Abstract
This study examined the role of Rab5a GTPase in regulating hCG-induced internalization and trafficking of the hCG-LH receptor complex in transfected 293T cells. Coexpression of wild-type Rab5a (WT) or constitutively active Rab5a (Q79L) with LHR significantly increased hCG-induced LHR internalization. Conversely, coexpression of dominant negative Rab5a (S34N) with LHR reduced internalization. Confocal microscopy showed LHR colocalizing with Rab5a (WT) and Rab5a (Q79L) in punctuate structures. Coexpression of Rab5a (WT) and Rab5a (Q79L) with LHR significantly increased colocalization of LHR in early endosomes. Conversely, dominant negative Rab5a (S34N) decreased this colocalization. While Rab5a stimulated internalization of LHR, it significantly decreased LHR recycling to the cell surface and increased degradation. Dominant negative Rab5a (S34N) increased LHR recycling and decreased degradation. These results suggest that Rab5a plays a role in LHR trafficking by facilitating internalization and fusion to early endosomes, increasing the degradation of internalized receptor resulting in a reduction in LHR recycling.
Keywords: LH receptor, Rab5a, Trafficking, Internalization, Recycling, Degradation, GPCR
Introduction
The luteinizing hormone receptor (LHR) is a G protein-coupled receptor (GPCR) that is critical for reproductive processes such as ovulation and maintenance of pregnancy [1–3]. It is now well established that interaction of LHR with luteinizing hormone (LH) or human chorionic gonadotrophin (hCG) leads to activation of adenylate cyclase mediated by the heterotrimeric G proteins, and that the receptor-bound hormone undergoes endocytosis through the arrestin and clathrin mediated pathway [4, 5]. After endocytosis, the receptor is targeted to endosomes, and unlike other GPCRs, a small portion of the LHR recycles back to the plasma membrane while the majority is routed to the lysosomes for degradation [6]. The steady-state level of the LHR undergoes significant changes during the ovarian cycle [2], and receptor trafficking to and from the cell surface might contribute to these changes.
Rab GTPases are G-proteins that are responsible for nearly every trafficking event of the cell [7]. They promote vesicle budding and fusion, thereby facilitating the transport of proteins to and from different cellular compartments [8]. Rab GTPases are members of the Ras superfamily, and like Ras, Rabs function as molecular switches that alternate between GDP and GTP-bound states [9, 10]. The nucleotide-bound state of Rab is regulated by guanine nucleotide exchange factors (GEFs), which promote binding of GTP, and GTPase activating proteins (GAPs), which stimulate GTP hydrolysis. At least 60 Rabs have been identified so far. Each Rab is localized to a specific membrane and regulates a specific transport step. For example, Rab5a is localized to early endosomes and mediates internalization [11]. Similarly, Rab4 and Rab11 localize to early and recycling endosomes [12], while Rab7 localizes to lysosomes and has been shown to mediate degradation of internalized proteins [13]. Rab5a GTPase is the most thoroughly characterized member of the Rab GTPase family, and it has been shown to be involved in the internalization of many GPCRs, including the β2 adrenergic receptor and oxytocin receptors [14–17], adeponectin receptor [18], D2 dopamine receptor [19], the neurokinin-1 receptor [20], angiotensin II type 1A receptor [21] and VEGF receptor [22].
Ligand-bound GPCRs can often stimulate similar downstream signaling pathways, yet their trafficking and fate can differ significantly. This diversity in GPCR trafficking likely contributes to the ability of these receptors to differentially regulate the extent and timing of cellular responses. The aim of this study was to examine the possible role of Rab5a GTPase in internalization and post-endocytic trafficking of the LHR. Here we demonstrate that Rab5a facilitate the internalization of LHR, colocalizes in early endosomes, increases its degradation and inhibits recycling.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium, Waymouth’s MB 752/1 medium and Hank's Buffered Saline Solution (HBSS) were purchased from Gibco (Carlsbad, CA). Anti-FLAG M2 antibodies and hCG were obtained from Sigma (St. Louis, MO). An ECL detection system was purchased from Amersham Pharmacia (Piscataway, NJ). Na 125I was purchased from Perkin Elmer (Boston, MA). The FuGENE6 transfection kit was obtained from Roche Diagnostics (Indianapolis, IN). Rab5a antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). EEA1 antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA). AlexaFluor-488 and AlexaFluor-594 secondary antibodies were purchased from Molecular Probes (Carlsbad, CA). Plasmids containing wild-type Rab5, dominant negative Rab5 and constitutively active Rab5 were provided by Dr. Stephen Ferguson (University of Western Ontario). GFP-tagged Rab5 constructs were provided by Dr. Jose Esteban (University of Michigan).
Methods
Transient expression of human LHR in 293T cells
293T cells were grown in DMEM as previously described [23]. Cells in exponential growth phase were plated 5–9 h before transfection at a density of 4–5 × 106 per 10-cm plate and 265,000–350,000 per well for 12-well plates. Cells were transfected using FuGENE6 transfection reagent or the calcium phosphate coprecipitation method with 10 μg of plasmid per 10-cm plate. For co-expression studies, 5 μg of each construct was used for transfections per 10-cm plate, as indicated in the figure legends.
Immunoprecipitation and Western blot analysis
Cells were harvested approximately 48 h after transfection and lysed in PBS containing triton X-100 and protease inhibitors. The cell lysates were precleared with protein A agarose and centrifuged at 14,000g for 5 min. The precleared lysate was incubated with Rab5 antibody followed by protein A agarose. After extensive washes, the proteins were eluted from the beads with SDS loading buffer containing 50 mM DTT and 5% β-mercaptoethanol. Proteins were separated by SDS-PAGE and transferred to nitrocellulose. Western blot analysis was performed using Rab5 antibody as the primary antibody and an HRP-conjugated secondary antibody (1:10,000). The blot was developed using ECL according to the manufacturer’s instruction (Amersham, IL).
Receptor-mediated 125I-hCG internalization assay
Internalization was performed as previously described from our laboratory [24, 25]. Briefly, 4–5 × 106 cells were transiently transfected in 10-cm cell culture dishes with plasmids containing hLHR with vector pcDNA4, wild-type Rab5a (WT), dominant negative Rab5a (S34N) or constitutively active Rab5a (Q79L) using FuGENE6 transfection reagent. The cells were harvested 48 h following transfection and incubated with 100 ng/ml of 125I-hCG for the indicated times at 37 or 4°C overnight. Non-specific binding was measured by adding excess, non-labeled hCG to designated tubes. The internalization reactions were terminated by the addition of 2 ml of cold Waymouth’s medium, and the cells were pelleted at 250g for 5 min at 4°C to remove unbound 125I-hCG. The supernatants were removed and the pellets washed with the same media. To remove cell surface-bound 125I-hCG, the pelleted cells were incubated with a low pH buffer (100 mM NaCl, 50 mM glycine pH 3.0) at 4°C for 4 min. This procedure was repeated, and both acid washes were pooled and assayed for 125I using a gamma counter. Washing the cells with the acidic buffer has been shown to releases approximately 90% of bound hormone. The radioactivity associated with the pellet was then determined. The radioactivity associated with the acid washes represents the surface-bound hormone, whereas the radioactivity associated with the pellet after acid washes represents internalized hormone. Non-specific binding was subtracted from total binding to calculate specific binding.
Assay of receptor recycling
The recycling of the internalized receptor was examined using a previously published procedure [26] with slight modification. Briefly, 293T cells were transfected with plasmids containing hLHR cloned into pcDNA4 with Rab5a (WT), dominant negative Rab5a (S34N) or constitutively active Rab5a (Q79L). After 48 h, the medium was replaced with 1 ml of Waymouth’s MB752/1 medium containing 1 mg/ml BSA and 20 mM HEPES, pH 7.4, and incubated for 20 min at 37°C. Cells were then incubated with 21 ng/ml 125I-hCG for 2 h at 37°C to allow for internalization. To account for non-specific binding, 1,000-fold excess of unlabeled hCG was added to designated plates. After 2 h of incubation, the cells were washed three times with cold HBSS containing 0.1% BSA to remove excess unbound ligand. Surface receptor-bound ligand was removed with acid washes performed two times at 4°C for 2 min. This acidic wash has been shown to remove approximately 90% of bound hormone (data not shown). The acid-washed cells were then rinsed once with acid followed by two washes with Waymouth’s medium containing BSA at 4°C. To follow the fate of the internalized receptor, cells were incubated at 37°C for the indicated times in Waymouth’s/BSA containing 100 ng/ml of non-labeled hCG to prevent reassociation of any undegraded 125I-hCG secreted into the media. After the indicated times, the medium was removed and saved. Cells were washed once with cold HBSS/BSA, and these washes as well as the saved media were counted. The radioactivity in the wash buffer was found to equal the background level showing that all of the non-specifically bound 125I-hCG had been removed. The total radioactivity in the saved media and the wash represents total secreted radioactivity. The 125I-hCG reappearing on the cell surface represents recycled receptor. To determine this amount of recycled receptor, surface-bound 125I-hCG was removed with two acid washes, 2 min each, followed by one rinse with acid wash and one rinse with Waymouth’s medium. These washes were counted to determine the amount of recycled hormone. Finally, cells were solubilized with 0.5 N NaOH, and the radioactivity associated with the cells was counted to determine the amount of cell-associated hormone.
Data analysis
The rate constants of internalization and recycling were calculated by fitting the data shown in Figs. 1b and 4 to a one-phase association equation, R = R max (1 − exp−kt), where the amount of receptor internalization starts at 0 and increases over time (t) to a maximum equal to R max with a rate constant of k. The initial rate of internalization was estimated by multiplying the rate constants shown in Table 1 by the maximum fraction of internalization. The initial rate of recycling was determined by plotting the fraction recycled (recycled/total) versus time (0–60 min). Linear regression analysis was performed to give the slope of the line, which was taken as the initial rate of recycling. Statistical significance was determined by t test with p < 0.05 considered significant.
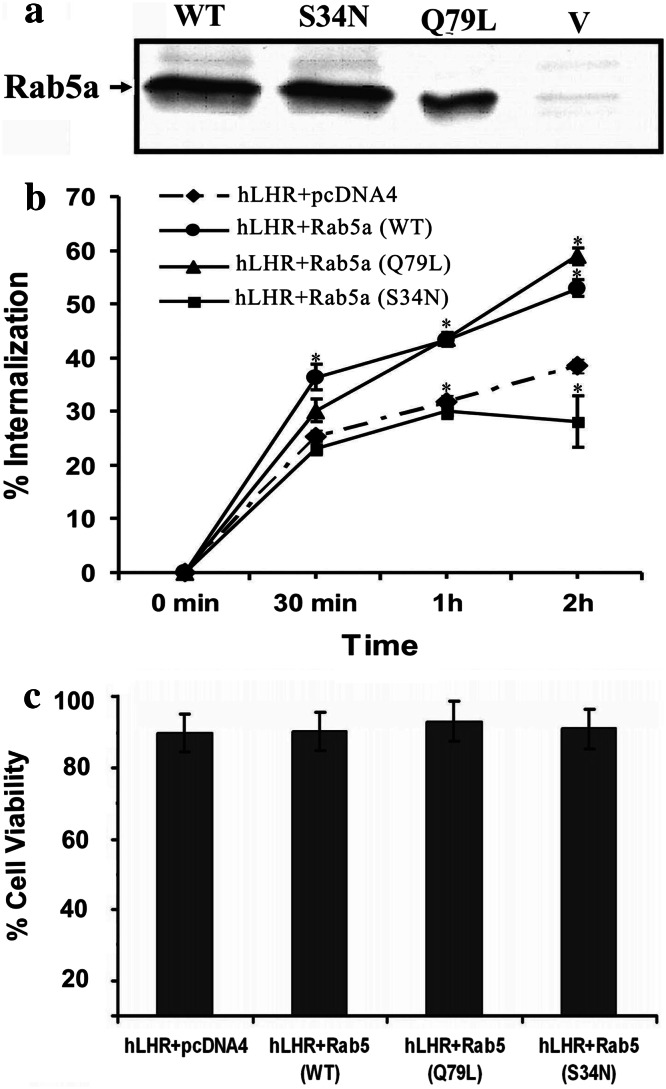
Fig. 1.
LH receptor trafficking. a Western blot analysis of Rab5a. Plasmids containing Rab5a cDNA were transiently transfected into 293T cells. After 48 h of transfection, the cells were lysed, and the Rab5a proteins were immunoprecipitated using Rab5a antibodies. The immunoprecipitants were run on SDS-PAGE, and Western blot analysis was done using Rab5a antibodies and HRP-conjugated secondary antibody to visualize the proteins. V represents the cells transfected with vector alone. b The effect of Rab5a on hLHR internalization when co-expressed with vector, Rab5a (WT), Rab5a (Q79L) or Rab5a (S34N). Internalization of 125I-hCG-bound hLHR was assessed as described in “Methods.” Each point represents the mean ± SEM, which was determined in triplicate for each experiment. The data were fitted to a one-phase exponential association equation using GraphPad Prism. Statistical significance (p < 0.05) compared to hLHR was assessed by t test and is indicated by asterisks at each time point. c The effect of Rab5a co-expression with hLHR on cell viability. 293T cells were transfected with hLHR plasmid plus vector (pcDNA4), Rab5a (WT) or Rab5a (Q79L) or Rab5a (S34N); 48 h after transfection, cells were harvested using PBS-EDTA and resuspended in complete DMEM. Trypan blue-excluded viable cells were counted with a hemocytometer. Triplicate wells were transfected for each construct(s), and four counts were done for each well. Data are expressed as mean ± SEM. There was no significant difference among the groups compared to hLHR plus vector (pcDNA4) alone
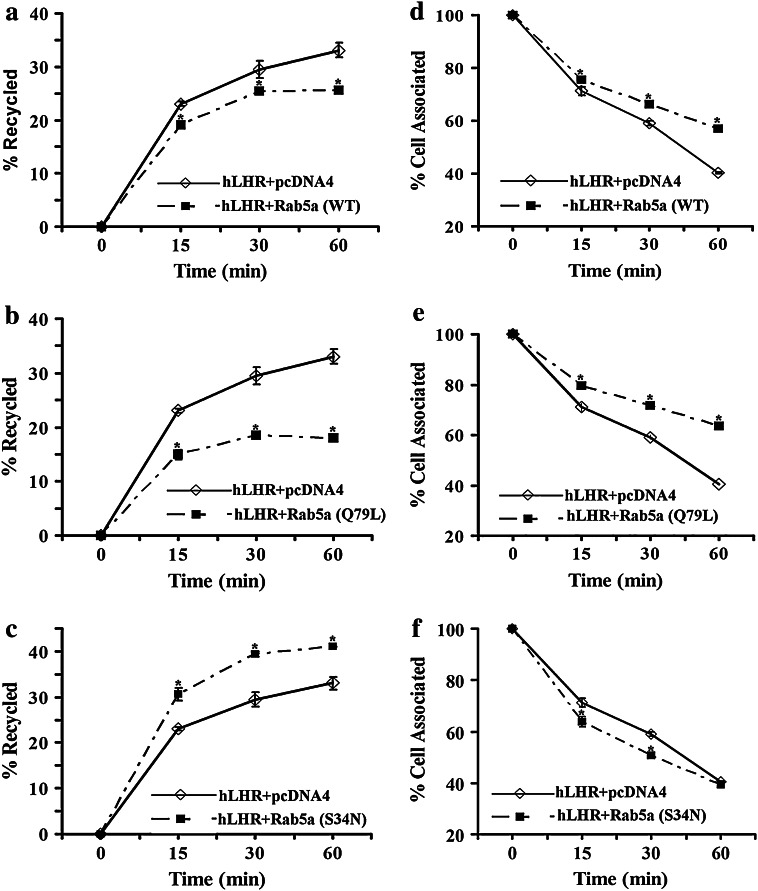
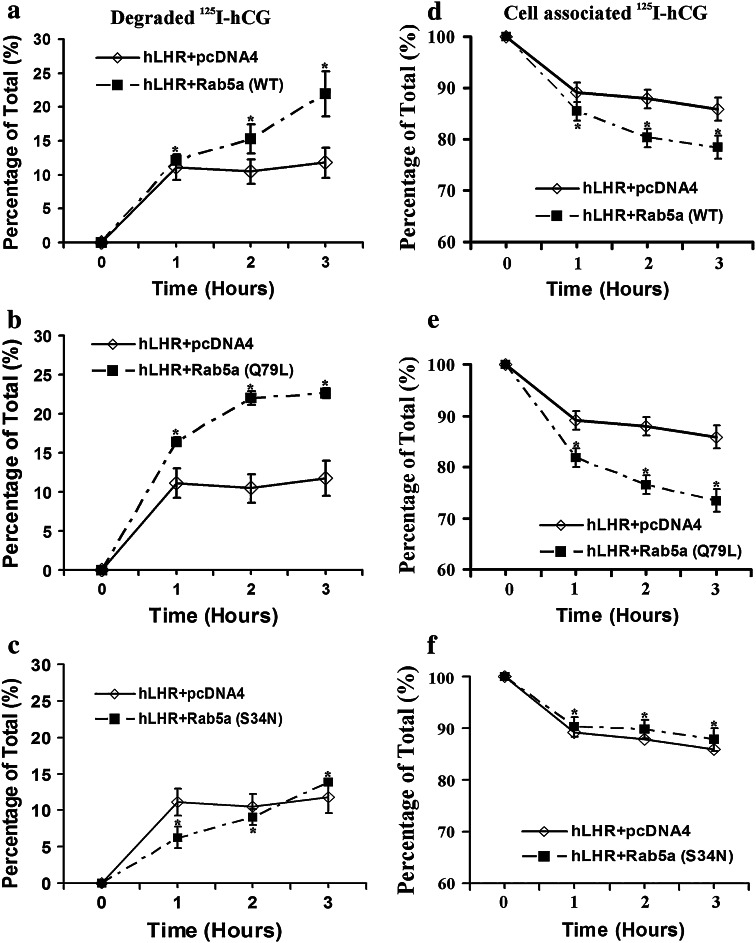
Fig. 4.
Effect of Rab5a on hLHR recycling. 293T cells were transiently cotransfected with plasmids containing hLHR and Rab5a (WT) or Rab5a (Q79L) or Rab5a (S34N) as indicated. After 48 h, the cells were incubated with 21 ng/ml of 125I-hCG for 2 h at 37°C. The receptor recycling experiments were performed as described in “Methods.” a Recycling of hLHR when coexpressed without or with Rab5a (WT). b Recycling of hLHR when coexpressed without or with Rab5a (Q79L). c Recycling of hLHR when coexpressed without or with Rab5a (S34N). d Cell-associated hLHR-hCG complex when coexpressed without or with Rab5a (WT). e Cell-associated hLHR-hCG complex when coexpressed without or with Rab5a (Q79L). f Cell-associated hLHR-hCG complex when coexpressed without or with Rab5a (S34N). The figures show the mean ± SEM of triplicate samples from two separate experiments. Statistical significance (p < 0.05) compared to hLHR + pcDNA4 was assessed by t test and is indicated by an asterisk at each time point
Table 1.
Analysis of hLHR internalization when coexpressed with vector or with Rab5a constructs
| Plasmid constructs | Rate constant of internalization (min−1) | Max % internalization | Estimated initial rate of internalization (min−1) |
|---|---|---|---|
| hLHR + pcDNA4 | 0.0338 ± 0.0018 | 38.40 ± 1.4 | 0.00326 ± 0.0003 |
| hLHR + Rab5a (WT) | 0.0297 ± 0.0013 | 53.90 ± 1.8* | 0.00443 ± 0.0001 |
| hLHR + Rab5a (Q79L) | 0.0320 ± 0.0030 | 61.80 ± 1.3* | 0.00484 ± 0.0005 |
| hLHR + Rab5a (S34N) | 0.0301 ± 0.0016 | 28.10 ± 1.9* | 0.00222 ± 0.0003* |
The rate constants of internalization and maximum percent internalization were obtained by fitting the data shown in Fig. 1b to a one-phase exponential association equation using GraphPad Prism. The results shown are the mean ± SEM of three independent experiments. The estimated initial rate of internalization was calculated by multiplying the rate constant and the maximum fraction of internalization. Statistical significance (p < 0.05) compared to hLHR + pcDNA4 was assessed by t test using Sigma Stat software and is indicated by an asterisk
Immunofluorescence and confocal microscopy
Cells (3–4 × 105) were seeded on glass coverslips in six-well cell culture dishes and allowed to attach overnight (60% confluence), and then transfected using FuGENE6 (Roche, Indianapolis, IN). For each transfection sample, 2 μg of cDNA was mixed with 12 μl of FuGENE6 reagent in 200 μl of DMEM without FBS and antibiotics, and incubated for 30 min at room temperature and added to the cells. The following plasmid constructs were used for cotransfections: hLHR and vector (pcDNA4), GFP-Rab5a (WT), GFP-Rab5a (S34N) or non-tagged Rab5a (WT), Rab5a (Q79L) and Rab5a (S34N). GFP-tagged Rab5a constructs affect hLHR trafficking similarly to the non-tagged Rab5a (data not shown). For hLHR and Rab5a colocalization studies, approximately 48 h after transfection the cells were rinsed with HBSS. The cells were exposed to 200 ng/ml hCG for 30 min at 37°C. The cells were washed three times with PBS, fixed in 4% paraformaldehyde for 20 min at room temperature and permeabilized with 0.2% Triton X-100 for 20 min. To detect hLHR-FLAG, cells were incubated with mouse anti-FLAG antibody overnight at 4°C followed by anti-mouse AlexaFluor 594 secondary antibody for 2 h at room temperature, and then the coverslips were mounted with anti-FADE reagent without DAPI.
For colocalization studies of FLAG-hLHR in early endosomes, transfected cells were incubated with and without 200 ng/ml hCG for 30 min at 37°C. The cells were washed three times with PBS, fixed, permeabilized and processed for double immunostaining after incubation with two primary antibodies (rabbit polyclonal anti-EEA1 to detect early endosomes and mouse anti-FLAG to detect FLAG-hLHR) overnight at 4°C. Cells were washed three times with PBS and incubated with two secondary antibodies (anti-rabbit AlexFluor 488 and anti-mouse AlexaFluor 594) for 2 h at room temperature. After extensive washing the coverslips were mounted using Anti-FADE reagent with DAPI (Molecular Probes) and analyzed for colocalization with an Olympus FluoView 500 Laser Scanning confocal microscope. Samples were scanned on an Olympus IX-71 inverted microscope using a 60× O (oil) objective. FluoView version 4.3 software was used to collect data using sequential scanning mode to minimize signal cross-over. Quantification of vesicular labeling was performed using the ImageJ program (NIH, version 1.43u). The samples were analyzed at different lower thresholds to determine the best fit. The upper threshold was always set at 255. Colocalization was quantified using the colocalization plugin of ImageJ. The channel ratio was always set at 90% for both channels; the best-fit lower threshold value to remove most background signal was determined using the threshold tool of the ImageJ program.
Degradation of the internalized receptor-hormone complex
This was measured using previously published procedures [24, 27]. Briefly, transfected cells were washed and then allowed to internalize by incubating with 50 ng/ml 125I hCG for 2 h at 37°C. The cells then were placed on ice and acid washed using pH 3.0 buffer to remove ligand bound to receptors on the cell surface. The cells were then incubated with assay medium at 37°C to allow the cells to process and degrade the hormone-receptor complex that had been internalized during the first incubation. At the time points indicated, the tubes were placed on ice and spun at 250g for 5 min. The media were saved, and the un-degraded and degraded hormone-receptor complexes were measured by precipitation with trichloroacetic acid (TCA, 10%) [28]. Radioactivity remaining in the pellets was assayed to determine the number of cell-associated receptors.
Results
Role of Rab5 in LH receptor internalization
LHR undergoes internalization following interaction with its ligand, LH, or hCG [3]. To examine if Rab5a GTPase mediates this process, 293T cells were transiently transfected with hLH receptor cDNA and vector pcDNA4, wild-type Rab5a (WT), dominant negative Rab5a (S34N) or constitutively active Rab5a (Q79L). Mutation of Gln79 to Leu reduces GTPase activity, maintaining Rab5 in a constitutively active state [29]. Conversely, dominant negative Rab5a (S34N) preferentially binds GDP, maintaining it in an inactive state [30]. The expression of the Rab5a constructs was verified by Western blot analysis (Fig. 1a), while trafficking of the Rab5a constructs was verified by following the fate of radiolabeled ligand, 125I-hCG. The binding of hCG to LHR is essentially irreversible (Kd is in the low nM range); thus, radiolabeled hCG has been used as a marker for hLHR trafficking in this study as well as in others [6, 24, 27, 31]. Figure 1b shows the time course of 125I-hCG internalization in cells expressing hLHR with and without overexpression of wild-type or mutant Rab5a. The results show that expression of either WT or constitutively active Rab5a (Q79L) significantly increased the extent of hLHR internalization, whereas dominant negative Rab5a (S34N) significantly decreased hLHR internalization (Table 1; Fig. 1b). The initial rate of hLHR internalization was estimated as described in the “Materials and methods.” Non-linear regression analysis of the data indicates that the hLHR is internalized with a rate constant of 0.0338 ± 0.0018 min−1 (Table 1). However, expression of the Rab5a constructs only affected the extent of internalization and not the rate constant of internalization. Furthermore, the expression of dominant negative Rab5a (S34N) significantly decreased the initial rate of internalization. While constitutively active and wild-type Rab5a (Q79L) appear to increase the initial rate of internalization, statistical analysis shows that the rates are not statistically different from hLHR expressed without exogenous Rab5a. Thus, only the extent of internalization is significantly affected when hLHR is expressed with wild-type Rab5a (WT) or constitutively active Rab5a (Q79L).
Since Rab5a activation has been shown to induce cell death in some cell types [32], the trypan blue exclusion method was used to determine if overexpression of Rab5a (WT), dominant negative Rab5a (S34N) or constitutively active Rab5a (Q79L) affects cell viability. Figure 1c shows that overexpression of all Rab5a constructs along with hLHR produced no effects on cell viability compared to cells transfected with vector and hLHR.
hCG-induced subcellular colocalization of the LHR and Rab5a (WT), Rab5a (S34N) or Rab5a (Q79L)
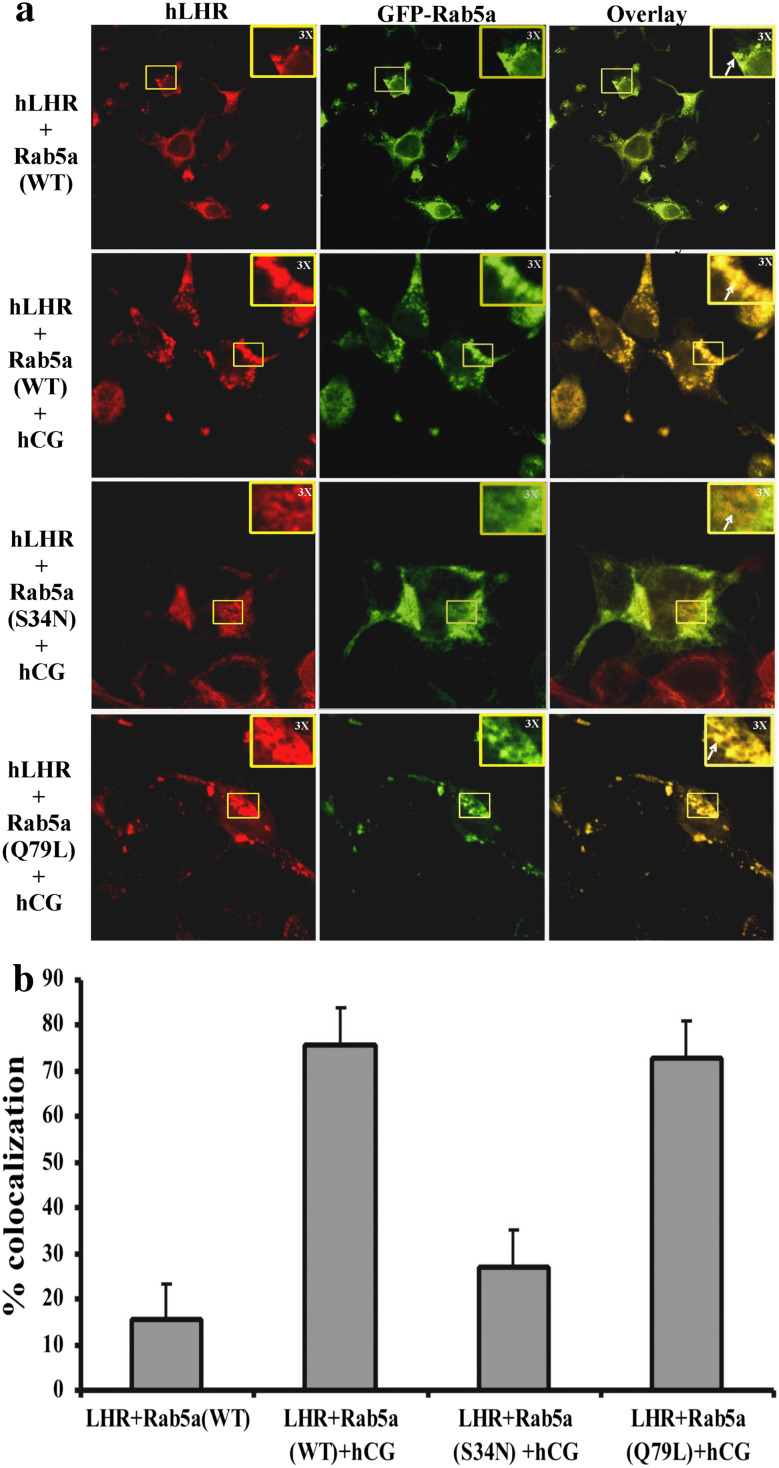
The stimulatory effect of Rab5a on LHR internalization suggests that wild-type Rab5a (WT) and constitutively active Rab5a (Q79L) may lead to a net increase in the intracellular levels of receptor, whereas dominant negative Rab5a (S34N) leads to a net decrease in intracellular LH receptor. These results were confirmed by direct visualization using confocal microscopy. 293T cells were transiently co-transfected with FLAG-tagged hLHR construct and plasmids encoding green fluorescent protein (GFP)-tagged forms of Rab5a (WT), Rab5a (S34N) or Rab5a (Q79L). After incubation with hCG to facilitate internalization, the colocalization of the FLAG-tagged LHR with Rab5a was examined by confocal microscopy as described in “Materials and methods.” The results show that GFP-Rab5a (Q79L) and GFP-Rab5 (WT) co-expression with FLAG-tagged LHR leads to the colocalization with LH receptor at the punctate structures in the sub-cellular region (Fig. 2a with overlay), whereas co-expression with dominant negative GFP-Rab5a (S34N) failed to show similar punctuate structures. As expected, little colocalization was visible at the cell surface (Fig. 2a with overlay). Furthermore, quantitation of colocalization data by ImageJ software with colocalization plugin showed 75–85% colocalization in the cells co-expressed with Rab5a (WT) or Rab5a (Q79L) and hLHR, whereas Rab5a (S34N) co-expression with hLHR reduced the colocalization to 28–35% (Fig. 2b).
Fig. 2.
a Subcellular colocalization of hLHR and Rab5a. 293T cells were plated on glass coverslips in six-well plates and transiently transfected with plasmids containing hLHR cDNA and GFP-Rab5a (WT), GFP-Rab5a (S34N) or GFP-Rab5a (Q79L); 48 h post-transfection, the cells were incubated with or without 200 ng/ml hCG for 30 min at 37°C. The cells were washed with HBSS, fixed and permeabilized, and hLHR was detected by incubation with mouse anti-FLAG M2 antibody and AlexaFluor 594-conjugated anti-mouse secondary antibody. Images were captured at 60× O (oil) magnification using an Olympus FluoView 500 Laser Scanning Confocal Microscope. These images show the colocalization (yellow), hLHR (red) and GFP-Rab5a (green). The colocalization area was magnified ×3 and is shown at the right corner of the panel. The figures shown are representative images of two experiments with each sample done in triplicate. b To quantify the level of colocalization, images were analyzed using the ImageJ colocalization plugin (as described in “Methods”). The graphs present the number of colocalization events normalized for the number of hLHR-positive compartments. After hCG treatment, ∼70–80% of hLHR positive compartments showed colocalization with Rab5a (WT). Values are the mean of at least two experiments. In each experiment, at least five random fields were analyzed for each point. Error bars represent the ±SEM
Rab5a regulates fusion of internalized hLHR endocytic vesicles with the early endosomes
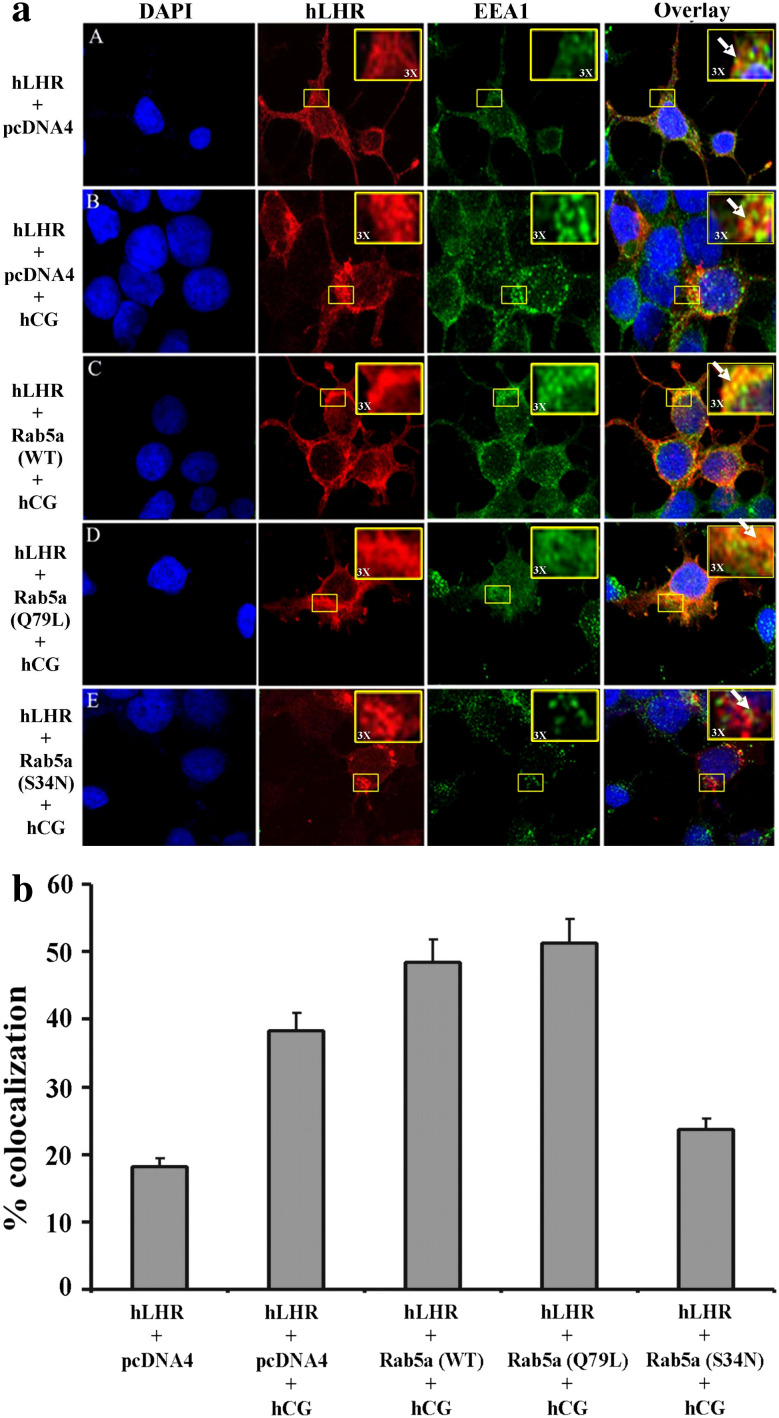
Endocytosis is characterized by vesicular transport along numerous pathways. Common steps in each pathway include membrane budding to form vesicles, transport to a particular destination, and ultimately docking and fusion with the target membrane. Specificity of vesicle targeting is rendered in part by associated Rab GTPases. Rab5a, a member of small GTPase family, plays a key role in the final fate of endocytosed membrane proteins and is usually recruited to the endocytic vesicles where it regulates fusion with the early endosomes [33]. To analyze whether Rab5a is involved in the fusion of hLHR to early endosomes, we co-expressed FLAG-hLHR and Rab5a (WT) or Rab5a (Q79L) or Rab5a (S34N). After incubation with 200 ng/ml hCG for 30 min at 37°C, the cells were analyzed for colocalization of hLHR in early endosomes using EEA1 as a marker of early endosomes. The results in Fig. 3a, C, D showed that Rab5a (WT) and Rab5a (Q79L) caused more colocalization of the hLHR in early endosomes compared to hLHR plus vector alone, whereas Rab5a (S34N) completely inhibited this colocalization (Fig. 3a, E). Since LHR internalization is ligand induced, no colocalization was seen in cells expressing hLHR and pcDNA4 not exposed to hCG (Fig. 3a, A), and little colocalization was seen in the cells expressing hLHR alone incubated with 100 ng/ml hCG (Fig. 3a, B). The quantitative measurement of colocalization of hLHR in early endosomes showed 50–55 and 55–58% in cells cotransfected with hLHR and Rab5a (WT) or Rab5a (Q79L), respectively, whereas hLHR with Rab5a (S34N) colocalization was reduced to 23–26% (Fig. 3b).
Fig. 3.
a Effect of Rab5a on colocalization of hLHR in early endosomes upon hCG treatment. 293T cells were plated on glass coverslips and transiently transfected with plasmids containing hLHR cDNA and Rab5a (WT), Rab5a (Q79L) or Rab5a (S34N); 48 h post-transfection, the cells were incubated with or without 200 ng/ml hCG for 30 min at 37°C. The cells were washed with PBS, fixed and permeabilized as explained in “Methods.” The hLHR was detected by incubation with mouse-anti-FLAG M2 antibody and AlexaFluor 594-conjugated anti-mouse secondary antibody. The early endosomes were detected using EEA1 antibody and AlexaFluor 488 secondary antibody. Images were captured at 60× O (oil) magnification using the Olympus FluoView 500 Laser Scanning Confocal Microscope. These images show the colocalization (yellow) or hLHR (red) in early endosomes (green). The colocalization area was magnified ×3 and is shown at the right corner of the panel. The figures shown are representative images of two separate experiments with each sample done in triplicate. b The colocalization was quantified as described in “Methods.” Values are the mean of at least two experiments. In each experiment at least five random fields were analyzed for each point. Error bars represent the ± SEM (p < 0.05)
Role of Rab5a in LHR recycling
The data in Fig. 1 suggest that Rab5a might have a role in regulating the steady-state levels of hLHR at the cell surface by affecting internalization of hLHR. However, since steady-state levels are dictated by both internalization and recycling, we next examined the role of Rab5a in hLHR recycling. The recycling assay was performed as described in the “Materials and methods.” The results (Fig. 4) show that overexpression of Rab5a (WT) or Rab5a (Q79L) significantly decreased receptor recycling (Fig. 4a, b), whereas expression of dominant negative Rab5a (S34N) increased hLHR receptor recycling (Fig. 4c). Consistent with the ability of constitutively active and wild-type Rab5a to decrease recycling, expression of each of these constructs increased the cell-associated hormone-receptor complex (Fig. 4d, e), whereas expression of dominant negative Rab5a (S34N) decreased cell associated receptor-hormone complex (Fig. 4f). The initial rates of recycling were determined by examining recycling at time points (0–60 min) and plotting the fraction of receptor recycled versus time. Linear regression analysis was used to obtain the slope of the lines to give the initial rates of recycling shown in Table 2. These results show that wild-type Rab5a (WT) and constitutively active Rab5a (Q79L) decreased the initial rate of LH receptor recycling, whereas dominant negative Rab5a (S34N) increased the initial rate.
Table 2.
Analysis of hLHR recycling when expressed with vector (pcDNA4) wild-type (WT), constitutively active (Q79L) or dominant negative (S34N) Rab5a
| Plasmid constructs | Initial rate of recycling (min−1) |
|---|---|
| hLHR + pcDNA4 | 0.00443 ± 0.0002 |
| hLHR + Rab5a (WT) | 0.00331 ± 0.0003* |
| hLHR + Rab5a (Q79L) | 0.00263 ± 0.0005* |
| hLHR + Rab5a (S34N) | 0.00508 ± 0.0005* |
Linear regression analysis was performed on a plot of the fraction of recycled receptor (recycled/total) versus time (0–60 min) to give a slope equal to the initial rate of recycling. The results shown here are the mean ± SEM of three samples from one representative experiment. Statistical significance (p < 0.05) compared to hLHR + pcDNA4 was determined by t test and is indicated by an asterisk
Role of Rab5a in the degradation of the internalized 125I-hCG–hLHR complex
The results of the recycling experiments presented above suggest that Rab5a inhibits hLHR recycling. Since there was a significant reduction in recycling, the possible effect of Rab5a on the degradation of the internalized 125I-hCG–hLHR complex was examined. The results showed that overexpression of Rab5a (WT) or Rab5a (Q79L) significantly increased degradation of internalized hormone-receptor complex (Fig. 5a, b), whereas expression of dominant negative Rab5a (S34N) decreased receptor degradation (Fig. 5c). Consistent with the ability of constitutively active and wild-type Rab5a to increase the degradation, expression of each of these constructs decreased cell-associated hormone-receptor complex (Fig. 5d, e). Conversely, the expression of dominant negative Rab5a (S34N) increased cell-associated receptor-hormone complex (Fig. 5f).
Fig. 5.
Time course of degradation of the internalized 125I-hCG-hLHR complex in 293T cells transiently cotransfected with hLHR and Rab5a (WT) or Rab5a (Q79L) or Rab5a (S34N). 293T cells were transiently cotransfected with the indicated plasmid constructs. After 48 h, the cells were incubated with 125I-hCG for 2 h at 37°C. At this time (time = 0), the cells were washed with a neutral buffer (to remove free hormone) and briefly treated with an isotonic pH-3 buffer (to remove the surface-bound hormone). The cells were incubated at 37°C in assay medium for the indicated times. At each time point, media were collected and saved. The media were precipitated by trichloroacetic acid (TCA, 10%), and used to measure the undegraded (TCA precipitated) and degraded (TCA soluble) hormone. The pelleted cells were used to determine the amount of radioactivity remaining cell associated. a, b and c represent the % degraded, and d, e and f represent % cell-associated radioactivity. The internalized radioactivity present at t = 0 was approximately 5,000–10,000 cpm/tube. Each value represents the mean ± SEM of triplicate determinations. The experiments were done two times with similar results. Statistical significance (p < 0.05) compared to hLHR + pcDNA4 was assessed by t test and is indicated by an asterisk at each time point
Discussion
The expression of LH/hCG receptors shows marked changes during the ovarian cycle in response to the changing hormonal milieu [34]. The steady-state level of the receptor is controlled by dynamic changes in the receptor turnover, and a number of membrane-associated and cytoplasmic proteins dictate this process. In this study, we used wild-type (WT) and mutant Rab5a constructs to determine whether Rab5a has any role in this process since Rab5a GTPase has been implicated in the internalization of some receptors of the GPCR family. Specifically, we examined the role of wild-type Rab5a (WT), constitutively active Rab5a (Q79L) and dominant negative Rab5a (S34N) on hLHR internalization, recycling and degradation. Our results show that these processes are regulated by Rab5a. Based on the role of Rab5a in the internalization of other GPCRs, [15–18], it was not surprising that Rab5a affects hLHR internalization. However, an unexpected finding was that Rab5a negatively affected recycling of the hLHR back to the cell surface, while increasing its degradation. Although the role of Rab5a in GPCR internalization has been reported previously for other GPCRs, to our knowledge, this is the first report that describes a role for Rab5a in GPCR recycling and degradation. Consistent with our results, a recent study reported that Rab5a specifically regulates the trafficking and degradation of epidermal growth factor receptor (EGFR) [35]. A role for Rab5a in the recycling of another GPCR, the corticotrophin-releasing factor (CRF) 1α receptor, also has been implicated, although recycling was not directly examined in that study [36]. Additional functions of Rab5a have been reported, including a role in the biogenesis of lysosomal compartments [37] and degradation [35]. Thus, while Rab5a is primarily thought to mediate internalization and endosome fusion, recent studies have suggested that Rab5a has a much wider role.
The role of Rab5a in LH receptor trafficking differs from its role in other GPCRs such as the β2-adrenergic receptor [38] in that Rab5a (WT) coexpression caused no change in internalization, but Rab5a (S34N) coexpression caused inhibition of internalization. It is of interest to note the similarities in the apparent role of Rab5a in the trafficking of the LH receptor reported here and the trafficking of the CRF1α receptor as reported by Holmes et al. [36], who showed that overexpression of Rab5 inhibited resensitization of the CRF1α receptor. In agreement with our findings, Dinneen and Ceresa have reported that Rab5 (Q79L) coexpression causes increased ligand-induced internalization and decreased recycling of the EGFR [39].
It is noteworthy that Rab5a caused increased association of LHR in the early endosomes, since the early endosome is known to be a hub for the sorting of membrane proteins after endocytosis [40]. In the endosome, cargo proteins can either be delivered to lysosomes through multivesicular bodies (MVBs) for degradation, recycled to the plasma membrane directly or indirectly through the recycling endosome, or recycled through retrograde transport from endosomes to the trans-Golgi network (TGN) [40]. Two distinct recycling pathways have been explained: rapid recycling from sorting endosomes or slow recycling from recycling endosomes. Rab4 partially colocalizes with Rab5 and governs the rapid cell surface recycling of proteins from early endosomes, whereas Rab11 controls the slow recycling route of proteins from perinuclear recycling endosomes back to the plasma membrane [41]. Lysosomal sorting and degradation of the cargo require transition of early endosomes to late endosomes by rapidly replacing Rab5 with Rab7 [42]. Our data show that cotransfection of hLHR with Rab5a (WT) or Rab5a (Q79L) resulted in colocalization into early endosomes. This suggests that internalized LHR is sorted to early endosomes before being delivered to lysosomes. In support of our findings, recently it has been reported that Rab5a is required in early endosomal fusion of vascular endothelial growth factor receptor 2 (VEGFR2) [22]. It is likely that additional proteins might be required to interact with Rab5 in order to direct the cargo to degradative or recycling pathways. For example, it has been shown recently that SAND-1/Mon1 is involved in the regulation of the transition of early endosome to late endosome by displacing RABX-5 from early endosomal membranes facilitating the recruitment of Rab7. This recruitment of Rab7 allows delivery of cargo to a degradative pathway [43, 44]. Similarly, Chen et al., have shown that Rab5a associates with Rin1 and contributes to the degradation of EGFR. This conclusion was based on studies using RNAi to inhibit Rab5a, Rab5b and Rab5c, which prevented the degradation of cargo by blocking the transition of early endosome to late endosome. They concluded from their studies that Rin1 associates preferably with Rab5a, but not with Rab5c [35]. In the present study, we show that cotransfections of hLHR with Rab5a (S34N) caused reduced degradation of hLHR. It is possible that a similar mechanism might be involved by recruiting other factors that contribute to the degradation of hLHR. It is interesting to note that Galet et al. have identified a GT motif in hLHR that directs the receptor from a degradative to a recycling pathway [45]. If the GT motif of hLHR plays a role in the interaction with any of these proteins that promote recycling, it is tempting to speculate that overexpression of Rab5a might prevent these interactions with GT motifs.
In summary, transport of the LH receptor to and from the cell surface and lysosomal degradation might contribute, in part, to the dynamic regulation of LH receptor expression during the ovarian cycle by permitting the entry of newly synthesized receptors to the cell surface.
Acknowledgments
This work was supported by National Institutes of Health grant R37 HD06656. We are grateful to Helle Peegel and other members of the laboratory for critical reading of the manuscript and many helpful suggestions. The confocal microscopic studies described here utilized the Morphology and Image Analysis Core (MIAC) facility at the University of Michigan Diabetes Research and training center.
Footnotes
T. Gulappa and C. L. Clouser contributed equally to this work.
References
- 1.Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev. 2002;23:141–174. doi: 10.1210/er.23.2.141. [DOI] [PubMed] [Google Scholar]
- 2.Menon KM, Clouser CL, Nair AK. Gonadotropin receptors: role of post-translational modifications and post-transcriptional regulation. Endocrine. 2005;26:249–257. doi: 10.1385/ENDO:26:3:249. [DOI] [PubMed] [Google Scholar]
- 3.Menon KM, Munshi UM, Clouser CL, Nair AK. Regulation of luteinizing hormone/human chorionic gonadotropin receptor expression: a perspective. Biol Reprod. 2004;70:861–866. doi: 10.1095/biolreprod.103.024471. [DOI] [PubMed] [Google Scholar]
- 4.Baratti-Elbaz C, Chinea N, Lahuna O, Lo-osfelt H, Pichon C, Milgrom E. Internalization and recycling pathways of the thyrotropin receptor. Mol Endocrinol. 1999;13:1751–1765. doi: 10.1210/me.13.10.1751. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura K, Liu X, Ascoli M. Seven non-contiguous intracellular residues of the lutropin/choriogonadotropin receptor dictate the rate of agonist-induced internalization and its sensitivity to non-visual arrestins. J Biol Chem. 2000;275:241–247. doi: 10.1074/jbc.275.1.241. [DOI] [PubMed] [Google Scholar]
- 6.Galet C, Ascoli M. A constitutively active mutant of the human lutropin receptor (hLHR-L457R) escapes lysosomal targeting and degradation. Mol Endocrinol. 2006;20:2931–2945. doi: 10.1210/me.2006-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–1017. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 8.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stenmark H. Seeing is believing. Nat Rev Mol Cell Biol. 2009;10:582. doi: 10.1038/nrm2747. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz SL, Cao C, Pylypenko O, Rak A, Wandinger-Ness A. Rab GTPases at a glance. J Cell Sci. 2007;120:3905–3910. doi: 10.1242/jcs.015909. [DOI] [PubMed] [Google Scholar]
- 11.Somsel Rodman J, Wandinger-Ness A. Rab GTPases coordinate endocytosis. J Cell Sci. 2000;113:183–192. doi: 10.1242/jcs.113.2.183. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Li HF, Felder RA, Periasamy A, Jose PA. Rab4 and Rab11 coordinately regulate the recycling of angiotensin II type I receptor as demonstrated by fluorescence resonance energy transfer microscopy. J Biomed Opt. 2008;13:031206. doi: 10.1117/1.2943286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Progida C, Cogli L, Piro F, De Luca A, Bakke O, Bucci C. Rab7b controls trafficking from endosomes to the TGN. J Cell Sci. 2010;123:1480–1491. doi: 10.1242/jcs.051474. [DOI] [PubMed] [Google Scholar]
- 14.Seachrist JL, Laporte SA, Dale LB, Babwah AV, Caron MG, Anborgh PH, Ferguson SS. Rab5 association with the angiotensin II type 1A receptor promotes Rab5 GTP binding and vesicular fusion. J Biol Chem. 2002;277:679–685. doi: 10.1074/jbc.M109022200. [DOI] [PubMed] [Google Scholar]
- 15.O’Keeffe MB, Reid HM, Kinsella BT. Agonist-dependent internalization and trafficking of the human prostacyclin receptor: a direct role for Rab5a GTPase. Biochim Biophys Acta. 2008;1783:1914–1928. doi: 10.1016/j.bbamcr.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunker CM, Kruk I, Hall J, Giambini H, Veisaga ML, Barbieri MA. Role of Rab5 in insulin receptor-mediated endocytosis and signaling. Arch Biochem Biophys. 2006;449:130–142. doi: 10.1016/j.abb.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Conti F, Sertic S, Reversi A, Chini B. Intracellular trafficking of the human oxytocin receptor: evidence of receptor recycling via a Rab4/Rab5 “short cycle”. Am J Physiol Endocrinol Metab. 2009;296:532–542. doi: 10.1152/ajpendo.90590.2008. [DOI] [PubMed] [Google Scholar]
- 18.Ding Q, Wang Z, Chen Y. Endocytosis of adiponectin receptor 1 through a clathrin-and Rab5-dependent pathway. Cell Res. 2009;19:317–327. doi: 10.1038/cr.2008.299. [DOI] [PubMed] [Google Scholar]
- 19.Iwata K, Ito K, Fukuzaki A, Inaki K, Haga T. Dynamin and rab5 regulate GRK2-dependent internalization of dopamine D2 receptors. Eur J Biochem. 1999;263:596–602. doi: 10.1046/j.1432-1327.1999.00549.x. [DOI] [PubMed] [Google Scholar]
- 20.Schmidlin F, Dery O, DeFea KO, Slice L, Patierno S, Sternini C, Grady EF, Bunnett NW. Dynamin and Rab5a-dependent trafficking and signaling of the neurokinin 1 receptor. J Biol Chem. 2001;276:25427–25437. doi: 10.1074/jbc.M101688200. [DOI] [PubMed] [Google Scholar]
- 21.Dale LB, Seachrist JL, Babwah AV, Ferguson SS. Regulation of angiotensin II type 1A receptor intracellular retention, degradation, and recycling by Rab5, Rab7, and Rab11 GTPases. J Biol Chem. 2004;279:13110–13118. doi: 10.1074/jbc.M313333200. [DOI] [PubMed] [Google Scholar]
- 22.Jopling HM, Odell AF, Hooper NM, Zachary IC, Walker JH, Ponnambalam S. Rab GTPase regulation of VEGFR2 trafficking and signaling in endothelial cells. Arterioscler Thromb Vasc Biol. 2009;29:1119–1124. doi: 10.1161/ATVBAHA.109.186239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munshi UM, Peegel H, Menon KM. Palmitoylation of the luteinizing Hormone/human chorionic gonadotropin receptor regulates receptor interaction with the arrestin-mediated internalization pathway. Eur J Biochem. 2001;268:1631–1639. doi: 10.1046/j.1432-1327.2001.02032.x. [DOI] [PubMed] [Google Scholar]
- 24.Bradbury FA, Menon KM. Evidence that constitutively active luteinizing hormone/human chorionic gonadotropin receptors are rapidly internalized. Biochemistry. 1999;38:8703–8712. doi: 10.1021/bi990169t. [DOI] [PubMed] [Google Scholar]
- 25.Kawate N, Menon KM. Palmitoylation of luteinizing hormone/human choriogonadotropin receptors in transfected cells. Abolition of palmitoylation by mutation of Cys-621 and Cys-622 residues in the cytoplasmic tail increases ligand- induced internalization of the receptor. J Biol Chem. 1994;269:30651–30658. [PubMed] [Google Scholar]
- 26.Lazari MF, Liu X, Nakamura K, Benovic JL, Ascoli M. Role of G protein-coupled receptor kinases on the agonist-induced phosphorylation and internalization of the follitropin receptor. Mol Endocrinol. 1999;13:866–878. doi: 10.1210/me.13.6.866. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura K, Ascoli M. A dileucine-based motif in the C-terminal tail of the lutropin/choriogonadotropin receptor inhibits endocytosis of the agonist-receptor complex. Mol Pharmacol. 1999;56:728–736. [PubMed] [Google Scholar]
- 28.Kishi M, Ascoli M. The C-terminal tail of the rat lutropin/choriogonadotropin (CG) receptor independently modulates human (h)CG-induced internalization of the cell surface receptor and the lysosomal targeting of the internalized hCG-receptor complex. Mol Endocrinol. 2000;14:926–936. doi: 10.1210/me.14.6.926. [DOI] [PubMed] [Google Scholar]
- 29.Barbieri MA, Li G, Mayorga LS, Stahl PD. Characterization of Rab5:Q79L-stimulated endosome fusion. Arch Biochem Biophys. 1996;326:64–72. doi: 10.1006/abbi.1996.0047. [DOI] [PubMed] [Google Scholar]
- 30.Stenmark H, Parton RG, Steele-Mortimer O, Lütcke A, Gruenberg J, Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munshi UM, Clouser CL, Peegel H, Menon KM. Evidence that palmitoylation of carboxyl terminus cysteine residues of the human luteinizing hormone receptor regulates postendocytic processing. Mol Endocrinol. 2005;19:749–758. doi: 10.1210/me.2004-0335. [DOI] [PubMed] [Google Scholar]
- 32.Laifenfeld D, Patzek LJ, McPhie DL, Chen Y, Levites Y, Cataldo AM, Neve RL. Rab5 mediates an amyloid precursor protein signaling pathway that leads to apoptosis. J Neurosci. 2007;27:7141–7153. doi: 10.1523/JNEUROSCI.4599-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soldati T, Schliwa M. Powering membrane traffic in endocytosis and recycling. Nat Rev Mol Cell Biol. 2006;7:897–908. doi: 10.1038/nrm2060. [DOI] [PubMed] [Google Scholar]
- 34.Nair AK, Menon KM. Regulation of luteinizing hormone receptor expression: evidence of translational suppression in vitro by a hormonally regulated mRNA-binding protein and its endogenous association with luteinizing hormone receptor mRNA in the ovary. J Biol Chem. 2005;280:42809–42816. doi: 10.1074/jbc.M503154200. [DOI] [PubMed] [Google Scholar]
- 35.Chen PI, Kong C, Su X, Stahl PD. Rab5 isoforms differentially regulate the trafficking and degradation of epidermal growth factor receptors. J Biol Chem. 2009;284:30328–30338. doi: 10.1074/jbc.M109.034546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holmes KD, Babwah AV, Dale LB, Poulter MO, Ferguson SS. Differential regulation of corticotropin releasing factor 1alpha receptor endocytosis and trafficking by beta-arrestins and Rab GTPases. J Neurochem. 2006;96:934–949. doi: 10.1111/j.1471-4159.2005.03603.x. [DOI] [PubMed] [Google Scholar]
- 37.Hirota Y, Kuronita T, Fujita H, Tanaka Y. A role for Rab5 activity in the biogenesis of endosomal and lysosomal compartments. Biochem Biophys Res Commun. 2007;364:40–47. doi: 10.1016/j.bbrc.2007.09.089. [DOI] [PubMed] [Google Scholar]
- 38.Seachrist JL, Anborgh PH, Ferguson SS. beta 2-adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases. J Biol Chem. 2000;275:27221–27228. doi: 10.1074/jbc.M003657200. [DOI] [PubMed] [Google Scholar]
- 39.Dinneen JL, Ceresa BP. Continual expression of Rab5 (Q79L) causes a ligand-independent EGFR internalization and diminishes EGFR activity. Traffic. 2004;5:606–615. doi: 10.1111/j.1398-9219.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 40.Shi A, Sun L, Banerjee R, Tobin M, Zhang Y, Grant BD. Regulation of endosomal clathrin and retromer-mediated endosome to Golgi retrograde transport by the J-domain protein RME-8. EMBO J. 2009;28:3290–3302. doi: 10.1038/emboj.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sonnichsen B, DeRenzis S, Nielson E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5 and Rab11. J Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 43.Del Conte-Zerial P, Brusch L, Rink JC, Collinet C, Kalaidzidis Y, Zerial M, Deutsch A. Membrane identity and GTPase cascades regulated by toggle and cut-out Switches. Mol Syst Biol. 2008;4:206. doi: 10.1038/msb.2008.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poteryaev D, Datta S, Ackema K, Zerial M, Spang A. Identification of the switch in early-to-late endosome transition. Cell. 2010;141:497–508. doi: 10.1016/j.cell.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Galet C, Min L, Narayanan R, Kishi M, Weigel NL, Ascoli M. Identification of transferable two-amino-acid motif (gt) present in the c-terminal tail of the human lutropin receptor that redirects internalized g protein-coupled receptors from a degradation to a recycling pathway. Mol Endocrinol. 2003;17:411–422. doi: 10.1210/me.2002-0161. [DOI] [PubMed] [Google Scholar]