Abstract
Setting: Free State Province, South Africa.
Objective: To examine sex-specific trends in 2-month sputum smear non-conversion in new sputum smear-positive tuberculosis (TB) cases during a period when the DOTS strategy was operative.
Design: A retrospective cohort study of TB cases registered between 2003 and 2009 was conducted. Non-conversion was indicated by a positive 2-month sputum smear result. Descriptive and generalised linear model analyses were performed and sex-specific trends in 2-month sputum smear non-conversion rates estimated.
Results: Overall, 2-month sputum smear non-conversion rates were 12.5% in males and 9.3% in females. Non-conversion was significantly associated with age in males (P < 0.001). Non-conversion rates declined significantly between 2003 and 2009: from 15.9% to 10.8% in males (P < 0.001) and from 12.0% to 6.6% in females (P < 0.001). The average rate of decline of non-conversion was higher among females (1.0%, 95%CI 0.8–1.2) than among males (0.8%, 95%CI 0.5–1.0). By 2009, males had a 60% higher risk of non-conversion than females (RR 1.60, CI 1.37–1.86).
Conclusion: The decline in the trend of 2-month sputum smear non-conversion confirms the relative success of the DOTS strategy in TB control, with better performance among females than males. Interventions should consider the sex and age of patients to improve the 2-month sputum smear-conversion rate.
Keywords: DOTS, HIV, MDR-TB, male-female differential
Abstract
Contexte : Province de l'Etat Libre, Afrique du Sud.
Objectif : Examiner les tendances en fonction du sexe de la nonconversion des frottis de crachats après 2 mois chez des nouveaux cas de tuberculose (TB) à frottis positifs pendant une période où la stratégie DOTS opérait.
Schéma : Réalisation d'une étude rétrospective de cohorte des cas de TB enregistrés entre 2003 et 2009. La non-conversion était définie par un résultat de frottis positif après 2 mois de traitement. Des analyses descriptives et de modèles linéaires généralisés ont été réalisées et les tendances de non conversion à 2 mois en fonction du sexe ont été estimées.
Résultats : Le taux d'ensemble de non conversion était de 12,5% chez les hommes et de 9,3% chez les femmes. La non conversion était significativement associée à l'âge chez les hommes (P < 0,001). Le taux de non conversion a significativement diminué entre 2003 et 2009 de 15,9% à 10,8% chez les hommes (P < 0,001) et de 12% à 6,6% chez les femmes (P < 0,001). Le taux moyen de déclin de la non-conversion était plus élevé chez les femmes à 1% (IC95% 0,8–1,2%) que chez les hommes à 0,8% (IC95% 0,5–1%). En 2009, le risque de non conversion était plus élevé de 60% chez les hommes (RR 1,60; IC95% 1,37–1,86).
Conclusion : Le déclin de la tendance à la non-conversion du frottis de crachats après 2 mois de traitement a mis en évidence le succès relatif de la stratégie DOTS dans la lutte contre la TB, avec un meilleur résultat chez les femmes que chez les hommes. Les interventions devraient tenir compte du sexe et de l'âge des patients afin d'améliorer le taux de conversion du frottis de crachats à 2 mois.
Abstract
Marco de referencia: La Provincia del Estado Libre en África del Sur.
Objetivo: Examinar las tendencias específicas de sexo, con respecto a la falta de conversión de la baciloscopia del esputo a los 2 meses de tratamiento, en los casos nuevos de tuberculosis (TB) con baciloscopia positiva, durante un período de aplicación de la estrategia DOTS.
Métodos: Se llevó a cabo un estudio retrospectivo de cohortes de los casos de TB registrados entre el 2003 y el 2009. La falta de conversión se definió como la obtención de un resultado positivo de la baciloscopia del esputo a los 2 meses. Se practicaron análisis con modelos generales lineales y se calculó la tendencia de la falta de conversión a los 2 meses, según las tasas específicas de sexo.
Résultados: En general, las tasas de falta de conversión fueron 12,5% en los hombres y 9,3% en las mujeres. La falta de conversión se asoció de manera significativa con la edad en los hombres (P < 0,001). El índice de falta de conversión disminuyó de manera considerable entre el 2003 y el 2009, de 15,9% a 10,8% en los hombres (P < 0,001) y de 12,0% a 6,6% en las mujeres (P < 0,001). La tasa promedio de disminución de la falta de conversión en las mujeres de 1,0 % (IC95% de 0,8% a 1,2%) fue más alta que la tasa de 0,8% en los hombres (IC95% de 0,5% a 1,0%). En el 2009, los hombres exhibieron un riesgo de falta de conversión superior en 60,0 % a las mujeres (RR 1,60; IC95% de 1,37 a 1,86).
Conclusión: La tendencia a la disminución de la falta de conversión de la baciloscopia a los 2 meses de tratamiento define la eficacia relativa de la estrategia DOTS en el control de la TB y ofrece un mejor rendimiento en las mujeres que en los hombres. Con el propósito de mejorar las tasas de conversión, las intervenciones deben tener en cuenta el sexo y la edad de los pacientes.
The internationally recommended DOTS strategy is the basic approach underpinning the Stop TB strategy for tuberculosis (TB) control.1,2 The wide implementation of DOTS is thought to have contributed to the global decline in TB mortality.2 South Africa initiated DOTS in 1997, and by 2006 all districts were implementing the strategy.3 However, treatment outcomes in the country have remained sub-optimal since 1996.2–4 In 2011, South Africa had the highest TB incidence (993 per 100 000 population) of the 22 high-burden countries, with a 73% cure rate and a 79% treatment success rate in new sputum smear-positive cases.4
Two-month sputum smear conversion is a useful indicator for management of new smear-positive cases, progress of anti-tuberculosis treatment and the performance of the DOTS strategy.5–7 Non-conversion of positive sputum smears of new pulmonary TB cases at the end of 2 months of treatment is associated with an unfavourable outcome, particularly default, later in the course of treatment.8 The World Health Organization compendium of indicators for monitoring and evaluating national TB programmes states that the majority of new smear-positive pulmonary TB patients should convert to smear-negative after 2 or 3 months of treatment. However, even if the initial phase of treatment is well supervised and good-quality drugs are used, skilled laboratory technicians can often still detect low grades of positivity at 2 months, and the non-conversion rate can still be as high as 25%.9 The most recent (2009) South African programme data show that 2-month sputum non-conversion across the nine provinces ranged from 5.8% to 10.9%, with the Free State Province ranking second highest, at 10.7%.10 These rates, however, exclude cases who were transferred out, interrupted treatment or died before the end of the intensive phase, and those for whom sputum smear results were unavailable.
Studies measuring associations between sex and 2-month sputum smear non-conversion have produced contradictory findings. While some studies have established higher rates of 2-month sputum smear conversion among males than females,11–14 others have not shown any statistically significant variation across the sexes.15,16 Longer delays before care seeking for TB by males have been reported in South Africa.17 Delayed treatment onset is associated with higher bacillary load at diagnosis, which in turn is related to higher sputum smear non-conversion.18,19
The current study was undertaken to examine sex-specific trends in 2-month sputum smear non-conversion among new sputum smear-positive TB cases in the Free State Province of South Africa between 2003 and 2009. The Free State has a population of approximately 2.9 million, of whom about 2.4 million are dependent on the public sector for health care.20 In 2009, the province recorded a cure rate of 71.4% among new sputum smear-positive TB cases, at a par with the country's cure rate of 71.1%, although lower than that of three other provinces.21 In the same year, human immunodeficiency virus (HIV) prevalence in the Free State was slightly higher than that for the country as a whole (30.1% vs. 29.4%).21
To our knowledge, no previous study on sex-specific trends in 2-month sputum smear non-conversion among new sputum smear-positive TB cases has been conducted in South Africa. However, several studies in other countries have explored causes and inhibitors of increased acquisition and progression of TB disease among males or females, and have recommended that sex disparities should be explained and addressed.22–26 The findings of the current study will inform the design and delivery of sex-specific TB services in the Free State.
METHODS
Study design and setting
This was a retrospective cohort study of routine data from the electronic TB register (ETR.net) of new smear-positive cases receiving regimen I, a 2-month combination of rifampicin (RMP, R), isoniazid (INH, H), pyrazinamide and ethambutol, followed by 4 months of RMP and INH, between 2003 and 2009 in the Free State Province. At clinic level, TB case data are first recorded in paper-based TB treatment registers, then electronically captured in the ETR.net at the sub-district level, and finally aggregated at the provincial level.
Study population and data management
The study population was defined as all new smear-positive TB cases aged ≥8 years registered in ETR.net between 2003 and 2009. Data were managed by the third author (SvM) who is responsible for the TB database in the Free State Province. Duplicate entries were deleted and patients' names were removed before data for all new smear-positive cases for the period 2003–2009 were extracted from the database. Data were excluded for cases who were transferred out, interrupted treatment or had died before the end of the intensive phase, as well as those whose sputum results were unavailable at the end of 2 months of intensive phase treatment.
Analysis
Data were analysed using Stata, version 11 (Stata Corp, College Station, TX, USA). Non-conversion was defined as a positive 2-month sputum smear result. The rate of non-conversion was calculated as the proportion of new smear-positive cases with a recorded positive 2-month sputum smear result. Results for descriptive analysis are depicted as absolute numbers, percentages and graphically. χ2 tests were performed to establish any association between 2-month sputum non-conversion, age at start of treatment, time in years, HIV status and multidrug-resistant TB (MDR-TB). These were followed by age group adjusted log binomial regression models for each year comparing males and females. Generalised linear models were also used to estimate the sex-specific rate of change in 2-month sputum smear non-conversion over the 7 years. The rates for males and females were compared for each year. The risk ratios (RRs), together with their corresponding 95% confidence intervals (CIs), were estimated using log binomial regression models. The significance level considered was 0.05. As there was significant statistical interaction between sex and sputum smear non-conversion at 2 months, stratification for sex and age was performed.
Ethics approval
The study was approved by the Health Research Ethics Committee of Stellenbosch University, Cape Town, South Africa, and the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France. A waiver of individual consent was granted.
RESULTS
Of the 54 164 new smear-positive cases recorded for the period 2003–2009, about a quarter (n = 14 177) had no record of 2-month sputum smear testing, with a large proportion (37.1%) having been transferred out of the province. This left 39 987 (73.8%) cases with a recorded 2-month sputum smear result, of which 11.0% had a positive smear result (Figure 1). The demographic variables, age and sex, were compared between those with and those without a recorded 2-month sputum smear result. No statistically significant sex and age differences were observed, except for the age group 28–37 years, where significantly more males lacked a recorded sputum smear result (P < 0.001).
FIGURE 1.
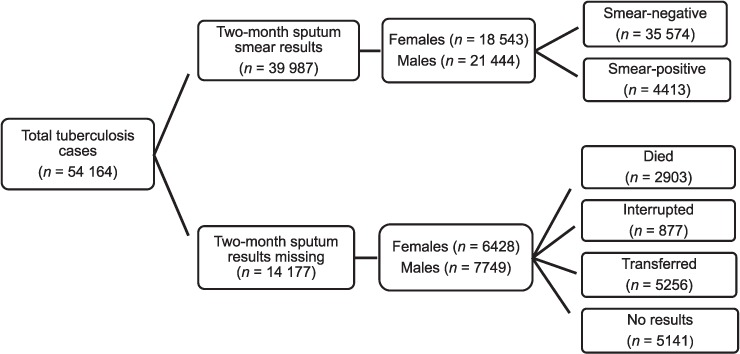
Flow diagram of inclusion of new sputum smear cases.
The overall 2-month sputum smear non-conversion rate over the period from 2003 to 2009 was 12.5% in males and 9.3% in females. The non-conversion rate was higher in males than in females across all age groups, except for the 8–17 years group. Contrary to females, a statistically significant association was established between age group and 2-month sputum smear conversion among males (P < 0.001) (Figure 2).
FIGURE 2.
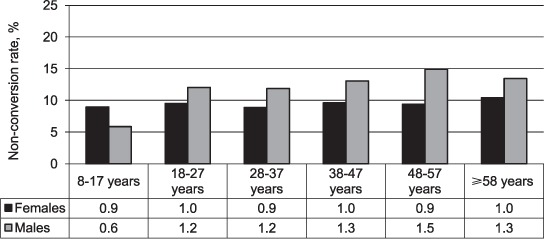
Two-month sputum smear non-conversion rate by age group for females (P = 0.667) and males (P < 0.001).
Two-month sputum smear non-conversion rates declined significantly between 2003 and 2009, from 15.9% to 10.8% in males (P < 0.001) and from 12.0% to 6.6% in females (P < 0.001) (Figure 3). The average rate of decline of 2-month sputum non-conversion was higher among females (1.0%, 95%CI 0.8–1.2) than males (0.8%, 95%CI 0.5–1.0), although this difference was not statistically significant.
FIGURE 3.
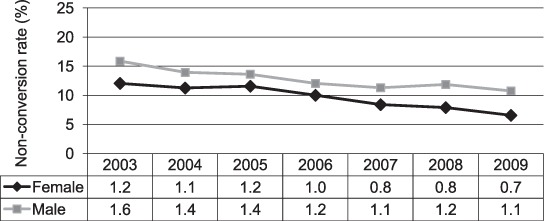
Two-month sputum smear non-conversion rate by year of diagnosis and sex (P < 0.001 for both females and males).
After adjusting for age, males had significantly higher risk of 2-month sputum non-conversion than females in 2003–2004 and 2006–2007 (all P values < 0.05). In 2007, males had higher risk of 2-month sputum smear non-conversion than females (RR 1.35, 95%CI 1.15–1.58). In 2008 and 2009, there were significant interactions between sex and age. Compared to their female counterparts, in 2008 males in the 48–57 years age group had higher risk of non-conversion (RR 1.64, 95%CI 1.06–2.55) and, in 2009 those in the 18–27 years age group had higher risk of non-conversion (RR 1.70, 95%CI 1.25–2.32) (Table 1).
TABLE 1.
Risk of sputum non-conversion for males compared to females, adjusted for age and MDR-TB, 2003–2009
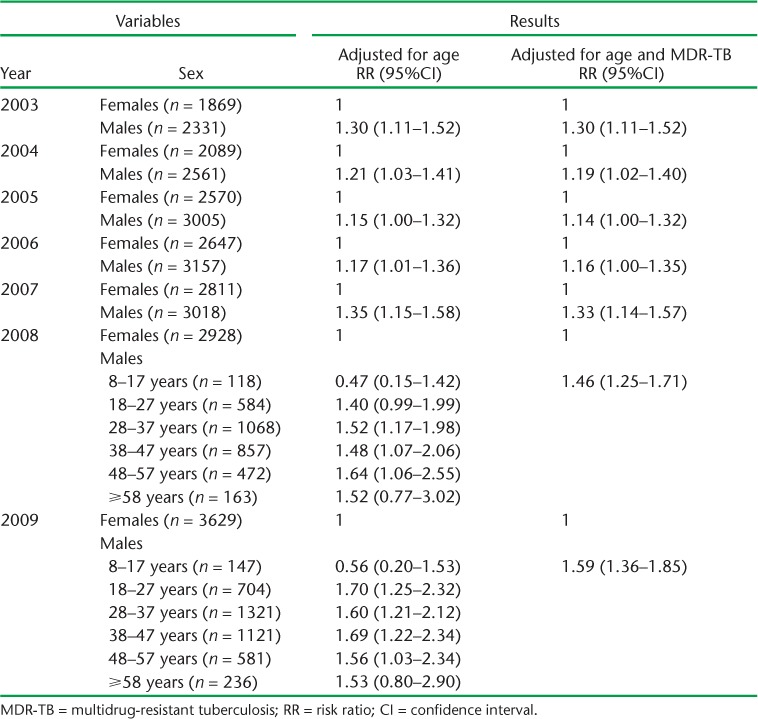
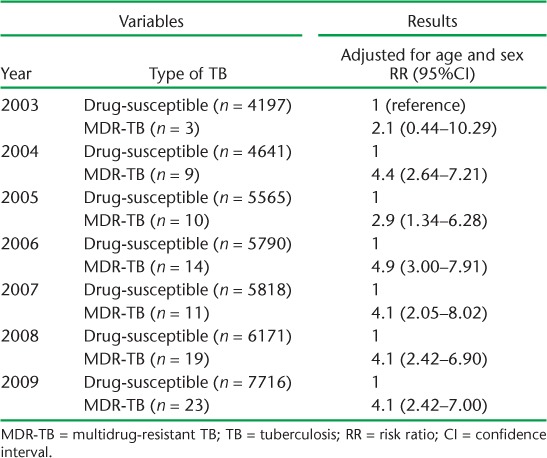
The documentation of resistance to both RMP and INH in the TB treatment register was defined as MDR-TB. Less than 1% of cases (males 0.27%, females 0.17%, P < 0.05) were resistant to both RMP and INH. Only 89 (0.22%) of the 39 987 cases were recorded as having MDR-TB. The risk ratios for 2-month sputum smear non-conversion were similar after adjusting for age only, and when adjusting for age as well as MDR-TB (Table 1). After controlling for sex and age, the highest risk for 2-month sputum smear non-conversion was established in 2006. In this year, MDR-TB cases had a risk ratio of 4.9 (95%CI 3.0–7.9) for sputum smear non-conversion compared to drug-susceptible cases (Table 2).
TABLE 2.
Risk of sputum non-conversion among drug-susceptible compared to MDR-TB cases, adjusted for age and MDR-TB, 2003–2009
Unexpectedly, we found that females in the 8–17 years age group had consistently poorer 2-month conversion rates than their male counterparts throughout the study period. The non-conversion rate consistently declined for the 28–37 years age group in males for all years, with the exception of 2008. Decline in non-conversion rates was less pronounced among males aged >47 years. Minimal decline in non-conversion was also observed among males aged ≥58 years, while in female cases in this age group there was in fact a slight increase in non-conversion (Figure 4).
FIGURE 4.
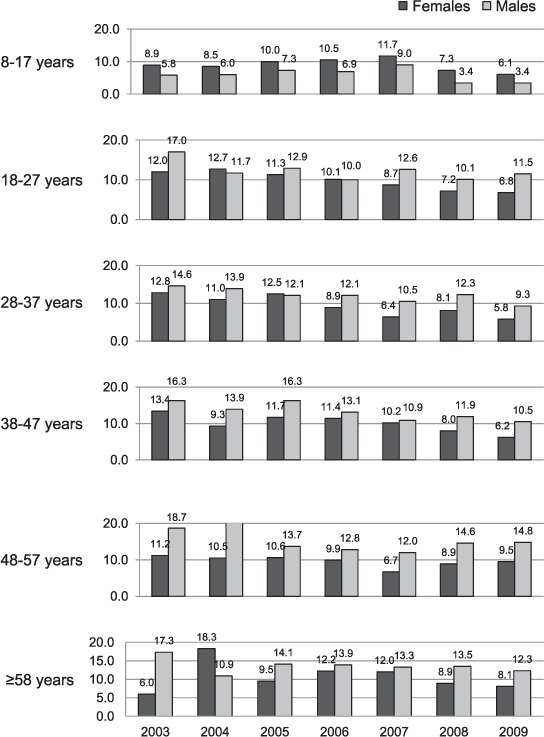
Two-month sputum smear non-conversion rate (%) by year, age and sex.
As HIV status based on rapid antibody tests was routinely captured as part of routine TB data only from 2006, and routine (opt-out) HIV testing for TB patients was effected only in 2007, only 8493 of the 39 987 TB cases with a 2-month sputum smear result had their HIV status recorded. Significantly higher HIV prevalence has been recorded among females than males since 2007 (P < 0.001). Of the 4162 females with recorded HIV test results, 77.9% had a positive status compared to 67.7% of their 4331 male counterparts. HIV status was statistically significantly associated with 2-month sputum smear non-conversion only among males (P < 0.001). After adjusting for age and HIV status, males had a higher risk of sputum smear non-conversion than females in 2008 (RR 1.58, 95%CI 1.25–1.99) and 2009 (RR 1.52, 95%CI 1.27–183).
DISCUSSION
For the overall study period (2003–2009), the 2-month smear non-conversion rates were 12.5% in males and 9.3% in females. There was a steady decline in 2-month smear non-conversion over this period for both males and females. This could probably be ascribed to the prioritisation of TB within the health system, improved TB case management through the DOTS strategy at the provincial and district levels, and better coordination of directly observed treatment at the facility level.27 Moreover, in 2005, the Free State Department of Health launched the TB Management Directorate to empower, manage, evaluate and coordinate TB services at the provincial level.
The overall 2-month sputum smear non-conversion rate was higher in males than females; however, the present study did not ascertain the reasons for this difference. A study among TB patients in India established that women were significantly more likely to access health facilities compared to males and were also more likely to adhere to treatment.28 Another Indian study found that males were less likely to be diagnosed early with TB, and further suggested that sputum non-conversion could be attributed to alcohol consumption and smoking habits.29 This might indicate a need to direct alternative TB control measures such as community-based intensified case finding at males.30,31 Our study results are also consistent with research in Burkina Faso,12 Portugal13 and Tanzania,14 finding an increase in 2-month sputum smear non-conversion with age among males. These findings signal the need for the TB programme to provide (more) support systems and follow-up for (older) male cases.
Future research should address our finding that HIV-negative cases, particularly males, were more likely not to convert to sputum smear-negative at 2 months of treatment. It would seem that integrated TB-HIV treatment and care might be leading to higher 2-month sputum smear non-conversion among HIV-negative than HIV-positive TB cases. In Tanzania, it was found that, after 2 weeks of treatment, conversion was significantly higher in HIV-positive (72.8%) than HIV-negative (63.3%) patients.32 On the other hand, a Ugandan study demonstrated that HIV-positive status is not a principal factor in delaying sputum conversion among patients receiving intensive phase anti-tuberculosis treatment.33
In line with previous research,34,35 our study — admittedly with only a small number (n = 89) of MDR-TB cases — showed that sputum conversion after 2 months was highly significantly associated with MDR-TB (resistance to both RMP and INH) when controlling for sex and age. As there were both higher 2-month sputum smear non-conversion rates and a slightly higher MDR-TB rate among males, future research should consider the programmatic implications of this finding, such as improved case finding and MDR-TB management among males. Future research should further investigate the unexpected finding that only in the 8–17 years age group did females record higher sputum smear non-conversion at 2 months of treatment.
This study has several limitations: first, a substantial proportion of the cases did not have 2-month sputum smear results recorded, although the total number of cases included in the study was still reasonably large. Second, the study concentrated only on 2-month sputum smear non-conversion to gauge the performance of the DOTS strategy. Third, analysis of risk for 2-month sputum smear non-conversion excluded the possible influence of other factors such as pre-treatment bacillary load, disease severity and time to treatment initiation. Further research is therefore needed to ascertain the association of these factors with 2-month sputum smear non-conversion, particularly with regard to male TB cases.
CONCLUSION
We conducted a retrospective cohort study of 2-month sputum smear non-conversion for male and female TB cases over a 7-year period. Age played a significant role in 2-month sputum smear non-conversion among males. Although the results showed a significant decline in 2-month sputum smear non-conversion for both sexes, male TB cases were at significantly higher risk of non-conversion. It would seem that the DOTS programme in the Free State Province, with more than 90% coverage, might not be as efficient among males as in females. Given the importance of 2-month sputum conversion as an indicator of TB programme performance, the male-female differential is of concern. These findings suggest that the TB DOTS programme in the Free State needs to pay closer attention and provide better support to male TB patients to adhere to treatment. Programme efforts should also be directed towards early MDR-TB diagnosis and improved management, in an attempt to reduce the rate of 2-month sputum smear non-conversion.
Acknowledgments
This research was supported by a United States Agency for International Development (USAID, Washington DC, USA) Cooperative Agreement (TREAT TB Agreement No GHN-A-00-08-00004-00). The contents are the responsibility of the authors and do not necessarily reflect the views of USAID. The Free State Department of Health is thanked for supporting this research. Professor C J Lombard from Medical Research Council, Cape Town, South Africa is thanked for his comments.
Footnotes
Conflict of interest: none declared.
References
- 1.World Health Organization. The Stop TB Strategy: building on and enhancing DOTS to meet the TB-related Millennium Development Goals. Geneva, Switzerland: WHO; 2006. WHO/HTM/TB/2006.368. [Google Scholar]
- 2.World Health Organization. Global tuberculosis control. Geneva, Switzerland: WHO; 2011. WHO Report 2011. WHO/HTM/TB/2011.16. [Google Scholar]
- 3.World Health Organization. Global tuberculosis control: epidemiology, strategy, financing. Geneva, Switzerland: WHO; 2009. WHO Report 2009. WHO/HTM/TB/2009.411. [Google Scholar]
- 4.World Health Organization. Global tuberculosis control, 2012. Geneva, Switzerland: WHO; 2012. WHO/HTM/TB/2012.6. [Google Scholar]
- 5.Trébucq A, Rieder H L. Two excellent tools for national tuberculosis programmes: history of previous treatment and sputum status at two-months. Int J Tuberc Lung Dis. 1998;2:184–186. [PubMed] [Google Scholar]
- 6.Department of Health, South Africa. National tuberculosis management guidelines. Pretoria: DoH; 2009. [Google Scholar]
- 7.Enarson D, Rieder H, Arnadottir T, Trébucq A. Management of tuberculosis. A guide for low-income countries. 5th ed. Paris, France: International Union Against Tuberculosis and Lung Disease; 2000. [Google Scholar]
- 8.Kuaban C, Bame R, Mouangue L, Djella S, Yomgni C. Non conversion of sputum smears in new smear positive pulmonary tuberculosis patients in Yaoundé, Cameroon. East Afr Med J. 2009;86:219–225. doi: 10.4314/eamj.v86i5.54192. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Compendium of indicators for monitoring and evaluating national tuberculosis programs. Geneva, Switzerland: WHO; 2006. WHO/HTM/TB/2004.344. [Google Scholar]
- 10.Department of Health, South Africa. Final tuberculosis report. Pretoria: DoH; 2009. [Google Scholar]
- 11.Güler M, Unsal E, Dursun B, Aydln O, Capan N. Factors influencing sputum smear and culture conversion time among patients with new case pulmonary tuberculosis. Int J Clin Pract. 2007;61:231–235. doi: 10.1111/j.1742-1241.2006.01131.x. [DOI] [PubMed] [Google Scholar]
- 12.Dembele S M, Ouedraogo H Z, Combary A, Saleri N, Macq J, Dujardin B. Conversion rate at two-month follow-up of smear-positive tuberculosis patients in Burkina Faso. Int J Tuberc Lung Dis. 2007;2:1339–1344. [PubMed] [Google Scholar]
- 13.Caetano Mota P, Carvalho A, Valented I, Bragad R, Duarte R. Predictors of delayed sputum smear and culture conversion among a Portuguese population with pulmonary tuberculosis. Rev Port Pneumol. 2012;18:72–79. doi: 10.1016/j.rppneu.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Senkoro M, Mfinanga S G, Mørkve O. Smear microscopy and culture conversion rates among smear positive pulmonary tuberculosis patients by HIV status in Dar es Salaam, Tanzania. BMC Infect Dis. 2010;10:210. doi: 10.1186/1471-2334-10-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J Y, Lee L N, Yu C J, Chien Y J, Yang P C, Tami Group Factors influencing time to smear conversion in patients with smear-positive pulmonary tuberculosis. Respirology. 2009;14:1012–1019. doi: 10.1111/j.1440-1843.2009.01598.x. [DOI] [PubMed] [Google Scholar]
- 16.Singla R, Osman M M, Khan N, Al-Sharif N, Al-Sayegh M O, Shaikh M A. Factors predicting persistent sputum smear positivity among pulmonary tuberculosis patients 2 months after treatment. Int J Tuberc Lung Dis. 2003;7:58–64. [PubMed] [Google Scholar]
- 17.Austin J F, Dick J M, Zwarenstein M. Gender disparity amongst TB suspects and new TB patients according to data recorded at the South African Institute of Medical Research laboratory for the Western Cape Region of South Africa. Int J Tuberc Lung Dis. 2004;8:435–439. [PubMed] [Google Scholar]
- 18.Caetano Mota P, Carvalho A, Valente I, Braga R, Duarte R. Predictors of delayed sputum smear and culture conversion among a Portuguese population with pulmonary tuberculosis. Rev Port Pneumol. 2012;18:72–79. doi: 10.1016/j.rppneu.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Gopi P G, Chandrasekaran V, Subramani R et al. Association of conversion & cure with initial smear grading among new smear positive pulmonary tuberculosis patients treated with Category I regimen. Indian J Med Res. 2006;123:807–814. [PubMed] [Google Scholar]
- 20.Day C, Gray A. Health and related indicators. In: Padarath A, English R, editors. South African Health Review 2012/13. Durban, South Africa: Health Systems Trust; 2013. pp. 207–322. [Google Scholar]
- 21.Day C, Gray A, Budgell E. Health and related indicators. In: Padarath A, English R, editors. South African Health Review 2011. Durban, South Africa: Health Systems Trust; 2011. pp. 119–247. [Google Scholar]
- 22.Dogar O F, Shah S K, Chughtai A A, Qadeer E. Gender disparity in tuberculosis cases in eastern and western provinces of Pakistan. BMC Infect Dis. 2012;12:244. doi: 10.1186/1471-2334-12-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukherjee A, Saha I, Sarkar A, Chowdhury R. Gender differences in notification rates, clinical forms and treatment outcome of tuberculosis patients under the RNTCP. Lung India. 2012;29:120–122. doi: 10.4103/0970-2113.95302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karim F, Islam A, Chowdhury A M R, Johansson E, Diwan V K. Gender differences in delays in diagnosis and treatment of tuberculosis. Health Policy Plan. 2007;22:329–334. doi: 10.1093/heapol/czm026. [DOI] [PubMed] [Google Scholar]
- 25.Neyrolles O, Quintana-Murci L. Sexual inequality in tuberculosis. PLOS MED. 2009;6:e1000199. doi: 10.1371/journal.pmed.1000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng J Y, Huang S F, Ting W Y et al. Gender differences in treatment outcomes of tuberculosis patients in Taiwan: a prospective observational study. Clin Microbiol Infect. 2012;18:E331–E333. doi: 10.1111/j.1469-0691.2012.03931.x. [DOI] [PubMed] [Google Scholar]
- 27.Van Rensburg H C J, Janse van Rensburg-Bonthuyzen E, Heunis J C, Meulemans H. Tuberculosis control in South Africa: reasons for persistent failure. Acta Academica. 2005:1–55. [Google Scholar]
- 28.Rekha B V V, Balasubramanian R, Swaminathan S et al. Sputum conversion at the end of intensive phase of Category 1 regimen in the treatment of pulmonary tuberculosis patients with diabetes mellitus or HIV infection. An analysis of risk factors. Indian J Med Res. 2007;126:452–458. [PubMed] [Google Scholar]
- 29.Balasubramanian R, Garg R, Santha T et al. Gender disparities in tuberculosis: report from a rural DOTS programme in south India. Int J Tuberc Lung Dis. 2004;8:323–332. [PubMed] [Google Scholar]
- 30.Becerra M C, Pacheo-Torreblanca I F, Boyona J et al. Expanding tuberculosis case detection by screening household contacts. Public Health Rep. 2005;120:271–277. doi: 10.1177/003335490512000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corbett E L, Bandason T, Duong T et al. Comparison of two active case-finding strategies for community based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DE-TECTB): a cluster-randomised trial. Lancet. 2010;376:1244–1253. doi: 10.1016/S0140-6736(10)61425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Senkoro M, Mfinanga S G, Mørkve O. Smear microscopy and culture conversion rates among smear positive pulmonary tuberculosis patients by HIV status in Dar es Salaam, Tanzania. BMC Infect Dis. 2010;10:210. doi: 10.1186/1471-2334-10-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bwire R, Borgdorff M W, Sticht-Groh V et al. Tuberculosis chemotherapy and sputum conversion among HIV-seropositive and HIV-seronegative patients in south-eastern Uganda. East Afr Med J. 1999;76:307–313. [PubMed] [Google Scholar]
- 34.Flora M S, Amin M N, Karim M R et al. Risk factors of multidrug-resistant tuberculosis in Bangladeshi population: a case control study. Bangladesh Med Res Counc Bull. 2013;39:34–41. doi: 10.3329/bmrcb.v39i1.15808. [DOI] [PubMed] [Google Scholar]
- 35.Streicher E M, Müller B, Chihota V et al. Emergence and treatment of multi-drug resistant (MDR) and extensively drug-resistant (XDR) tuberculosis in South Africa. Infect Genet Evol. 2012;12:686–694. doi: 10.1016/j.meegid.2011.07.019. [DOI] [PubMed] [Google Scholar]


