Abstract
Setting: Two subdistricts in Bangladesh, Fulbaria and Trishal, which are hyperendemic for leishmaniasis.
Objective: To determine 1) the numbers of patients diagnosed with visceral leishmaniasis (VL) and post-kala azar dermal leishmaniasis (PKDL) using an active case detection (ACD) strategy in Fulbaria and a passive case detection (PCD) strategy in Trishal, and 2) the time taken from symptoms to diagnosis in the ACD subdistrict.
Design: A cross-sectional descriptive study of patients diagnosed from May 2010 to December 2011. The ACD strategy involved community education and outreach workers targeting households of index patients using symptom-based screening and rK-39 tests for suspected cases.
Results: In the ACD subdistrict (Fulbaria) and PCD sub-district (Trishal), respectively 1088 and 756 residents were diagnosed with VL and 1145 and 37 with PKDL. In the ACD subdistrict, the median time to diagnosis for patients directly referred by outreach workers or self-referred was similar, at 60 days for VL and respectively 345 and 360 days for PKDL.
Conclusion: An ACD strategy at the subdistrict level resulted in an increased yield of VL and a much higher yield of PKDL. As PKDL acts as a reservoir for infection, a strategy of ACD and treatment can contribute to the regional elimination of leishmaniasis in the Indian sub-continent.
Keywords: Bangladesh, visceral leishmaniasis, post-kala azar dermal leishmaniasis, case detection strategies
Abstract
Contexte : Deux sous-districts du Bangladesh, Fulbaria et Trishai, où la leishmaniose est hyper-endémique.
Objectif : Déterminer 1) le nombre de patients ayant eu un diagnostic de leishmaniose viscérale (VL) et de leishmaniose dermique post-kala azar (PKDL) grâce à une stratégie de détection active des cas (ACD) à Fulbaria et à une stratégie de détection passive (PCD) à Trishai, et 2) le temps écoulé entre les symptômes et le diagnostic dans le sous-district à ACD.
Schéma : Etude descriptive transversale des patients diagnostiqués entre mai 2010 et décembre 2011. La stratégie ACD comportait une éducation des communautés et des stratégies avancées ciblant les foyers des patients index grâce à un dépistage basé sur les symptômes et au test rK-39 pour les patients suspects.
Résultats : Dans les districts de stratégie ACD (Fulbaria) et le sous-district de stratégie PCD (Trishai), 1088 et 756 patients respectivement ont eu un diagnostic de VL et 1145 et 37 respectivement ont eu un diagnostic de PKDL. Dans ce sous-district, le délai médian de diagnostic était de 60 jours pour tous les patients atteints de VL, qu'ils soient référés par du personnel des stratégies avancées ou viennent d'eux-mêmes. Il était respectivement de 345 et 360 jours pour la PKDL.
Conclusion : Une stratégie ACD au niveau d'un sous-district permet de dépister un nombre accru de VL et encore plus de PKDL. Comme la PKDL constitue un réservoir d'infection, la stratégie d'ACD et le traitement des cas dépistés peuvent contribuer à l'élimination régionale de la leishmaniose du sous-continent indien.
Abstract
Marco de referencia: Los subdistritos de Fulbaria y Trishal en Bangladesh, donde es hiper-endémica la leishmaniasis.
Objetivo: Determinar: 1) el número de pacientes con diagnóstico de leishmaniasis visceral (VL) y de leishmaniasis cutánea pos kala azar (PKDL) mediante una estrategia de detección activa de casos (ACD) en Fulbaria y una detección pasiva (PCD) en Trishal; y 2) y el lapso entre la aparición de los síntomas y el diagnóstico en el subdistrito que aplicó la estrategia ACD.
Método: Se llevó a cabo un estudio transversal descriptivo de los pacientes en quienes se estableció el diagnóstico de leishmaniasis entre mayo del 2010 y diciembre del 2011. Como parte de la estrategia ACD se impartió educación a la comunidad y participaron trabajadores extrainstitucionales que contactaban a los hogares de los casos iniciales y practicaban una detección basada en los síntomas y la prueba rK-39 de diagnóstico rápido en los casos de presunción diagnóstica.
Resultados: En el subdistrito de Fulbaria la estrategia ACD permitió el reconocimiento de 1088 residentes con VL y 1145 con PKDL; en el subdistrito de Trishal se detectaron mediante la estrategia PCD 756 y 37 casos, respectivamente. La mediana del lapso necesario hasta establecer el diagnóstico de VL en los pacientes remitidos directamente por los trabajadores periféricos fue análoga a la mediana del lapso en los que se presentaron por su propia iniciativa y correspondió a 60 días en casos de VL; en los pacientes con PKDL la mediana del lapso fue 360 días en los pacientes remitidos por los trabajadores periféricos y 360 en los pacientes que acudieron por su propia cuenta.
Conclusión: Una estrategia ACD a escala del subdistrito ofrece un mayor rendimiento diagnóstico de la VL y un incremento aun mayor del diagnóstico de PKDL. Dado que la PKDL representa un reservorio de la infección, una estrategia ACD y tratamiento puede contribuir a la eliminación regional de la leishmaniasis en el subcontinente indio.
Visceral leishmaniasis (VL, also known as kala-azar) is the second largest parasitic killer in the world after malaria, responsible for an estimated 200 000 – 400 000 cases each year worldwide;1 90% of the global burden occurs in just six countries: India, Bangladesh, Sudan, South Sudan, Ethiopia and Brazil. The infecting parasite is of the Leishmania donovani complex, and infection occurs in humans as a result of being bitten by an infected female Phlebotomine sandfly. In VL, the disease is visceral, with the parasites migrating to internal organs; signs and symptoms include fever, weight loss, cachexia and anaemia.
The gold standard for the diagnosis of VL is the demonstration of amastigotes in tissue aspirates from the spleen, followed by bone marrow or lymph nodes; however, due to technical constraints, serology is now more frequently used.2 In areas where leishmaniasis is endemic, the rK-39 dipstick test (Kalazar Detect® Rapid Test; InBios International, Seattle, WA, USA) is therefore used with finger-prick blood samples; in SouthEast Asia, this has excellent sensitivity (99–100%) and specificity (96–98%).3,4 The treatment of choice was traditionally with pentavalent antimonials; however, this therapy is toxic, associated with iatrogenic mortality, and resistance has developed, particularly in India, resulting in treatment failure. Current treatment regimens now include various preparations of amphotericin B and miltefosine.
Post-kala azar dermal leishmaniasis (PKDL) is a sequela of VL that manifests as a skin rash in a patient after successful treatment for VL who is otherwise well. The rash is characteristic and usually macular in nature, and the diagnosis is mainly clinical. Available confirmatory tests are not very sensitive for macular lesions. PKDL is a potential reservoir for ongoing transmission of infection,5 particularly in the Indian subcontinent.
In Bangladesh, humans are the only known reservoir of the infection and therefore, with early diagnosis and treatment of VL and PKDL, it should be possible to reduce the incidence rates of these diseases drastically. The Government of Bangladesh aims to reduce the annual incidence of VL at the subdistrict level to below 1 per 10 000 by 2015.6 The detection and diagnosis of VL and PKDL in Bangladesh is mainly through a passive case detection strategy. In the case of PKDL, few patients come forward for diagnosis and treatment, as in most cases it presents only as hypopigmented macules that do not cause physical impairment. As a result, many PKDL cases in the community are undiagnosed and untreated and remain a source of Leishmania transmission.
Médecins Sans Frontières (MSF) started working in the Fulbaria subdistrict of Mymensingh District in Bangladesh in April 2010, with the specific aim of controlling VL and PKDL through an active case detection (ACD) strategy combined with provision of free treatment for both VL and PKDL with short-course liposomal amphotericin B.
The aim of the present study was to describe the results of the ACD strategy for VL and PKDL at the subdistrict level in Bangladesh, and to compare the results with those obtained in a neighbouring subdistrict where a passive case detection (PCD) strategy has remained standard practice. Specific objectives were to determine 1) the numbers of patients, stratified by age and sex, diagnosed with VL and PKDL in two subdistricts, one with an ACD and the other with a PCD strategy, and 2) in the ACD subdistrict, the length of time from onset of symptoms to diagnosis.
METHODS
Study design
This is a cross-sectional descriptive study based on record review.
Setting
General
Bangladesh is a country with a population of 170 million, 40% of whom live below the poverty line.7 VL is endemic in 41 districts in the country, with an annual VL case burden of approximately 6000 over the past 10 years.8,9 However, the epicentre of the disease is in Mymensingh District, which accounts for approximately 60% of all cases in the country.
Study sites
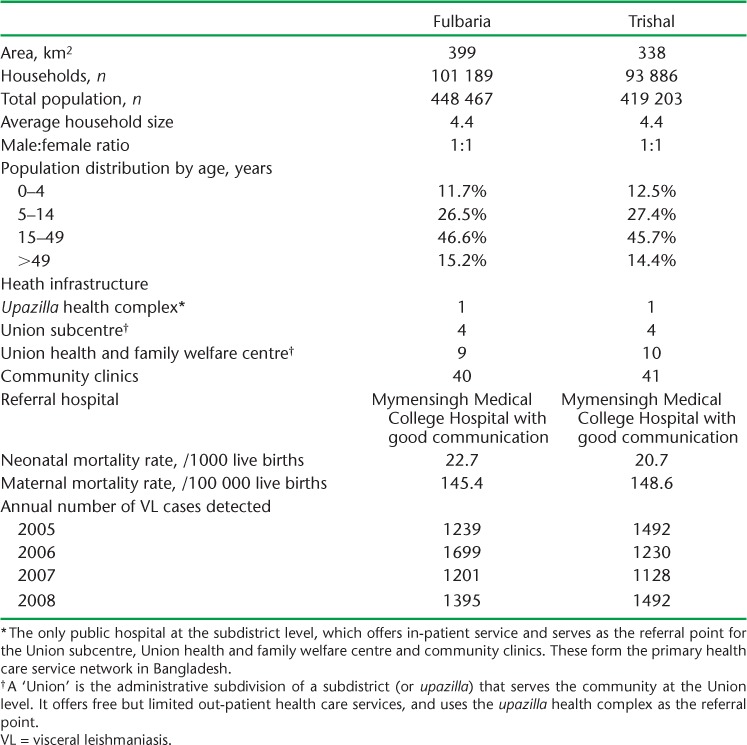
Mymensingh District, the focus of the study, is situated approximately 150 km north of Dhaka, the capital city. In 2007, 80% of VL cases from Mymensingh District occurred in just two subdistricts, Fulbaria and Trishal. These two subdistricts are adjacent to each other and comparable socio-demographically and geographically. The population of each of these subdistricts at the time of the study was approximately 400 000,7 with most people living in rural settings. Table 1 shows the socio-demographic characteristics of the population, the health care infrastructure of the two subdistricts and the annual case burden of VL in the two subdistricts between 2005 and 2008.
TABLE 1.
Socio-demographic characteristics, access to health care and annual case burden of VL in Fulbaria and Trishal subdistricts, Bangladesh
The PCD subdistrict (Trishal)
In Trishal subdistrict, patients were diagnosed and treated using a PCD strategy. Patients self-referred to health centres, where diagnosis and treatment were carried out using the same diagnostic tests as the intervention district but with different treatment regimens based on pentavalent antimony and miltefosine. Leishmaniasis-specific community education was non-existent. In Trishal, all data were kept in a general VL and PKDL register in the Upazilla health complex (UHC).
The ACD subdistrict (Fulbaria)
In May 2010, the MSF started a leishmaniasis project in Fulbaria subdistrict with an ACD strategy superimposed on the PCD system. The MSF Operational Centre Amsterdam recruited and trained outreach workers (ORWs) on how to identify a suspected case of VL and PKDL and perform rK-39 tests in the field. The ORWs were based at village level and visited households on a regular basis to screen for VL and PKDL. ORWs with at least 12 years of formal school education received both theoretical and hands-on training from the MSF. The number of ORWs over the course of the project varied from 25 to 35.
Screening was carried out in the households of index patients treated at the MSF clinic. Using this approach, the house of an index patient was visited, as were all other households within a 200 m radius, called a cluster. The approach is based on the flight characteristics of the sandfly.10 In this targeted approach, MSF ORWs visited each cluster every 2 months up to three or four times until it was decided that no further cases could be found. When multiple patients came from the same household or from a close neighbourhood, instead of creating multiple new clusters, only one cluster was created around that household or neighbourhood, which was subsequently visited three or four times. During the study period, 1858 new clusters (37 160 households with a population of 163 504) were covered. Of all the clusters, 68% were visited ⩾3 times, and 75% of the new clusters were formed within 14 days after the index case was diagnosed as having either VL or PKDL.
Screening was carried out using a symptom- and sign-based strategy. After identification of a clinically suspect person, a serological test was performed in the home using an rK39 dipstick. The positivity rate of the rK-39 tests performed by the ORWs in the community was approximately 40% for VL and >75% for suspected PKDL cases. If positive, the patient was referred to the MSF-OCA clinic for clinical assessment and diagnosis by a physician and treatment. PKDL was diagnosed on clinical grounds and by a positive rK-39 test. If VL and PKDL were confirmed either clinically or by identifying amastigotes in tissue aspirates, treatment was given with liposomal amphotericin B.
In addition to screening, health education on VL and PKDL was provided to the community.
Since the project started in April 2010, the MSF has treated approximately 1400 VL and 1300 PKDL patients. All clinical, diagnostic and treatment data are kept in medical files, and a special electronic database is maintained. In addition, the outreach data were kept in a register that also served as a source of information.
Study population
All patients, irrespective of age and sex, who were diagnosed in the period between May 2010 and December 2011 with VL and PKDL and were residents of Fulbaria and Trishal were included in the study.
VL and PKDL were defined as follows: a person from an endemic area with a history of fever for >2 weeks, weight loss and splenomegaly and either a positive serological test rK-39 or with parasites found in aspirated specimens of spleen, was considered to have VL.8 A person with a previous history of VL with typical PKDL lesions (hypopigmented dermal lesions which can be macular, papular, nodular or of mixed type), with no loss of skin sensation and with a positive rK-39 test was diagnosed as PKDL.8
Data variables, data collection instrument and sources of data
The variables collected for confirmed VL and PKDL cases included location, age, weight, sex, address, date of onset of symptoms, date of diagnosis, diagnosis, date of start of treatment, the number of households visited by ORWs for each case and the number of patients diagnosed and admitted for treatment. For PKDL, the time between onset of symptoms and diagnosis was collected in months and converted to days for presentation. Data were collected into a structured proforma. The sources of data were the patient database and outreach registration books for Fulbaria (ACD), and the VL/PKDL register kept in the Trishal Upazilla Health Complex and the annual overview record kept at the Ministry of Health in Dhaka for Trishal (PCD).
Analysis and statistics
Data from the structured proforma were double entered into an EpiData version 3.1 (EpiData Association, Odense, Denmark) and analysed using SPSS version 11.5 (Statistical Product and Service Solutions, Chicago, IL, USA) and Microsoft Excel 2007 (Microsoft, Redmond, WA, USA). Simple frequency analysis was conducted and tables were constructed in a categorical way and presented in charts and diagrams to describe the study population. Some of the continuous variables were converted into categorical variables for further statistical tests using the χ2 test with 95% confidence intervals as appropriate. Levels of significance were set at 5% (P < 0.05).
Ethics approval
This study met the MSF (Geneva, Switzerland) Ethics Review Board-approved criteria for analysis of routinely collected program data. It was also approved by the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France. Permission was obtained from the Director of Communicable Disease Control, Ministry of Health, Dhaka, Bangladesh.
RESULTS
Respectively 2181 and 1316 patients were diagnosed with VL and PKDL in the two subdistricts. Figure 1 shows the number of patients diagnosed with VL who were residents, and of these, the number identified in the ACD and PCD subdistricts. Of all the residential patients, 59% were detected in the ACD subdistrict. However, there was contamination of patients between the two subdistricts, with 170 (16%) VL patients living on the border of the PCD subdistrict being diagnosed in the ACD subdistrict and three (<1%) patients living on the border of the ACD subdistrict being diagnosed in the PCD subdistrict. Figure 2A shows the number of patients diagnosed with VL each month during the pre-study period in 2008 in Fulbaria (n = 1395) and Trishal (n = 1492), when PCD was the normal form of screening in both the subdistricts. More VL cases were detected overall in Trishal, but the seasonal variation was similar in the two subdistricts. Figure 2B shows the number of patients diagnosed with VL during the study period in Fulbaria and in Trishal with the ACD and PCD strategies, respectively. Although seasonal variation was again similar, this time more VL cases were diagnosed in the ACD subdistrict.
FIGURE 1.
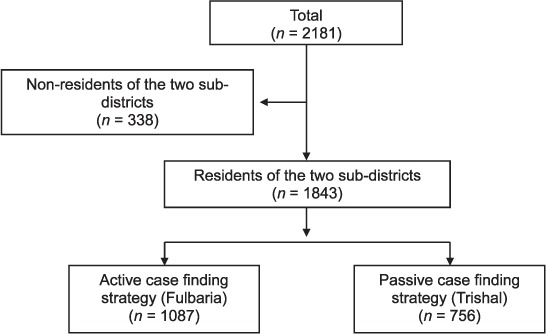
Patients with visceral leishmaniasis detected using active and passive case-finding strategies in two hyperendemic subdistricts of Bangladesh, May 2010 – December 2011.
FIGURE 2.
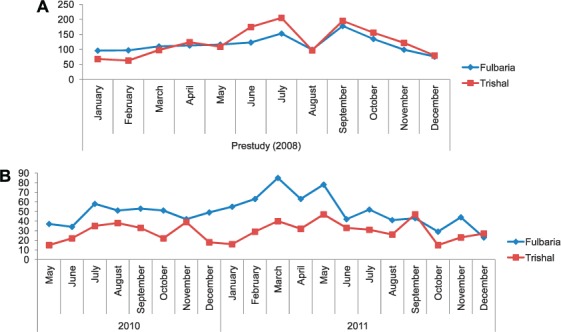
Number of patients detected each month with VL in Fulbaria and Trishal subdistricts before and during the study periods. A) Monthly VL cases detected in 2008 (passive case detection by MoH in both subdistricts); B) monthly VL cases detected in 2010 and 2011 in Fulbaria (active case detection by Médecin Sans Frontières) and in Trishal (passive case detection by MoH) during the study period. VL = visceral leishmaniasis; MoH = Ministry of Health.
Figure 3 shows the number of patients diagnosed with PKDL who were residential, and of these, the number identified in the ACD and PCD subdistricts. Of all the residential patients, 97% were detected in the ACD subdistrict. There was also contamination of patients between the two subdistricts, with 70 (6%) PKDL patients from the PCD subdistrict who lived on the border being diagnosed in the ACD subdistrict and none vice versa. In the ACD subdistrict, 195 (18%) patients with VL and 667 (58%) with PKDL were directly referred by ORWs to the MSF treatment centre, while the remaining patients were self-referred.
FIGURE 3.
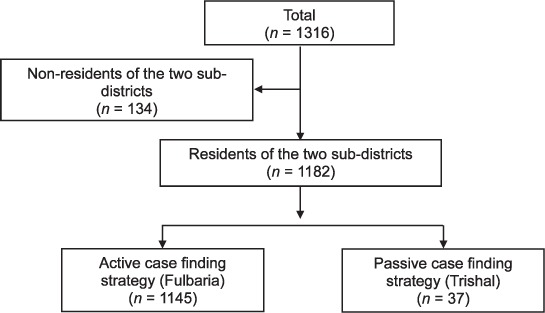
The number of patients with post-kala azar dermal leishmaniasis detected using active and passive case-finding strategies in the two hyperendemic subdistricts of Bangladesh, May 2010 – December 2011.
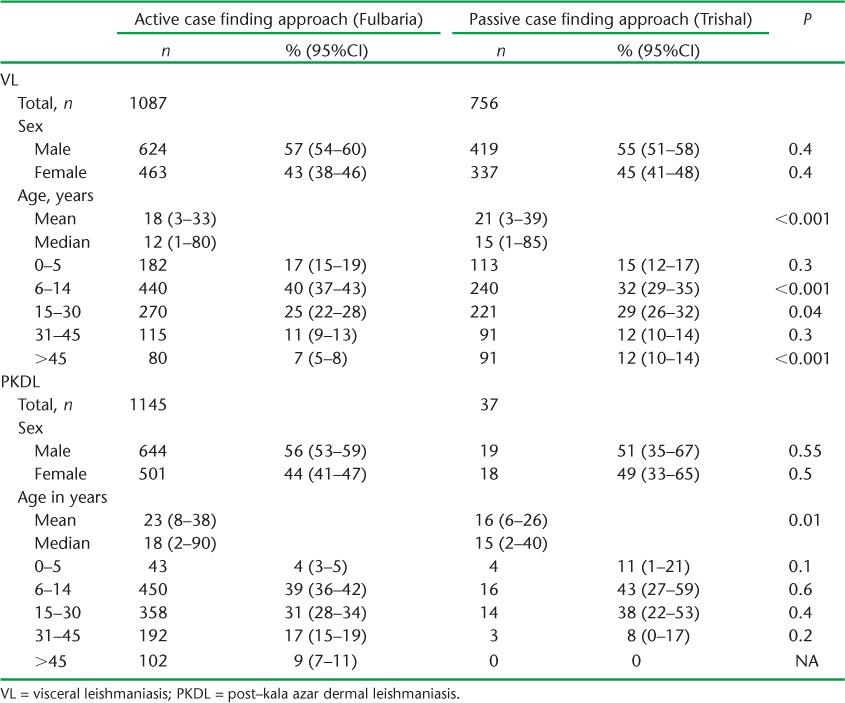
The mean age of the patients with VL and PKDL was respectively 18 (standard deviation [SD] ± 15) and 23 years (SD ± 15) in the ACD subdistrict, and 21 (SD ± 18) and 16 years (SD ± 10) in the PCD subdistrict. Demographic characteristics in terms of sex and age groups between the two subdistricts in patients with VL and PKDL are shown in Table 2. With VL, the proportions of males and females were similar, but there were some significant (although not important) differences in proportions of patients in the different age groups. With PKDL, no differences were found.
TABLE 2.
Patients detected with VL and PKDL, stratified by sex and age according to active and passive case-finding approaches in Fulbari and Trishal subdistricts, Bangladesh, May 2010 to December 2011
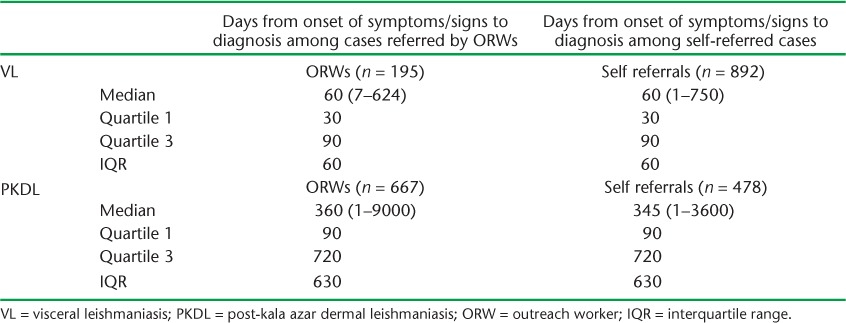
The median time from onset of symptoms and/or signs to diagnosis in patients referred by ORWs and those self-referred in the ACD subdistrict is shown in Table 3. In patients with VL, the median time to diagnosis in the two groups was similar, at 60 days. In patients with PKDL, the median time to diagnosis was also similar, at 360 and 345 days for the two groups.
TABLE 3.
Time from onset of symptoms to diagnosis in patients with VL and PKDL in a subdistrict of Bangladesh using an active case-finding strategy
DISCUSSION
This is the first report to compare the use of ACD and PCD strategies in the detection of VL and PKDL in a routine setting of two subdistricts in Bangladesh. The number of cases diagnosed with VL was generally similar in the two subdistricts when a PCD strategy was used in both. However, the number of VL cases increased when an ACD strategy was introduced. A striking finding was the considerably higher number of cases diagnosed with PKDL when an ACD strategy was introduced.
These findings make intuitive sense. VL is a systemic disease characterised by fever, weight loss and wasting, and these symptoms are likely to drive patients to seek health care whether or not they have been screened by ORWs, who in any case conduct targeted outreach activities once every 2 months. It was interesting to note that the median time from onset of symptoms to diagnosis in patients in the ACD subdistrict was 2 months whether they were referred directly by an ORW or self-referred, which corroborates this interpretation.
In contrast, PKDL is a skin disease, and the patient feels otherwise well; an ACD strategy may thus be more likely to result in patients being referred to health facilities for diagnosis and care. In the ACD subdistrict, nearly 60% of the patients with PKDL were directly referred by ORWs to the treatment centre, but the remainder were self-referred, which is probably a positive effect of the health education conducted in the community. The long delay of almost 1 year between onset of skin lesions and diagnosis reflects the benign nature of this disease.
The strengths of this study were the large size and comparability of the subdistricts in terms of socio-demographic characteristics and health infrastructure, the large number of patients diagnosed with VL and PKDL and the excellent database and monitoring system set up in the ACD subdistrict. The limitations relate to the operational nature of the study, in which patients from Trishal (the PCD subdistrict) living on the border areas came over to Fulbaria (ACD subdistrict), providing some degree of cross-contamination between the two strategies. However, this type of practice, with transferrals from one administrative area to another to seek diagnosis and care, is common in low- and middle-income countries, and is an operational reality in the field. The other limitations relate to the different treatment regimens used in the two subdistricts, and the record keeping of the PCD subdistrict, which was inferior to that of the other subdistrict and also did not allow collection of data on time taken from onset of symptoms to diagnosis for comparison with the ACD subdistrict.
ACD has been recommended as one of the five pillars of VL elimination, combined with early diagnosis and treatment, integrated vector management and surveillance, social mobilisation and building partnerships and clinical and operational research.11 Our study shows that ACD is superior to PCD in the detection of VL, although the head-to-head comparison between the two districts was difficult due to the limitations mentioned above. Studies assessing ACD for VL in Asia, including Bangladesh, have shown more positive yields than in our study.12,13 However, these were conducted in research settings and sometimes used incentives. Screening of the whole community and using incentives may be difficult to implement and sustain at the routine level.
In contrast, we found that an ACD strategy using ORWs was associated with a considerably higher yield of PKDL. Similar results under research settings and using trained village volunteers have been reported from elsewhere in Bangladesh,14 and this confirms our belief that such an approach is effective. As PKDL acts as a reservoir of infection, this strategy can contribute to the elimination of leishmaniasis.
In conclusion, this study conducted in leishmaniasis-endemic subdistricts in Bangladesh shows that an ACD strategy using ORWs and a symptom- or sign-based approach results in an increase yield of VL and a considerably higher yield of PKDL. This approach should be promoted more widely, as it may contribute substantially to the elimination of leishmaniasis in South-East Asia.15
Acknowledgments
This research was supported through an operational research course that was jointly developed and run by the Operational Research Unit (Luxembourg), Médecins Sans Frontières, Brussels-Luxembourg, The Centre for Operational Research, International Union Against Tuberculosis and Lung Disease (The Union), Paris, France, and The Union South-East Asia Regional Office, New Delhi, India. Additional support for running the course was provided by the Centre for International Health, University of Bergen, Bergen, Norway, and the Institute of Tropical Medicine, Antwerp, Belgium. Funding for the course was from an anonymous donor, the Department for International Development, London, UK and MSF, Luxembourg.
Footnotes
Conflict of interest: none declared.
References
- 1.Alvar J, Velez I D, Bern C et al. Leishmaniasis worldwide and global estimates of its incidence. PLOS ONE. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sundar S, Rai M. Laboratory diagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol. 2002;9:951–958. doi: 10.1128/CDLI.9.5.951-958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohapatra T M, Singh D P, Sen M R, Bharti K, Sundar S. Comparative evaluation of rK9, rK26 and rK39 antigens in the serodiagnosis of Indian visceral leishmaniasis. J Infect Dev Ctries. 2010;4:114–117. doi: 10.3855/jidc.544. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham J, Hasker E, Das P et al. A global comparative evaluation of commercial immunochromatographic rapid diagnostic tests for visceral leishmaniasis. Clin Infect Dis. 2012;55:1312–1319. doi: 10.1093/cid/cis716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahman K M, Islam S, Rahman M W et al. Increasing incidence of post-kala azar dermal leishmaniasis in a population-based study in Bangladesh. Clin Infect Dis. 2010;50:73–76. doi: 10.1086/648727. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization South-East Asia Regional Office. New Delhi, India: 2005. Intercountry Meeting of National Programme Managers for Kala-azar Elimination. Behror, India, 1–2 September 2005. SEA–VBC–89. http://209.61.208.233/LinkFiles/Kala_azar_VBC-89.pdf Accessed March 2014. [Google Scholar]
- 7.Bangladesh Bureau of Statistics. Dhaka, Bangladesh: Bangladesh Bureau of Statistics; 2011. http://www.bbs.gov.bd/WebTestApplication/userfiles/Image/Census2011/Dhaka/Dhaka/Dhaka_C01.pdf Accessed January 2014. [Google Scholar]
- 8.Bern C, Chowdhury R. The epidemiology of visceral leishmaniasis in Bangladesh: prospects for improved control. Indian J Med Res. 2006;123:275–288. [PubMed] [Google Scholar]
- 9.Rahman R, Bangali M, Kabir H, Naher F B, Mahboob S. Kala-azar situation in Bangladesh. National guideline and training module for kala-azar elimination in Bangladesh. 1st ed. Dhaka, Bangladesh: Centers for Disease Control and Prevention, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of the Peoples' Republic of Bangladesh; 2008. [Google Scholar]
- 10.Bern C, Hightower A W, Chowdhury R et al. Risk factors for kala-azar in Bangladesh. Emerg Infect Dis. 2005;11:655–662. doi: 10.3201/eid1105.040718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joshi A, Narain J P, Prasittisuk C et al. Can visceral leishmaniasis be eliminated from Asia? J Vector Borne Dis. 2008;45:105–111. [PubMed] [Google Scholar]
- 12.Singh S P, Hirve S, Huda M M et al. Options for active case detection of visceral leishmaniasis in endemic districts of India, Nepal, and Bangladesh, comparing yields, feasibility and costs. PLOS Negl Trop Dis. 2011;5:e960. doi: 10.1371/journal.pntd.0000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huda M M, Hirve S, Siddiqui N A et al. Active case detection in national visceral leishmaniasis elimination programs in Bangladesh, India, and Nepal: feasibility, performance and costs. BMC Public Health. 2012;12:1001. doi: 10.1186/1471-2458-12-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mondal D, Nasrin K N, Huda M M et al. Enhanced case detection and improved diagnosis of PKDL in a kala-azar endemic area of Bangladesh. PLOS Negl Trop Dis. 2010;4:e832. doi: 10.1371/journal.pntd.0000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Regional strategic framework for elimination of kala-azar from the South-East Asia region (2005 – 2015) New Delhi, India: South-East Asia Regional Office, WHO; 2012. http://www.searo.who.int/entity/vector_borne_tropical_diseases/documents/SEA-CD-239/en/index.html Accessed January 2014. [Google Scholar]


