Abstract
The prefrontal cortex undergoes dramatic, sex-specific maturation during adolescence. Adolescence is a vulnerable window for developing mental illnesses that show significant sexual dimorphisms. Gestational stress is associated with increased risk for both schizophrenia, which is more common among men, and cognitive deficits. We have shown that male, but not female, rats exposed to prenatal stress develop post-pubertal deficits in cognitive behaviors supported by the prefrontal cortex. Here, we tested the hypothesis that repeated variable prenatal stress during the third week of rat gestation disrupts peri-adolescent development of prefrontal neurons in a sex-specific fashion. Using Golgi Cox stained tissue, we compared dendritic arborization and spine density of prelimbic layer III neurons in prenatally stressed and control animals at juvenile (day 20), pre-pubertal (day 30), post-pubertal (day 56), and adult (day 90) ages (N=115). Dendritic ramification followed a sex-specific pattern that was disrupted during adolescence in prenatally stressed males, but not females. In contrast, the impact of prenatal stress on the female PFC was not evident until adulthood. Prenatal stress also caused reductions in brain and body weights, and the latter effect was more pronounced among males. Additionally, there was a trend towards reduced testosterone levels for adult prenatally stressed males. Our findings indicate that, similar to humans, the rat prefrontal cortex undergoes sex-specific development during adolescence, and furthermore that this process is disrupted by prenatal stress. These findings may be relevant to both the development of normal sex differences in cognition as well as differential male-female vulnerability to psychiatric conditions.
Keywords: adolescence, schizophrenia, sex differences, puberty, cognition, estrogen
Introduction
Longitudinal neuroimaging studies have revealed that the prefrontal cortex (PFC) undergoes significant sex-specific changes in volume during adolescence (Lenroot et al., 2007; Raznahan et al., 2010; Raznahan et al., 2011). Differences between the adolescent and adult brain are greatest in the frontal cortex (Sowell et al., 2004), and the post-adolescent reduction in PFC volume has been positively correlated with I.Q. (Shaw et al., 2006). We have previously shown that the rat PFC also undergoes sex-specific, peri-adolescent changes in PFC volume, which are due in part to a late wave of neuronal apoptosis (Markham et al., 2007). Synaptic density in the PFC is also reduced between adolescence and adulthood in both humans and rhesus macaques (Huttenlocher, 1979; Bourgeois et al., 1994). While Golgi studies have made it clear that synaptic pruning occurs in the primate cortex over adolescence (Boothe et al., 1979; Anderson et al., 1995), the finding of continued neuronal apoptosis in the rodent cortex during adolescence (Nuñez et al., 2001; Markham et al., 2007) raises the additional possibility that loss of neurons contributes, at least in part, to the post-pubertal reduction in PFC synapses per unit volume of neuropil that is observed in primates. Although the changes in rat PFC gray and white matter volumes during adolescence mirror what is seen in humans (Markham et al., 2007), it is not known whether neurons in the rat PFC undergo a similar process of peri-adolescent synaptic pruning. Furthermore, although previous studies have documented sex differences in dendritic spine density and/or dendritic complexity in the adult PFC (Kolb and Stewart, 1991; Markham et al., 2001), no study has followed sex-specific dendritic development in the PFC across multiple ages. Potential sex-specific changes in these measures are important to study, because they could contribute to the sex-specific changes in PFC volume and PFC-supported cognition that occur during adolescence in both humans and rodents (Shaw et al., 2006; Lenroot et al., 2007; Markham et al., 2007; Markham et al., 2010).
Sex-specific patterns of peri-adolescent PFC development are also relevant to the neurobiology of psychiatric illnesses. For instance, schizophrenia is more prevalent among men (Aleman et al., 2003; McGrath et al., 2004), it typically emerges in late adolescence (Angermeyer and Kuhn, 1988; Hafner et al., 1998), and PFC pathology is implicated in the cognitive deficits associated with the illness (reviewed by Beneyto and Lewis, 2011). It is possible that estrogen is protective for women, given that illness onset is somewhat earlier for men, and that earlier puberty is associated with delayed onset among girls (Hafner et al., 1998; Cohen et al., 1999; Markham, 2012). Essentially, however, it remains puzzling why a psychiatric illness with neurodevelopmental origins does not fully emerge until adolescence, and why men are more likely to develop this illness than women. An increased understanding of the mechanisms underlying normal brain maturation during adolescence, and how these processes differ between males and females, can contribute to the body of work attempting to address this important question.
Gestational stress has been consistently associated with both impaired cognitive development (Buitelaar et al., 2003; Laplante et al., 2004; Bergman et al., 2007; Entringer et al., 2009) and an increased risk for schizophrenia (reviewed by Koenig et al., 2002; Markham and Koenig, 2011). Some studies confirm that gestational stress-induced cognitive deficits are at least partially mediated by prenatal cortisol exposure (Bergman et al., 2010; Davis and Sandman, 2010). Beyond the link with glucocorticoid exposure, the neurobiological mechanisms underlying the relationship between gestational stress, cognitive impairment, and psychiatric illness are not well understood. Because the majority of excitatory synapses occur on dendritic spines and the plasticity of these structures, including their sensitivity to stress hormones during adulthood, is well established (Markham and Greenough, 2004; McEwen et al., 2012), several studies have used rodent models to test the impact of gestational stress on dendritic complexity and spine density. Prenatal stress alters dendritic complexity and spine density of pyramidal neurons in several brain regions that support cognition (Weinstock, 2011). The greatest number of studies has focused on the hippocampus, where prenatal stress reduces both dendritic complexity and spine density in adult male offspring (Hosseini-Ishiwata et al., 2005; Sharifabad and Hadinedoushan, 2007; Martinez-Tellez et al., 2009; Bustamante et al., 2010; Suenaga et al., 2012). Examination of females and males at other ages has sometimes upheld this pattern (Fujioka et al., 2006; Hayashi et al., 1998; Jia et al., 2010), while other studies have reported either no change in these measures or even increases in spine density, depending on the hippocampal subarea (Martinez-Tellez et al., 2009; Bock et al., 2011; Mychasiuk et al., 2012).
Comparatively few studies have examined the impact of prenatal stress on the PFC. Although these studies have demonstrated sensitivity of PFC neurons to gestational stress in immature animals of both sexes (Murmu et al., 2006; Muhammad and Kolb, 2011; Mychasiuk et al., 2012), it is not known whether prenatal stress exerts a persistent sex-specific impact on dendritic complexity of these neurons. Michelsen et al. (2007) did quantify spine density in adult rats, but found no impact of prenatal stress (although they did report a reduced ratio of mushroom type spines). This study did not include females and was further limited by the fact that the subjects had all been exposed to prior behavioral testing (of an unspecified nature), experience which could have interacted with the effect of prenatal stress to impact spine morphology and/or density. Of the studies to date that have investigated the impact of prenatal stress on PFC neuronal morphology, none has followed development of these neurons over more than one time point, and none has tested whether the sex-specific impact of prenatal stress on dendritic complexity persists into adulthood. (Although one study did examine both sexes during adulthood, for some reason males and females were never compared in the same statistical analysis (Suenaga et al., 2012)). However, information is scant regarding maturation of PFC neurons even under normal developmental conditions. For instance, although significant sex differences exist in both PFC-supported cognition and peri-adolescent changes in PFC gray and white matter volume in both rats and humans (Lenroot et al., 2007; Markham et al., 2007; Christakou et al., 2009; Markham et al., 2010; Rubia et al., 2010), the possibility that PFC neurons may follow sex-specific patterns of development has not previously been examined in any species. From a public health perspective, this is an important question to address, given the striking sex differences that exist for psychiatric conditions known to involve PFC dysfunction, including schizophrenia, depression, and risk for suicide and drug abuse (Aleman et al., 2003; Kessler, 2003; McGrath et al., 2004; Forum on Child and Family Statistics, 2009).
We have previously shown that repeated variable prenatal stress during the third week of rodent gestation reorganizes the hypothalamic-pituitary-adrenal axis, brain development, and behavior of post-pubertal male offspring in ways that are consistent with what is observed in schizophrenia (Kinnunen et al., 2003; Koenig et al., 2005; Lee et al., 2007; Markham et al., 2010). Recently, we demonstrated that male, but not female, rats exposed to prenatal stress develop post-adolescent deficits in cognitive behaviors supported by the PFC, such as working memory and behavioral flexibility (Markham et al., 2010). In rats, the prelimbic area of the medial PFC (area 32) supports many cognitive abilities that are disrupted in schizophrenia, including those we have shown to be disrupted in prenatally stressed adult male rats (Ragozzino et al., 1999; 2002; Holmes and Wellman, 2009; Markham et al., 2010). Based on a number of criteria including cytoarchitectonic features and connectivity with the mediodorsal nucleas of the thalamus, there is general consensus that the rodent medial PFC represents the primate PFC (although rodents do not possess an exact anatomical homologue to the primate dorsalateral PFC) (e.g., Uylings et al., 2003). Therefore, the present study was designed to test the hypothesis that sex-specific, peri-adolescent development of the PFC is disrupted by repeated variable prenatal stress. We chose to examine PFC pyramidal neuron morphology because sex-specific patterns of PFC pyramidal neuron maturation have not previously been examined in any species, and because these neurons consistently show abnormalities in schizophrenia (Garey et al., 1998; Glantz and Lewis, 2000; Kalus et al., 2000; Broadbelt et al., 2002; Black et al., 2004).
Materials and Methods
Subjects
Male and female Sprague-Dawley rats, exposed either to prenatal stress (PS) or control conditions, were examined at one of four postnatal ages: day 20, 30, 56, or 90. Our selection of ages was based on their relationship to the hormonal events of rodent puberty, as reviewed by Ojeda and Urbanski (1994), as well as their comparison with human stages of development, which has also been nicely reviewed elsewhere (Andersen, 2003; Sisk and Zehr, 2005). Because the age of puberty onset is more variable in humans compared to rats and moreover has been occurring at increasingly younger ages over the last century (DiVall and Radovick, 2008), we list comparisons with human stages of development rather than exact ages in years. For both humans and rats, puberty is defined as the point at which sexual maturity is reached, whereas adolescence is defined more broadly as the period of time between childhood and adulthood. Postnatal day 20 is a juvenile and pre-adolescent timepoint because it precedes the rise of serum gonadal hormones; it corresponds roughly to young childhood in humans. Serum gonadal hormones begin to rise around postnatal day 25 but day 30 is still prior to puberty in both sexes, so we refer to the period between days 20 and 30 as early adolescence. We refer to the period between days 30 and 56 as late adolescence (relative to the earlier period between days 20–30) because it encompasses the onset of puberty for both females (defined as first estrus and marked by vaginal opening, occurs ~day 38) and males (marked preputial separation, occurs ~day 40–45, followed ~10 days later by the appearance of mature spermatozoa in the vas deferens) (Clegg, 1960; Korenbrot et al., 1977; Ojeda and Urbanski, 1994). Day 56 old rats are post-pubertal, and the period between days 56 and 90 is considered comparable to young adulthood in humans.
A total of 115 subjects were included in this experiment, with 6–8 per treatment/sex/age condition (Control males: day 20 (n=8), day 30 (n=6), day 56 (n=7), day 90 (n=8); Control females: day 20 (n=8), day 30 (n=8), day 56 (n=8), day 90 (n=7); PS males: day 20 (n=7), day 30 (n=6), day 56 (n=7), day 90 (n=7); PS females: day 20 (n=7), day 30 (n=7), day 56 (n=8), day 90 (n=6)). Animals were housed in same-treatment/sex/age groups of 2–3 beginning at weaning (day 25). Following the standard for studies of prenatal treatment effects, only one animal per sex/age condition was included from each litter (Holson and Pearce, 1992). Animals were maintained on a 12:12 hour light/dark cycle (lights on 0700). Food (Harlan Teklad 7012) and water were available ad libitum. All procedures conformed to guidelines for animal research established by the NIH, and were approved by the University of Maryland – Baltimore IACUC.
Repeated Variable Prenatal Stress
Timed pregnant females arrived from Charles River Laboratories (Raleigh, NC) on day 2 of gestation and were individually housed. On days 14–21 of gestation, dams were exposed to a repeated variable stress paradigm according to our previously published protocol (Kinnunen et al., 2003; Koenig et al., 2005; Lee et al., 2007; Markham et al., 2010). The stressors used in this paradigm were: (1) restraint for one hour, (2) exposure to a cold environment (4°C) for six hours, (3) overnight food deprivation, (4) prevention of sleep during the light (inactive) portion of the cycle for 90 minutes, (5) 15 minutes of swim stress, and (6) social stress induced by overcrowded housing conditions during the dark (active) phase of the cycle. Two to three stressors were administered daily in a randomized order. Following delivery, litters remained undisturbed until weaning. Some litters contributed offspring to more than one experiment, so the total number of dams contributing offspring to this experiment was 28 (12 PS and 16 Control). Similar to what we have previously shown (Lee et al., 2007; Markham et al., 2010), repeated variable prenatal stress did not impact litter size (Control: 12 ± 0.3 pups, PS: 11.5 ± 0.7) or sex ratio (Control: 1.3, PS: 1.2 males: females).
Radioimmunoassay
Animals were weighed prior to sacrifice by rapid decapitation. Trunk blood was collected, and the serum fraction stored at −80°C until analysis (all samples run in the same assay). Serum 17β-estradiol and total testosterone levels were assayed in animals using Coat-a-Count Radioimmunoassay kits (Siemens Diagnostics).
Golgi Cox Histology
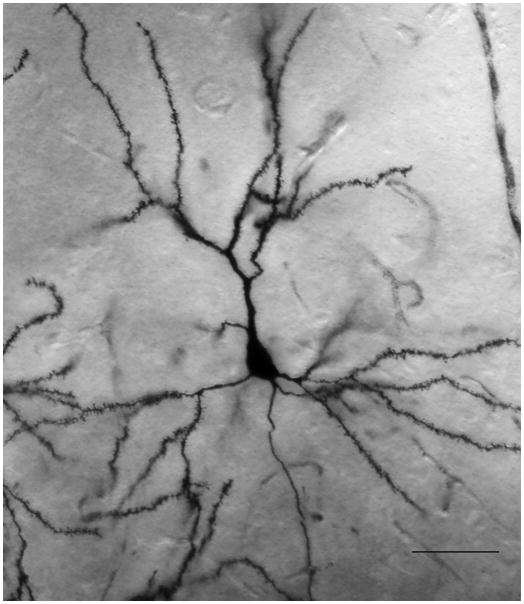
Tissue histology followed our previously published protocol (Markham and Juraska, 2002; Markham et al., 2005). Whole brains were removed, weighed, and immersed in Golgi-Cox solution. Beginning one week later, tissue test slices were taken to verify that neurons were well filled (Figure 1). Brains were coronally blocked at the optic chiasm, and the two parts dehydrated separately (with acetone and ether immersion), then embedded in celloidin and coronally sectioned at 160 μm using a sliding microtome. Free-floating sections were developed according to (Glaser and Van der Loos, 1981) and mounted onto slides. Tissue was coded and anatomical measurements were conducted blind to the animal’s group. Pyramidal neurons in layer III of the prelimbic cortex (Vogt and Peters, 1981; Zilles, 1985) that met the following criteria were selected for measurement: 1) completely filled with the stain, 2) unobscured and 3) not truncated by the section. Neurons were drawn (625X) using a microscope equipped with a camera lucida (see Figure 2A for representative drawings from Control males). Typically eight neurons per animal (minimum of six) were drawn in their entirety. Dendritic complexity was estimated by Sholl ring analysis (Sholl, 1956), in which a grid of concentric rings (spaced 20μm apart) is placed over the drawing of the dendritic field and the number of intersections between dendrites and rings is counted. The number of primary dendrites was also recorded. Distance between the soma and the pial surface was measured for each neuron, and average distance was then compared across groups. Because prefrontal volume continues to change over postnatal development (Markham et al., 2007), a significant effect of age on this measure was anticipated (F3,98= 34.2, p<.01 × 10−12; day 20 (256 ± 5μm) < d30 (290 ± 5μm) p<.000001, day 30 = day 56, day 56 (293 ± 4μm) < day 90 (322 ± 5μm) p<.0001). PS did not influence this measure, however, indicating that neurons were sampled from equivalent laminar depths across treatment groups. To evaluate group differences in the amount of dendritic material according to location, the data were broken down into proximal, middle, and distal regions, based on the average arbor extent observed for adult neurons.
Figure 1.
A Golgi Cox stained pyramidal neuron in the medial PFC, showing multiple dendritic branches (scale bar = 50μm) and dendritic spines (B; scale bar = 5μm).
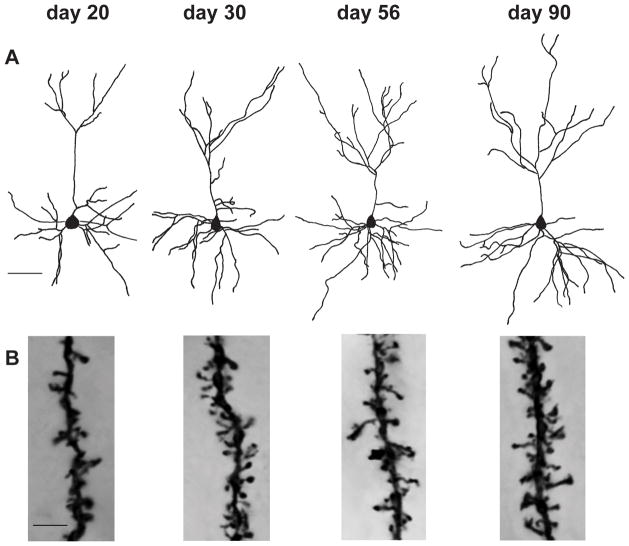
Figure 2.
Reconstructions of Golgi-impregnated pyramidal neurons in layer III of the medial PFC (A), and images showing dendritic spines on segments located on the apical tree of these neurons (B), taken from each of the four Control male age groups. Scale bar = 50μm (A) or =5μm (B).
Dendritic segments (>20 μm long) were selected for measurement of spine density from the same neurons from which dendritic complexity was estimated (16–20 measurements per animal; apical and basilar trees analyzed separately) (see Figure 2B for representative pictures from Control males). Segments were traced at 1562.5X, and the total number of dendritic spines visible along both sides of the segment was counted. Each measurement of spine density was taken from a segment that was approximately 1 μm thick and remained in a single plane of focus. Basilar spine density was measured on 3°–5° centrifugal order terminating dendrites and apical measurements were taken from 1° centrifugal order terminating dendrites. Soma diameter was also measured for each neuron.
Photomicrographs of Golgi impregnated neurons were taken using a Zeiss Axioplan microscope equipped with an Olympus DP70 camera and operated under the guidance of Olympus DP Controller image acquisition software. Images were saved as high resolution tiff files and were cropped to size and adjusted for brightness, contrast, and noise (using despeckling and sharpening filters) using Adobe Photoshop software. Each image of dendritic spines in Figure 2 was compiled from a stack of images taken through regular focal intervals using Helicon Focus software. The multi-paneled Figure 2 was constructed using Adobe Illustrator software.
Statistical Analyses
Average measurements were calculated for each subject, so the N equaled the number of subjects (not the number of measurements). One animal was dropped from the branching portion of the experiment due to an insufficient number of neurons that met criteria, and spine density measurements could not be collected from three subjects because slide thickness prevented desired tissue resolution. Two animals generated insufficient trunk blood to permit RIA analysis. Separate 2×2×4 (treatment, sex, age) Analyses of Variance were conducted for each outcome measure. Analyses examining regional changes in dendritic complexity also included the within subjects factor of dendritic location (2×2×4×3 Analyses of Variance). Due to our previous finding of a sex difference in spine density on neurons in prefrontal layer V (Markham et al., 2001), we chose to analyze development of spine density in layer III in males and females separately here. Rather than conducting post hoc analyses for all possible pairwise comparisons, analysis was limited to the following planned pairwise comparisons: between-age comparisons within a given treatment/sex condition, and between treatment/sex conditions within a given age. Post hoc analyses were conducted using l-matrix contrast statements in SPSS. All analyses were conducted using SPSS statistical software (version 17.0), and p<0.05 was considered significant.
Results
Brain and Body Weight
Both brain (F3,99= 140.3, p<0.01 × 10−33) and body (F3,99= 1936.2, p<0.01 × 10−86) weight increased with age, and males had greater brain (F1,99= 23.1, p<0.00001) and body (F1,99= 597.9, p<0.01 × 10−44) weight compared to females (Table 1). The sex difference in body weight became greater with increasing age (age by sex interaction: F3,99= 193.1, p<0.01 × 10−38), while there was no change in the magnitude of the sex difference in brain weight across ages.
Table 1.
Brain and Body Weights
Brain and body weights increased over adolescence, were greater in males compared to females, and were reduced by prenatal stress. Group means (in grams) ± standard errors of the mean are shown.
| Control Male | PS Male | Control Female | PS Female | |
|---|---|---|---|---|
| Brain Weight T,A,S | ||||
| day 20 | 1.46 ± .03 | 1.39 ± .04 | 1.36 ± .03 | 1.34 ± .11 |
| day 30 | 1.70 ± .03 | 1.62 ± .04 | 1.63 ± .03 | 1.54 ± .03 |
| day 56 | 1.94 ± .07 | 1.94 ± .04 | 1.81 ± .04 | 1.76 ± .04 |
| day 90 | 2.14 ± .05 | 2.07 ± .05 | 2.00 ± .05 | 1.92 ± .08 |
| Body Weight T,A,S,A* S,A* T,S* T | ||||
| day 20 | 59.5 ± 3.5 | 51.8 ± 2.9 | 50.8 ± 2.8 | 59.3 ± 5.7 |
| day 30 | 112.2 ± 2.7 | 106.5 ± 5.3 | 98.0 ± 3.3 | 97.3 ± 3.0 |
| day 56 | 345.9 ± 15.8 | 326.1 ± 16.6 | 216.8 ± 3.7 | 201.1 ± 8.4 |
| day 90 | 527.9 ± 5.1 | 477.1 ± 12.0* | 303.7 ± 7.5 | 281.6 ± 3.1* |
Symbols indicate significant effects: T prenatal stress treatment, A age, S sex, A*S interaction between age and sex, A*T interaction between age and prenatal stress treatment, S*T trend towards an interaction between sex and treatment.
vs. same-sex day 90 control animals: males PS < Control (p<0.001), females PS < Control (p<0.02).
PS reduced brain weight by ~3% in both sexes (F1,99= 5.7, p<0.02) (Table 1). Body weight was also reduced by PS (F1,99= 15.9, p<0.001) (Table 1). This treatment effect interacted with age (F3,99= 5.4, p<0.002), such that differences between Control and PS animals were greatest at day 90 (males: p<0.001, females: p<0.02). There was also a trend towards an interaction between treatment and sex (F1,99= 3.5, p<0.063), such that PS males showed more than twice the reduction in body weight (~9%) that was exhibited by PS females (under 4%), relative to same-sex controls.
Serum Gonadal Steroid Levels
As expected, females had higher circulating levels of estradiol compared to males (F1,97= 18.4, p<0.0001), especially as adults (sex by age interaction F3,97= 6.5, p<0.001) (Table 2). Among Control females, a significant increase in serum estradiol was observed early in adolescence (day 20 < day 30, p<0.03); in fact, at day 20 estradiol levels were actually higher in Control males compared to Control females (p<0.003). Interestingly, PS females also showed higher levels of serum estradiol compared to Control females at day 20 (p<0.004), and also similar to males, they did not show a change in estradiol levels between days 20 and 30. Both Control and PS males showed reduced serum estradiol levels between days 56 and 90, although this reduction only approached significance for Control males (p<0.06).
Table 2.
Serum gonadal steroid levels.
Serum estradiol (pg/ml) and testosterone (ng/ml) levels followed expected sex-specific patterns over adolescence. Group means ± standard errors of the mean are shown.
| Control Male | PS Male | Control Female | PS Female | |
|---|---|---|---|---|
| Estradiol S,A* S | ||||
| day 20 | 17.1 ± 2.4 | 16.7 ± 7.8 | 7.7 ± 1.4 # | 24.6 ± 5.9 |
| day 30 | 17.2 ± 2.8 | 16.3 ± 3.7 | 23.3 ± 6.6 | 24.2 ± 4.2 |
| day 56 | 17.0 ± 4.5 | 20.1 ± 10.0 | 26.3 ± 3.5 | 25.0 ± 5.0 |
| day 90 | 7.6 ± 2.3+ | 11.8 ± 3.1 | 41.5 ± 11.1 | 34.5 ± 7.9 |
| Testosterone A,S,A* S | ||||
| day 20 | .15 ± .08 | .16 ± .09 | .05 ± .02 | .16 ± .16 |
| day 30 | .04 ± .02 | .22 ± .10 | .01 ± .01 | .01 ± .01 |
| day 56 | 1.91 ± .65 | 2.52 ± .46 | .01 ± .01 | .01 ± .01 |
| day 90 | 2.08 ± .48 | 1.38 ± .35 * | .08 ± .04 | .05 ± .04 |
Symbols indicate significant effects: A age, S sex, A*S interaction between age and sex.
day 20 Control females < day 20 Control males (p<0.03) and < day 20 PS females (p<0.004);
day 90 < day 56 Control males (p<0.06);
day 90 < day 56 PS males (p<0.053).
Circulating levels of testosterone increased with postnatal age (F3,97= 19.2, p<0.01 × 10−7). As expected, males had higher testosterone levels compared to females (F1,97= 69.2, p<0.01 × 10−10), but only as adults (sex by age interaction F3,97= 20.4, p<0.01 × 10−7) (Table 2). Interestingly, while both Control (p<0.02) and PS (p<0.001) males showed the expected increase in serum testosterone levels between days 30 and 56, PS males showed a trend towards an uncharacteristic reduction in circulating testosterone levels between days 56 and 90 (p<0.053).
Dendritic Complexity
Apical dendritic tree complexity increased dramatically with age (F3,98= 61.4, p<0.01 × 10−19) (Figures 2, 3). A significant interaction of treatment and sex (F1,98= 4.3, p<0.04) and a more robust three-way interaction of treatment, sex, and age (F1,98= 4.7, p<0.004) were also found to impact apical tree complexity. Post hoc analyses revealed sex-specific patterns of apical dendritic maturation (see Figure 3). In males, normal development was characterized by a gradual increase in dendritic complexity over the entire adolescent period, with no further refinement during young adulthood (Control males: day 20 < day 30 (p<0.04); day 30 < day 56 (p<0.006); day 56 = day 90). Normal female development was characterized by a near doubling of dendritic complexity early in adolescence, followed by a trend towards further growth during young adulthood (Control females: day 20 < day 30 (p<0.00001); day 30 = day 56; day 56 < day 90 (p<0.06)). Females’ more dramatic increase in complexity during early adolescence resulted in a trend towards a sex difference at day 30 (Control females > Control males p<0.053), which did not persist after the period of growth observed in males later in adolescence. In males, PS disrupted the pattern of apical dendritic development such that it no longer resembled control male development, resembling instead the maturation pattern observed in control females (PS males: day 20 < day 30 (p<0.0001); day 30 = day 56; day 56 < day 90 (p<0.08)). Also similar to control females, prenatally stressed males showed greater dendritic complexity than control males at day 30 (p<0.02). Peri-adolescent maturation of the apical tree was not disrupted in females exposed to PS, but this group failed to show continued maturation of apical dendrites during young adulthood (PS females: day 20 < day 30 (p<0.001); day 30 = day 56 = day 90). At day 90, this resulted in lower dendritic complexity for PS females relative to both Control females (p<0.01) and PS males (p<0.03).
Figure 3.
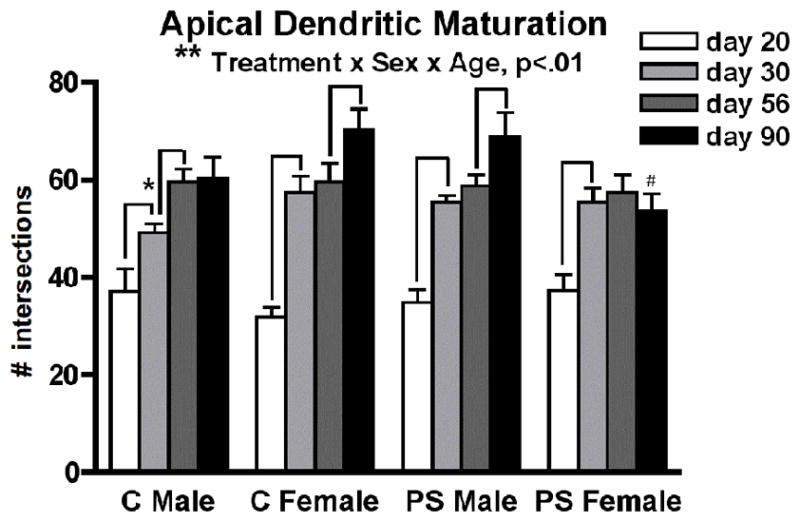
Peri-adolescent maturation of apical dendritic complexity on layer III pyramidal neurons in the medial PFC follows a sex-specific pattern in control (C) animals that is disrupted by prenatal stress (PS) (**treatment by sex by age interaction, p<0.01). Mean number of Sholl ring intersections for each group ± standard errors of the mean are shown. Lines indicate significant age-related changes. *day 30 Control males < Control females (p<0.053) and < PS males (p<0.02). #day 90 PS females < Control females (p<0.01) and < PS males (p<0.03).
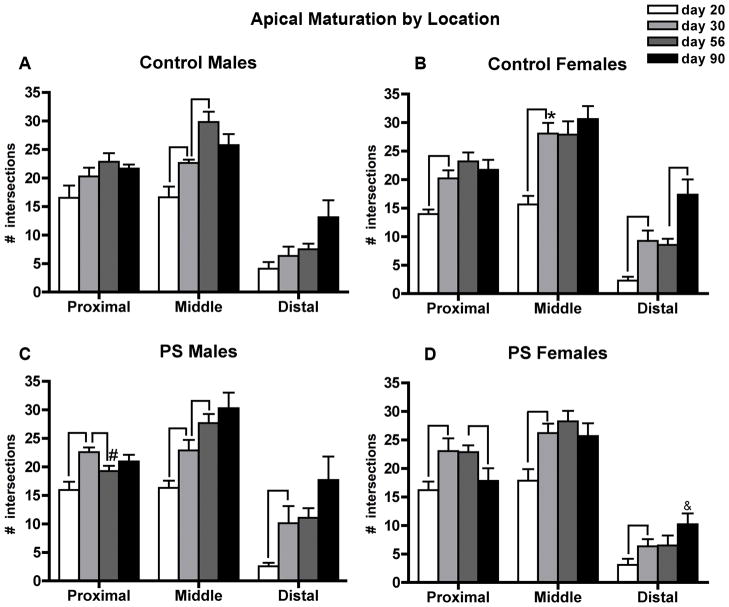
The amount of dendritic material differed according to location along the apical tree (F2,196= 439.2, p<0.01 × 10−70; middle > proximal > distal for all groups). In addition to the interactions that were expected for this analysis given the above results for total apical dendritic complexity (treatment x sex (F1,98= 3.6, p<0.06); treatment x sex x age (F3,98= 4.5, p<0.005)), there was also a significant interaction between location and age (F6,196= 8.6, p<0.01 × 10−5) and a three-way interaction between location, treatment, and sex (F2,196= 4.0, p<0.02). In Control males (Figure 4A), significant growth was limited to the middle portion of the apical tree throughout adolescence (day 20 < day 30 (p<0.02); day 30 < day 56 (p<0.003)), whereas Control females showed dramatic growth in all three locations early in adolescence (day 20 < day 30: proximal p<0.001, middle p<0.001, distal p<0.002), but no further changes in dendritic complexity during late adolescence (day 30 = day 56 for all locations) (Figure 4B). Although all groups showed an increase in distal dendritic complexity between days 56 and 90, this growth was only significant for Control females (day 56 < day 90 (p<0.004)). The sex-specific pattern of apical dendritic tree maturation resulted in Control females having more complexity than control males in the middle portion of the apical tree at day 30 (p<0.03). The pattern for PS males resembled that of Control females early in adolescence, with growth occurring along all locations of the apical dendritic tree (day 20 < day 30: proximal p<0.002, middle, p<0.007, distal, p<0.02) (Figure 4C). However, PS males actually showed a significant reduction in complexity of proximal branches late in adolescence (day 30 > day 56 (p<0.02)), with the result being that at day 56 they had significantly less proximal dendritic complexity than control males (p<0.05). The reduction in proximal branches for PS males during late adolescence was somewhat offset by an increase in complexity in the middle portion of the apical tree during this time (day 30 < day 56 (p<0.055)). The growth pattern of PS females resembled that of Control females throughout adolescence (growth between days 20 and 30 for all locations: proximal p<0.02, middle p<0.005, distal p<0.05; no further growth between days 30 and 56) (Figure 4D). During young adulthood, however, PS females underwent a reduction in proximal dendritic complexity (day 56 > day 90 (p<0.04)) which was somewhat offset by a nonsignificant increase in distal branches. This latter increase was not of the magnitude shown by Control females, which resulted in PS females showing less dendritic complexity in the distal portion of the apical tree at day 90 (p<0.05).
Figure 4.
Apical dendritic ramification according to dendritic location relative to the soma: proximal (20–120μm), middle (140–240μm), and distal (>260μm)) in control (A,B) and prenatal stress (PS) (C,D) animals of both sexes. Mean number of Sholl ring intersections for each group ± standard errors of the mean are shown. Lines indicate significant age-related changes. *day 30 Control females > Control males (p<0.03). # day 56 PS males < Control males (p<0.05). & day 90 PS females < Control females (p<0.05).
Basilar dendritic complexity also increased with age (F3,98=43.7, p<0.01 × 10−15) (Figure 5A). Normal maturation of basilar dendritic complexity followed sex-specific patterns similar to those observed for the apical tree; i.e., gradual maturation for males (Control males: day 20 < day 30 (p<0.02); day 30 < day 90 (p<0.04)), contrasted with females’ pattern of an early adolescent increase followed by later maturation during young adulthood (Control females: day 20 < day 30 (p<0.002); day 30 = day 56; day 56 < day 90 (p<0.03). Both PS males and females showed the normal increase in basilar dendritic complexity between days 20 and 30 (p<0.001 for both), but only males showed a trend for continued dendritic growth after that (days 30 vs 56 p<0.07).
Figure 5.
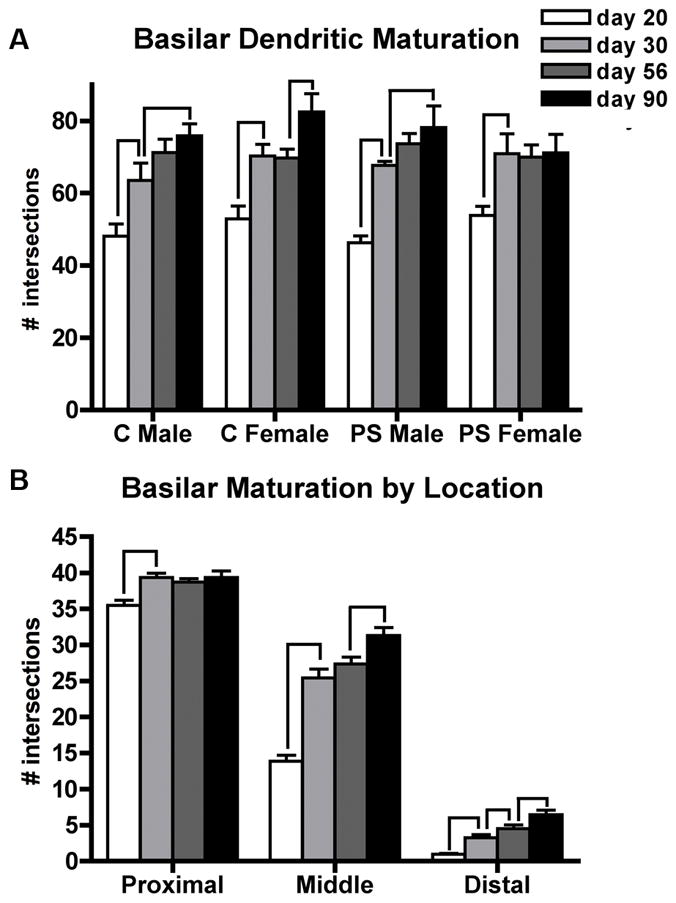
Peri-adolescent maturation of basilar dendritic complexity on layer III pyramidal neurons in the medial PFC follows a sex-specific pattern in control (C) and prenatally stress (PS) animals. Mean number of Sholl ring intersections for each group ± standard errors of the mean are shown. (A) total intersections, (B) intersections by dendritic location: proximal (20–60μm), middle (80–120μm), distal (>140μm). For (B), data from all four sex/treatment groups were collapsed for best presentation of the age x location interaction. Lines indicate significant age-related changes.
The amount of dendritic material differed according to location along the basilar tree (F2,196= 4165.2, p<0.01 × 10−158; proximal > middle > distal for all groups). Age-dependent growth depended on location (location x age interaction (F6,196= 38.0, p<0.01 × 10−27), although there were no further interactions between location and either treatment or sex. Therefore, the data from all four sex/treatment groups were combined to allow comparisons between ages (Figure 5B). Early in adolescence, increases in dendritic complexity were observed at all locations of the basilar tree (day 20 < day 30: proximal (p<0.0001), middle (p<0.01 × 10−8), distal (p<0.000001)). Minimal increases in complexity were observed during late adolescence, which only approached significance in the distal portion of the tree (day 30 < day 56 (p<0.06)). Significant growth continued during young adulthood in both the middle (p<0.01) and distal (p<0.02) portions of the basilar tree.
Number of Primary Basilar Dendrites
There were no main effects or interactions for this measure; all groups had 4–5 primary basilar dendrites.
Soma Diameter
The average soma diameter increased with age (F3,96= 7.9, p<0.04), with the largest increase during late adolescence: 15.1 ± .2 μm (day 20), 15.7 ± .2 μm (day 30), 17.1 ± .2 μm (day 56), 16.6 ± .2 μm (day 90) (mean ± SEM) (day 20 < 30 (p<0.05), day 30 < d56 (p<0.00001), day 56 = day 90). Soma diameter was not affected by sex or PS.
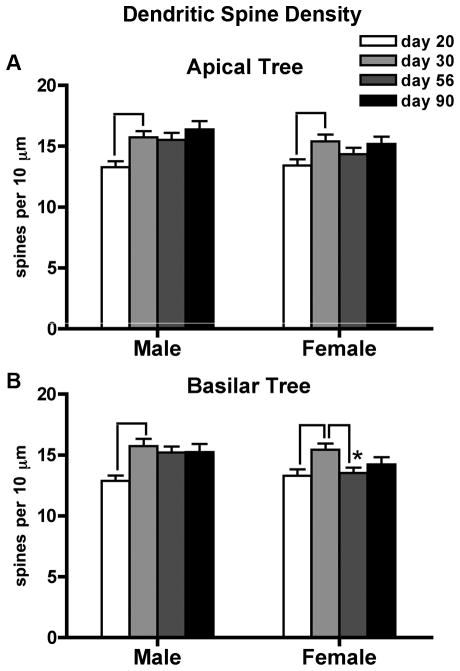
Dendritic Spine Density
PS did not alter dendritic spine density in either sex. For both dendritic tree fields, spine density was robustly impacted by postnatal age (apical: F3,96= 7.2, p<0.001; basilar: F3,96= F=7.0, p<0.001). Spinogenesis on the apical tree was limited to early adolescence for both sexes (day 20 < day 30, females p<.02, males p<0.001) (Figure 6A). On the basilar tree, however, sex-specific patterns of spine density maturation were evident (Figure 6B). Males showed a robust increase in basilar spine density between days 20 and 30 (p<0.001) followed by no further changes in spine density. In contrast, spinogenesis during early adolescence in females (day 20 < day 30, p<0.01) was followed by spine pruning late in adolescence (day 30 > day 56 p<0.01). This resulted in a significant sex difference in basilar spine density during young adulthood (day 56 males > females p<0.02).
Figure 6.
Peri-adolescent maturation of dendritic spine density on the apical (A) and basilar (B) dendritic trees of layer III pyramidal neurons in the medial PFC takes place primarily during early adolescence. Group means ± standard errors of the mean are shown. Lines indicate significant age-related changes. Only females show post-adolescent spine pruning, resulting in an adult sex difference in spine density (*day 56 females < males (p<0.02)).
Discussion
Our findings demonstrate that maturation of pyramidal neurons in the PFC continues very late in postnatal development, and that a major period of dendritic growth and synaptic refinement in this region coincides with adolescence, a period of vulnerability for development of psychiatric illness. Additionally, we show for the first time that normal peri-adolescent maturation of PFC pyramidal neurons follows sex-specific patterns, a finding which may be relevant to sex differences in psychiatric illnesses that emerge during adolescence such as schizophrenia, depression, and drug abuse (Angermeyer and Kuhn, 1988; Hafner et al., 1998; Kessler, 2003; Forum on Child and Family Statistics, 2009). Considerable dendritic ramification of PFC neurons occurred during adolescence in both sexes, but earlier for females compared to males, consistent with their relatively earlier timing of puberty. The location of peri-pubertal refinement also differed between the sexes, with females showing dendritic ramification across all regions of the apical arbor and males showing the most dramatic growth in the middle portion of the apical tree. Both sexes showed pre-pubertal increases in spines, but only females showed evidence of post-pubertal spine pruning. Collectively, these findings indicate that adolescence is an important time for maturation of the PFC, and may suggest a role for pubertal hormones in normal sex-specific PFC development, a possibility that warrants direct testing in future experiments.
Late Maturation of the PFC
The PFC is one of the last structures in the brain to mature. During adolescence, gray matter in most cortical regions shows an inverted U-shaped trajectory, with pre-adolescent increases followed by post-adolescent decreases (Giedd et al., 1999; Lenroot et al., 2007; Raznahan et al., 2010). PFC areas are the last to complete this trajectory (Giedd et al., 1999; Lenroot et al., 2007; Raznahan et al., 2010). Consequently, differences between the adolescent and adult brain are greatest in the frontal cortex (Sowell et al., 2004). Additionally, maturation of cerebral energy metabolism in the PFC lags behind other cortical areas, and patterns of cerebral blood flow in the frontal cortex do not reach those of the adult until late adolescence (Chugani et al., 1987; Chugani and Phelps, 1991; Chiron et al., 1992). Likewise, the period of synaptic pruning occurs later in the human PFC compared to both visual and auditory cortices (Huttenlocher, 1979; Huttenlocher and de Courten, 1987; Huttenlocher and Dabholkar, 1997).
The rat PFC also undergoes significant maturation over adolescence. In addition to the post-adolescent loss of neurons and accompanying reduction in gray matter volume mentioned earlier, there is significant refinement of afferent neurotransmitter systems, reduced projections to the basolateral amygdala, pruning of dopamine receptors, dramatic changes in dopamine modulation of PFC interneurons, and evidence of continued myelination during adolescence (Leslie et al., 1991; Benes et al., 1996; Andersen et al., 2000; Markham et al., 2007; Tseng and O’Donnell, 2007; Cressman et al., 2010; Heng et al., 2011). Our findings indicate that dendritic ramification of layer III pyramidal neurons in the PFC continues in both sexes until postnatal day 90. By comparison, dendritic branching in both the rat visual and somatosensory cortices reaches adult levels much earlier (Juraska and Fifkova, 1979; Juraska, 1982)(Eayrs and Goodhead, 1959; Petit et al., 1988). Therefore, our findings support the notion that, similar to the human PFC, the rat PFC may have a relatively protracted period of maturation compared to other cortical areas.
Peri-adolescent Synaptic Pruning
Post-pubertal synaptic pruning has been well documented in both humans and monkeys, including in the prefrontal, motor, inferotemporal, visual, and somatosensory cortices (Huttenlocher, 1979; Boothe et al., 1979; Huttenlocher and de Courten, 1987; Zecevic et al., 1989; Zecevic and Rakic, 1991; Missler et al., 1993; Bourgeois and Rakic, 1993; Bourgeois et al., 1994; Anderson et al., 1995; Huttenlocher and Dabholkar, 1997; Elston et al., 2009). Findings have been less consistent in other species. In the rat somatosensory cortex, spine density on basilar branches of layer III pyramidal neurons is reduced between late adolescence (43 days) and adulthood (four months) (Wise et al., 1979). However, in layer V, only spines on the apical tree are pruned during adolescence; basilar spine density is stable after day 20 (Galofre and Ferrer, 1987; Petit et al., 1988). In the rat visual cortex, spine density was found to be reduced on the apical shaft of pyramidal neurons in both layers III and V between days 30–90 (Miller, 1981). A subsequent study, however, revealed that the developmental pattern varied within a cortical layer and depended not only on the dendritic tree (apical vs. basilar) but also on dendritic location (bifurcating vs. terminating); i.e., some spine populations showed pre-adolescent synaptogenesis, some showed post-adolescent pruning, some showed both, and still others showed no changes over adolescence (Juraska, 1982). This kind of variability between cell populations is evident in the rabbit cortex as well; some dendritic populations show the pattern of pre-pubertal increase followed by post-pubertal decrease in spine density, whereas others reach adult levels before adolescence (Murphy and Magness, 1984)(McMullen et al., 1988).
In the present study, the most common pattern we found for layer III pyramidal neurons in the PFC was that spine density increased before day 30 and then remained stable thereafter. Only basilar dendrites in females showed a reduction between days 30 and 56, and this effect, though significant, was modest (an approximate 13% reduction). The dendritic population from which we sampled was exclusively terminating dendrites, and the stability of spine density in post-pubertal ages is consistent with previous findings for terminating dendrites in the rat visual cortex (Juraska, 1982). Combined with our finding that layer III PFC neurons continue to ramify rather than retract over adolescence, our data indicate that excitatory synapses are not pruned from the terminating dendrites of these cells. However, given the significant variability in the pattern of effects for different dendritic locations that has previously been observed, the possibility remains open that spines on neurons in another cortical layer within this region, or indeed on bifurcating branches or along the apical shaft of these same cells, could undergo pruning during adolescence.
Sex Differences in Dendritic Maturation in the PFC
Some of the most interesting findings to emerge from this study involve sex differences. Rather than manifesting as adult sex differences in total dendritic complexity or spine density, the most obvious sex differences in layer III PFC neurons emerged in the pattern of dendritic ramification during adolescence. Considerable dendritic ramification of PFC neurons occurred during adolescence in both sexes, but the most intense period of growth occurred earlier for females compared to males. Furthermore, only females showed continued significant dendritic growth during adulthood, and this took place on both apical and basilar trees. For males, there was a gradual increase in dendritic complexity between days 30 and 90 but the comparison between days 56 and 90 was not significant for either the apical or basilar tree. The absence of sex differences in either dendritic complexity or spine density during adulthood is somewhat surprising, given our previous finding that neurons in layer V of the male rat PFC are more complex than those of females and have greater spine density (Markham et al., 2001). Because layer II/III neurons in the prelimbic cortex have previously been reported to be equivalently complex between males and females during adulthood (Kolb and Stewart, 1991), it appears that neurons in layer V of the PFC show greater male-female differences in size during adulthood than neurons in layer III. Although estimates of dendritic complexity are equivalent for adult males and females, the impact of sex was reflected in other ways. Consider that, in addition to the differences in timing, the location of refinement also differed between the sexes, with females showing dendritic ramification across all regions of the apical arbor and males showing the most dramatic growth in the middle portion of the apical tree. Given the considerable refinement in afferent and efferent connections, as well as neurotransmitter systems, that is occurring in the PFC during adolescence (discussed above), it seems likely that functional differences between male and female layer III neurons exist that cannot be appreciated from the perspective of estimates of aggregate complexity and spine density.
Sex-specific Impact of Prenatal Stress
We also found evidence for sex differences in the vulnerability to prenatal stress, consistent with what we have previously found and what others have reported using different prenatal stress paradigms (Bowman et al., 2004; Richardson et al., 2006; Weinstock, 2007; 2011). Prenatal stress disrupted maturation of the apical dendritic tree during adolescence in males, but not females. Prenatal stress can disrupt the late prenatal (E18–19) testosterone surge (Ward and Weisz, 1980; Ward et al., 2003), so consequently brain regions that are normally masculinized during this time, such as sexually dimorphic nuclei of the hypothalamus and the spinal cord, are disrupted by co-occurring prenatal stress (Anderson et al., 1985; Anderson et al., 1986; Grisham et al., 1991). In contrast to fetal testosterone levels, early postnatal levels of testosterone are not altered by prenatal stress (Ward et al., 2002; Bowman et al., 2004), and our findings indicate that serum levels of testosterone are also normal in prenatally stressed male rats during adolescence. Therefore it is not clear whether sex differences that normally develop postnatally are affected by changes in prenatal testosterone. For instance, sex differences in the medial amygdala which normally develop in the early postnatal period are impervious to the reduction in late prenatal testosterone caused by maternal stress (Kerchner et al., 1995). On the other hand, prenatal stress can mute sex differences in cerebral cortical asymmetries (Fleming et al., 1986). In any case there would appear to be more at work in the prenatally stressed male PFC than simply an impaired process of masculinization, since dendritic development in these animals showed some patterns not observed in controls of either sex (such as loss of proximal dendrites).
Females exposed to prenatal stress showed normal patterns of development during adolescence, but during adulthood failed to show the continued dendritic ramification observed in control females. Although speculative, this could be relevant to the delayed onset for schizophrenia that is observed for women (Hafner et al., 1998). Some evidence suggests that estrogen may be protective against schizophrenia, and that women with the illness suffer from hypoestrogenism; similarly, men with schizophrenia often show lower than normal levels of testosterone (reviewed in Markham, 2012). Our findings support a potential role for prenatal stress in the latter finding; as adults, prenatally stressed males showed a trend towards lower than normal testosterone levels. Finally, prenatal stress resulted in a small but significant reduction in brain weight as well as a reduction in body weight that was evident earlier in males. A similarly small and consistent reduction in brain volume is evident in post-mortem tissue from individuals with schizophrenia, and is also detected in neuroimaging studies of first-episode patients (Harrison et al., 2003; Steen et al., 2006), suggesting both that reduced brain weight is a persistent feature of the illness and that it is not a consequence of antipsychotic drug treatment. Furthermore, reduced body weight during childhood and adolescence is predictive of schizophrenia (Wahlbeck et al., 2001). Therefore, our findings may speak to the neurodevelopmental origins of schizophrenia and the greater male vulnerability to this psychiatric illness.
Our previous work using this paradigm has suggested a greater vulnerability of males to prenatal stress, reflected in phenotypes that are relevant to schizophrenia (reviewed by Markham, 2012). For instance, male rats exposed to prenatal stress show a greater response to amphetamine and have exacerbated deficits in social behavior, compared to prenatally stressed females (Koenig et al., 2005; Markham et al., 2009)(Lee et al., 2007; Markham et al., 2008). We have also found that males are more vulnerable to prenatal stress-induced impairments in cognitive abilities, particularly those supported by the PFC (Markham et al., 2010). Importantly, we have found that prenatal stress-induced behavioral abnormalities either initially appear or are magnified following puberty (Koenig et al., 2005; Markham et al., 2010). In adulthood, stress-induced changes in PFC pyramidal neuron morphology are associated with deficits in PFC-supported tasks (Izquierdo et al., 2006; Liston et al., 2006); therefore, disrupted peri-adolescent maturation of PFC neurons in prenatally stressed males may partially underlie the functional deficits we have observed in post-pubertal males, but not females, exposed to prenatal stress (Markham et al., 2010). Importantly, our previous behavioral work has generally been conducted in 56-day-old animals; therefore, the potential impact of repeated variable prenatal stress at later ages, such as day 90 (when neurons in the female PFC reflect changes), is not known at this time.
Prenatal Stress and Dendritic Morphology in the PFC
A few previous studies have examined the impact of prenatal stress on dendritic complexity in the rat PFC. Murmu and colleagues (2006) reported reduced dendritic complexity in prenatally stressed males, but not females, at 23 days of age, whereas our (20 day old) animals did not show this effect. The cell populations differed between the two studies differed both in terms of PFC subregion and layer; therefore what is most striking is the similarity in the overall pattern of results between the studies, with males being more affected by prenatal stress than females. Kolb’s group (Mychasiuk et al., 2012) examined the same cell population as we did and, similarly, did not find an impact of prenatal stress on branching complexity in juvenile (21 day old) animals. In a very recent study, Suenega et al. (2012) examined three subregions of the adult PFC and reported reduced apical dendritic complexity on several Sholl rings in the prelimbic cortex of the prenatally stressed male. However, each Sholl ring intersection appears to have been analyzed using a separate t-test (resulting in hundreds of individual comparisons, seven of which reached significance), and males and females were never compared in the same statistical analysis, making conclusions from this study intriguing but difficult to interpret.
Our finding that prenatal stress does not have a lasting impact on dendritic spine density in the PFC is consistent with the results of Michelsen et al. (2007) in adult male rats, and the present study extends this finding to females. In contrast, Kolb’s group has recently reported an increase in PFC spine density among juvenile (21 day old) prenatally stressed animals of both sexes (Mychasiuk et al., 2012), while in an earlier study they reported a reduction in this measure for adult (80 day old) animals (Muhammad and Kolb, 2011). Importantly, in addition to the strain of rats used, both the nature and the timing of the stress paradigm used differ greatly between our studies and those conducted by Kolb’s group. Repeated versus variable prenatal stress paradigms are known to induce very distinct behavioral and neuroendocrine phenotypes in offspring (e.g., Koenig, 2006; Richardson et al., 2006), and differences in the timing of an environmental insult during gestation are known to result in widely disparate outcomes for offspring as well (Meyer et al., 2006). Additionally, in our study pregnant dams were shipped from a supplier on gestational day 2, whereas in the above studies they were bred in-house. Although both control and stressed dams were shipped for our study, it is nevertheless possible that an interaction occurred between the early stress of shipping and the later stress administered in our laboratory.
The present study is unique in several important ways. It is the first study to have followed the impact of prenatal stress across more than one postnatal age in any neocortical region. Additionally, the possibility that PFC dendritic maturation may follow sex-specific patterns, under either normal conditions or in response to a developmental insult such as prenatal stress, has not previously been examined in any species. Finally, no prior study has directly tested whether a sex-specific impact of prenatal stress on dendritic complexity in the PFC persists into adulthood. (In the only other study to examine dendritic ramification in the adult PFC, males and females were never compared in the same statistical analysis (Suenaga et al., 2012)).
Summary
In summary, the key novel findings of this study are 1) that PFC neurons undergo significant maturation during adolescence, 2) this process is sex-specific, and 3) prenatal stress disrupts adolescent development of the male, but not female PFC, whereas changes in the female PFC do not emerge until adulthood. Our findings of sex-specific development of the PFC during adolescence compliment the growing pediatric neuroimaging literature, which indicates that the human PFC undergoes sex-specific changes in volume during adolescence (e.g., Sowell et al., 1999; Lenroot et al., 2007). Volumetric changes in the PFC during adolescence have been related to cognitive ability (Shaw et al., 2006). Thus, it is possible that sex differences in PFC neuronal development during adolescence contribute to the development of sex differences in PFC-supported cognition (Christakou et al., 2009; Rubia et al., 2010) as well as sex differences in psychopathology (Kessler, 2003; Forum on Child and Family Statistics, 2009). Furthermore, it is significant that adverse prenatal events such as prenatal stress can alter peri-adolescent development of the PFC, because pathology of this region has been linked to psychiatric illnesses that often emerge during adolescence, including schizophrenia. It is not known why men are more likely to develop schizophrenia than women, but some epidemiological work suggests that a sex difference in the vulnerability to prenatal stress may be partially accountable (van Os and Selten, 1998). In support of this notion, our findings indicate that post-adolescent deficits in PFC-supported cognition (Markham et al., 2010) and, now, peri-adolescent maturation of PFC neurons are disrupted in males but not females exposed to prenatal stress.
Acknowledgments
Grant Support: This work was supported by National Institutes of Health grants K12HD043489 (JM), T32 MH067533 (JM), MH73826 (JK), and P50 MH082999 (Project 1 and Core 3 - JK).
The authors wish to thank Brooke Kanaskie for technical assistance.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Role of Authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data. Study concept and design: JM, JK. Acquisition of data: JM, SM. Statistical analysis and interpretation of data: JM. Drafting of the manuscript: JM. Critical revision of the manuscript for intellectual content: JM, JK, SM. Obtaining funding: JM, JK.
Literature Cited
- Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Archives of general psychiatry. 2003;60(6):565–571. doi: 10.1001/archpsyc.60.6.565. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neuroscience and biobehavioral reviews. 2003;27(1–2):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse (New York, NY. 2000;37(2):167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Anderson DK, Rhees RW, Fleming DE. Effects of prenatal stress on differentiation of the sexually dimorphic nucleus of the preoptic area (SDN-POA) of the rat brain. Brain Res. 1985;332(1):113–118. doi: 10.1016/0006-8993(85)90394-4. [DOI] [PubMed] [Google Scholar]
- Anderson RH, Fleming DE, Rhees RW, Kinghorn E. Relationships between sexual activity, plasma testosterone, and the volume of the sexually dimorphic nucleus of the preoptic area in prenatally stressed and non-stressed rats. Brain Res. 1986;370(1):1–10. doi: 10.1016/0006-8993(86)91098-x. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Classey JD, Conde F, Lund JS, Lewis DA. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67(1):7–22. doi: 10.1016/0306-4522(95)00051-j. [DOI] [PubMed] [Google Scholar]
- Angermeyer MC, Kuhn L. Gender differences in age at onset of schizophrenia. An overview. European archives of psychiatry and neurological sciences. 1988;237(6):351–364. doi: 10.1007/BF00380979. [DOI] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Molloy R, Khan Y. Increased interaction of dopamine-immunoreactive varicosities with GABA neurons of rat medial prefrontal cortex occurs during the postweanling period. Synapse (New York, NY. 1996;23(4):237–245. doi: 10.1002/(SICI)1098-2396(199608)23:4<237::AID-SYN1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Lewis DA. Insights into the neurodevelopmental origin of schizophrenia from postmortem studies of prefrontal cortical circuitry. Int J Dev Neurosci. 2011;29(3):295–304. doi: 10.1016/j.ijdevneu.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, Glover V, O’Connor TG. Maternal prenatal cortisol and infant cognitive development: moderation by infant-mother attachment. Biological psychiatry. 2010;67(11):1026–1032. doi: 10.1016/j.biopsych.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, O’Connor TG, Modi N, Glover V. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(11):1454–1463. doi: 10.1097/chi.0b013e31814a62f6. [DOI] [PubMed] [Google Scholar]
- Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, Vostrikov V, Uranova N, Greenough WT. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. The American journal of psychiatry. 2004;161(4):742–744. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- Bock J, Murmu MS, Biala Y, Weinstock M, Braun K. Prenatal stress and neonatal handling induce sex-specific changes in dendritic complexity and dendritic spine density in hippocampal subregions of prepubertal rats. Neuroscience. 2011;193:34–43. doi: 10.1016/j.neuroscience.2011.07.048. [DOI] [PubMed] [Google Scholar]
- Boothe RG, Greenough WT, Lund JS, Wrege K. A quantitative investigation of spine and dendrite development of neurons in visual cortex (area 17) of Macaca nemestrina monkeys. J Comp Neurol. 1979;186(3):473–489. doi: 10.1002/cne.901860310. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4(1):78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13(7):2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman RE, MacLusky NJ, Sarmiento Y, Frankfurt M, Gordon M, Luine VN. Sexually dimorphic effects of prenatal stress on cognition, hormonal responses, and central neurotransmitters. Endocrinology. 2004;145(8):3778–3787. doi: 10.1210/en.2003-1759. [DOI] [PubMed] [Google Scholar]
- Broadbelt K, Byne W, Jones LB. Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex. Schizophrenia research. 2002;58(1):75–81. doi: 10.1016/s0920-9964(02)00201-3. [DOI] [PubMed] [Google Scholar]
- Buitelaar JK, Huizink AC, Mulder EJ, de Medina PG, Visser GH. Prenatal stress and cognitive development and temperament in infants. Neurobiology of aging. 2003;24(Suppl 1):S53–60. doi: 10.1016/s0197-4580(03)00050-2. discussion S67-58. [DOI] [PubMed] [Google Scholar]
- Bustamante C, Bilbao P, Contreras W, Martinez M, Mendoza A, Reyes A, Pascual R. Effects of prenatal stress and exercise on dentate granule cells maturation and spatial memory in adolescent mice. Int J Dev Neurosci. 2010;28(7):605–609. doi: 10.1016/j.ijdevneu.2010.07.229. [DOI] [PubMed] [Google Scholar]
- Chiron C, Raynaud C, Maziere B, Zilbovicius M, Laflamme L, Masure MC, Dulac O, Bourguignon M, Syrota A. Changes in regional cerebral blood flow during brain maturation in children and adolescents. J Nucl Med. 1992;33(5):696–703. [PubMed] [Google Scholar]
- Christakou A, Halari R, Smith AB, Ifkovits E, Brammer M, Rubia K. Sex-dependent age modulation of frontostriatal and temporo-parietal activation during cognitive control. NeuroImage. 2009;48(1):223–236. doi: 10.1016/j.neuroimage.2009.06.070. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME. Imaging human brain development with positron emission tomography. J Nucl Med. 1991;32(1):23–26. [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Annals of neurology. 1987;22(4):487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- Clegg EJ. The age at which male rats become fertile. Journal of reproduction and fertility. 1960;1:119–120. [Google Scholar]
- Cohen RZ, Seeman MV, Gotowiec A, Kopala L. Earlier puberty as a predictor of later onset of schizophrenia in women. The American journal of psychiatry. 1999;156(7):1059–1064. doi: 10.1176/ajp.156.7.1059. [DOI] [PubMed] [Google Scholar]
- Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, Moore H. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J Comp Neurol. 2010;518(14):2693–2709. doi: 10.1002/cne.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child development. 2010;81(1):131–148. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiVall SA, Radovick S. Pubertal development and menarche. Annals of the New York Academy of Sciences. 2008;1135:19–28. doi: 10.1196/annals.1429.026. [DOI] [PubMed] [Google Scholar]
- Eayrs JT, Goodhead B. Postnatal development of the cerebral cortex in the rat. Journal of anatomy. 1959;93:385–402. [PMC free article] [PubMed] [Google Scholar]
- Elston GN, Oga T, Fujita I. Spinogenesis and pruning scales across functional hierarchies. J Neurosci. 2009;29(10):3271–3275. doi: 10.1523/JNEUROSCI.5216-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Kumsta R, Hellhammer DH, Wadhwa PD, Wust S. Prenatal psychosocial stress exposure is associated with subsequent working memory performance in young women. Behavioral neuroscience. 2009;123(4):886–893. doi: 10.1037/a0016265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming DE, Anderson RH, Rhees RW, Kinghorn E, Bakaitis J. Effects of prenatal stress on sexually dimorphic asymmetries in the cerebral cortex of the male rat. Brain research bulletin. 1986;16(3):395–398. doi: 10.1016/0361-9230(86)90062-6. [DOI] [PubMed] [Google Scholar]
- Forum on Child and Family Statistics; Statistics FoCaF, editor. America’s Children: Key National Indicators of Well-Being. Washington, D.C: US Government Printing Office; 2009. [Google Scholar]
- Fujioka A, Fujioka T, Ishida Y, Maekawa T, Nakamura S. Differential effects of prenatal stress on the morphological maturation of hippocampal neurons. Neuroscience. 2006;141(2):907–915. doi: 10.1016/j.neuroscience.2006.04.046. [DOI] [PubMed] [Google Scholar]
- Galofre E, Ferrer I. Development of dendritic spines in the Vth’s layer pyramidal neurons of the rat’s somatosensory cortex. A qualitative and quantitative study with the Golgi method. Journal fur Hirnforschung. 1987;28(6):653–659. [PubMed] [Google Scholar]
- Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TR, Hirsch SR. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. Journal of neurology, neurosurgery, and psychiatry. 1998;65(4):446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Archives of general psychiatry. 2000;57(1):65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Van der Loos H. Analysis of thick brain sections by obverse-reverse computer microscopy: application of a new, high clarity Golgi-Nissl stain. Journal of neuroscience methods. 1981;4(2):117–125. doi: 10.1016/0165-0270(81)90045-5. [DOI] [PubMed] [Google Scholar]
- Grisham W, Kerchner M, Ward IL. Prenatal stress alters sexually dimorphic nuclei in the spinal cord of male rats. Brain Res. 1991;551(1–2):126–131. doi: 10.1016/0006-8993(91)90922-i. [DOI] [PubMed] [Google Scholar]
- Hafner H, Maurer K, Loffler W, an der Heiden W, Munk-Jorgensen P, Hambrecht M, Riecher-Rossler A. The ABC Schizophrenia Study: a preliminary overview of the results. Social psychiatry and psychiatric epidemiology. 1998;33(8):380–386. doi: 10.1007/s001270050069. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Freemantle N, Geddes JR. Meta-analysis of brain weight in schizophrenia. Schizophrenia research. 2003;64(1):25–34. doi: 10.1016/s0920-9964(02)00502-9. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Nagaoka M, Yamada K, Ichitani Y, Miake Y, Okado N. Maternal stress induces synaptic loss and developmental disabilities of offspring. Int J Dev Neurosci. 1998;16(3–4):209–216. doi: 10.1016/s0736-5748(98)00028-8. [DOI] [PubMed] [Google Scholar]
- Heng LJ, Markham JA, Hu XT, Tseng KY. Concurrent upregulation of postsynaptic L-type Ca(2+) channel function and protein kinase A signaling is required for the periadolescent facilitation of Ca(2+) plateau potentials and dopamine D1 receptor modulation in the prefrontal cortex. Neuropharmacology. 2011;60(6):953–962. doi: 10.1016/j.neuropharm.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neuroscience and biobehavioral reviews. 2009;33(6):773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicology and teratology. 1992;14(3):221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Hosseini-Sharifabad M, Hadinedoushan H. Prenatal stress induces learning deficits and is associated with a decrease in granules and CA3 cell dendritic tree size in rat hippocampus. Anatomical science international. 2007;82(4):211–217. doi: 10.1111/j.1447-073X.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C. The development of synapses in striate cortex of man. Human neurobiology. 1987;6(1):1–9. [PubMed] [Google Scholar]
- Ishiwata H, Shiga T, Okado N. Selective serotonin reuptake inhibitor treatment of early postnatal mice reverses their prenatal stress-induced brain dysfunction. Neuroscience. 2005;133(4):893–901. doi: 10.1016/j.neuroscience.2005.03.048. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26(21):5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia N, Yang K, Sun Q, Cai Q, Li H, Cheng D, Fan X, Zhu Z. Prenatal stress causes dendritic atrophy of pyramidal neurons in hippocampal CA3 region by glutamate in offspring rats. Developmental neurobiology. 2010;70(2):114–125. doi: 10.1002/dneu.20766. [DOI] [PubMed] [Google Scholar]
- Juraska JM. The development of pyramidal neurons after eye opening in the visual cortex of hooded rats: a quantitative study. J Comp Neurol. 1982;212(2):208–213. doi: 10.1002/cne.902120210. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Fifkova E. A Golgi study of the early postnatal development of the visual cortex of the hooded rat. J Comp Neurol. 1979;183(2):247–256. doi: 10.1002/cne.901830203. [DOI] [PubMed] [Google Scholar]
- Kalus P, Muller TJ, Zuschratter W, Senitz D. The dendritic architecture of prefrontal pyramidal neurons in schizophrenic patients. Neuroreport. 2000;11(16):3621–3625. doi: 10.1097/00001756-200011090-00044. [DOI] [PubMed] [Google Scholar]
- Kerchner M, Malsbury CW, Ward OB, Ward IL. Sexually dimorphic areas in the rat medial amygdala: resistance to the demasculinizing effect of prenatal stress. Brain Res. 1995;672(1–2):251–260. doi: 10.1016/0006-8993(94)01378-u. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. Journal of affective disorders. 2003;74(1):5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kinnunen AK, Koenig JI, Bilbe G. Repeated variable prenatal stress alters pre- and postsynaptic gene expression in the rat frontal pole. Journal of neurochemistry. 2003;86(3):736–748. doi: 10.1046/j.1471-4159.2003.01873.x. [DOI] [PubMed] [Google Scholar]
- Koenig JI. Schizophrenia: a unique translational opportunity in behavioral neuroendocrinology. Hormones and behavior. 2006;50(4):602–611. doi: 10.1016/j.yhbeh.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, Hercher E, Brady DL. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behavioural brain research. 2005;156(2):251–261. doi: 10.1016/j.bbr.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Koenig JI, Kirkpatrick B, Lee P. Glucocorticoid hormones and early brain development in schizophrenia. Neuropsychopharmacology. 2002;27(2):309–318. doi: 10.1016/S0893-133X(01)00396-7. [DOI] [PubMed] [Google Scholar]
- Kolb B, Stewart J. Sex-related differences in dendritic branching of cells in the prefrontal cortex of rats. Journal of neuroendocrinology. 1991;3(1):95–99. doi: 10.1111/j.1365-2826.1991.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biology of reproduction. 1977;17(2):298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- Laplante DP, Barr RG, Brunet A, Galbaud du Fort G, Meaney ML, Saucier JF, Zelazo PR, King S. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatric research. 2004;56(3):400–410. doi: 10.1203/01.PDR.0000136281.34035.44. [DOI] [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Prenatal stress generates deficits in rat social behavior: Reversal by oxytocin. Brain Res. 2007;1156:152–167. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie CA, Robertson MW, Cutler AJ, Bennett JP., Jr Postnatal development of D1 dopamine receptors in the medial prefrontal cortex, striatum and nucleus accumbens of normal and neonatal 6-hydroxydopamine treated rats: a quantitative autoradiographic analysis. Brain research. 1991;62(1):109–114. doi: 10.1016/0165-3806(91)90195-o. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26(30):7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA. Sex Steroids and Schizophrenia. Rev Endocr Metab Dis. 2012;13(3):187–207. doi: 10.1007/s11154-011-9184-2. [DOI] [PubMed] [Google Scholar]
- Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron glia biology. 2004;1(4):351–363. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Juraska JM. Aging and sex influence the anatomy of the rat anterior cingulate cortex. Neurobiology of aging. 2002;23(4):579–588. doi: 10.1016/s0197-4580(02)00004-0. [DOI] [PubMed] [Google Scholar]
- Markham JA, Koenig JI. Prenatal stress: role in psychotic and depressive diseases. Psychopharmacology. 2011;214(1):89–106. doi: 10.1007/s00213-010-2035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, McKian KP, Stroup TS, Juraska JM. Sexually dimorphic aging of dendritic morphology in CA1 of hippocampus. Hippocampus. 2005;15(1):97–103. doi: 10.1002/hipo.20034. [DOI] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144(3):961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Markham JA, Mullins SE, Koenig JI. Peri-adolescent maturation of object recognition memory and associative memory is disrupted in male, but not female, rats exposed to prenatal stress. Society for Neuroscience Abstracts. 2009:341.326. [Google Scholar]
- Markham JA, Taylor AR, Shelton S, Brady-Bell D, Koenig JI. Neurobiology of Stress Workshop Abstracts. San Rafael, CA: 2008. The repeated variable prenatal stress paradigm as a rodent model for schizophrenia. [Google Scholar]
- Markham JA, Taylor AR, Taylor SB, Bell DB, Koenig JI. Characterization of the cognitive impairments induced by prenatal exposure to stress in the rat. Frontiers in Behavioral Neuroscience. 2010;4:Article 173. doi: 10.3389/fnbeh.2010.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Ward JE, Juraska JM. Sex differences in the pattern of aging: spine density in the rat primary motor cortex. 2001:101.108. [Google Scholar]
- Martinez-Tellez RI, Hernandez-Torres E, Gamboa C, Flores G. Prenatal stress alters spine density and dendritic length of nucleus accumbens and hippocampus neurons in rat offspring. Synapse (New York, NY. 2009;63(9):794–804. doi: 10.1002/syn.20664. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62(1):3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Saha S, Welham J, El Saadi O, MacCauley C, Chant D. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC medicine. 2004;2:13. doi: 10.1186/1741-7015-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen NT, Goldberger B, Glaser EM. Postnatal development of lamina III/IV nonpyramidal neurons in rabbit auditory cortex: quantitative and spatial analyses of Golgi-impregnated material. J Comp Neurol. 1988;278(1):139–155. doi: 10.1002/cne.902780109. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26(18):4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen KA, van den Hove DL, Schmitz C, Segers O, Prickaerts J, Steinbusch HW. Prenatal stress and subsequent exposure to chronic mild stress influence dendritic spine density and morphology in the rat medial prefrontal cortex. BMC neuroscience. 2007;8:107. doi: 10.1186/1471-2202-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. Maturation of rat visual cortex. I. A quantitative study of Golgi-impregnated pyramidal neurons. Journal of neurocytology. 1981;10(5):859–878. doi: 10.1007/BF01262658. [DOI] [PubMed] [Google Scholar]
- Missler M, Wolff A, Merker HJ, Wolff JR. Pre- and postnatal development of the primary visual cortex of the common marmoset. II. Formation, remodelling, and elimination of synapses as overlapping processes. J Comp Neurol. 1993;333(1):53–67. doi: 10.1002/cne.903330105. [DOI] [PubMed] [Google Scholar]
- Muhammad A, Kolb B. Mild prenatal stress-modulated behavior and neuronal spine density without affecting amphetamine sensitization. Developmental neuroscience. 2011;33(2):85–98. doi: 10.1159/000324744. [DOI] [PubMed] [Google Scholar]
- Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. The European journal of neuroscience. 2006;24(5):1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- Murphy EH, Magness R. Development of the rabbit visual cortex: a quantitative Golgi analysis. Experimental brain research. Experimentelle Hirnforschung. 1984;53(2):304–314. doi: 10.1007/BF00238159. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Gibb R, Kolb B. Prenatal stress alters dendritic morphology and synaptic connectivity in the prefrontal cortex and hippocampus of developing offspring. Synapse (New York, NY. 2012;66(4):308–314. doi: 10.1002/syn.21512. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Lauschke DM, Juraska JM. Cell death in the development of the posterior cortex in male and female rats. J Comp Neurol. 2001;436(1):32–41. [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF. Puberty in the rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2. New York: Raven Press, Ltd; 1994. pp. 363–409. [Google Scholar]
- Pascual R, Ebner D, Araneda R, Urqueta MJ, Bustamante C. Maternal stress induces long-lasting Purkinje cell developmental impairments in mouse offspring. European journal of pediatrics. 2010;169(12):1517–1522. doi: 10.1007/s00431-010-1258-8. [DOI] [PubMed] [Google Scholar]
- Petit TL, LeBoutillier JC, Gregorio A, Libstug H. The pattern of dendritic development in the cerebral cortex of the rat. Brain Res. 1988;469(1–2):209–219. doi: 10.1016/0165-3806(88)90183-6. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19(11):4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. The effects of prelimbic and infralimbic lesions on working memory for visual objects in rats. Neurobiology of learning and memory. 2002;77(1):29–43. doi: 10.1006/nlme.2001.4003. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Lee Y, Stidd R, Long R, Greenstein D, Clasen L, Addington A, Gogtay N, Rapoport JL, Giedd JN. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc Natl Acad Sci U S A. 2010;107(39):16988–16993. doi: 10.1073/pnas.1006025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Lerch JP, Lee N, Greenstein D, Wallace GL, Stockman M, Clasen L, Shaw PW, Giedd JN. Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling. Neuron. 2011;72(5):873–884. doi: 10.1016/j.neuron.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology. 2006;147(5):2506–2517. doi: 10.1210/en.2005-1054. [DOI] [PubMed] [Google Scholar]
- Rubia K, Hyde Z, Halari R, Giampietro V, Smith A. Effects of age and sex on developmental neural networks of visual-spatial attention allocation. NeuroImage. 2010;51(2):817–827. doi: 10.1016/j.neuroimage.2010.02.058. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Sholl DA. The measurable parameters of the cerebral cortex and their significance in its organization. Progress in neurobiology. 1956;(2):324–333. [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Frontiers in neuroendocrinology. 2005;26(3–4):163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- Suenaga T, Yukie M, Gao S, Nakahara D. Sex-specific effects of prenatal stress on neuronal development in the medial prefrontal cortex and the hippocampus. Neuroreport. 2012;23(7):430–435. doi: 10.1097/WNR.0b013e3283529805. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17(5):1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behavioural brain research. 2003;146(1–2):3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- van Os J, Selten JP. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. Br J Psychiatry. 1998;172:324–326. doi: 10.1192/bjp.172.4.324. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Peters A. Form and distribution of neurons in rat cingulate cortex: areas 32, 24, and 29. J Comp Neurol. 1981;195(4):603–625. doi: 10.1002/cne.901950406. [DOI] [PubMed] [Google Scholar]
- Wahlbeck K, Forsen T, Osmond C, Barker DJ, Eriksson JG. Association of schizophrenia with low maternal body mass index, small size at birth, and thinness during childhood. Archives of general psychiatry. 2001;58(1):48–52. doi: 10.1001/archpsyc.58.1.48. [DOI] [PubMed] [Google Scholar]
- Ward IL, Ward OB, Affuso JD, Long WD, 3rd, French JA, Hendricks SE. Fetal testosterone surge: specific modulations induced in male rats by maternal stress and/or alcohol consumption. Hormones and behavior. 2003;43(5):531–539. doi: 10.1016/s0018-506x(03)00061-8. [DOI] [PubMed] [Google Scholar]
- Ward IL, Weisz J. Maternal stress alters plasma testosterone in fetal males. Science (New York, NY. 1980;207(4428):328–329. doi: 10.1126/science.7188648. [DOI] [PubMed] [Google Scholar]
- Ward OB, Ward IL, Denning JH, Hendricks SE, French JA. Hormonal mechanisms underlying aberrant sexual differentiation in male rats prenatally exposed to alcohol, stress, or both. Archives of sexual behavior. 2002;31(1):9–16. doi: 10.1023/a:1014018931977. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochemical research. 2007;32(10):1730–1740. doi: 10.1007/s11064-007-9339-4. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Sex-dependent changes induced by prenatal stress in cortical and hippocampal morphology and behaviour in rats: an update. Stress (Amsterdam, Netherlands) 2011;14(6):604–613. doi: 10.3109/10253890.2011.588294. [DOI] [PubMed] [Google Scholar]
- Wise SP, Fleshman JW, Jr, Jones EG. Maturation of pyramidal cell form in relation to developing afferent and efferent connections of rat somatic sensory cortex. Neuroscience. 1979;4(9):1275–1297. doi: 10.1016/0306-4522(79)90157-x. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Bourgeois JP, Rakic P. Changes in synaptic density in motor cortex of rhesus monkey during fetal and postnatal life. Brain research. 1989;50(1):11–32. doi: 10.1016/0165-3806(89)90124-7. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Rakic P. Synaptogenesis in monkey somatosensory cortex. Cereb Cortex. 1991;1(6):510–523. doi: 10.1093/cercor/1.6.510. [DOI] [PubMed] [Google Scholar]
- Zilles K. The cortex of the rat: a stereotaxic atlas. Berlin: Apringer-Verlag; 1985. [Google Scholar]