Abstract
The proportion of parents aged ≥35 years at the birth of their child continues to increase, but long-term health consequences for these children are not fully understood. A recent prospective study of 110,999 adult women showed an association between paternal—but not maternal—age at birth and sporadic hematological cancer risk. To further investigate this topic, we examined these associations in women and men in the American Cancer Society Cancer Prevention Study-II Nutrition Cohort. Among 138,003 Cancer Prevention Study-II participants, 2,532 incident hematological cancers were identified between 1992 and 2009. Multivariable-adjusted hazard ratios and 95% confidence intervals were computed by using Cox proportional hazards regression. There was no clear linear trend in the risk of hematological malignancies by either paternal or maternal age. However, there was a strong, positive association with paternal age among participants without siblings. In that group, the hazard ratio for fathers aged ≥35 years compared with <25 years at birth was 1.63 (95% confidence interval: 1.19, 2.23), and a linear dose-response association was suggested (Pspline = 0.002).There were no differences by subtype of hematological cancer. Results of this study support the need for further research to better understand the association between paternal age at birth and hematological malignancies.
Keywords: hematological neoplasms, leukemia, lymphoma, maternal age, myeloma, paternal age, prospective cohort studies
The average age of parents at the birth of their children has steadily increased in recent years. In the United States, first-time mothers were, on average, aged 21.4 years in 1970 and 25.8 years in 2012, and the proportion of first-time mothers aged 35 years or more increased 9 times in that time period (1). This trend is not just among mothers; the average paternal age is also increasing. The rate of babies born to fathers aged 35–39 years in the United States jumped more than 50% between 1980 and 2009 (2).
The short- and long-term health consequences for children of older parents are not fully understood, but studies have found higher risk of congenital anomalies, schizophrenia, autism spectrum disorders, and several childhood (3) and adult-onset (4–8) cancers in the offspring of older parents. Both older maternal (9–13) and paternal (11, 13) ages have been associated with pediatric (or young adult) leukemia or lymphoma in some (but not all) previous studies. However, in the 1 study of parental age and risk of hematological cancer in older adults, an association was observed for paternal age only. In this recent analysis (8) of 110,999 women in the California Teachers Study cohort, participants whose fathers were aged 40 years or older at their birth had a 50% higher risk of adult-onset, sporadic (nonfamilial) non-Hodgkin lymphoma than did participants whose fathers were younger than 25 years, controlling for race, birth order, participant age, and maternal age (hazard ratio (HR) = 1.51, 95% confidence interval (CI): 1.08, 2.13; P for trend = 0.01); in analyses limited to women without siblings, there was a 3-fold higher risk of non-Hodgkin lymphoma. The risk associated with paternal age was highest for chronic lymphocytic leukemia/small lymphocytic lymphoma. Maternal age was not independently associated with all hematological cancers but was associated with a 3-fold higher risk of multiple myeloma.
Expanding on the previous findings from the California Teachers Study, we used data from the American Cancer Society Cancer Prevention Study II (CPS-II) Nutrition Cohort to examine the associations of maternal and paternal age with risk of adult, sporadic hematological malignancies in both women and men. Furthermore, we examined whether these associations differed on the basis of whether or not the participant had siblings.
METHODS
Study population
The CPS-II Nutrition Cohort (n = 184,185) is a prospective study of cancer incidence in 21 states in the United States initiated in 1992. The recruitment, characteristics, and follow-up of the cohort are described in greater detail elsewhere (14). It is a subset of a larger CPS-II cohort (nearly 1.2 million participants) recruited by American Cancer Society volunteers in 1982 and followed for mortality. At enrollment in the larger cohort in 1982 and in the subcohort in 1992/1993, participants completed self-administered questionnaires that included information on demographics, family characteristics, personal and family history of cancer and other diseases, reproductive history, and various behavioral, environmental, occupational, and dietary exposures. Beginning in 1997, follow-up questionnaires were sent to subcohort members every 2 years to update exposure information and to ascertain newly diagnosed cancers. Response rates for follow-up surveys were at least 86%. The Emory University Institutional Review Board has approved all aspects of this study.
Participants were excluded from the analytical cohort for 1 of the following reasons: lost to follow-up (alive after 1997 but did not return any survey after 1992) (n = 6,251, 3.4%); history of cancer at baseline other than nonmelanoma skin cancer (n = 22,863, 12.4%); missing 1 or both parents’ age at birth (n = 12,037, 6.5%); and report of hematological cancer on the first returned follow-up survey that could not be verified (n = 61, 0.03%). To limit the analysis to sporadic (rather than familial) hematological malignancies, we also excluded participants who reported a family history of hematological malignancy (n = 4,970, 2.7%). After exclusions, the final analytical cohort consisted of 138,003 individuals (64,421 men and 73,582 women; 74.9% of the full CPS-II Nutrition Cohort). Participants in this cohort were aged 40–93 years at enrollment in 1992/1993.
Participants who developed cancer during the follow-up period were censored at their date of diagnosis unless they were previously censored for another reason. Reasons for additional censoring included reported hematological cancer that could not be verified or failure to return subsequent follow-up surveys (unless deceased). Participants who died during follow-up were censored at their death date unless they were previously censored for1 of the reasons listed above. For participants who did not develop a hematological malignancy or were not otherwise censored during the follow-up period, person-time was calculated as the number of years between the date of survey return in 1992/1993 and June 30, 2009.
Case ascertainment
This analysis included 2,532 incident hematopoietic cancer cases (1,423 men, 1,109 women) diagnosed between the date of enrollment and June 30, 2009. Most cases were identified by self-report on 1 of the follow-up surveys (1997–2009) and subsequently verified by medical record (n = 1,279) or by linkage with state cancer registries (n = 456). In addition, 755 incident cases were identified as interval hematological cancer deaths through biennial automated linkage of the entire cohort with the National Death Index. Finally, 42 cases were identified through the process of verifying another cancer reported by the participant. Hematological cancer subtypes were defined by using the 2008 World Health Organization classification scheme (15) adapted for epidemiologic studies by the International Lymphoma Epidemiology Consortium Pathology Working Group (16). Histology codes from the International Classification of Diseases for Oncology, second and third editions, were used to group these cancers into the following subtypes: lymphoid malignancies (n = 2,071) including diffuse large B-cell lymphoma (n = 410), chronic lymphocytic leukemia/small lymphocytic lymphoma (n = 484), follicular lymphoma (n = 271), multiple myeloma (n = 376), and myeloid malignancies (n = 322) including acute myeloid leukemia (n = 222). Because of sample size limitations of other subtypes, all other hematological malignancies were grouped into an “other hematological malignancies” (n = 669) category.
Exposure ascertainment
On the 1982 enrollment questionnaire, participants were asked the write-in question, “When you were born, 1) how old was your mother? 2) How old was your father?” Maternal and paternal ages at birth were modeled as both a continuous and a categorical variable. Categories for both maternal and paternal age were as follows: <25, 25–29, 30–34, and ≥35 years.
Statistical analysis
Cox proportional hazards regression (17) was used to calculate multivariable-adjusted hazard ratios and corresponding 95% confidence intervals. All Cox models were stratified on the single year of age at the time of baseline in 1992. The variables included in multivariable models were race (white, other, missing); sex; education (<high school, high school, some college, college/graduate school, missing); and sibling status (only child, ≥1 siblings). Restricted cubic splines were used to evaluate linear and nonlinear associations (18). Multiplicative interaction terms were created to test for statistical interaction between maternal and paternal age and 2 covariates, sex and sibling status, by using a likelihood ratio test (significant P ≤ 0.05). In addition, statistical interaction between maternal and paternal age was explored. The proportional hazards assumption was assessed, and no violations were observed. Statistical Analysis System (SAS Institute, Inc., Cary, North Carolina), version 9.3, software was used for all analyses.
RESULTS
The 138,003 participants included in this analysis contributed 1,756,446 person-years over 17 years of follow-up (median, 15.8 years). On average, participants were aged 63 years at the start of follow-up in 1992 or 1993. At birth, participants’ mothers tended to be younger (median age, 27 years) than fathers (median age, 31 years), and almost one-third of the fathers were aged 35 years or more when the participant was born (compared with 17% of the mothers). Participants with the youngest parents at birth were most likely to be obese and to have no siblings (Table 1).
Table 1.
Distribution of Baseline Characteristics According to Paternal and Maternal Age at Birth, Cancer Prevention Study II Nutrition Cohort, 1992–2009
| Characteristic | Paternal Age at Birth, % |
Maternal Age at Birth, % |
||||||
|---|---|---|---|---|---|---|---|---|
| <25 years (n = 20,981) | 25–29 years (n = 38,340) | 30–34 years (n = 35,668) | ≥35 years (n = 43,014) | <25 years (n = 44,510) | 25–29 years (n = 40,698) | 30–34 years (n = 29,791) | ≥35 years (n = 23,004) | |
| Age at cohort entry, years | ||||||||
| ≤60 | 37.3 | 36.9 | 37.3 | 35.7 | 37.1 | 37.1 | 37.0 | 34.8 |
| 61–66 | 33.7 | 33.7 | 33.0 | 34.2 | 33.3 | 33.6 | 33.5 | 34.8 |
| ≥67 | 29.1 | 29.4 | 29.6 | 30.0 | 29.5 | 29.3 | 29.5 | 30.4 |
| Sex | ||||||||
| Male | 47.1 | 46.9 | 46.7 | 46.3 | 46.8 | 47.1 | 46.5 | 46.1 |
| Female | 52.9 | 53.1 | 53.3 | 53.7 | 53.2 | 52.9 | 53.5 | 53.9 |
| Race | ||||||||
| White | 97.0 | 97.8 | 97.9 | 97.3 | 96.9 | 97.9 | 97.9 | 97.7 |
| Other | 2.8 | 1.9 | 1.9 | 2.5 | 2.9 | 1.9 | 1.9 | 2.0 |
| Missing | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Education | ||||||||
| <High school | 7.3 | 5.6 | 5.2 | 6.5 | 7.1 | 5.1 | 5.3 | 6.6 |
| High school | 29.9 | 25.0 | 23.5 | 25.9 | 28.4 | 23.6 | 23.5 | 26.8 |
| Some college | 32.0 | 29.0 | 27.5 | 27.8 | 31.1 | 28.0 | 26.9 | 27.8 |
| College graduate | 30.1 | 39.8 | 43.2 | 39.1 | 32.9 | 42.8 | 43.7 | 38.0 |
| Missing | 0.7 | 0.6 | 0.6 | 0.7 | 0.6 | 0.6 | 0.6 | 0.7 |
| Body mass indexa | ||||||||
| <18.5 | 1.2 | 1.2 | 1.3 | 1.3 | 1.2 | 1.3 | 1.2 | 1.3 |
| 18.5 to <25 | 39.5 | 43.0 | 44.7 | 44.5 | 40.6 | 44.5 | 45.7 | 43.7 |
| 25 to <30 | 40.8 | 39.6 | 38.8 | 38.8 | 40.1 | 39.2 | 38.4 | 39.4 |
| ≥30 | 17.2 | 14.8 | 14.0 | 14.0 | 16.8 | 13.7 | 13.4 | 14.1 |
| Missing | 1.4 | 1.4 | 1.4 | 1.3 | 1.4 | 1.3 | 1.3 | 1.4 |
| Alcohol consumption, servings/day | ||||||||
| Nondrinker | 42.3 | 39.1 | 38.0 | 40.1 | 41.6 | 38.1 | 38.2 | 40.4 |
| <1 | 36.6 | 38.3 | 38.5 | 37.6 | 37.2 | 38.8 | 38.4 | 36.9 |
| 1–2 | 9.5 | 10.8 | 11.5 | 10.7 | 9.8 | 11.5 | 11.3 | 10.6 |
| >2 | 8.2 | 8.9 | 9.1 | 8.6 | 8.2 | 9.0 | 9.3 | 8.7 |
| Missing | 3.4 | 2.8 | 2.9 | 3.0 | 3.3 | 2.7 | 2.8 | 3.3 |
| Smoking status | ||||||||
| Never smoker | 40.3 | 40.7 | 40.4 | 40.9 | 40.7 | 40.4 | 40.1 | 41.5 |
| Ever smoker | 58.9 | 58.6 | 59.0 | 58.3 | 58.6 | 58.9 | 59.2 | 57.8 |
| Missing | 0.7 | 0.6 | 0.6 | 0.8 | 0.7 | 0.7 | 0.7 | 0.8 |
| No. of siblings | ||||||||
| 0 | 30.4 | 29.1 | 27.4 | 22.4 | 29.2 | 28.0 | 25.9 | 21.1 |
| ≥1 | 69.6 | 70.9 | 72.6 | 77.6 | 70.8 | 72.0 | 74.1 | 78.9 |
| Sitting, hours/day | ||||||||
| <3 | 42.0 | 43.4 | 44.3 | 44.1 | 42.7 | 44.0 | 44.4 | 44.0 |
| 3–5 | 43.2 | 42.5 | 41.7 | 41.8 | 42.9 | 41.9 | 41.6 | 42.2 |
| ≥6 | 12.1 | 11.5 | 11.4 | 11.4 | 11.8 | 11.5 | 11.4 | 11.1 |
| Missing | 2.7 | 2.6 | 2.7 | 2.7 | 2.7 | 2.6 | 2.5 | 2.7 |
a Weight (kg)/height (m)2.
In the categorical analysis, there was a positive association between older paternal age at birth and risk of hematological malignancies in men (Table 2) (paternal age ≥35 vs. <25 years: HR = 1.35, 95% CI: 1.07, 1.69) but not in women. However, when paternal age was modeled as a continuous variable, there was no evidence of a clear linear association with hematological cancer risk, and there was no difference by sex (P for interaction = 0.28). In models that did not control for maternal age, associations with paternal age were slightly weaker and not statistically significant (Table 2). There was no association between maternal age at birth and risk of hematological cancer in men or in women. In addition, associations of paternal and maternal age at birth with risk of hematological cancer did not differ by histological subtype (P for heterogeneity, paternal P = 0.22, maternal P = 0.78). (Refer to Web Table 1, available at http://aje.oxfordjournals.org/.)
Table 2.
Parental Age and Risk of All Hematological Malignancies, Cancer Prevention Study II Nutrition Cohort, 1992–2009a
| Variable | Men |
Women |
All Participants |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Cases (n = 1,423) | HR | 95% CI | HRb | 95% CI | No. of Cases (n = 1,109) | HR | 95% CI | HRb | 95% CI | No. of Cases (n = 2,532) | HRc | 95% CI | HRb,c | 95% CI | |
| Paternal age at birth, years | |||||||||||||||
| <25d | 187 | 1.00 | Referent | 1.00 | Referent | 176 | 1.00 | Referent | 1.00 | Referent | 363 | 1.00 | Referent | 1.00 | Referent |
| 25–29 | 431 | 1.23 | 1.04, 1.46 | 1.29 | 1.08, 1.55 | 286 | 0.87 | 0.72, 1.05 | 0.91 | 0.74, 1.11 | 717 | 1.06 | 0.93, 1.20 | 1.11 | 0.97, 1.27 |
| 30–34 | 344 | 1.06 | 0.88, 1.26 | 1.14 | 0.92, 1.40 | 303 | 0.99 | 0.82, 1.20 | 1.05 | 0.84, 1.32 | 647 | 1.03 | 0.90, 1.17 | 1.10 | 0.94, 1.28 |
| ≥35 | 461 | 1.19 | 1.00, 1.41 | 1.35 | 1.07, 1.69 | 344 | 0.92 | 0.77, 1.11 | 1.04 | 0.81, 1.34 | 805 | 1.06 | 0.94, 1.20 | 1.20 | 1.01, 1.42 |
| Continuous, per 5 years | 1.02 | 0.98, 1.06 | 1.04 | 0.98, 1.10 | 0.99 | 0.95, 1.03 | 1.02 | 0.96, 1.09 | 1.01 | 0.98, 1.04 | 1.03 | 0.99, 1.08 | |||
| Maternal age at birth, years | |||||||||||||||
| <25d | 463 | 1.00 | Referent | 1.00 | Referent | 370 | 1.00 | Referent | 1.00 | Referent | 833 | 1.00 | Referent | 1.00 | Referent |
| 25–29 | 419 | 0.96 | 0.84, 1.10 | 0.89 | 0.77, 1.04 | 313 | 0.93 | 0.80, 1.08 | 0.92 | 0.77, 1.09 | 732 | 0.95 | 0.86, 1.05 | 0.91 | 0.81, 1.01 |
| 30–34 | 312 | 1.00 | 0.86, 1.15 | 0.92 | 0.77, 1.11 | 253 | 1.01 | 0.86, 1.19 | 0.95 | 0.77, 1.16 | 565 | 1.00 | 0.90, 1.12 | 0.93 | 0.81, 1.07 |
| ≥35 | 229 | 0.95 | 0.81, 1.12 | 0.83 | 0.66, 1.03 | 173 | 0.87 | 0.73, 1.05 | 0.81 | 0.63, 1.05 | 402 | 0.92 | 0.82, 1.04 | 0.82 | 0.70, 0.97 |
| Continuous, per 5 years | 1.01 | 0.97, 1.05 | 0.97 | 0.91, 1.04 | 0.98 | 0.93, 1.02 | 0.96 | 0.89, 1.03 | 0.99 | 0.96, 1.03 | 0.97 | 0.92, 1.02 | |||
Abbreviations: CI, confidence interval; HR, hazard ratio.
a All models were adjusted for race, sibling status, and education and stratified by age in years at cohort entry.
b The model was further adjusted for age of the other parent at the time of participant's birth.
c The model was further adjusted for sex.
d Referent category.
There was evidence of multiplicative interaction by sibling status on the association between paternal age at birth—but not maternal age at birth—and risk of non-Hodgkin lymphoma (Table 3). Among all participants with no siblings, there was a statistically significant, linear positive association with paternal age (P = 0.002) (Figure 1). Among female participants, the positive association was suggestive (paternal age ≥35 years vs. <25 years, HR = 1.40, 95% CI: 0.86, 2.29), whereas among male participants it was statistically significant (paternal age ≥35 vs. <25 years, HR = 1.84, 95% CI: 1.22, 2.78). However, the linear spline (data not shown) was statistically significant for both men (P = 0.01) and women (P = 0.04). No association was observed between paternal age at birth and risk of hematological malignancies among participants with at least 1 sibling (paternal age ≥35 vs. <25, HR = 1.06, 95% CI: 0.87, 1.30).
Table 3.
Parental Age and Risk of Hematological Malignancies, by Sex and Sibling Status, in the Cancer Prevention Study II Nutrition Cohort, 1992–2009a
| Variable | Men |
Women |
All Participants |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Cases (n = 405) | HR | 95% CI | HRb | 95% CI | No. of Cases (n = 280) | HR | 95% CI | HRb | 95% CI | No. of Cases (n = 685) | HRc | 95% CI | HRb,c | 95% CI | |
| No Siblings | |||||||||||||||
| Paternal age at birth, years | |||||||||||||||
| <25d | 55 | 1.00 | Referent | 1.00 | Referent | 44 | 1.00 | Referent | 1.00 | Referent | 99 | 1.00 | Referent | 1.00 | Referent |
| 25–29 | 121 | 1.20 | 0.87, 1.66 | 1.25 | 0.89, 1.75 | 73 | 0.93 | 0.64, 1.36 | 0.94 | 0.63, 1.41 | 194 | 1.09 | 0.85, 1.39 | 1.11 | 0.86, 1.44 |
| 30–34 | 109 | 1.25 | 0.90, 1.74 | 1.38 | 0.94, 2.01 | 80 | 1.16 | 0.80, 1.68 | 1.22 | 0.78, 1.90 | 189 | 1.21 | 0.95, 1.55 | 1.30 | 0.98, 1.73 |
| ≥35 | 120 | 1.50 | 1.09, 2.07 | 1.84 | 1.22, 2.78 | 83 | 1.21 | 0.84, 1.74 | 1.40 | 0.86, 2.29 | 203 | 1.37 | 1.07, 1.74 | 1.63 | 1.19, 2.23 |
| Continuous, per 5 years | 1.09 | 1.01, 1.16 | 1.13 | 1.03, 1.25 | 1.07 | 0.99, 1.16 | 1.13 | 1.01, 1.27 | 1.08 | 1.03, 1.14 | 1.13 | 1.05, 1.22 | |||
| Maternal age at birth, years | |||||||||||||||
| <25d | 134 | 1.00 | Referent | 1.00 | Referent | 93 | 1.00 | Referent | 1.00 | Referent | 227 | 1.00 | Referent | 1.00 | Referent |
| 25–29 | 133 | 1.09 | 0.86, 1.39 | 0.94 | 0.72, 1.24 | 88 | 1.09 | 0.81, 1.47 | 1.01 | 0.72, 1.41 | 221 | 1.09 | 0.91, 1.32 | 0.97 | 0.78, 1.20 |
| 30–34 | 87 | 1.11 | 0.84, 1.46 | 0.85 | 0.61, 1.18 | 62 | 1.10 | 0.80, 1.53 | 0.89 | 0.59, 1.34 | 149 | 1.11 | 0.90, 1.37 | 0.87 | 0.68, 1.13 |
| ≥35 | 51 | 1.07 | 0.77, 1.48 | 0.71 | 0.47, 1.08 | 37 | 1.03 | 0.70, 1.51 | 0.76 | 0.46, 1.27 | 88 | 1.05 | 0.82, 1.35 | 0.74 | 0.54, 1.02 |
| Continuous, per 5 years | 1.04 | 0.96, 1.13 | 0.93 | 0.82, 1.05 | 1.02 | 0.92, 1.13 | 0.91 | 0.79, 1.06 | 1.03 | 0.97, 1.10 | 0.92 | 0.84, 1.01 | |||
| ≥1 Sibling | |||||||||||||||
| Paternal age at birth, years | |||||||||||||||
| <25d | 132 | 1.00 | Referent | 1.00 | Referent | 132 | 1.00 | Referent | 1.00 | Referent | 264 | 1.00 | Referent | 1.00 | Referent |
| 25–29 | 310 | 1.23 | 1.00, 1.51 | 1.30 | 1.05, 1.62 | 213 | 0.85 | 0.68, 1.06 | 0.89 | 0.71, 1.13 | 523 | 1.04 | 0.90, 1.21 | 1.10 | 0.94, 1.29 |
| 30–34 | 235 | 0.97 | 0.78, 1.20 | 1.04 | 0.81, 1.34 | 223 | 0.93 | 0.75, 1.16 | 0.99 | 0.76, 1.29 | 458 | 0.95 | 0.82, 1.11 | 1.02 | 0.85, 1.22 |
| ≥35 | 341 | 1.09 | 0.89, 1.33 | 1.18 | 0.90, 1.56 | 261 | 0.84 | 0.68, 1.04 | 0.93 | 0.70, 1.25 | 602 | 0.97 | 0.84, 1.12 | 1.06 | 0.87, 1.30 |
| Continuous, per 5 years | 1.00 | 0.96, 1.04 | 1.00 | 0.94, 1.07 | 0.97 | 0.92, 1.01 | 0.98 | 0.91, 1.06 | 0.98 | 0.95, 1.02 | 0.99 | 0.94, 1.05 | |||
| Maternal age at birth, years | |||||||||||||||
| <25d | 329 | 1.00 | Referent | 1.00 | Referent | 277 | 1.00 | Referent | 1.00 | Referent | 606 | 1.00 | Referent | 1.00 | Referent |
| 25–29 | 286 | 0.90 | 0.77, 1.05 | 0.87 | 0.73, 1.04 | 225 | 0.87 | 0.73, 1.04 | 0.89 | 0.73, 1.09 | 511 | 0.89 | 0.79, 1.00 | 0.88 | 0.77, 1.00 |
| 30–34 | 225 | 0.95 | 0.80, 1.13 | 0.96 | 0.77, 1.20 | 191 | 0.97 | 0.81, 1.17 | 0.96 | 0.76, 1.23 | 416 | 0.96 | 0.85, 1.09 | 0.96 | 0.82, 1.13 |
| ≥35 | 178 | 0.91 | 0.76, 1.09 | 0.88 | 0.68, 1.14 | 136 | 0.83 | 0.67, 1.02 | 0.84 | 0.63, 1.13 | 314 | 0.87 | 0.76, 1.00 | 0.86 | 0.71, 1.05 |
| Continuous, per 5 years | 1.00 | 0.95, 1.05 | 0.99 | 0.92, 1.07 | 0.96 | 0.91, 1.02 | 0.98 | 0.90, 1.07 | 0.98 | 0.95, 1.02 | 0.99 | 0.93, 1.05 | |||
Abbreviations: CI, confidence interval; HR, hazard ratio.
a All models were adjusted for race and education and stratified by age in years at cohort entry.
b The model was further adjusted for age of the other parent at the time of participant's birth.
c The model was further adjusted for sex.
d Referent category.
Figure 1.
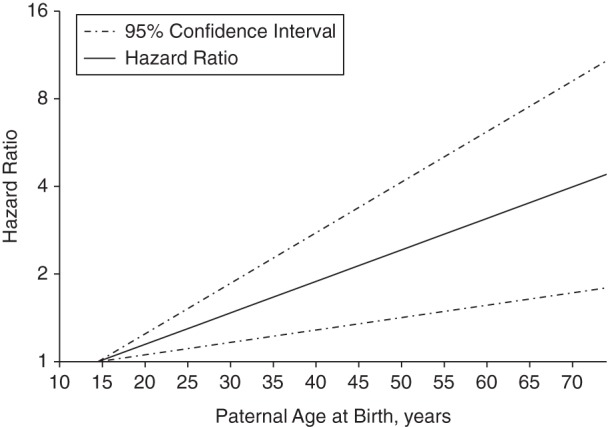
Linear association between paternal age and risk of hematological malignancies among participants with no siblings (n = 36,958; P = 0.002), Cancer Prevention Study II Nutrition Cohort, 1992–2009.
DISCUSSION
The results of this study support an association between paternal—but not maternal—age at birth and risk of adult, sporadic hematological malignancies among men and women with no siblings. No differences by hematological cancer subtypes were detected.
Our main findings are consistent with those from the California Teachers Study, the only other epidemiologic study of adult-onset, sporadic hematological cancer on the topic (8). In both studies, there was no association between maternal age at birth and risk of adult hematological cancer. The California Teachers Study results were slightly stronger than ours for the overall association with paternal age, but both studies observed a strong, positive association in the subset of participants who had no siblings. Of note, the California Teachers Study included only women, and in our study we found stronger results in men. The reasons for a stronger association in men compared with women in our study are unclear. It is possible that, in our study, power was limited among women without siblings, since the association between paternal age and hematological cancer appears to be limited to participants with no siblings. In fact, when the paternal age analysis was restricted to female participants with no siblings, we observed a statistically significant, linear positive association of the same magnitude as was observed in men (HR = 1.13, 95% CI: 1.01, 1.27; P = 0.04).
Our analyses showed no differences in association by non-Hodgkin lymphoma subtype. In contrast, in the California Teachers Study analysis, associations of maternal age with risk of multiple myeloma and of paternal age with risk of chronic lymphocytic leukemia/small lymphocytic lymphoma were stronger than those observed with hematological cancers overall. In both studies, the case numbers for specific subtypes were small, and differences between studies might be due to random error. A pooled analysis might help to clarify if there are differences in associations of parental age at birth by histological subtype of hematological malignancies.
An association between older paternal age and higher risk of hematological cancer is biologically plausible. Recent data from mouse models suggested that advanced paternal age was associated with an increased rate of de novo single nucleotide and copy number mutations during spermatogenesis (19, 20). Furthermore, according to the American College of Medical Genetics, the typical mutation rate for base substitutions and subsequent chromosomal aberrations in humans is much higher in men than women and increases with paternal age (3). A cross-sectional study of 66 men aged 20–57 years (21) found that age was positively correlated with percentage of sperm with highly damaged DNA (ρ = 0.56; P < 0.0001) and negatively correlated with percentage of apoptotic sperm (ρ = −0.28; P = 0.028). In particular, statistical comparisons of different age groups showed that men aged 36–57 years had both higher percentage of sperm with DNA damage (P < 0.005) and lower percentage of apoptotic sperm (P < 0.02) than men aged 20–35 years (21). It is plausible that genetic alterations associated with paternal age might contribute to the risk of hematological cancers in children of older fathers as there is established evidence of a genetic component to the etiology of these cancers (22–24).
Heritable epigenetic alterations in the sperm are another possible reason for the observed association between paternal age and hematological cancer risk. A recent study compared methylation patterns in 2 sperm samples collected from 17 men, 9–19 years apart (25), and then tested the findings in an independent cross-sectional sample of 66 men in 2 age groups (<25 and ≥45 years). The authors found convincing evidence of age-related methylation changes (both hypermethylation globally and hypomethylation regionally (i.e., gene associated)) in several regions involving more than 100 genes. Furthermore, in a mouse model study, changes in sperm DNA methylation patterns with aging were also documented, and these age-related methylation abnormalities, as well as gene expression changes, were found in the offspring of the older mice (23). A study of human umbilical cord blood also demonstrated an association between parental age and the levels of DNA methylation in the next generation, and many of the loci where methylation changes occurred have been linked to oncogenesis and cancer progression (26). Methylation pattern changes have been hypothesized to be an early step in the development of hematological cancers, via promotion of overexpression of proto-oncogenes, chromosomal translocations, and mutations (27).
Alterations in telomere length with age also support a possible biological link between paternal (but not maternal) age and risk of hematological cancer. Recent studies consistently found that human paternal age is associated with longer telomere length in offspring and that the association with maternal age is considerably weaker (28–30). It has been hypothesized that the increased cell survival that results from longer telomeres may promote the accumulation of genetic mutations that lead to the development of cancer (31). A recent prospective study (32) from the European Prospective Investigation into Cancer and Nutrition cohort showed a statistically significant 3-fold higher risk of B-cell lymphoid neoplasms for those with the longest compared with those with the shortest telomeres. This finding is consistent with an earlier, smaller prospective study from the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (33).
The specificity of our observed association to participants with no siblings is intriguing and needs further study to elucidate the possible biological explanation for this finding. One possibility is the “hygiene hypothesis,” the idea that exposure to mild infections in childhood is important to immune system development and may reduce the risk of immune-related diseases (34). Therefore, it is possible that the combination of having an older father and no siblings can create an in vivo environment that promotes cell proliferation in an underdeveloped immune system and, as such, favors lymphomagenesis.
Strengths of this study include the large cohort of men and women, the prospective design, the ability to control for a range of potential confounders, and the available data on hematological cancer subtypes. Possible limitations include the self-reported paternal and maternal ages at birth. However, recalled parental age has been shown to be highly reliable and is unlikely to be differential on the basis of subsequent risk of hematological cancer (6). Additional limitations include a possible lack of power in the hematological histological subtype results and the lack of information on birth order or other possible confounding or association-modifying factors.
In summary, our results suggest a positive association between older paternal age and risk of hematological malignancies among men and women who have no siblings. Further research to confirm these findings and to clarify the biological underpinning for this association is warranted, given the growing number of children born to older fathers in the United States and worldwide.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Epidemiology Research Program, Department of Intramural Research, American Cancer Society, Atlanta, Georgia (Lauren R. Teras, Mia M. Gaudet, Jennifer L. Blase, Susan M. Gapstur).
The American Cancer Society funds the creation, maintenance, and updating of the Cancer Prevention Study II Nutrition Cohort.
We would like to acknowledge the contribution to this study from central cancer registries supported through the National Program of Cancer Registries by the Centers for Disease Control and Prevention and also cancer registries supported through the Surveillance, Epidemiology, and End Results program by the National Cancer Institute.
Conflict of interest: none declared.
REFERENCES
- 1.Mathews T, Hamilton BE. First Births to Older Women Continue to Rise. Hyattsville, MD: National Center for Health Statistics; 2014:1–8. (NCHS data brief no. 152). [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2012. Natl Vital Stat Rep. 2013;629:1–68. [PubMed] [Google Scholar]
- 3.Toriello HV, Meck JM; Professional Practice and Guidelines Committee. Statement on guidance for genetic counseling in advanced paternal age. Genet Med. 2008;106:457–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi JY, Lee KM, Park SK, et al. Association of paternal age at birth and the risk of breast cancer in offspring: a case control study. BMC Cancer. 2005;5:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodgson ME, Newman B, Millikan RC. Birthweight, parental age, birth order and breast cancer risk in African-American and white women: a population-based case-control study. Breast Cancer Res. 2004;66:R656–R667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue F, Colditz GA, Willett WC, et al. Parental age at delivery and incidence of breast cancer: a prospective cohort study. Breast Cancer Res Treat. 2007;1043:331–340. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Kreger BE, Dorgan JF, et al. Parental age at child's birth and son's risk of prostate cancer. The Framingham Study. Am J Epidemiol. 1999;15011:1208–1212. [DOI] [PubMed] [Google Scholar]
- 8.Lu Y, Ma H, Sullivan-Halley J, et al. Parents’ ages at birth and risk of adult-onset hematologic malignancies among female teachers in California. Am J Epidemiol. 2010;17112:1262–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crump C, Sundquist K, Sieh W, et al. Perinatal and family risk factors for non-Hodgkin lymphoma in early life: a Swedish national cohort study. J Natl Cancer Inst. 2012;10412:923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson KJ, Carozza SE, Chow EJ, et al. Parental age and risk of childhood cancer: a pooled analysis. Epidemiology. 2009;204:475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yip BH, Pawitan Y, Czene K. Parental age and risk of childhood cancers: a population-based cohort study from Sweden. Int J Epidemiol. 2006;356:1495–1503. [DOI] [PubMed] [Google Scholar]
- 12.Hemminki K, Kyyrönen P, Vaittinen P. Parental age as a risk factor of childhood leukemia and brain cancer in offspring. Epidemiology. 1999;103:271–275. [PubMed] [Google Scholar]
- 13.Dockerty JD, Draper G, Vincent T, et al. Case-control study of parental age, parity and socioeconomic level in relation to childhood cancers. Int J Epidemiol. 2001;306:1428–1437. [DOI] [PubMed] [Google Scholar]
- 14.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;949:2490–2501. [DOI] [PubMed] [Google Scholar]
- 15.Swerdlow S, Campo E, Harris N. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. [Google Scholar]
- 16.Turner JJ, Morton LM, Linet MS, et al. InterLymph hierarchical classification of lymphoid neoplasms for epidemiologic research based on the WHO classification (2008): update and future directions. Blood. 2010;11620:e90–e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox DR. Regression models and life tables (with discussions) J R Stat Soc Series B. 1972;342:187–220. [Google Scholar]
- 18.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;85:551–561. [DOI] [PubMed] [Google Scholar]
- 19.Kong A, Frigge ML, Masson G, et al. Rate of de novo mutations and the importance of father's age to disease risk. Nature. 2012;4887412:471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flatscher-Bader T, Foldi CJ, Chong S, et al. Increased de novo copy number variants in the offspring of older males. Transl Psychiatry. 2011;1:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh NP, Muller CH, Berger RE. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil Steril. 2003;806:1420–1430. [DOI] [PubMed] [Google Scholar]
- 22.Conde L, Riby J, Zhang J, et al. Copy number variation analysis on a non-Hodgkin lymphoma case-control study identifies an 11q25 duplication associated with diffuse large B-cell lymphoma. PLoS One. 2014;98:e105382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berndt SI, Skibola CF, Joseph V, et al. Genome-wide association study identifies multiple risk loci for chronic lymphocytic leukemia. Nat Genet. 2013;458:868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong S, Liu Y, Chen J. MTHFR gene polymorphism and risk of myeloid leukemia: a meta-analysis. Tumour Biol. 2014;359:8913–8919. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins TG, Aston KI, Pflueger C, et al. Age-associated sperm DNA methylation alterations: possible implications in offspring disease susceptibility. PLoS Genet. 2014;107:e1004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adkins RM, Thomas F, Tylavsky FA, et al. Parental ages and levels of DNA methylation in the newborn are correlated. BMC Med Genet. 2011;12:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson B. Impact of aging on DNA methylation. Ageing Res Rev. 2003;23:245–261. [DOI] [PubMed] [Google Scholar]
- 28.Kimura M, Cherkas LF, Kato BS, et al. Offspring's leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008;42:e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Meyer T, Rietzschel ER, De Buyzere ML, et al. Paternal age at birth is an important determinant of offspring telomere length. Hum Mol Genet. 2007;1624:3097–3102. [DOI] [PubMed] [Google Scholar]
- 30.Unryn BM, Cook LS, Riabowol KT. Paternal age is positively linked to telomere length of children. Aging Cell. 2005;42:97–101. [DOI] [PubMed] [Google Scholar]
- 31.Noy A. Telomeres: the long and short of developing non-Hodgkin lymphoma. Clin Cancer Res. 2009;1523:7114–7115. [DOI] [PubMed] [Google Scholar]
- 32.Hosnijeh FS, Matullo G, Russo A, et al. Prediagnostic telomere length and risk of B-cell lymphoma: results from the EPIC cohort study. Int J Cancer. 2014;13512:2910–2917. [DOI] [PubMed] [Google Scholar]
- 33.Lan Q, Cawthon R, Shen M, et al. A prospective study of telomere length measured by monochrome multiplex quantitative PCR and risk of non-Hodgkin lymphoma. Clin Cancer Res. 2009;1523:7429–7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rook GA, Dalgleish A. Infection, immunoregulation, and cancer. Immunol Rev. 2011;2401:141–159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


