Abstract
BACKGROUND
Necrotizing enterocolitis (NEC) is an immature intestinal condition resulting in devastating intestinal inflammation due to unknown mechanisms. Evidence has suggested that intestinal maturation attenuates the severity of NEC and TLR4 has been suggested to play a critical role in its pathogenesis. We investigated whether maturational effects of TLR4 expression in immature colon might contribute to the development of NEC.
METHODS
TLR4 colonocyte expression was detected by immunofluorescence confocal microscopy. Interleukin-6 (IL-6) levels were assayed by an enzyme-linked immunosorbent assay (ELISA).
RESULTS
TLR4 expression was high in fetal colonic epithelium in human and mouse, with earlier gestation having a higher surface/cytoplasm distribution. TLR4 remained high in mouse postnatal day 1 but the surface/cytoplasm distribution was reduced. TLR4 decreased in amount and then was expressed in crypts in the mature human and mouse colon. Hydrocortisone (HC) reduced the surface/cytoplasm distribution of TLR4 in human fetal colon. Elevated IL-6 levels in immature colon after LPS was attenuated by HC in human and mouse.
CONCLUSION
Expression, localization and signaling of TLR4 in colonic epithelium may be developmentally regulated. HC may accelerate the TLR developmental pathway change to an adult type which may account for its impact on TLR4 signaling.
INTRODUCTION
Necrotizing enterocolitis (NEC) is the leading cause of death from gastrointestinal disease in the preterm infant (1). Up to forty percent of afflicted premature infants require intestinal resection with a mortality rate of almost fifty percent and a significant subsequent morbidity (e.g., short bowel syndrome, etc) (2). The approach to management of the infant with NEC has not changed in the past 30 years and the outcome is generally as poor today as it was three decades ago (3). These dismal results in current therapy for NEC highlight the urgent need for a better understanding of its pathogenesis and the importance of establishing novel, new therapies.
It has been suggested that an abnormal response by the premature infant to colonizing intestinal microbiota may contribute to the susceptibility of developing NEC. The incidence and mortality of NEC is highest in premature infants (1, 4, 5) implicating gut immaturity as an additional risk factor. Intestinal maturation is affected by multiple factors such as intrinsic timing, exposure to trophic factors and cytokines in amniotic fluid and the initial interaction with colonizing microbes (6, 7). For example, amniotic fluid and breast milk contain hydrocortisone (HC) that interacts with the gut during the perinatal period to stimulate a rapid transition to an adult enterocyte phenotype as reported with the induction of sucrase and galactosyltransferase and enterocyte plasma membrane maturation in previous studies (6,8, 9) which may attenuate the expression of the disease.
NEC is characterized by a severe inflammation and necrosis of the intestine. Recent evidence has suggested that the nature of the Toll-like receptor 4 (TLR4) expression within epithelium may contribute to the inflammatory response to enteric bacteria resulting in the development of NEC (3, 10). The function of TLR 4 signaling is determined, in part, by the cell in which it is expressed, by its selective use of signal transduction and by its cellular location and trafficking capacity (11-15). Although NEC can cause damage throughout the gut, it primarily evokes inflammation and necrosis in the distal small intestine and colon (4, 5). Unfortunately, the cellular distribution and trafficking of TLR4 in colonic cells is not well understood. Accordingly, in this study we determined the location and degree of expression of TLR4 in immature human and mouse colonocytes and investigated whether a trophic hormone (hydrocortisone [HC]) could affect its distribution and response.
RESULTS
TLR4 expression in human fetal colonic epithelium
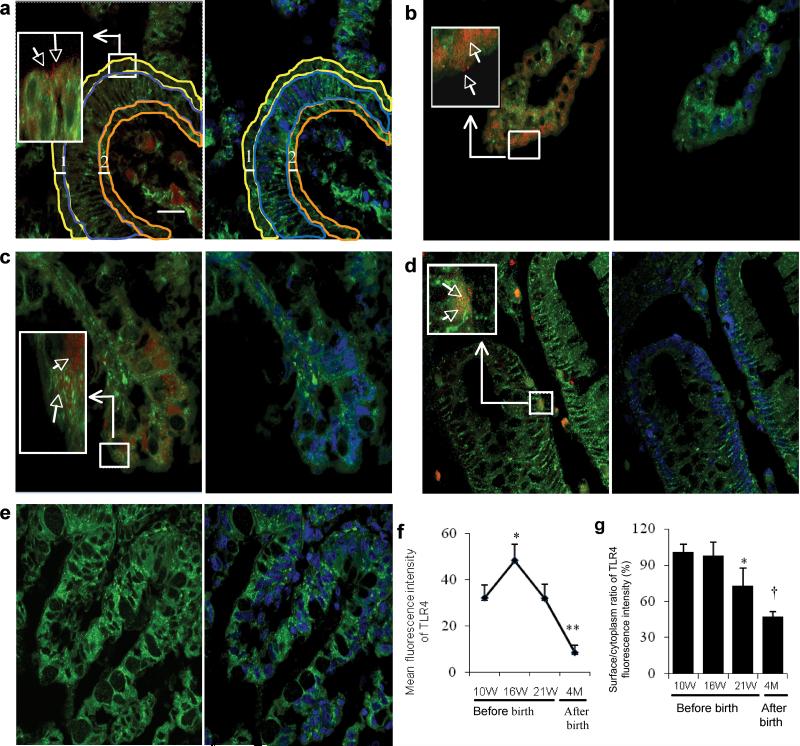
To investigate TLR4 expression in developing human colonic epithelium, we localized and quantitated the receptor at gestational ages 10 (Figure 1a), 16 (Figure 1b), and 21 weeks (Figure 1c) and at 4 months postpartum (considered mature tissue) (Figure 1d) by immunofluorescence confocal microscopy. TLR4 was expressed on the apical surface, within the cytoplasm, at the basal cellular level of fetal colonic epithelium and in the lamina propria from 10-21 weeks. At 4 months postpartum, human colonic TLR4 was scattered in crypt epithelial cells, lamina propria (Figure 1d) and occasionally detected on the apical surface and within the cytoplasm (Data not shown). The quantity of TLR4 in fetal colonic epithelium increased to its highest level at gestational age 16-week and then steadily decreased, reaching its lowest level at 4 months postpartum (Figure 1f). The surface to cytoplasm ratio of TLR4 was highest at gestational age 10 and 16 weeks, but decreased 27% by gestational age 21 weeks and an additional 26% by 4 months postpartum (Figure 1g). These results suggest an increased expression of TLR4 in human fetal colonic epithelium that may be developmentally regulated, perhaps due to an intrinsic timing mechanism. A larger percentage of TLR4 was present on the surface of enterocytes at earlier gestational ages.
Figure 1.
Location and quantity of TLR4 expression in human colonic epithelium from 10 weeks gestation to 4 months postpartum. Confocal microscopy image (a-e) left pane: overlayer of human colon tissues stained with a phycoerythrin (PE) labeled anti-human TLR4 antibody (red) and a fluorescein isothiocyanate (FITC) labeled anti- phospholipid antibody (green) (as a cell membrane marker); right pane: overlayer of phospholipid-FITC and DRAQ5(blue)(as a cell nuclear marker). Magnification × 1000; scale bar-20μm. (a) An area classification of colonic epithelium in a 10 week human colon. The thickness of the surface (line 1) and basal membrane (line 2) both are 3.75 μm. Surface area is surrounded by a yellow line, cytoplasm area is surrounded by a blue line and basal area was surrounded by a dark yellow line. (b) TLR4 expression at gestational age 16 weeks, (c) 21 weeks and (d) 4 months after birth. (e) Negative control for anti-human TLR4 antibody in a human fetal 16 week colonic section. (f) The average mean fluorescence intensity of TLR4 expression within the whole epithelial area (n=3-4) for each group, error bars represent SD, compared to a 10-week gestational age group * p<0.05, **p<0.01). (g) Histogram of surface to cytoplasm ratio of TLR4 fluorescence intensity in colonic sections (n=3-4) for each group, error bars represent SD, compare to a 10-week gestational age group * p<0.05, †P<0.001).
TLR4 expression in mouse colonic epithelium
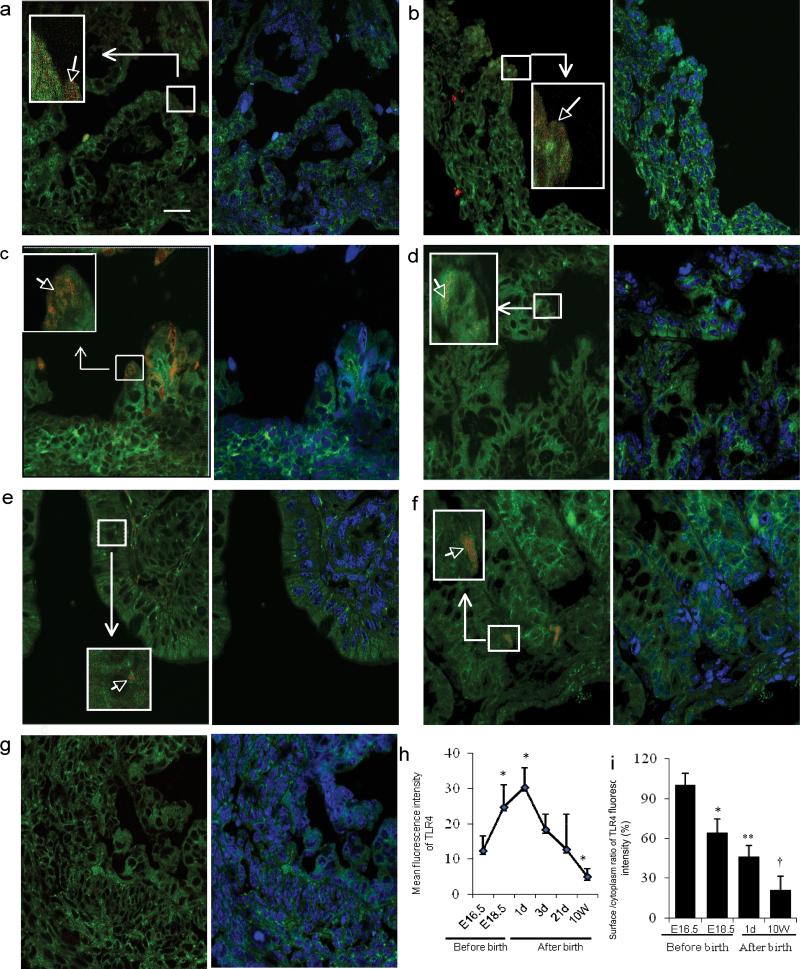
To study the ontogeny of TLR4 expression in mouse colon, we localized and quantitated TLR4 in C57BL/6J mice at embryonic day E 16.5 (Figure 2a), E 18.5 (Figure 2b), and postnatal days 1(Figure 2c), 3(Figure 2d), 21(Figure 2e) and at 10 weeks (Figure 2f) by immunofluorescent confocal microscopy. The quantity of TLR4 was high at E 16.5 and continued to increase until postnatal day 1 when it reached its peak and then decreased from postnatal day 3 to reach its lowest level at 10 weeks (Figure 2h). However, the surface to cytoplasm ratio of TLR4 was highest at E 16.5, and decreased at E18.5 through postnatal day 1 and continued to decrease until it reached the lowest level by 10 weeks after birth (Figure 2i). These results suggest that the quantity and distribution of TLR4 also appears to be developmentally regulated in mouse colonic epithelium as evidenced by the observation showing a higher surface to cytoplasm distribution of TLR4 at the earlier gestational age. However, after birth presumably as the colon was exposed to colonizing microbiota, surface distribution of TLR4 rapidly decreased at the first day of the life and continued to decrease until 10 weeks.
Figure 2.
The ontogeny of TLR4 expression in mouse colonic epithelium during gestation and the postpartum period. Representative confocal image (a-g) left pane: overlayer of mouse colonic tissue stained with a PE labeled anti-mouse TLR4 antibody (red) and counter stained with FITC labeled anti-phospholipids antibody as a cell membrane marker (green). right pane: overlayer of phospholipid-FITC and DRAQ5(blue)(as a cell nuclear marker). The colonic epithelium classification uses the same measurement units as shown in Figure 1-a, but with 1.75 μm as the thickness of surface and basal membranes. Magnification × 1000; scale bar-20μm. (a) Representative image of TLR4 expression E16.5, (b) E18.5 and, (c) postnatal days 1, (d) 3, (e) 21 and 10 weeks (f). (g) Negative control of an anti-mouse TLR4 in an E18.5 mouse fetal colonic section. (h) The average mean fluorescent intensity of TLR4 expression within the entire epithelial area (n=3-4 for each group, error bars represent SD, * p<0.05 vs. E16.5). (i) Histogram of the surface to cytoplasm ratio of TLR4 fluorescent intensity in mouse colonic sections (n=3-4 for each group, error bars represent SD, * p<0.05, **p<0.01, †P<0.001 vs. E16.5).
HC exposure alters the TLR4 response to LPS in fetal enterocytes
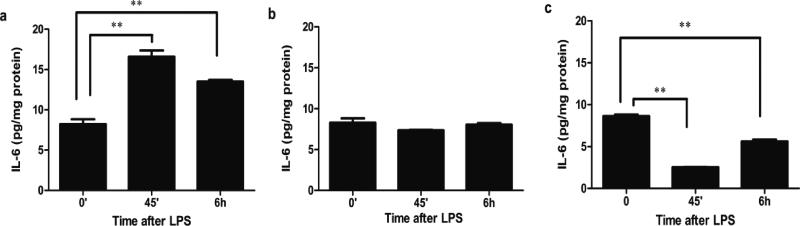
To investigate if the trophic factor HC can alter the TLR4 signaling response to LPS in immature colon, FHC cells were stimulated with or without HC at doses found in amniotic fluid for 5 days and then exposed to LPS (15μg/ml). After 45 minutes, supernatant IL-6 levels significantly increased in the control group and remained high at 6 hours (Figure 3a). In contrast, LPS-stimulation failed to induce an IL-6 response in HC-treated (Figure 3b). We further determined the impact of maturation on LPS-TLR4 signaling by exposure of an adult human primary colonic epithelial cell line (NCM 460) to LPS. After 45 minutes, supernatant IL-6 levels significantly decreased and only partially recovered at 6 hours (Figure 3c). These results suggest that the trophic hormone HC can alter the LPS-induced TLR4 response from an immature to one comparable to a mature pattern.
Figure 3.
Hydrocortisone (HC) induces a shift in the TLR4 response to LPS in human fetal colonic epithelial cells. FHC with or without HC pretreatment and adult human colonic epithelial cells (NCM 460) were stimulated with LPS for 45 minutes and for 6 hours (a) a FHC cell line, (b) FHC pre-treated with HC (1μM) for 5 days, and (c) a NCM 460 cell line. The means and SD are from triplicate wells and are representative of three separate experiments, **p<0.01 vs. 0 minute.
HC exposure to LPS in fetal mouse colon
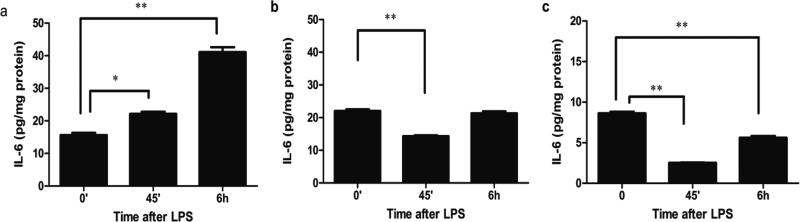
To investigate if HC can affect TLR4 signaling in immature mouse colon, pregnant C57BL/6J mice were subcutaneous injected with HC once at a dose of 50 mg/kg on E 14.5 or with the same volume of PBS. A fetal colonic organ culture was established at E 18.5 and the colonic tissues exposed to LPS. In the PBS-treated control group, supernatant IL-6 levels increased significantly at 45 minutes and continued to increase at 6 hours (Figure 4a). However, in the HC pre-treated group, IL-6 levels decreased significantly at 45 minutes and only recovered to baseline at 6 hours (Figure 4b). To compare the LPS-induced TLR4 reaction in mature mouse colonic tissue, 10 week postpartum C57BL/6J colonic tissues were challenged with LPS in organ culture. After 45 minutes the supernatant IL-6 levels decreased significantly and returned to baseline levels at 6 hours (Figure 4c). These results suggest that the TLR4response to LPS was different between immature and mature colon. Immature colon, expressing a greater quantity and more surface TLR4, had a greater IL-6 response to LPS stimulation than did mature colon which expressed less total and more intracellular TLR4. Furthermore, pre-treatment with HC resulted in the immature colon exhibiting a more mature response to LPS. These observations suggest that with intestinal maturation LPS-induced TLR4 signaling is reduced to a more mature response.
Figure 4.
Hydrocortisone (HC) induced a shift in the response of TLR4 to LPS in mouse fetal colonic organ culture. C57BL/6J fetal and adult mouse colonic tissues were incubated with LPS for 45 minutes and for 6 hours and IL-6 levels measured in the supernatant (a) E 18.5, (b) E18.5 from a mother treated with HC, and (c) 12 week adult mouse colonic tissues. The mean and SD are from triplicate wells, and are representative of three separate experiments, *p<0.05, **p<0.01vs.0 minute.
HC effect on the surface to cytoplasm TLR4 ratio in human fetal colonic tissue
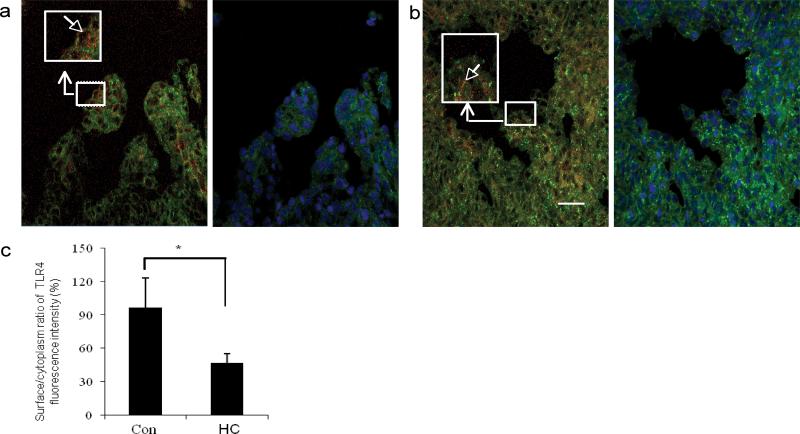
To investigate if HC reduced LPS-induced TLR4 signaling by affecting the surface TLR4 expression, human fetal colonic tissues at 16 weeks gestation (n=3) were exposed to PBS (Figure 5a) or HC (Figure 5b) in organ culture. After 20 hours, the surface to cytoplasm distribution of TLR4 was noted to be significantly decreased in HC treated organ culture (Figure 5c). This observation suggests that the HC effect appears to reduce the surface to cytoplasm ratio of TLR4 and may account for the reduction of the LPS- induced TLR4 signaling in immature colonic epithelial cells exposed to trophic factors in utero.
Figure 5.
Hydrocortisone (HC) reduces surface TLR4 expression in human fetal colonic epithelium. Human 16 week fetal colonic tissues incubated for 2 hours and then stimulated with HC or not for 20 hours. TLR4 expression was detected by the methods described. Original magnification ×1000; scale bar-20 μm. (a) TLR4 expression in control colonic sections. (b) HC pre-treated colonic sections. (c) Histogram of the surface to cytoplasm ratio of TLR4 fluorescent intensity in control and HC pre-treated colonic sections. The results represent the mean±SD, n=3, *p<0.05 vs. control group.
HC effects on the surface to cytoplasm ratio of TLR4 in human fetal colonic cells
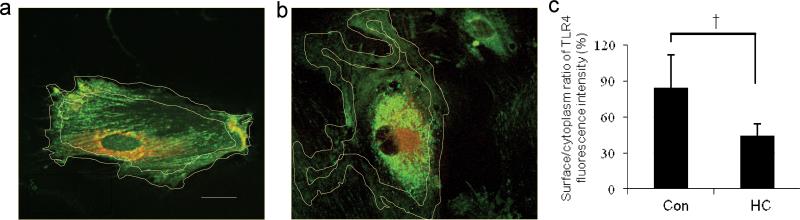
To provide further evidence that HC can reduce the surface to cytoplasm ratio of TLR4 in immature colon, FHC cells were exposed to PBS (Figure 6a) or HC (Figure 6b). After 5 days, the surface to cytoplasm ratio of TLR4 was noted to be significantly reduced in HC treated cells (Figure 6c) suggesting that this response may account for its effect in reducing LPS- induced TLR4 signaling.
Figure 6.
Hydrocortisone (HC) reduces surface to cytoplasm ratio of TLR4 in fetal human colonic epithelial cells. Original magnification ×1000; scale bar-20 μm measured as previously described. (a) Control FHC cells. (b) HC pre-treated FHC cells. (c) Histogram of the surface to cytoplasm ratio of TLR4 fluorescence intensity in control and HC pre-treated FHC cells, results represent a mean±SD, n=10, †p<0.001 vs. control group.
DISCUSSION
In this study, we have systematically examined TLR4 expression in fetal and neonatal human and mouse colonic epithelium. Our observations by morphologic quantitation and by a functional TLR4 response to LPS stimulation suggests that the TLR response to LPS stimulation appears to be developmentally regulated. Further support of this conclusion comes from the effect of a trophic hormone, hydrocortisone (HC, (known to be present in both amniotic fluid and breast milk) on the surface to cytoplasmic ratio of TLR4 and a functional response of TLR4 to LPS stimulation in human fetal colonic tissue and a colonic cell line. In these experiments exposure to HC (moved to results section on p.6-second paragraph line 2), resulted in maturation of TLR4 expression and function to that seen in the mature colon.
Since greater than 90% of NEC cases occur in premature infants weighing less than 1500 g and involves severe inflammation and necrosis, principally of the distal small intestine and colon, it is generally assumed that a major risk factor is the interaction of colonizing bacteria with the immature intestine (1, 2). In studies from this laboratory, using intestinal models of fetal human intestine (16), we have reported that unlike mature enterocytes, the immature enterocyte responds excessively to an exogenous (LPS) and endogenous (IL-1β) stimulus (17, 18). More recently, we have reported that an immature expression of innate immune response genes may contribute to this excessive inflammatory response (19). As part of these studies, we have reported an increased expression of TLR4 in immature human fetal intestine. This study was designed to determine the developmental role of TLR4 expression and its response to LPS stimulation in human and mouse colonocytes. In a previous publication, we have reported for the first time that TLR4 was expressed on the basal lateral surface of a human primary fetal small intestinal cell line (H4 cells) and transcription was upregulated by inflammation (14).
Since that observation was published, other investigators have provided evidence implicating TLR4 in the pathogenesis of NEC. The Hackam group have reported that the expression of TLR4 is significantly greater in the premature mouse and human small intestine compared with their full-term counterpart and the expression of TLR4 is significantly elevated under conditions that are particularly relevant to the pathogenesis of NEC, namely the presence of hypoxia and exogenous bacteria expression of LPS (15,20-22). In premature infants, TLR4 signaling within the small intestinal epithelium has been shown to regulate apoptosis, proliferation and migration of enterocytes, the differentiation of goblet cells, and to reduce microcirculatory perfusion conditions, which collectively contribute to the development of NEC (3,12-17, 19-23). Furthermore, the Caplan research group has shown that a TLR4 knockout mouse model cannot develop experimental necrotizing enterocolitis (24) suggesting a prominent role for this receptor and its signaling molecules in the pathogenesis of this disease. Despite these observations, the expression of TLR4 in developing colon has not been extensively studied. Accordingly, in this study we have shown that fetal and early neonatal expression of TLR4 is much higher than in mature intestine. Furthermore, the earlier fetal colonic specimens have a higher surface to cytoplasmic ratio. We believe that this developmental expression of TLR4 in colonic cells likely contributes to the increased IL-6 response to LPS stimulation between immature and mature colon. Since the intrauterine environment is free of bacteria, the increased surface expression of this receptor may serve another function, e.g., as a developmental regulator in a manner similar to that which exists in the Drosophila.Toll was first discovered in Drosophila as a gene that controled the dorsal-ventral axis of the developing embryo. Subsequently, TLR4 in the more mature Drosophila was noted to have an innate immune function. However the functional significance of TLR4 other than for innate immunity in human intestine remains largely unknown (25). It is possible that TLR4 in human fetal intestine may have another function.
We know that the gastrointestinal tract in the extrauterine environment downregulates the transcription of TLR4 and the receptor is internalized to minimize unnecessary inflammation (12,25). To prepare for full term delivery, the fetus in the later stages of gestation is exposed to trophic factors in amniotic fluid which cause maturation of the intestine This deleted part has been combined into intoduction p.6 Previous observations(6,8,9,26) suggest that HC affects intestinal maturation at multiple levels. Thus we hypothesized that HC may play a role in colonic TLR4 expression. To address this hypothesis we treated human fetal colon tissues with HC at gestational age 16 weeks leading to the reduction of the TLR4 surface to cytoplasm ratio. A similar result was observed in a fetal human colonic epithelial cell line (FHC). These observations suggest that HC affects the regulation of TLR4 expression causing the immature colon to express a mature colonocyte phenotype.
It has been suggested that TLR4 location and cellular trafficking affects its function (27, 28,29). TLR4 engages two distinct adaptor proteins: MyD88 which is recruited by TIRAP and elicits the production of proinflammatory cytokinesand TRIF which is recruited by the adaptor TRAM and activates the production of type 1 interferon as well as proinflammatory cytokines. Kagan et al (13) have shown that TLR4 signals through TIRAP-MyD88 and TRAM-TRIF sequentially rather than simultaneously. Their data indicate that endocytosis of TLR4 terminates the initial phase of MyD88-dependent signaling and heralds the start of a second phase of TRIF - dependent signal transduction from TLR4 molecules located in endosome activated IRF3 for production of type 1 interferon (11-13). These reports suggest that the TLR4 activity is closely related to its location. Based on the difference of regional and spatial localization of TLR4 in immature and mature colon, we compared the pro-inflammatory response of TLR4 in human and mouse colon and found that with both LPS induced IL-6 induction was only observed in the immature colon which expressed high levels of TLR4 and more surface expression. In addition, IL-6 induction can be inhibited by pretreatment with HC which alters TLR4 expression from an immature to a mature pattern by reducing the surface to cytoplasm ratio. Our results suggest that the quantity, location of TLR4 and maturation effector HC are important for TLR4 signaling function in the immature colon.
Furthermore, in mouse colon, although the quantity of TLR4 continued to be high until the first day after birth, the surface to cytoplasm ratio of TLR4 was decreased. This result suggests that colonizing microbiota may in part regulate the distribution of TLR4 immediately after birth as has been previously suggested (3,29). Although it is inappropriate to obtain the colonic tissue from infants at day one after birth, our murine model observations might be extrapolated to suggest what happens in humans.
In these experiments, TLR4 expression and its proinflammatory response to LPS may be influenced by fetal age, exposure to trophic amniotic factors and early neonatal colonization. However, NEC is complex and may be caused by multiple additional factors that affect intestinal injury and repair. For example in mature cells TLR4 and an intracellular receptor TLR9 interact to affect proliferation and repair of tissue damage (3). Why the relationship does not exist in premature infants with its effect favoring inflammation must be determined in future studies.
In summary, the quantity and location of TLR4 in colonic epithelium appears to be developmentally regulated by a combination of genetic preprogramming, trophic factors and luminal microbiota which in turn affect TLR4 function in the developing colon. This study for the first time provides insight into TLR4 expression in developing colon and may contribute to a better understanding of TLR4 specific contributions to NEC development and possibly provide a new TLR4 - signaling specific therapeutic approach to preventing NEC.
METHODS
Human intestinal tissue
All specimens were collected with informed consent approved by the Human Studies Committee at the Massachusetts General Hospital (protocol # 1999-P- 003833). Human fetal colonic tissues were obtained from prostaglandin/saline-induced aborted fetuses at gestational ages of 10, 16 and 21 weeks (n= 3-4 per age group). Surgically resected marginal colonic tissues (n=3) were obtained from patients undergoing intestinal resection for clinical indications. Tissues were maintained in 1066 media (Connaught Medical Research Laboratories, CMRL) containing 100 U/ml penicillin and 100 μg/ml streptomycin (17). The fetal large intestine was identified as intestine between the appendix and anus. Tissues were washed with cold phosphate buffered saline (PBS) at 4°C, fixed in 4% paraformaldehyde at 4 °C overnight, then washed with PBS containing 30% sucrose, balanced in the same solution at 4°C overnight and embedded in optimal cutting temperature compound (OCT)(Sakura Finetek USA, Torrance, CA). Frozen sections (5 μm thick) were kept at −20 °C for later use.
Human colonic organ culture
16 week gestational aged human colonic tissues were collected (n= 3), cut into 3 mm pieces and cultured in BD-Falcon Tissue Culture Plates (Becton, Dickinson and Company, Franklin Lakes, NJ) with OptiMEM media (Life Technologies, Grand Island, NY) supplemented with 10 mM HEPES, 2.5 mM glutamine, 20 ng/ml human epidermal growth factor, 10 μg/ml insulin, 4% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin, 2 mM GlutaMAX-I at 37°C with 95% O2, 5% CO2 (16) for 2 hours and then exposed to hydrocortisone (HC) (1 μM) or PBS for 20 hours. Fixation and frozen sections were prepared as described above.
Animals
C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME). All mice were bred and housed in a specific pathogen–free facility. Animals were given water and standard laboratory chow ad libitum. Timed pregnant mice were set up by pairing 10 to 12 week old female mice with proven breeder males just prior to the end of the daily light cycle. The following morning, each female was examined for the presence of an ejaculatory plug in the vagina. When noted, the female was placed in a dated cage and considered pregnant, embryonic day (E)0.5. Pups were delivered by caesarean delivery between day E16.5 and 18.5. Colonic tissues were collected for experiments. In addition viable mice were sacrificed on day 1, 3, 21 and 10 weeks after birth and colon tissues collected. Animal procedures were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care and Use committee (A3596-01).
Prenatal hydrocortisone (HC) treatment
Pregnant C57BL/6J mice were injected subcutaneously (SC) with a single dose of HC (50 mg/kg body weight, n=3) (6) or an equal volume of saline (n=3) at day E14.5 and the fetal colonic tissues obtained at day E18.5 for experimental use.
Mouse colonic organ culture
Mouse fetal colonic tissues were obtained at E 18.5 with or without HC treatment during pregnancy and 10-week old adult mice colonic tissues were cut into 3 mm pieces and cultured under conditions described in the human colonic organ culture section. After 2 hours at 37°C, tissues were stimulated with ultra purified LPS 0111:B4 from Escherichia coli (E.coli) (List Biological Laboratories, Campbell, CA) or PBS for 45 minutes and 6 hours. Supernatants were collected and stored at - 20°C for enzyme-linked immunosorbent assay (ELISA) analysis.
Cell cultures
Normal non-immortalized epithelial human fetal colon cells (FHC) were purchased from American Type Culture Collection (ATCC, Manassas, VA) and maintained in the prescribed media. Briefly, 90% Dulbecco's Modified Eagle Medium: Nutrient mixture F-12 (DMEM:F-12)media (ATCC) and 10% fetal bovine serum (FBS) (Mediatech, Manassa, VA), supplemented with 25 mM HEPESL (Life Technologies, Grand Island, NY); 10 ng/ml cholera toxin (Sigma Aldrich, St. Louis, MO); 0.005 mg/ml insulin (Eli Lilly and Company, Indianapolis, Indiana); 0.005 mg/ml transferrin and 100 ng/ml (equal 0.2 μM) hydrocortisone(HC) (Sigma Aldrich); 100 U/ml penicillin and 100 μg/ml streptomycin (Life Technologies). Cells were maintained at 37°C in 5% CO2, 95% O2 in a humidified incubator. Human adult NCM460 colonocytes derived from normal colon were provided by INCELL (San Antonio, TX) and cultured in M3:Base A ™ (M3A) media (INCELL), supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin. The cells were cultured at 37 °C in 5% CO2, and 95% O2 in a humidified incubator.
LPS and HC cellular exposure
FHC and NCM 460 cells were cultured in 24 well BD-Falcon tissue culture plates (Becton, Dickinson and Company, Franklin lakes, NJ). FHC cells, at 70% confluence, were treated with or without HC (1μM) for 5 days. The cells were then treated with or without 15μg/ml ultra purified LPS 0111:B4 from E.coli for 45 minutes and 6 hours. Supernatants were collected and stored at −20°C for ELISA analysis. NCM 460 cells at 90% confluence were also treated with or without LPS (15μg/ml) for 45 minutes and 6 hours and supernatants were then collected and stored at −20°C for ELISA analysis.
Immunofluorescent staining
The human or mouse colonic frozen sections were washed with PBS and blocked with 3% bovine serum albumin( BSA) in PBS for 1 h. They were then incubated with Phycoerythrin (PE)-conjugated anti-human or mouse TLR4 antibody (1:20) (both eBioscience, San Diego, CA) or with mouse IgG2a kappa isotype antibody (1:20) as a negative control for human samples (eBioscience,) and anti-mouse IgG1 kappa isotype antibody (1:20) (eBioscience) as a negative control for mouse samples, all in 1% BSA containing 0.3% saponin at 37°C for 1 h, then washed 3 times with PBS and counter stained with fluorescein isothiocyanate (FITC) labeled anti phospholipid antibody (1:400) as a cell membrane marker, in 1% BSA containing 0.3% saponin (Life Technologies, Grand Island, NY) at room temperature (RT) for 1 hour. After 3 times washing with PBS, overstained with 1,5-bis {[α-(di-methylamino) ethyl] amino}-4,8-dihydroxyanthracene-9,10-dione (DRAQ5) (1:1000) (Life Technologies) for 20 minutes at room temperature (RT) for nuclear visualisation. Specimens were then washed three times with PBS, mounted with ProLong® Antifade Reagent (Life Technologies) and analyzed with a fluorescent Leica confocal microscope. FHC cells cultured on glass coverslips in 6 well tissue culture plates at 70% confluence were treated with or without HC(1μM) for 5 days, washed with PBS and fixed in 4% paraformaldehyde for 15 minutes at RT, washed with PBS, then using blocking and staining steps as described.
Analysis of TLR4 quantity and location
Total TLR4 (represented by a mean TLR4 epithelial fluorescent intensity) and the surface to cytoplasm ratio of TLR4 were measured by confocal micro-photo analysis with Image J (fiji-win64) software. The scale set up for measurement was based on specific scanning conditions, e.g., distance in pixels. Color channels were used as a measure of fluorescent intensity of TLR4 (PE-TLR4) in red and FITC-labeled antiphospholipid in green as a measure of the cell membranes. Phospholipid staining was used to measure the thickness of cell membrane because the main component of the cell membrane lipid bilayer is phospholipid. Figure 1a depicts in color the thickness of the cell, the cell membrane (yellow line), the basal area (dark yellow line) and the cytoplasm, e.g., the area between cell membrane and basal area markers. The whole colonic epithelial area represents the sum of surface, cytoplasm and basal areas. Total TLR4 was measured by determining fluorescent intensity of the whole epithelial area minus background (BG) from 3 to 4 images (one image/sample) and the surface to cytoplasmic TLR4 ratio was measured by (fluorescent intensity of the surface area minus BG)/(cytoplasm area minus BG)X 100 (n=3 to 4). These methods were also used in mouse colon TLR4 quantitation and localization.
Enzyme-linked immunosserbent assay (Elisa)
Levels of Interleukin-6 (IL-6) were measured in culture supernatants using ELISA kits (R & D system Inc, Minneapolis, MN) according to the manufacturer's instructions. IL-6 was quantified in each supernatant in triplicate. Colorimetric results were read at a wavelength of 450 nm. Values were normalized to total protein in cells or organ cultures. Protein was determined by the bicinchoninic acid protein assay (Pierce, Rockford, IL) modified for 96-well microtiter plates according to the manufacturer's protocol.
Statistical Analysis of Data
Results were expressed as the mean ± SE and were analyzed by a two way ANOVA and with a post-hoc two-tailed unpaired t test. Differences of a P value <0.05 were considered significant (*p<0.05, **p<0.01, †p<0.001).
ACKNOWLEDGMENTS
We express our gratitude to the coordinating medical and research staff at Mucosal Immunology and Biology Research Center, Massachusetts General Hospital for Children, Boston, MA, including special thanks to Dr. Bobby Cherayil for technical support;
Suzzette McCarron for manuscript organization; Meiqian Weng and Kriston Ganguli for coordination and Maureen Garron for administrative support. We also have special thanks to Kathleen Sirois at Brigham and Women's Hospital, Boston, MA for sample collection.
Statement of financial support: This work was supported by grants from National Institutes of Health (NIH) (Bethesda, Maryland, USA): P01DK033506 and P30 DK040561, RO1-HD059126, RO1-HD12437 (W. Allan Walker) and R01 DK08427 (Haining Shi)
Footnotes
Contributions of authorship: Di Meng: contributed to experimental design, experiments and manuscript preparation; Weishu Zhu: helped with experiments and sample collection; Haining Shi: made suggestions for experimental design, and provided technical assistance; Lei Lu: made suggestions for experimental design and technical approach; Vasuki Wijendran: contributed to experiments; Vinber Xu: contributed to morphologic studies; W. Allan Walker: made substantial contribution to concept and design and manuscript preparation.
Competing Interests: The authors have no competing interests.
REFERENCES
- 1.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364:255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grave GD, Nelson SA, Walker WA, et al. New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr Res. 2007;62:510–514. doi: 10.1203/PDR.0b013e318142580a. [DOI] [PubMed] [Google Scholar]
- 3.Afrazi A, Sodhi CP, Hackam DJ. New insights into the pathogenesis and treatment of necrotizing enterocolitis: toll-like receptors and beyond. Pediatr Res. 2011;69:183–188. doi: 10.1203/PDR.0b013e3182093280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosloske AM. Epidemiology of necrotising enterocolitis. Acta Paediatr Suppl. 1994;396:2–7. doi: 10.1111/j.1651-2227.1994.tb13232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beeby PJ, Jeffery H. Risk factors for necrotising enterocolitis: the influence of gestational age. Arch Dis Child. 1992;67:432–435. doi: 10.1136/adc.67.4_spec_no.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nanthakumar NN, Dai D, Meng D, Chaudry N, Newburg DS, Walker WA. Regulation of intestinal ontogeny: effect of glucocoeticoids and luminal microbes on galactosyltransferase and trehalase induction in mice. Glycobiology. 2005;15:221–232. doi: 10.1093/glycob/cwi004. [DOI] [PubMed] [Google Scholar]
- 7.Hooper LV, Bry L, Flk PG, Gordon JL. Host-microbial symbiosis in the mammalian intestine: exploring an internal ecosystem. Bioessays. 1998;20:336–343. doi: 10.1002/(SICI)1521-1878(199804)20:4<336::AID-BIES10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Nanthakumar NN, Young C, Ko JS, et al. Glucocorticoid responsiveness in developing human intestine: possible role in prevention of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G85–G92. doi: 10.1152/ajpgi.00169.2004. [DOI] [PubMed] [Google Scholar]
- 9.Lu L, Bao Y, Khan A, et al. Hydrocortisone modulates cholera toxin endocytosis by regulating immature enterocyte plasma membrane phospholipids. Gastroenterology. 2008;135:185–193. doi: 10.1053/j.gastro.2008.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harju K, Ojaniemi M, Rounioja S, et al. Expression of toll-like receptor 4 and endotoxin responsiveness in mice during perinatal period. Pediatr Res. 2005;57:644–8. doi: 10.1203/01.PDR.0000156212.03459.A9. [DOI] [PubMed] [Google Scholar]
- 11.Chaturvedi A, Pierce SK. How location governs toll-like receptor signaling. Traffic. 2009;10:621–628. doi: 10.1111/j.1600-0854.2009.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watts C. Location, location, location: identifying the neighborhoods of LPS signaling. Nat Immunol. 2008;9:343–345. doi: 10.1038/ni0408-343. [DOI] [PubMed] [Google Scholar]
- 13.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fusunyan RD, Nanthakumar NN, Baldeon ME, Walker WA. Evidence for an innate immune response in the immature human intestine: toll-like receptors on fetal enterocytes. Pediatr Res. 2001;49:589–593. doi: 10.1203/00006450-200104000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Lu P, Sodhi CP, Hackam DJ. Toll-like receptor regulation of intestinal development and inflammation in the pathogenesis of necrotizing enterocolitis. Pathophysiology. 2014;21:81–93. doi: 10.1016/j.pathophys.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanderson I, Ezzell R, Kedinger M, et al. Human fetal enterocytes in vitro: modulation of the phenotype by extracellular matrix. Proc Natl Acad Sci USA. 1996;93:7717–7722. doi: 10.1073/pnas.93.15.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nanthakumar NN, Fusunyan RD, Sanderson I, Walker WA. Inflammation in the developing human intestine: A possible pathophysiologic contribution to necrotizing enterocolitis. PNAS. 2000;97:6043–6048. doi: 10.1073/pnas.97.11.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Claud EC, Lu L, Anton PM, Savidge T, Walker WA, Cherayil BJ. Developmentally-regulated IκB expression in intestinal epithelium and susceptibility to flagellin-induced inflammation. PNAS. 2004;101:7404–7408. doi: 10.1073/pnas.0401710101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nanthakumar NN, Meng D, Goldstein AM, et al. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PLoS One. 2011;6:e17776. doi: 10.1371/journal.pone.0017776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gribar SC, Anand RJ, Sodhi CP, Hackam DJ. The role of epithelial Toll-like receptor signaling in the pathogenesis of intestinal inflammation. JLB. 2008;83:493–498. doi: 10.1189/jlb.0607358. [DOI] [PubMed] [Google Scholar]
- 21.Hackam DJ, Afrazi A, Good M, Sodhi CP. Innate immune signaling in the pathogenesis of necrotizing enterocolitis. Clin DEV Immunol. 2013 doi: 10.1155/2013/475415. Article ID 475415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leaphart CL, Cavallo J, Gribar SC, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007;179:4808–4820. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 23.Richardson WM, Sodhi CP, Russo A, et al. Nucleotide-binding oligomerization domain-2 inhibits toll-like receptor-4 signaling in the intestinal epithelium. Gastroenterology. 2010;139:904–917. doi: 10.1053/j.gastro.2010.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jilling T, Simon D, Lu K, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol. 2006;177:3273–3282. doi: 10.4049/jimmunol.177.5.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abreu MT. Toll-like receptor signaling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nature Rev Immunol. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 26.Bio MC, Martin A, Richard M, Louishot P. Developmental changes in intestinal glycosyl-transferase activities. Pediatr Res. 1987;22:250–256. doi: 10.1203/00006450-198709000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Hornef MW, Frisan T, Vandewalle A, Normark S, Dahlfors AR. Toll-like receptor 4 resides in the golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med. 2002;195:559–570. doi: 10.1084/jem.20011788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zanoni I, Ostuni R, Marek LR, et al. CD14 controls the LPS-induced endocytosis of toll-like receptor 4. Cell. 2011;147:868–880. doi: 10.1016/j.cell.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aberu MT, Vora P, Faure E, Thomas LS, Arnond ET, Arditi M. Decresed expression of toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bactrial lipopolysaccharid. J Immunol. 2001;167:1609–1613. doi: 10.4049/jimmunol.167.3.1609. [DOI] [PubMed] [Google Scholar]