and therefore recorded in the CRL register. The arrival of sputum specimens at the CRL, with subsequent culture and reporting of results back to the INF-NTRC, is shown in Table 3. Better overall results were obtained in failure and RAD patients compared with relapse patients. In failure patients, 44% of sputum specimens arrived at the CRL, which was significantly higher than that of relapse patients (11%; OR 6.6, 95%CI 3.0–14.3, P < 0.001). For RAD patients, 23% of specimens arrived; this, too, was significantly higher than in relapse patients (OR 2.6, 95%CI 1.0–6.4, P = 0.04).
TABLE 3.
Patients with retreatment TB in mid-west Nepal by retreatment category and the number and proportion of patients whose sputum specimens and culture and DST results were recorded at the CRL
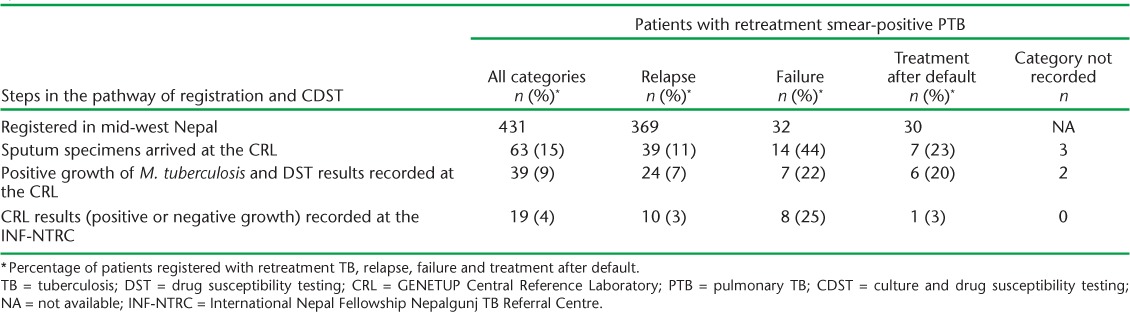
CDST results are shown in Table 4. Resistance to RMP and INH (MDR-TB) was demonstrated in 13 (33%) of 39 patients, with one other patient showing resistance to RMP but susceptibility to INH. Of these 14 patients, 7 (50%) were started on MDR-TB treatment. Of the remainder, 4 died before CDST results were available, 1 was lost to follow-up, 1 had no record of treatment and 1 patient who had been started on a retreatment regimen continued on this regimen. Where there were positive cultures, MDR-TB was most common in patients with failure (86%), followed by patients on treatment after default (50%) and relapses (17%). Of the 13 MDR-TB patients, the results of eight (62%) were reported back to the INF-NTRC.
TABLE 4.
Culture and DST from sputum specimens of retreatment TB patients that grew Mycobacterium tuberculosis at the CRL, mid-west Nepal
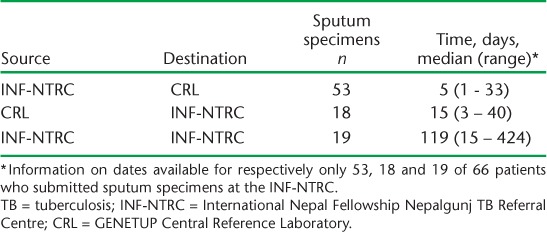
The time taken in days for the sputum specimens of retreatment TB patients to move along the pathway from patient registration to CDST to reporting of results is shown in Table 5. Among those with information on date available, the median time was 5 days for sputum specimens to journey from INF-NTRC to the CRL, 15 days for CDST results to move from the CRL to INF-NTRC and 119 days for the total journey of sputum specimens sent from the NTRC to CDST results arriving back at the INF-NTRC.
TABLE 5.
Time taken for sputum specimens to move along the pathway from patient registration to culture and drug susceptibility testing to reporting of results in retreatment TB patients
DISCUSSION
The study confirms our hypothesis that poor MDR-TB case detection in Nepal is a result of the failure of the whole CDST health service package. In the mid-west region of Nepal, only 15% of retreatment TB patients submitted sputum specimens, 15% of sputum samples arrived at the CRL, and less than 10% of patients had a CDST result. In the 39 patients whose sputum specimens showed a positive culture, one third showed resistance to RMP and INH (i.e., MDR-TB). If all samples from the 431 retreatment patients registered in mid-west Nepal had arrived at the CRL and shown positive CDST results, based on extrapolation of our findings just over 140 cases of MDR-TB might have been detected compared with the 13 cases that were in fact detected – a 10-fold difference.
The strengths of this study were the rigorous assessment of each step of the CDST pathway, the large number of retreatment patients assessed and the attention paid to following internationally agreed recommendations for reporting on observational studies.13,14 Limitations relate to the operational nature of the study, the use of registers at various sites to collect data and the necessity to match names that might have been recorded differently from one register to another. It is also possible that some samples were sent to the CRL after more than 6 months following registration, in which case they would have been missed, with the patient's sample inadvertently being recorded as missing.
This is the first study in Nepal to examine how the total CDST delivery system works in retreatment TB patients. The failures identified here are not confined to Nepal. Despite national guidelines in many countries recommending CDST for all retreatment patients, this does not happen. A country-wide survey in Malawi showed that in retreatment patients only 40% of specimens arrived at the CRL and less than 15% showed positive mycobacterial growth.3 A programme audit in Tanzania in over 40 000 retreatment patients showed that only 10% had sputum specimens arrive at the CRL for CDST.6 A study in China showed that less than one third of patients who should have undergone CDST actually did so.4 In India, only 55% of MDR-TB suspects had positive culture results,5 and in a recent study in presumptive MDR-TB patients in Cambodia, less than 50% were examined for culture and only 21% had positive M. tuberculosis cultures.15
How do we begin to improve the system? First, the submission of sputum specimens for CDST among all smear-positive retreatment TB patients should be made mandatory. Failure to seriously try and identify MDR-TB cases in this group of patients, particularly those who have failed treatment, is akin to public health malpractice. This action may persuade peripheral health facilities to ensure that retreatment patients report to the INF-NTRC. Second, more attention needs to be paid to recording. In the INF-NTRC register, the majority of retreatment patients were not classified as relapse, failure or RAD, and this must change. Third, solutions need to be found to the problem of performing CDST at a single CRL in the country. Serious consideration should be paid to strengthening laboratory capacity and fostering decentralisation to regional laboratories with the use of rapid DST methods, such as Xpert® MTB/RIF (Cepheid Inc, Sunnyvale, CA, USA).16,17 While Xpert is a revolutionary advance in diagnostics for TB and particularly MDR-TB, there are issues of cost and feasibility of use in health facilities where infrastructure is poor,18 and these need to be resolved before the assay can be made widely available and brought as close to the patient as possible. Finally, CDST results need to be more rapidly communicated so that patients with MDR-TB are rapidly started on appropriate treatment.
In conclusion, this study shows that one third of positive sputum cultures from retreatment TB patients in the mid-west region of Nepal had MDR-TB; however, less than 10% of these patients had positive sputum cultures recorded at the CRL. This is a serious shortcoming in Nepal's CDST system, and steps need to be taken urgently to correct the situation.
Acknowledgments
This research was supported through an operational research course that was jointly developed and run by the International Union Against Tuberculosis and Lung Disease (The Union) South-East Asia Regional Office, New Delhi, India; the Centre for Operational Research, The Union, Paris, France; and the Operational Research Unit, Médecins Sans Frontières, Brussels Operational Centre, Luxembourg. This course is under the umbrella of the World Health Organization Special Programme for Research and Training in Tropical Diseases programme (structured operational research and training initiative) for capacity building in low- and middle-income countries. We are also thankful to R Basnet for giving information about the course, B B Thapa and P Roche (INF), R Pant (Director, National TB Centre Nepal) and A Shah (National TB Centre) for support; other staff members of INF-NTRC Nepalgunj and GENETUP Central Reference Laboratory in Kathmandu for providing necessary records for review. Funding for the course was from Bloomberg Philanthropies, New York, NY, USA, and the Department for International Development, London, UK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest: none declared.
* S = streptomycin; H = isoniazid; R = rifampicin; Z = pyrazinamide; E = ethambutol. Numbers before the letters indicate the duration in months of the phase of treatment.
References
- 1.World Health Organization. Global tuberculosis report, 2012. Geneva, Switzerland: WHO; 2012. WHO/HTM/TB/2012.6. [Google Scholar]
- 2.National Tuberculosis Centre, Ministry of Health, Nepal. National Tuberculosis Guideline 2007. Kathmandu, Nepal: MoH; 2007. [Google Scholar]
- 3.Harries A D, Michongwe J, Nyirenda T E et al. Using a bus service for transporting sputum specimens to the Central Reference Laboratory: effect on the routine TB culture service in Malawi. Int J Tuberc Lung Dis. 2004;8:204–210. [PubMed] [Google Scholar]
- 4.Qi W, Harries A D, Hinderaker S G. Performance of culture and drug susceptibility testing in pulmonary tuberculosis patients in northern China. Int J Tuberc Lung Dis. 2011;15:137–139. [PubMed] [Google Scholar]
- 5.Chadha S S, Sharath B N, Reddy K et al. Operational challenges in diagnosing multi-drug resistant TB and initiating treatment in Andhra Pradesh, India. PLOS ONE. 2011;6:e26659. doi: 10.1371/journal.pone.0026659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilale A M, Ngowi B J, Mfinanga G S et al. Are sputum samples of retreatment tuberculosis reaching the reference laboratories? A 9-year audit in Tanzania. Public Health Action. 2013;3:156–159. doi: 10.5588/pha.12.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Central Bureau of Statistics, National Planning Commission Secretariat, Government of Nepal. National Population and Housing Census 2011 (National Report) Kathmandu, Nepal: CBS; 2012. [Google Scholar]
- 8.World Health Organization. Treatment of tuberculosis: guidelines. 4th ed. Geneva, Switzerland: WHO; 2009. WHO/HTM/TB/2009.420. [Google Scholar]
- 9.Ministry of Health and Population, Health Service Division, National Tuberculosis Centre, Nepal. Training manual for treatment and management of drug-resistant tuberculosis. Kathmandu, Nepal: Ministry of Health and Population; 2012–2013. [Google Scholar]
- 10.Canetti G, Fox W, Khomenko A et al. Advances in techniques of testing mycobacterial drug sensitivity and the use of sensitivity tests in tuberculosis control programs. Bull World Health Organ. 1969;41:21–43. [PMC free article] [PubMed] [Google Scholar]
- 11.Rieder H L, Chonde T M, Myking H The public health service national tuberculosis reference laboratory and the national laboratory network. Paris, France: International Union Against Tuberculosis and Lung Disease; 1998. [Google Scholar]
- 12.Ministry of Health and Population, Department of Health Services, National Tuberculosis Centre, Nepal. National Tuberculosis Programme Annual Report 2011/2012. Kathmandu, Nepal: Ministry of Health and Population; 2013. [Google Scholar]
- 13.von Elm E, Altman D G, Egger M, Pocock S J, Gotzsche P, Vandenbroucke J P, for the STROBE initiative The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 14.Edginton M, Enarson D, Zachariah R et al. Why ethics is indispensible for good-quality operational research. Public Health Action. 2012;2:21–22. doi: 10.5588/pha.12.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khann S, Mao E T, Rajendra Y P, Satyanarayana S, Nagaraja S B, Kumar A M V. Linkage of presumptive multidrug-resistant tuberculosis (MDR-TB) patients to diagnostic and treatment services in Cambodia. PLOS ONE. 2013;8:e59903. doi: 10.1371/journal.pone.0059903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Checklist of prerequisites to country implementation of Xpert MTB/RIF and key action points at country level. Geneva, Switzerland: WHO; 2011. WHO/HTM/TB/2011.12. [Google Scholar]
- 17.Vassall A, van Kampen S, Sohn H et al. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high burden countries: a cost-effectiveness analysis. PLOS MED. 2011;8:e1001120. doi: 10.1371/journal.pmed.1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trébucq A, Enarson D A, Chiang C Y et al. Xpert MTB/RIF for national tuberculosis programmes in low-income countries: when, where and how? Int J Tuberc Lung Dis. 2011;15:1567–1571. doi: 10.5588/ijtld.11.0392. [DOI] [PubMed] [Google Scholar]


