Abstract
Utilizing a combination of neuropsychological and cognitive neuroscience approaches may be essential for characterizing cognitive deficits in schizophrenia and eventually assessing cognitive outcomes. This study was designed to compare the stability of select exemplars for these approaches and their correlations in schizophrenia patients with stable treatment and clinical profiles. Reliability estimates for serial order processing were comparable to neuropsychological measures and indicate that experimental serial order processing measures may be less susceptible to practice effects than traditional neuropsychological measures. Correlations were moderate and consistent with a global cognitive factor. Exploratory analyses indicated a potentially critical role of the Met allele of the Catechol-O-methyltransferase (COMT) Val158Met polymorphism in externally paced sequential recall. Experimental measures of serial order processing may reflect frontostriatal dysfunction and be a useful supplement to large neuropsychological batteries.
Keywords: COMT, Reliability, neuropsychology, working memory, serial order processing
Deficient storage and manipulation of information in working memory has been well established in schizophrenia (Lee and Park, 2005;Silver et al., 2003). Deficits have been observed across a wide range of neuropsychological (Blanchard and Neale, 1994;Dickinson et al., 2004;Saykin et al., 1991;Reichenberg and Harvey, 2007;Keefe et al., 2004;Lee and Park, 2005) and neurophysiological paradigms (Clementz et al., 1998;Reilly et al., 2005;Reilly et al., 2008). Working memory impairments for temporal order have also been observed (Cellard et al., 2007;Dreher et al., 2001;Fraser et al., 2004) and may have implications for making causal inferences and executing behavior plans. Using a spatial serial order recall paradigm adapted from the primate literature (Barone and Joseph, 1989), we recently reported a unique phenomenon in which internal processing disrupted working memory for temporal order (Hill et al., 2011). Specifically, when responding was “unpaced” and participants were allowed to reproduce spatial targets at their own pace, performance was equivalent to demographically similar controls. However, when responding was interrupted by a two second pause between responses, schizophrenia patients were impaired. Unpaced trials may be supported by simple rehearsal storage and chunked retrieval while paced responding may involve frequent working memory updates. This was attributed to deficient frontostriatal communications (Dickinson and Elvevag, 2009) responsible for updating working memory (Hill et al., 2011) and/or Catechol-O-methyltransferase (COMT) mediated maintenance of working memory in the prefrontal cortex. Specifically, the COMT Val158Met polymorphism (rs4680) accounts for significant variability in COMT function whereby the rare (Met) allele is associated with enzyme instability resulting in lower dopamine metabolism and increased cortical dopamine (Lachman et al., 1996). Indeed, the Met allele was associated with more efficient prefrontal activity during working memory processing (Egan et al., 2001).
Approaches to characterizing cognitive deficits in schizophrenia are typically rooted in the neuropsychological or cognitive neuroscience disciplines. By virtue of wide availability of well-trained neuropsychologists, standardized administration and scoring procedures, established reliability, published normative data, portable and low cost instrumentation, and reliable links to functional status (Green, 1996), the neuropsychological approach has become engrained in treatment-related studies in schizophrenia. Indeed, the NIMH – Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) program was commissioned to develop a consensus neuropsychological battery for evaluating cognitive outcomes (Nuechterlein et al., 2008). However, based on tests of highly specific cognitive processes with closer ties to animal models, neurotransmitter systems, and brain neurophysiology, the cognitive neuroscience approach may be more sensitive to pharmacological treatments (Barrett et al., 2004;Burke and Reveley, 2002;Hill et al., 2010).
Understanding the relationship between the neuropsychological and cognitive neuroscience approaches is essential to integrating these approaches and developing efficient and valid tools for characterizing cognitive deficits in schizophrenia and eventually assessing cognitive outcomes. One goal of the present study was to establish reliability of spatial serial order recall (Hill et al., 2011) and investigate possible practice effects compared to the consensus neuropsychological battery. Although subtests for the MATRICS Consensus Cognitive Battery (MCCB) were selected, in part, on reliability and limited practice effects (Keefe et al., 2011;Nuechterlein et al., 2008), traditional neuropsychological tests may be more susceptible to practice effects compared to cognitive neuroscience measures (Hill et al., 2007). Spatial serial order processing paradigms are rooted in animal models and highly specific compared to the MCCB. Thus, the stability of this experimental spatial serial order task is expected to be equivalent with MCCB reliability estimates.
A second aim of this investigation was to determine the neuropsychological correlates of this novel serial order processing test with the MATRICS consensus neuropsychological battery. Prior studies of spatial working memory in schizophrenia have shown positive associations with visual orientation, processing speed, attention, memory for objects and faces, executive functioning, and auditory verbal learning (Silver et al., 2003;Silver and Goodman, 2008;Snitz et al., 1999). Thus, one might expect serial order processing to correlate moderately with MCCB domains. However, our prior report indicated significant patient deficits in the paced responding condition beyond a general cognitive deficit (patients were comparable to controls on age, education, parental SES, and intelligence) (Hill et al., 2011), so the paced condition may be more sensitive and specific in patients. Thus, paced and unpaced serial order response conditions were predicted to show distinct correlation patterns in which few MCCB domains uniquely predict unpaced responding while multiple MCCB domains predict paced responding. Finally, as an exploratory analysis the impact of the COMT Val158Met polymorphism (rs4680) was assessed for the two response output conditions. In general, one would expect the Met allele to facilitate performance during unpaced responding when maintenance demands are essential. However, when frequent updating is also required, such as during the paced responding condition, the Met allele may not be optimal.
Methods
Participants
The patient sample included 27 individuals who met criteria for schizophrenia spectrum disorders (24 schizophrenia, 3 schizoaffective) based on the Structured Clinical Interview for DSM-IV (SCID). To limit effects of both acute illness and recent changes to medication treatments, all patients were clinically stable, meaning there was no acute symptomatology, significant change in positive symptom severity, or change in pharmacotherapy regimen during the prior month. All patients were treated with antipsychotics. Those treated with a second-generation antipsychotics (n=23) were prescribed low-dose risperidone (1–3 mg), while a few patients were treated with first-generation antipsychotics (n=4). Concomitant medications included SSRIs (n=3), mood stabilizers (n=2), lithium (n=1), anticholinergic (n=1), and benzodiazepine (n=1). Neither chlorpromazine equivalents (Andreasen et al., 2010) nor clinical symptom ratings were significantly related to overall neuropsychological performance. Thus, neither medication status nor clinical symptom ratings were used as covariates. A sample of 25 healthy individuals, recruited from the community via local advertisements and research registry, were free of SCID Axis I diagnosis. All participants had normal range intelligence (WASI IQ>79) and were free of substance abuse within the last three months, lifetime history of substance dependence, neurological disease, significant head injury, and systemic disorders known to affect brain function. Written consent was provided by participants and the study was approved by the Institutional Review Board at the University of Illinois at Chicago. There were no group differences for age, education, parental socioeconomic status, estimated premorbid intelligence, and current intelligence (Table 1).
Table 1.
Group demographics and clinical data (for schizophrenia group)
| Healthy Controls (CTL) n=25 |
Schizophrenia (SZ) n=27 |
Analysis | ||||
|---|---|---|---|---|---|---|
| Demographics | F/x2 | df | p | |||
| Age (years) | 39.44 (10.94) | 35.00(9.98) | 2.34 | 1,50 | .13 | |
| Sex | ||||||
| Male | 56.0% | 66.7% | .62 | 1 | .43 | |
| Female | 44.0% | 33.3% | ||||
| Race | ||||||
| Caucasian | 24.0% | 22.2% | .77 | 2 | .68 | |
| African American | 60.0% | 51.9% | ||||
| Asian/Latino/Other | 16.0% | 25.9% | ||||
| Dominant | ||||||
| Right | 88.0% | 96.3% | 1.26 | 1 | .26 | |
| Left | 12.0% | 3.7% | ||||
| Education | 13.80(1.83) | 14.57(3.24) | 1.10 | 1,50 | .30 | |
| Parental SES | 3.20(1.19) | 3.12(0.99) | .08 | 1,49 | .78 | |
| WRAT-III Reading | 95.17(12.16) | 98.3(14.11) | .71 | 1,49 | .40 | |
| WASI-IQ | 101.36(11.07) | 102.0 (14.72) | .03 | 1,50 | .86 | |
| Clinical Data | ||||||
| Illness Duration (years) | 11.85(10.78) | |||||
| PANSS Total | 38.00(6.57) | |||||
| PANSS Positive | 17.73 (4.06) | |||||
| PANSS Negative | 19.27(5.16) | |||||
| Side Effect Ratings | ||||||
| AIMS Total | 0.80(1.32) | |||||
| ESRS Total | 4.07 (4.37) | |||||
WRAT-III: Wide Range Achievement Test: Third Edition
WASI: Wechsler Abbreviated Scale of Intelligence
PANSS: Positive and Negative Syndrome Scale
AIMS: Abnormal Involuntary Movement Scale
ESRS: Extrapyramidal Symptom Rating Scale
WASI-IQ: 2-Subtest (Vocabulary and Matrix Reasoning) estimate
Measures
All participants were given the MCCB, which consists of 10 subtests covering six domains including, speed of processing, attention/vigilance, working memory, verbal learning, visual learning, reasoning and problem solving, and social cognition.
Spatial Serial Order Paradigm
The serial order task adapted from animal models was previously described (Hill et al., 2011). Briefly, participants were asked to retrieve and reconstruct a complete sequence of spatial locations at their own speed by clicking on target locations in succession (unpaced condition). In the paced responding condition, a two second delay was imposed after each response, then participants were cued to click on the next location in the series. Processing demand for both response output conditions was varied according to the number locations to be remembered (3 to 5 targets).
Genotyping
Genomic DNA was isolated from EDTA-treated whole blood using standard protocols, quantified, and quality checked with Picogreen (Invitrogen, Eugene,OR) and Nanodrop assays (Thermo Scientific, Wilmington, DE). Samples were genotyped for the rs4680 SNP within the COMT gene using a validated Pyrosequencing assay. Genotyping was done blind to case/diagnostic status and clinical ratings. Genotype calls were 100%.
Procedures and Statistical Analysis
The translational spatial serial order task was run on a 15.4 inch laptop screen using E-Prime software (Schneider et al., 2002). To reduce the potential for paced condition instructions to interfere with the unpaced condition, the conditions were administered in separate sessions on separate days. The unpaced condition was always administered first and no subject was administered both conditions on the same day. Both the MCCB and serial order processing tasks were administered at two separate time points approximately four weeks apart. Test-retest reliability estimates were calculated for each MCCB subtest and each level of working memory demand for serial order processing. Regression analyses were used to assess the baseline neuropsychological correlates of serial order processing accuracy. Separate analyses were conducted for each response condition (paced and unpaced). The six MCCB domains were entered together as potential predictors and the F to enter was set at p<.05. Because the groups were demographically similar, no covariates were used. To assess for mean changes over time and the potential for differential sensitivity to practice effects two separate ANOVA were conducted. A two-way repeated measures ANOVA was conducted for the MCCB composite with diagnosis as the between subjects variable and time as the within subject variable. For response accuracy on the translational paradigm a four-way repeated measures ANOVA was conducted with output condition (paced, unpaced), working memory load (3–5 items), and time as within subjects variables and diagnosis as the between subjects variable. Allele and genotype frequencies and Hardy Weinberg Equilibrium (HWE) were assessed with Haploview version 4.2 software with no evidence for significant deviation from HWE in all patients or within Caucasian and African American subgroups. Regardless, the exploratory analyses involving genotype were repeated with race as a covariate and a similar pattern of findings were observed. Participants with Met/Met or Val/Met genotypes had similar performance patterns and were grouped together for the exploratory genetic analyses.
Results
Test-retest Reliability
Serial order tasks
For the paced condition, test-retest reliability estimates for healthy controls ranged from .89 to .96 and ICCs ranged from .81 to .92 (Table 2). Paced test-retest reliability estimates for patients ranged from .91 to .95 and ICCs ranged from .83 to .91. For the unpaced condition, test-retest reliability estimates for healthy controls ranged from .89 to .96 and ICCs ranged from .80 to .93. Unpaced test-retest reliability estimates for patients ranged from .77 to .89 and ICCs ranged from .62 to .80 (Table 2).
Table 2.
Test-Retest reliability of MATRICS battery and experimental serial order recall response conditions.
| MATRICS | Patients | Controls | |||
|---|---|---|---|---|---|
| Cognitive Domain | Subtest | R | ICC | R | ICC |
| Speed of Processing | .93 | .88 | .87 | .77 | |
| Category Fluency | .83 | .72 | .70 | .53 | |
| Trails A | .89 | .80 | .60 | .43 | |
| Symbol Coding | .91 | .83 | .90 | .82 | |
| Working Memory | .91 | .83 | .85 | .74 | |
| Letter-Number | .85 | .74 | .85 | .74 | |
| Spatial Span | .86 | .81 | .81 | .68 | |
| Verbal Learning | HVLT | .85 | .74 | .67 | .50 |
| Attention | CPT | .89 | .80 | .94 | .88 |
| Visual Learning | BVMT | .88 | .79 | .84 | .73 |
| Reasoning/Problem Solving | Mazes | .82 | .72 | .90 | .82 |
| General Composite | .96 | .93 | .96 | .92 | |
| Experimental Tasks | Working Memory Load | ||||
| Paced Accuracy | 3 items | .92 | .85 | .91 | .84 |
| 4 items | .91 | .83 | .89 | .81 | |
| 5 items | .92 | .85 | .96 | .92 | |
| All trials | .95 | .91 | .95 | .92 | |
| Unpaced Accuracy | 3 items | .77 | .62 | .89 | .80 |
| 4 items | .87 | .78 | .91 | .84 | |
| 5 items | .87 | .77 | .94 | .88 | |
| All trials | 89 | .80 | .96 | .93 | |
MATRICS: Measurement and Treatment Research to Improve Cognition in Schizophrenia
HVLT: Hopkins Verbal Learning Test-Revised
CPT-IP: Continuous Performance Test-Identical Pairs Version
BVMT: Brief Visuospatial Memory Test-Revised
MCCB
Test-retest reliability estimates for healthy controls ranged from .60 to .96 and ICCs ranged from .43 to .92. Test-retest reliability estimates for patients ranged from .82 to .96 and ICCs ranged from .72 to .93 (Table 2).
Regression
Stepwise regression of the paced condition revealed that speed of processing was the strongest predictor of serial order recall accuracy [Schizophrenia patients: R=.64, R2=.40,F(1,23)=15.58, p=.001; healthy controls: R=.69, R2=.47, F(1,19)=17.00, p=.001]. Zero-order correlations indicated medium sized correlations for MCCB domains and paced recall accuracy (SZ: r = .40 to .65; HC: r = .35 to .70) and a high rate of multi-colinearity as no other domain added significantly to the regression equation. Stepwise regression of the unpaced condition revealed that for patients, speed of processing [R=.62, R2=.39, F(1,25)=15.88, p=.001] was the strongest predictor and adding verbal learning to the regression [R=.70, R2=.49, F(1,24)=11.52, p<.001] significantly increased the explained variance. For healthy controls, working memory was the best predictor of unpaced serial order recall accuracy [R=.64, R2=.41, F(1,22)=15.06, p<.001] and no other domain score significantly increased the amount of explained variance.
Analysis of Variance
A two-way repeated measures ANOVA was used to assess mean MCCB composites scores. There were significant main effects of diagnosis [Wilks’ Lambda F(1,44)=3.88, p=0.05; d=0.70] and time [F(1,44)=10.43, p<0.01; d=0.23] but the diagnosis by time interaction was nonsignificant [F(1,44)=0.20, p=0.66]. These findings indicated mild overall impairments in patients, but similar improvement over time in both groups. To assess the accuracy of serial order recall, a four-way repeated measures ANOVA was conducted. There was a significant main effect for only working memory load [F(1,38)=94.83, p<0.001], but no significant main effect of time [F(1,38)=0.03, p=0.88] and no significant interactions involving time. This indicated modest but uniform practice effects for both groups on neuropsychological measures and negligible practice effects for serial order recall.
To address the utility of supplementing neuropsychological testing with measures of serial order processing, a logistic regression was conducted with diagnosis as the criterion. Composite scores from the MCCB were moderately correlated with the serial order processing conditions (r = .63-.70) and on par, although somewhat lower, than the regression findings. Thus, the MCCB composite was included in a logistic regression with the two serial order order conditions using a stepwise method. The MCCB composite was the best predictor of diagnosis (−2LL = 42.84, p = 0.06), with the model fit statistic improving when accuracy for the paced and unpaced conditions were added to the model (−2LL = 40.08; p=0.06).
Incidental findings
The repeated measures ANOVA of serial order recall accuracy revealed a trend for the three-way interaction of task by working memory load by diagnosis [F(1,41)=3.34, p=0.07]. Given the limited power of this small sample size, particularly for higher-order interactions, the probability of a Type II error is increased and exploratory analyses to clarify this interaction were conducted. Post-hoc comparisons indicated that although performance dropped as working memory load increased for both groups, schizophrenia patients showed an exaggerated decline for paced responding at higher working memory loads.
Exploratory genetic analyses
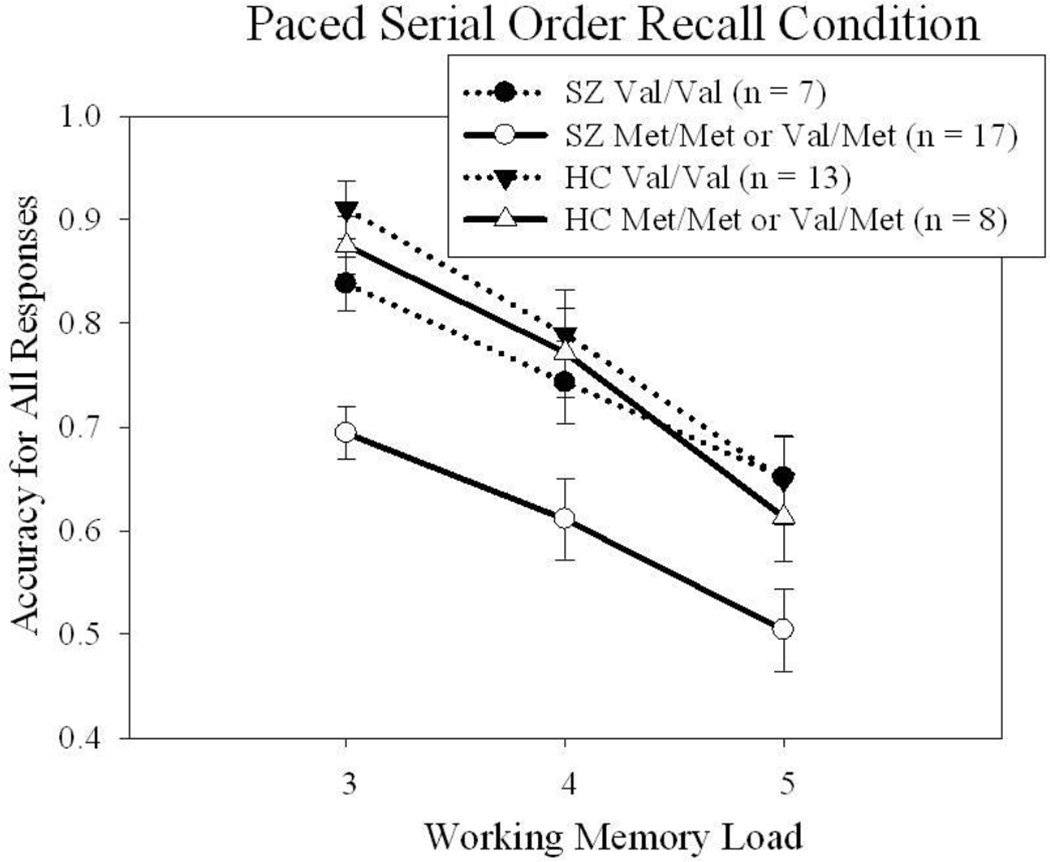
To further explore the serial order task by working memory load by diagnosis interaction further, COMT subtype was added to the repeated measures ANOVA and run separately for each group. The serial order task by COMT subtype was nonsignificant for controls [F(1,19)=0.04, p=0.85] and trend level for schizophrenia patients [F(1,33)=1.91, p=0.18]. This was not a by-product of selective COMT subtype cognitive deficits as there were no differences among COMT subtypes on the MCCB composite [F(1,44)=1.05, p=0.31]. Likewise, there was no difference in chlorpromazine equivalents or clinical symptom ratings as a function of COMT subtype. As illustrated in Figure 1, the presence of at least one Met allele may play a role in the differential disruption of response accuracy during paced output in schizophrenia patients. Given the small sample and the trend findings, a bootstrapping technique was used to assess whether adding more subjects, and increasing power, would be likely to produce statistically meaningful findings. Bootstrapping techniques randomly resample the data (typically to the equivalent of n=1000) to estimate standard error based on a larger sample size, then adjust the confidence interval accordingly (Shrout and Bolger, 2002). After resampling, the bootstrapped model was examined at the 95th percentile confidence interval and indicated a potentially meaningful finding in which schizophrenia patients with at least one Met allele were differentially impaired during paced response output.
Figure 1.
Serial order recall accuracy for paced and unpaced response conditions across working memory load was notable for decreased accuracy in schizophrenia patients with a Met variant of COMT (Val/Met, Met/Val, or Met/Met) during the paced response condition. In contrast, performance was similar for schizophrenia patients with a Val/Val allele combination and both control groups.
Discussion
This study was designed to assess the reliability and neuropsychological correlates of a translational serial order processing measure relative to the consensus neuropsychological battery in a sample of schizophrenia patients who were demographically similar to healthy volunteers. Reliability estimates for serial order processing was at least on par with the MCCB. Correlational analyses indicated moderate associations between serial order processing and neuropsychological domains that were similar for patients and controls. Finally, when predicting diagnostic group the addition of serial order processing to neuropsychological measures enhanced the model, supporting the notion that experimental measures may have some utility as a supplement to ‘classical’ neuropsychological batteries.
Reliability
For the MCCB, estimated reliability was similar for schizophrenia patients and psychiatrically healthy controls and comparable to those reported by the MATRICS initiative (Nuechterlein et al., 2008). Reliability estimates for the experimental serial order processing measure were at least on par with MCCB subtests for both patients and controls. This was consistent with prior reports that compared traditional neuropsychological measures with experimental oculomotor measures and found that experimental measures were somewhat more consistent over time (Hill et al., 2007) and may reflect an increased tendency for participants to benefit from exposure to neuropsychological test procedures over time in contrast to a lower likelihood of carry over within or between experimental serial order processing measures. Indeed, the present findings indicated a main effect of time for MCCB subtests, but not the experimental serial order processing measure (in either group). There was no time by diagnosis interaction, hence no evidence of differential practice effects. This may be a useful point of reference for future studies assessing neuropsychological measures as outcomes and disentangling treatment-related effects from practice effects.
Neuropsychological correlates
When predicting serial order processing, MCCB domains accounted for 39–49% of variance. This was consistent with our expectation of moderate correlations between serial order processing and neuropsychological domains as well as prior reports (Silver et al., 2003;Silver and Goodman, 2008;Snitz et al., 1999). The notion that unique correlation patterns would be associated with specific response output conditions during serial order recall was weakly supported. Although two of the regressions produced multiple predictors, there was a high degree of multi-colinearity among neuropsychological domains that was more consistent with a general cognitive factor. This may reflect the breadth of neuropsychological measures which concurrently evaluate several discrete cognitive processes and their integration. Given this level of integration and the overlap across domains (Dickinson and Harvey, 2009;Dickinson et al., 2011;Hill et al., 2008) removing the variance associated with one domain may limit the predictive utility of the remaining domains. Overall, both reliability and regression findings indicate that experimental serial order processing measures may be less susceptible to practice effects than neuropsychological measures and may offer a useful supplement to the global cognitive factor characterizing neuropsychological batteries, particularly for detailed assessment of working memory.
Genetics
Although exploratory, there was evidence that the paced read-out failure in schizophrenia patients is related to the COMT gene and its Met allele variant of the Val158Met polymorphism. One account for this finding is that an attenuated caudate signal for updating the contents of working memory interacts with prefrontal dopamine. COMT plays a critical role in the maintenance of working memory due to its role in the breakdown of dopamine. The high activity (Val) functional polymorphism, associated with lower synaptic dopamine, has been associated with increased risk for schizophrenia and less efficient working memory related prefrontal activity when compared to the low activity (Met) allele (Egan et al., 2001). The Met allele has been associated with greater deficits in shifting from one task to another (Nolan et al., 2004). Thus, for those with a Met allele, paced output of serial order may entail a challenging shift from maintenance to updating and/or require greater cognitive control.
Limitations
The experimental serial order processing measure is highly specific and limited in scope, potentially resulting in a restricted range that limits the magnitude of correlations with a broader neuropsychological battery. It is also possible that the extended maintenance duration during the paced task relative to the unpaced task may accelerate degradation of maintained information and reduce accuracy in a subset of schizophrenia patients vulnerable to disruptions of working memory stores. With appropriate regard for inferential limitations imposed by the small sample sizes the present findings were consistent with the larger MCCB report (Nuechterlein et al., 2008) and offer a demographically similar healthy control group to anchor reliability estimates and expectations for practice effects. Although the groups were demographically similar, the present patient sample was high functioning with mean current intelligence estimates above 100 and numerically (but not significantly) higher than controls. Thus, this sample may not be representative of a broader schizophrenia sample with more severe overall cognitive impairments and the generalizability of the present findings may be limited. Additional caution should be used when interpreting the exploratory analyses. This should be addressed prospectively in a large study. Another limitation lies in the potential impact of antipsychotic treatments. The sample was primarily chronic schizophrenia patients with long-term exposure to antipsychotic medications. Treatment with antipsychotics may play an important role in dopamine availability, particularly in the striatum, and the frontostriatal communication supporting both working memory maintenance and updating the contents of working memory. Regardless of the nature of these deficits, the present findings suggest that parametric manipulation of response output pacing during serial order recall represents a novel, reliable, and specific approach for investigating frontostriatal systems dysfunction in schizophrenia.
Supplementary Material
Acknowledgements
Role of funding source
This study was supported by funds received from NIH/NIMH (MH072767 & MH083888). The funding source played no role in data analysis or interpretation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
There are no conflicts of interest to report.
Contributors
Peter Weiden, MD: Subject recruitment services.
Ellen Herbener, PhD: and Clinical ratings and independent diagnostic evaluations
Reference List
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone P, Joseph JP. Prefrontal cortex and spatial sequencing in macaque monkey. Exp Brain Res. 1989;78:447–464. doi: 10.1007/BF00230234. [DOI] [PubMed] [Google Scholar]
- Barrett SL, Bell R, Watson D, King DJ. Effects of amisulpride, risperidone and chlorpromazine on auditory and visual latent inhibition, prepulse inhibition, executive function and eye movements in healthy volunteers. J Psychopharmacol. 2004;18:156–172. doi: 10.1177/0269881104042614. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Neale JM. The neuropsychological signature of schizophrenia: generalized or differential deficit? Am J Psychiatry. 1994;151:40–48. doi: 10.1176/ajp.151.1.40. [DOI] [PubMed] [Google Scholar]
- Burke JG, Reveley MA. Improved antisaccade performance with risperidone in schizophrenia. J Neurol Neurosurg Psychiatry. 2002;72:449–454. doi: 10.1136/jnnp.72.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellard C, Tremblay S, Lehoux C, Roy MA. Processing spatial-temporal information in recent-onset schizophrenia: the study of short-term memory and its susceptibility to distraction. Brain Cognition. 2007;64:201–207. doi: 10.1016/j.bandc.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Geyer MA, Braff DL. Multiple site evaluation of P50 suppression among schizophrenia and normal comparison subjects. Schizophr Res. 1998;30:71–80. doi: 10.1016/s0920-9964(97)00122-9. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Elvevag B. Genes, cognition and brain through a COMT lens. Neuroscience. 2009;164:72–87. doi: 10.1016/j.neuroscience.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Goldberg TE, Gold JM, Elvevag B, Weinberger DR. Cognitive factor structure and invariance in people with schizophrenia, their unaffected siblings, and controls. Schizophr Bull. 2011;37:1157–1167. doi: 10.1093/schbul/sbq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Harvey PD. Systemic hypotheses for generalized cognitive deficits in schizophrenia: a new take on an old problem. Schizophr Bull. 2009;35:403–414. doi: 10.1093/schbul/sbn097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Iannone VN, Wilk CM, Gold JM. General and specific cognitive deficits in schizophrenia. Biol Psychiatry. 2004;55:826–833. doi: 10.1016/j.biopsych.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Banquet JP, Allilaire JF, Paillere-Martinot ML, Dubois B, Burnod Y. Temporal order and spatial memory in schizophrenia: a parametric study. Schizophr Res. 2001;51:137–147. doi: 10.1016/s0920-9964(00)00151-1. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D, Park S, Clark G, Yohanna D, Houk JC. Spatial serial order processing in schizophrenia. Schizophr Res. 2004;70:203–213. doi: 10.1016/j.schres.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Hill SK, Bishop JR, Palumbo D, Sweeney JA. Effect of second-generation antipsychotics on cognition: current issues and future challenges. Expert Rev Neurother. 2010;10:43–57. doi: 10.1586/ern.09.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Griffin GB, Houk JC, Sweeney JA. Differential effects of paced and unpaced responding on delayed serial order recall in schizophrenia. Schizophr Res. 2011;131:192–197. doi: 10.1016/j.schres.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Harris MS, Khine T, Sweeney JA. Oculomotor and Neuropsychological Effects of Antipsychotic Treatment for Schizophrenia. Schizophr Bull. 2007 doi: 10.1093/schbul/sbm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Sweeney JA, Hamer RM, Keefe RS, Perkins DO, Gu H, McEvoy JP, Lieberman JA. Efficiency of the CATIE and BACS neuropsychological batteries in assessing cognitive effects of antipsychotic treatments in schizophrenia. J Int Neuropsychol Soc. 2008;14:209–221. doi: 10.1017/S1355617708080570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Buchanan RW, Marder SR, Schooler NR, Dugar A, Zivkov M, Stewart M. Clinical Trials of Potential Cognitive-Enhancing Drugs in Schizophrenia: What Have We Learned So Far? Schizophr Bull. 2011 doi: 10.1093/schbul/sbr153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Nolan KA, Bilder RM, Lachman HM, Volavka J. Catechol O-methyltransferase Val158Met polymorphism in schizophrenia: differential effects of Val and Met aleles on cognitive stability and flexibility. Am J Psychiatry. 2004;161:359–361. doi: 10.1176/appi.ajp.161.2.359. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, III, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS Consensus Cognitive Battery, Part 1: Test Selection, Reliability, and Validity. Am J Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychol Bull. 2007;133:833–858. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Harris MS, Keshavan MS, Sweeney JA. Abnormalities in visually guided saccades suggest corticofugal dysregulation in never-treated schizophrenia. Biol Psychiatry. 2005;57:145–154. doi: 10.1016/j.biopsych.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Harris MS, Khine TT, Keshavan MS, Sweeney JA. Reduced attentional engagement contributes to deficits in prefrontal inhibitory control in schizophrenia. Biol Psychiatry. 2008;63:776–783. doi: 10.1016/j.biopsych.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Kester DB, Stafiniak P. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry. 1991;48:618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschmann A, Zuccolotto A. E-prime user's guide. Pittsburgh, PA: Psychology Software Tools, Inc.; 2002. [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- Silver H, Feldman P, Bilker W, Gur RC. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. Am J Psychiatry. 2003;160:1809–1816. doi: 10.1176/appi.ajp.160.10.1809. [DOI] [PubMed] [Google Scholar]
- Silver H, Goodman C. Verbal as well as spatial working memory predicts visuospatial processing in male schizophrenia patients. Schizophr Res. 2008;101:210–217. doi: 10.1016/j.schres.2007.12.478. [DOI] [PubMed] [Google Scholar]
- Snitz BE, Curtis CE, Zald DH, Katsanis J, Iacono WG. Neuropsychological and oculomotor correlates of spatial working memory performance in schizophrenia patients and controls. Schizophr Res. 1999;38:37–50. doi: 10.1016/s0920-9964(98)00178-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.